Significance
Human activities can drive both species’ range contractions and expansions. Species’ responses to human activities are suggested to be influenced by their geographic range sizes. Here, we test the idea that human impacts cause range declines in small-range species but promote range expansion of common species, using distribution data for 9,701 plants across China to examine the relation of human activities to the degree to which species fill their climatic potential ranges. We found that narrow-ranged and widespread species indeed exhibited opposing responses to human activities, with their range filling decreasing and increasing with human influence, respectively. These findings suggest that human activities have reduced ranges of narrow-ranged species but expanded those of widespread species, causing biotic homogenization across China.
Keywords: biotic homogenization, land use, plant species distribution, range filling, range size
Abstract
Human activities have shaped large-scale distributions of many species, driving both range contractions and expansions. Species differ naturally in range size, with small-range species concentrated in particular geographic areas and potentially deviating ecologically from widespread species. Hence, species’ responses to human activities may be influenced by their geographic range sizes, but if and how this happens are poorly understood. Here, we use a comprehensive distribution database and species distribution modeling to examine if and how human activities have affected the extent to which 9,701 vascular plants fill their climatic potential ranges in China. We find that narrow-ranged species have lower range filling and widespread species have higher range filling in the human-dominated southeastern part of China, compared with their counterparts distributed in the less human-influenced northwestern part. Variations in range filling across species and space are strongly associated with indicators of human activities (human population density, human footprint, and proportion of cropland) even after controlling for alternative drivers. Importantly, narrow-ranged and widespread species show negative and positive range-filling relationships to these human indicators, respectively. Our results illustrate that floras risk biotic homogenization as a consequence of anthropogenic activities, with narrow-ranged species becoming replaced by widespread species. Because narrow-ranged species are more numerous than widespread species in nature, negative impacts of human activities will be prevalent. Our findings highlight the importance of establishing more protected areas and zones of reduced human activities to safeguard the rich flora of China.
Biodiversity is important in itself as well as for society, providing a variety of ecosystem services, from numerous products and regulation of climate to cultural and even psychological benefits (1). However, human activities have strongly affected not just local biodiversity but also large-scale species distributions, with intensifying impacts across the last centuries and decades due to the exponential increases in the human population size, resource consumption, and technological capabilities (2, 3). Hence, there is an increasing need for understanding the effects of human activities on species distributions, such as in terms of extinction risks (3–7).
Anthropogenic activities can drive both species’ range contractions and expansions. Many species have lost substantial distribution areas due to intensifying land use and associated habitat loss as well as other human activities (3, 4, 8), with substantial numbers even becoming globally extinct (6, 7). About 20% of plant species globally are considered threatened with extinction (9). Human activities have also driven range expansions in many other species, exemplified by the spread of alien species, promoted by global transport (10). However, many regionally native species have also experienced range increases through human-mediated dispersal and the ability to thrive in anthropogenic landscapes (11). These opposing processes, range contraction and expansion, come together to cause biotic homogenization, where biotic assemblages are becoming more taxonomically similar through the loss of rare and distinct species (“losers”) and the expansion of alien or common native species (“winners”) (12, 13). Biotic homogenization has been shown to be a common outcome of land-use intensification and human disturbance (11, 14).
Species differ naturally in range size, with small-range species concentrated in particular geographic areas and potentially deviating ecologically from widespread species (15), for example, having more specialized habitat requirements (16). In a human-dominated world, narrow-ranged, specialist species are more likely to be losers, whereas widespread, generalist species should have a higher probability of being winners (13). By comparing distribution atlases of 736 plant species in the United Kingdom and Estonia surveyed at 2 periods with an interval of about 30 y, Laanisto et al. (4) found that those species with smaller range sizes at the first survey lost higher proportions of their distribution areas. The more specialized bird species in France have also been found to show a more negative response to landscape disturbance and fragmentation (17). Conversely, in the severely disturbed Atlantic rain forest of Brazil, tree species with increasing occurrences between pre-1980 and post-1980 periods tend to be widely distributed (11). The probability of a species becoming naturalized outside its native range has also been shown to be positively related to its native range size and habitat range (18, 19). Still, while it is clear that species’ responses to human activities may be influenced by their geographic range sizes, if and how this happens are still poorly understood.
Although species distributions can be influenced by human activities, their distributions at broad scales are primarily determined by contemporary climate and their dispersal abilities (20). Specifically, a species’ ecological niche and the climatic conditions over the Earth’s surface determine its climatic potential distribution areas. These potential ranges, however, are often not fully occupied due to dispersal limitation as well as biotic interactions (21, 22). Therefore, realized ranges of species are often in disequilibrium with current climate, filling a limited portion of their climatic potential ranges. For example, European tree species are found to occupy collectively 38% of their potential ranges, which in large part can be attributed to postglacial dispersal limitation (23). The degree of range equilibrium with climate determined by natural factors may be distorted by human activities. Range filling (RF; realized/potential range size ratio) of species in a region may increase or decrease due to anthropogenic activities. In eastern North America, distributions of multiple tree species have been shown to be associated with Native American settlements, with their probabilities of presence increased or reduced close to villages and trails (24), likely having led to greater or lower RF.
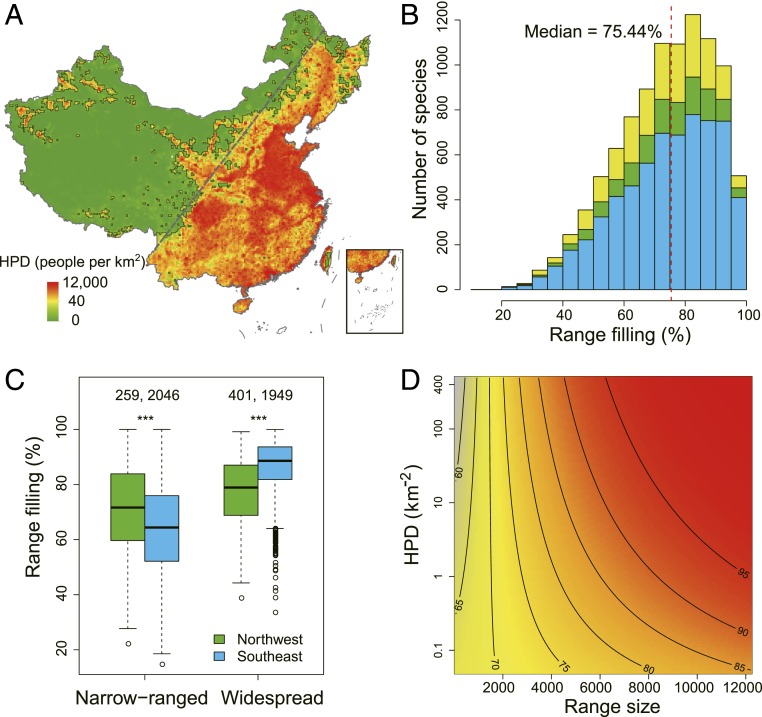
China is one of the most species-rich countries, with a latest estimate of ∼36,000 vascular plants, due to its diverse ecological characteristics and unique evolutionary history (25, 26). In the latest Red List for higher plants in China, however, 3,879 species, or 11% of the assessed species, were identified as threatened (27). China has overall suffered from high human pressures for several millennia (28, 29). Due to the lack of extensive historical distribution data for plants in China, as for most regions in the world, no study has hitherto directly evaluated human impacts on distributions for many plant species at the national scale. Despite the large human population in China, there is a clear national-scale differentiation in human population density (HPD), with most people living in the southeastern part and the northwestern part much more thinly settled (Fig. 1A). The 2 parts are roughly separated by a straight line, known as the Hu Huanyong Line (HHL), stretching from Heihe City (Heilongjiang Province) to Tengchong County (Yunnan Province) (30). The southeastern part only occupies 43% of Chinese terrestrial area but supports 94% of the total human population. Comparing these 2 regions offers an opportunity to investigate human impacts on plant distributions at large scales, given adequate control for environmental differences, with the less intensely settled northwestern part providing a baseline for the extent to which species fill their potential ranges under relatively natural conditions.
Fig. 1.
Human impacts on species range filling of vascular plants in China. (A) HPD across China at the 20 × 20-km resolution. The dashed line indicates the Hu Huanyong Line, which separates China into the northwestern and southeastern parts. Grid cells with low and high HPD (classified by the median, 13.5 people per square kilometer) are separated with black outlines. (B) Histogram of species range filling. Blue and green show those species with >80% of their ranges in the southeastern or northwestern parts, respectively, and yellow shows the remaining species. The red vertical dashed line shows the median of range filling. (C) Comparisons of range filling between the northwestern and southeastern species within the 30% most narrow-ranging species (≤1,145 grid cells) and the 30% most wide-ranging species (≥2,475 grid cells). Numbers above the boxplot show the number of species in each category. ***P < 0.001. (D) Contour plot showing the interaction effect between HPD and range size on species range filling. The range filling in the plot is the predicted values by a beta-regression model while keeping other predictors as their mean observed values.
Here, we assembled distribution data across China at a 20 × 20-km resolution for 9,701 vascular plant species and estimated each species’ climatic potential ranges using species distribution models (SDMs). We then calculated the RF of each species by overlaying its potential range with its observed range. RF was then compared between species that were primarily distributed in the northwestern or southeastern parts of China. We also calculated geographic patterns of grid-cell mean RF (MRF), namely the mean RF of the species occurring in each 200 × 200–km grid cell. Variation in RF across species and space was then modeled against 3 indicators of human activities (HPD; human footprint, HFP; proportion of cropland, cropland) and other potential determinants, such as topography, current climate, and Quaternary climate change. To evaluate whether species’ responses to human activities are influenced by their geographic range sizes, we tested for an interaction effect between range size and human impact factors as well as performed analyses for the 30% most narrow-ranging and 30% most wide-ranging species, respectively. We hypothesize that 1) the RF of narrow-ranged species is reduced, while that of widespread species is increased in southeastern China due to intensive human influence; and 2) variation in RF across species and space is strongly associated with human activities, even after controlling for alternative drivers, with negative relations for narrow-ranged species but positive relations for widespread species.
Results
Range Filling across Species.
Using ensemble SDMs, we estimated that species occupied high proportions of their climatic potential ranges (Fig. 1B). The median RF was 75.4% and the mean was 73.4% (SD 15.7%). There were 870 species (9.0% of total) having RF less than 50%. The threatened species had significantly lower RF (median 66.1%) than nonthreatened species (median 75.5%; SI Appendix, Fig. S1).
Overall, species with over 80% of their distribution ranges in either northwestern or southeastern China filled similar percentages of their potential ranges, with median values of 74.2 and 76.2%, respectively (Fig. 1B). However, narrow-ranged and widespread species showed opposing differences in RF between northwestern and southeastern species. Southeastern species had lower RF than the northwestern species for the 30% most narrow-ranging species but higher RF for the 30% most wide-ranging species (Fig. 1C), consistent with range size-dependent effects of human activities. The above results were confirmed by beta-regression models with consideration of multiple explanatory variables (Table 1 and SI Appendix, Tables S1 and S2), as the interaction term between human impact and range size was positive and larger than the human impact main effect, indicating that human activities have negative impacts on narrow-ranged species but positive impacts on widespread species (Fig. 1D, Table 1, and SI Appendix, Tables S1 and S2).
Table 1.
Beta regression of species range filling against the explanatory variables
| Estimate | SE | z | P value | |
| HPD | 0.041 | 0.014 | 3.06 | 0.002 |
| EleR | 0.175 | 0.011 | 16.22 | <0.001 |
| Anomaly | 0.070 | 0.015 | 4.66 | <0.001 |
| MAT | 0.104 | 0.019 | 5.43 | <0.001 |
| RZ | 0.499 | 0.009 | 58.68 | <0.001 |
| HPD × RZ | 0.086 | 0.007 | 11.60 | <0.001 |
Estimate, standardized regression coefficients; pseudo R2 = 0.344. Mean annual precipitation was excluded by model selection. Anomaly, temperature anomaly since the Last Glacial Maximum; EleR, elevation range within grid cells; RZ, species observed range size.
Geographic Range-Filling Patterns.
Grid-cell MRF of all, narrow-ranged, and widespread species exhibited strong and distinct spatial patterns (Fig. 2). Considering all species, high MRF mainly occurred in southeastern China, whereas low MRF was observed in the northwest, with widespread species showing a similar spatial pattern. Narrow-ranged species, however, showed a divergent pattern, with high MRF mainly occurring in northwestern China. The patterns of grid-cell MRF were different from the geographic patterns of observed and potential range sizes, where high-range sizes were generally observed in higher latitudes (SI Appendix, Fig. S2), showing that the patterns in RF were not simply driven by variation in range size. This independence was also supported by the geographic patterns in the grid-cell mean of the residuals of species RF after controlling for range size, as these were highly correlated with corresponding MRF patterns (Pearson’s r = 0.997 and 0.966 for narrow-ranged and widespread species, respectively; SI Appendix, Fig. S3).
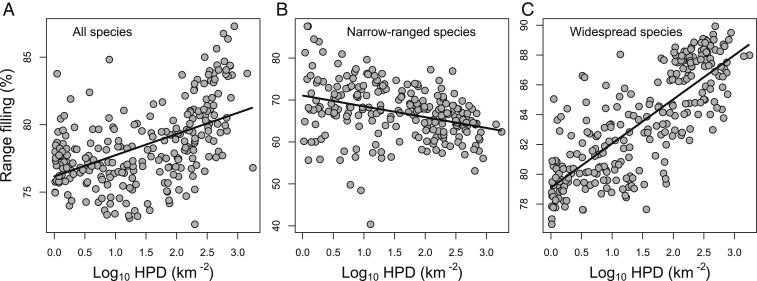
Fig. 2.
Geographic patterns of grid-cell mean range filling for all species (A), the 30% most narrow-ranging species (B), and the 30% most wide-ranging species (C). Grid cells without data are shown in gray.
Both simple regression and multiple regression analyses showed that geographic patterns of MRF had strong associations with human activities, which were even comparable to or stronger than those with natural factors such as topography, current climate, and paleoclimate change (Fig. 3, Table 2, and SI Appendix, Fig. S4 and Tables S3–S5). However, as for the species-level analyses, human activities had opposing effects on narrow-ranged and widespread species, with their MRF decreasing and increasing with the intensity of human activities, respectively (Fig. 3 and SI Appendix, Fig. S4). For narrow-ranged species, human impact factors had the strongest association with MRF among the explanatory variables. For widespread species, human impact factors had strong and positive effects but weaker than current climate. The relations for all species were similar to those for widespread species.
Fig. 3.
Relationships between grid-cell mean range filling and HPD for all species (A), the 30% most narrow-ranging species (B), and the 30% most wide-ranging species (C). The lines are fitted with simple linear regressions. HPD is log10-transformed.
Table 2.
Multiple linear regressions of grid-cell mean range filling of all species, the 30% most narrow-ranging species, and the 30% most wide-ranging species against the explanatory variables and the selected eigenvector-based spatial filters
| Estimate | SE | t | P value | R2 | Partial R2 | |
| All species | ||||||
| Full model | 0.719 | |||||
| HPD | 0.29 | 0.07 | 4.41 | <0.001 | 0.077 | |
| EleR | −0.35 | 0.04 | −7.69 | <0.001 | 0.203 | |
| Anomaly | 0.04 | 0.07 | 0.51 | 0.611 | 0.001 | |
| MAT | 0.22 | 0.10 | 2.25 | 0.025 | 0.021 | |
| MAP | 0.01 | 0.09 | 0.08 | 0.937 | 0.000 | |
| 14 spatial filters | <0.05 | |||||
| Narrow-ranged species | ||||||
| Full model | 0.586 | |||||
| HPD | −0.28 | 0.08 | −3.67 | <0.001 | 0.059 | |
| EleR | 0.08 | 0.05 | 1.55 | 0.123 | 0.011 | |
| Anomaly | 0.23 | 0.10 | 2.29 | 0.023 | 0.024 | |
| MAT | 0.23 | 0.11 | 2.16 | 0.032 | 0.021 | |
| MAP | −0.11 | 0.07 | −1.49 | 0.137 | 0.010 | |
| 11 spatial filters | <0.05 | |||||
| Widespread species | ||||||
| Full model | 0.898 | |||||
| HPD | 0.15 | 0.05 | 3.17 | 0.002 | 0.040 | |
| EleR | −0.13 | 0.03 | −5.16 | <0.001 | 0.100 | |
| Anomaly | −0.10 | 0.04 | −2.52 | 0.012 | 0.026 | |
| MAT | 0.31 | 0.05 | 5.96 | <0.001 | 0.129 | |
| MAP | 0.31 | 0.04 | 7.62 | <0.001 | 0.195 | |
| 8 spatial filters | <0.05 | |||||
Estimate, standardized regression coefficients; R2, R2 of full models; partial R2, partial R2 of each variable in the models. Anomaly, temperature anomaly since the Last Glacial Maximum; EleR, elevation range within grid cells.
Range-Filling Patterns across Plant Growth Forms and Orders.
The opposite RF patterns and associations with human activities between narrow-ranged and widespread species were generally consistent across plant growth forms (SI Appendix, Figs. S5 and S7 and Table S6) and orders (SI Appendix, Figs. S6 and S8 and Table S7). Southeastern species tended to have higher median RF than the northwestern species for widespread species, but lower RF for narrow-ranged species in most species groups (SI Appendix, Figs. S5 and S6). Further, widespread species had consistently positive MRF correlations with indicators of human activities, while these correlations were negative for narrow-ranged species for most species groups (SI Appendix, Figs. S7 and S8 and Tables S6 and S7).
Discussion
Our results suggest that the distributions of vascular plants in human-dominated regions of China are strongly shaped by anthropogenic activities, with narrow-ranged and widespread species experiencing range reductions and expansions, respectively. The lower RF for narrow-ranged species and higher values for widespread species in the human-dominated southeast compared with their counterparts in the less intensely settled northwest can be attributed, at least partly, to human activities. Human impacts on plant distributions are further confirmed by the strong associations between geographic patterns of grid-cell MRF and human activities for both narrow-ranged and widespread species, even after controlling for topography, current climate, paleoclimate, and spatial autocorrelation.
Although environmental conditions differ across the studied regions, a strong association between RF across both species and space and human activities remained even after controlling for major environmental differences. Additionally, RF patterns and associations with human activities were consistent across major plant growth forms and orders, showing that the regions’ contrasting RF patterns cannot be explained from differences in functional or phylogenetic composition. The opposing responses of narrow-ranged and widespread plants to anthropogenic activities may be related to their different sensitivities to human-induced habitat change. Narrow-ranged species may be more vulnerable to land-use change and tend to be excluded from disturbed habitats (31, 32). For example, epiphyte assemblages in young secondary forests in Ecuador are dominated by species with larger geographic ranges and broader ecological niches compared with those in primary forests (32). Furthermore, a recent global multiclade study found more widespread species in assemblages in disturbed habitats compared with natural habitats and also increased abundances of widespread species but reduced abundances of narrow-ranged species (31). This is probably because environmental conditions in the disturbed habitats are not tolerated by many specialist species (32). Additionally, winner tree species in the context of human disturbance are overrepresented by pioneer species because of their high capacity to establish in disturbed habitats (11). These pioneer species generally have larger ranges (15). Further, human-mediated dispersal of useful plants may selectively target widespread species due to factors such as recognition and availability. A recent study on palms in South America indeed found that widespread species are preferentially used by people relative to narrow-ranged species (33).
Although it is widely accepted that species' realized ranges are often in disequilibrium with contemporary climate, there are few studies investigating the degree to which species fill their climatic potential ranges and the underlying determinants (23). Postglacial dispersal limitation and dispersal capacity of species have been found to be important for RF (23, 34, 35). Besides these natural factors, our results showed that human activities can also be important. This shows that the RF metric is useful in the evaluation of human impacts on large-scale species distributions. We indeed found that threatened plant species in China have lower RF than nonthreatened species (SI Appendix, Fig. S1A). Even though China has suffered from high but heterogeneous human pressures, human activity descriptors only explained a limited variation in species richness of woody plants in China, which in contrast is mainly coupled to climate (36). Because RF accounts for current climate through potential ranges, the human effects become more apparent in the geographic patterns of RF. Due to the limited availability of temporal distribution data, human impacts on plant distributions at large scales have not been frequently investigated (4). The RF approach used in this study provides a possible way to estimate human impacts on species ranges for the many species without dynamic distribution data but with good coverage in terms of distribution data.
Species range size is widely used to assess species extinction risk (37). The common idea is that narrow-ranged species have higher extinction risk under stochastic threats because of their small distribution areas (38). As a complement, our results suggest that the narrow-ranged species are more sensitive to anthropogenic activities, thus having a higher probability to be loser species. Our results emphasize the importance of narrow-ranged species in the assessment of spatial patterns of extinction risk and the decision regarding conservation priority areas. Because widespread species contribute disproportionately more distribution records, narrow-ranged species are underrepresented in the overall patterns of biological summary metrics such as species richness (39) or grid-cell MRF. To ensure representativeness, assessment of priority areas for conservation should specifically consider biodiversity patterns of narrow-ranged species.
In this study, we did not consider species interactions or edaphic and other nonclimatic environmental factors, which may affect plant distributions. We note that the extent and grain of this study are beyond the scale domain where these factors typically limit plant ranges (20). Additionally, this study focused on contrasts in RF between different regions rather than absolute values. Because of widespread mountainous terrain in China, grid-cell mean climatic conditions may not capture topoclimate well, potentially resulting in larger predicted potential distributions (40) and thus lower RF. However, elevation range within grid cells is comparable between southeastern and northwestern China at 20 × 20-km resolution (SI Appendix, Fig. S9). Compared with previous studies focused on European plants (23, 35), vascular plants in China are estimated as having higher RF, probably due to methodological differences. First, we only examined species having at least 20 occurrences, while over 20,000 species with narrower ranges were not included (41). Because narrow-ranged species tend to have low RF (42), the RF estimates of examined species thus likely represent relatively high levels of RF relative to the whole flora. Second, sophisticated SDM algorithms, rather than a rectilinear climatic envelope modeling, were utilized here, which may produce conservative potential ranges (23). We note that conservative potential ranges may be more appropriate for measuring human impacts on absences in potential ranges, by better excluding areas of relatively low climatic suitability. Third, the observed ranges at a coarse resolution (200 × 200 km) may contain unoccupied areas even though climatically unsuitable areas have been removed by overlaying them with the predicted distributions at the 20 × 20-km resolution. The geographic patterns of RF, however, are not likely to be distorted as the distribution data had the same resolution across the study area.
In summary, this study measured the extent to which 9,701 vascular plant species fill their climatic potential ranges across China and analyzed the human impacts on RF of narrow-ranged and widespread species, respectively. We found that narrow-ranged and widespread species exhibited opposite relations to human activities, with their RFs decreasing and increasing with human influence, respectively. These results are consistent with floras experiencing biotic homogenization due to human activities, with narrow-ranged species being replaced by widespread species. Narrow-ranged species as defined in this study actually are not even the rarest, because they were defined as having at least 20 occurrences to allow for modeling. Moreover, there are about 2-fold more vascular plants in China with narrower range sizes (41). As it is a general pattern that narrow-ranged species are more numerous than widespread species in nature (43), negative impacts of human activities on plant distributions are therefore likely very prevalent. Our findings highlight the importance of establishing more protected areas and zones of reduced human activities to help mitigate negative human impacts on China’s rich diversity of plants, especially the narrow-ranged species, as well as a need to promote the recovery of narrow-ranged species as a goal in China’s extensive programs for ecosystem restoration.
Materials and Methods
Species Data.
The species distribution data came from the Chinese Vascular Plant Distribution Database, which was compiled from over 6 million specimens and more than 1,000 published floras, checklists, and inventory reports (41). All records in this database were georeferenced to a spatial unit of county level, and most of them also provided collection locality descriptions. Because these records with the resolution of county were relatively imprecise for building SDMs, we further georeferenced these records to such higher resolutions as towns, villages, and specific sampling sites according to the locality descriptions and then obtained latitude and longitude information. These georeferenced records were then aggregated within grid cells of a 20 × 20-km resolution. Species with more than 20 presences were selected for further analyses, leaving 9,784 study species belonging to 1,929 genera and 264 families, with a total of 974,596 presence records, aggregated from 4,287,352 records with coordinates, georeferenced from 7,034,587 records at the county level. The presence records at the resolution of 20 × 20 km were used for SDMs, whereas the records at the county level were projected to the resolution of 200 × 200 km, which were used to describe observed species distributions. We chose the 200 × 200–km resolution because species inventories at this coarse resolution were relatively complete whereas they were likely undersampled at the county-level and finer resolutions (44) (SI Appendix, Fig. S10).
Environmental Data.
We extracted current climatic variables from the WorldClim 1.4 database at a 2.5′ resolution for the period of 1960 to 1990, including 19 bioclimatic variables (bio1 to bio19) and monthly mean temperature and precipitation (45). Based on monthly mean temperature and precipitation, we also derived 2 commonly used bioclimatic variables, growing degree days (with 5 °C as base temperature) and water balance (calculated as the difference between annual precipitation and potential evapotranspiration) (23). These 2 variables plus the above 19 bioclimatic variables were used as predictor candidates to predict species potential distributions. Because of strong multicollinearity among these variables, we performed variable selection based on the intensity of collinearity and predictive capacity of variables (SI Appendix). Finally, 5 climatic variables were selected (SI Appendix, Fig. S11): temperature seasonality (bio4), minimum temperature of coldest month (bio6), precipitation seasonality (bio15), precipitation of warmest quarter (bio18), and coldest quarter (bio19).
We used 3 indicators of human activities to explain variations in species RF and geographic patterns of grid-cell MRF including HPD, HFP, and cropland from open sources (SI Appendix, Fig. S12 and Table S8). These variables were strongly correlated (SI Appendix, Table S9) and thus included in statistical models below individually. Besides human impacts, RF may also be affected by topography and paleoclimatic change (23). For topography, we used elevation range, which was defined as the range of elevation within each grid cell using elevation data at a 1-km spatial resolution (SI Appendix, Fig. S12). For paleoclimatic change, we used temperature anomaly since the Last Glacial Maximum (LGM), which was calculated as the difference between current mean annual temperature (MAT) and the average of 2 estimates of MAT during the LGM from the simulation of the models CCSM4 (46) and MIROC-ESM (47) from WorldClim (SI Appendix, Fig. S12). We also included current MAT and mean annual precipitation (MAP) to control for environmental differences across China even though current climates had been used in predicting potential ranges of species and hence to some extent was already considered in the computation of RF (SI Appendix, Fig. S12).
Species Distribution Modeling.
An ensemble approach was used to forecast species potential distributions at grid cells of 20 × 20 km (48). We used 4 modeling algorithms: generalized linear model, generalized additive mode, random forest, and maximum entropy. Because these algorithms require background data or pseudoabsence data, we generated 20 sets of pseudoabsences for each species with the same size of each as the number of presences (49). Due to spatial biases in the observed presences, we selected pseudoabsences with a similar bias as found in the occurrence data, using a target-group method rather than selecting pseudoabsences randomly from across the whole study region (SI Appendix) (50). The probability of a grid cell to be chosen was weighted by its mean number of records per species (SI Appendix, Fig. S13). We then calibrated models using a 70% random sample of initial data and evaluated them against the remaining 30% of the data using true skill statistics (TSS) and area under the receiver operating characteristic curve, which were repeated 5 times. Those models with TSS >0.5 were included to build an ensemble model. A total of 9,701 species passed the evaluation and were used for further analyses. The generated ensemble SDMs have generally good performance (SI Appendix, Fig. S14). The calibrated models were then projected to current climatic space and the ensemble forecasts were classified to presence/absence with the threshold by maximizing TSS (51). All of the modeling was performed using the biomod2 package in the R language (52).
Calculation of Species Range Filling.
For each species, we calculated the ratio of observed to potential range sizes as RF to measure range equilibrium with current climate (Dataset S1). Both observed and potential range sizes were measured as numbers of grid cells at the 20 × 20-km resolution. However, reliable plant distribution data at fine resolutions across China are not available presently. We therefore overlaid the observed species distributions at the 200 × 200–km resolution with the potential distributions at the 20 × 20-km resolution. In this way, all climatically suitable grid cells of 20 × 20-km within a 200 × 200–km grid cell were assumed as occupied when a species was observed in the 200 × 200–km grid cell.
In this study, RF was used to detect human impacts on species distributions. Besides anthropogenic activities, absences within climatic potential ranges at broader scales might also be caused via large-scale dispersal limitation. Such absences were more likely to occur for climatically suitable areas outside of observed ranges. We therefore used a 200-km buffered minimum convex polygon around the observed ranges to clip climatic potential ranges. Then, we repeated the calculation and analyses using these clipped potential ranges. The produced RF estimates and spatial patterns of RF were similar to those using the unclipped potential ranges (SI Appendix, Figs. S15–S17). Therefore, we did not perform further analyses using RF based on the clipped potential ranges.
We further examined whether RF varied with threat-level categories (27), endemism status (53), plant phylum, and growth form (41) using Wilcoxon rank-sum and Kruskal–Wallis rank-sum tests. Because there were few species in each category labeled as threatened or near-threatened in our studied species, we combined the species within the IUCN categories critically endangered (16 species), endangered (82 species), vulnerable (251 species), and near-threatened (293 species) as threatened species, and then compared them with the least-concern species (7,477 species). Endemism status represented whether or not a species was endemic to China (endemics: 3,141 species; nonendemics: 6,560 species) (53). Plant phyla were categorized as pteridophytes (715 species), gymnosperms (102 species), and angiosperms (8,884 species). For angiosperms, species were further divided as annual herbs (639 species), perennial herbs (3,583 species), climbers (950 species), shrubs (2,048 species), and trees (1,441 species).
Range Filling across Species and Determinants.
To test whether human activities have opposing effects on narrow-ranged and widespread species, we first compared RF between species from low and high human-influenced regions for narrow-ranged and widespread species, respectively. The northwestern and southeastern parts of HHL in China represent 2 contiguous regions with contrasting intensities of human activities. Although regions with low or high human activities can be defined directly based on human activity variables, the generated regions will be fragmented. The species with their ≥80% observed ranges in the northwest and in the southeast were defined as northwestern and southeastern species, respectively, which represented 2 species groups affected by low versus high human activities. We then ranked all studied species by observed range size regardless of regions where species were primarily distributed. The species with the 30% lowest range sizes (≤1,145 grid cells) were categorized as narrow-ranged species and the species with the 30% highest range sizes (≥2,475 grid cells) as widespread species (SI Appendix, Fig. S18). Within narrow-ranged and widespread species, the northwestern and southeastern species had similar range sizes and their RFs were compared using the Wilcoxon rank-sum test. We note that narrow-ranged species may be specialists that persist in limited habitats within their geographic ranges and thus have lower RF compared with widespread species (42) (SI Appendix).
Second, we tested for associations between species RF and explanatory variables with beta regression using the betareg package of R (54). Beta regression is commonly used to model proportional data restricted between 0 and 1 that are typically nonnormal and heteroskedastic (54). Explanatory variables included human impact factors (HPD, HFP, and cropland), elevation range, temperature anomaly, MAT, MAP, and species observed range size. The interaction term between human impact factors and range size was included to test the range size-dependent effect of human activities. Here, the environmental variables were calculated as the median value across species potential ranges. We performed backward model selection based on the Akaike information criterion to select predictors. HPD and cropland were log10-transformed to improve linearity and goodness of fit of models. All of the explanatory variables were standardized for comparing regression coefficients.
Here, we did not account for phylogenetic relatedness of species in significance tests, which had the potential to inflate type I error. The nested ANOVA was then applied to determine the proportions of variation in RF occurring at different taxonomic levels. We found that the majority of variation (77.3%) occurred at the species level, 18.8% at the genus level, and 4.0% at the family level, suggesting that species-level RF was not strongly phylogenetically dependent.
Geographic Range-Filling Patterns and Determinants.
Besides the species-level analyses, we also used an assemblage-based approach to summarize RF of species in each 200 × 200–km grid cell and explore spatial patterns of RF. By integrating species RF estimates with distribution data at the 200 × 200–km resolution, we calculated the mean value of RF of species observed in each grid cell for all, narrow-ranged and widespread species, respectively. To check whether geographic variations of MRF were associated with range size patterns, we also calculated the grid-cell median of both observed and potential range sizes, and grid-cell mean residuals of species RF from the regression against range size for all, narrow-ranged and widespread species, and compared them with MRF (SI Appendix).
We then used simple and multiple linear regressions to explore the associations between MRF and the explanatory variables, including human impact factors (HPD, HFP, and cropland), elevation range, temperature anomaly, MAT, and MAP. However, spatial correlograms and global Moran’s I showed strong spatial autocorrelations presented in residuals of the fitted multiple regression models, which could inflate type I error and bias coefficient estimates (SI Appendix, Fig. S19). We then used an eigenvector-based spatial filtering approach to account for the spatial autocorrelation (55) (SI Appendix). Explanatory variables together with the selected spatial filters were used as predictors of multiple regression models, the residuals of which were therefore without spatial autocorrelation (SI Appendix, Fig. S19). As a supplement, we provide the results without controlling for spatial autocorrelation in SI Appendix, Tables S10–S12; these were consistent with the results from the modeling with spatial filters.
The standardized regression coefficients and partial R2 were calculated to measure the relative importance of the explanatory variables. In the statistical analyses, we removed the grid cells with areas smaller than 12,000 km2, leaving a total of 253 grid cells. To improve linearity and normality of model residuals, HPD, cropland, temperature anomaly, and elevation range were log10-transformed. All statistical analyses were performed using R 3.4.3 (56). The spatial filtering analysis was performed using the function pcnm in the vegan package of R (57).
Range-Filling Patterns across Plant Growth Forms and Orders.
To investigate if it can be generalized that narrow-ranged and widespread species have opposite RF patterns and associations with human activities, we performed comparisons across growth forms and evolutionarily independent lineages. Different growth forms and evolutionary lineages have distinct geographic distributions, which are jointly determined by both ecological and evolutionary processes (25, 41). Similarity in RF patterns and associations with human activities across growth forms and lineages would suggest similar mechanisms involved in generating RF patterns across species groups.
Four growth forms (annual herbs, perennial herbs, shrubs, and trees) were evaluated. To represent evolutionarily independent lineages, we chose angiosperm orders with >100 studied species overall and at least 5 narrow-ranged and widespread species in both the southeastern and northwestern regions, resulting in 13 orders: Asparagales, Asterales, Brassicales, Caryophyllales, Ericales, Fabales, Gentianales, Lamiales, Malpighiales, Poales, Ranunculales, Rosales, and Saxifragales. We examined differences of RF between the northwestern and southeastern species within narrow-ranged and widespread species across these growth forms and orders. We also calculated geographic patterns of MRF and analyzed their Pearson correlations with 3 indicators of human activities (HPD, HFP, and cropland) using Dutilleul et al.’s modified t test (58) to test the significance (controlling for spatial autocorrelation) for narrow-ranged and widespread species, respectively.
Data Accessibility.
The main source of the species distribution data used in this study, the specimen information, is accessible through the Chinese Virtual Herbarium (http://www.cvh.ac.cn). The products that are based on the species distribution data (species observed and potential range sizes and range filling) are indicated in Dataset S1.
Supplementary Material
Acknowledgments
We thank the editors and anonymous reviewers for their constructive comments that greatly improved the manuscript. We are grateful to Drs. Canran Liu and Jian Zhang for helpful discussions on data analysis, and to Dr. Tiemei Chen for help with data preparation. This study was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA19050404). J.-C.S. was supported by the European Research Council (ERC-2012-StG-310886-HISTFUNC). J.-C.S. also considers this work a contribution to his VILLUM Investigator project (VILLUM FONDEN Grant 16549). M.-G.Z. was supported by the National Natural Science Foundation of China (31700465) and Applied Basic Research Project of Shanxi Province (201701D221217). Datasets used in this study are partially provided by Project NSII (National Specimen Information Infrastructure of China), which was supported by the Ministry of Science and Technology of China (Y5217G1001).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. A.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911851116/-/DCSupplemental.
References
- 1.Isbell F., et al. , Linking the influence and dependence of people on biodiversity across scales. Nature 546, 65–72 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newbold T., et al. , Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Di Marco M., Santini L., Human pressures predict species’ geographic range size better than biological traits. Glob. Change Biol. 21, 2169–2178 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Laanisto L., Sammul M., Kull T., Macek P., Hutchings M. J., Trait-based analysis of decline in plant species ranges during the 20th century: A regional comparison between the UK and Estonia. Glob. Change Biol. 21, 2726–2738 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Alroy J., Effects of habitat disturbance on tropical forest biodiversity. Proc. Natl. Acad. Sci. U.S.A. 114, 6056–6061 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimm S. L., et al. , The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Humphreys A. M., Govaerts R., Ficinski S. Z., Nic Lughadha E., Vorontsova M. S., Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 3, 1043–1047 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Ceballos G., Ehrlich P. R., Mammal population losses and the extinction crisis. Science 296, 904–907 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Brummitt N. A., et al. , Green plants in the red: A baseline global assessment for the IUCN Sampled Red List Index for Plants. PLoS One 10, e0135152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kleunen M., et al. , Global exchange and accumulation of non-native plants. Nature 525, 100–103 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Lôbo D., Leão T., Melo F. P. L., Santos A. M. M., Tabarelli M., Forest fragmentation drives Atlantic forest of northeastern Brazil to biotic homogenization. Divers. Distrib. 17, 287–296 (2011). [Google Scholar]
- 12.Olden J. D., Biotic homogenization: A new research agenda for conservation biogeography. J. Biogeogr. 33, 2027–2039 (2006). [Google Scholar]
- 13.McKinney M. L., Lockwood J. L., Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Gossner M. M., et al. , Land-use intensification causes multitrophic homogenization of grassland communities. Nature 540, 266–269 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Morin X., Chuine I., Niche breadth, competitive strength and range size of tree species: A trade-off based framework to understand species distribution. Ecol. Lett. 9, 185–195 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Slatyer R. A., Hirst M., Sexton J. P., Niche breadth predicts geographical range size: A general ecological pattern. Ecol. Lett. 16, 1104–1114 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Devictor V., Julliard R., Jiguet F., Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117, 507–514 (2008). [Google Scholar]
- 18.Kalusová V., et al. , Naturalization of European plants on other continents: The role of donor habitats. Proc. Natl. Acad. Sci. U.S.A. 114, 13756–13761 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyšek P., et al. , The global invasion success of Central European plants is related to distribution characteristics in their native range and species traits. Divers. Distrib. 15, 891–903 (2009). [Google Scholar]
- 20.Pearson R. G., Dawson T. P., Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 12, 361–371 (2003). [Google Scholar]
- 21.Soberón J., Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Wisz M. S., et al. , The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. Camb. Philos. Soc. 88, 15–30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svenning J. C., Skov F., Limited filling of the potential range in European tree species. Ecol. Lett. 7, 565–573 (2004). [Google Scholar]
- 24.Tulowiecki S. J., Larsen C. P., Native American impact on past forest composition inferred from species distribution models, Chautauqua County, New York. Ecol. Monogr. 85, 557–581 (2015). [Google Scholar]
- 25.Lu L. M., et al. , Evolutionary history of the angiosperm flora of China. Nature 554, 234–238 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Lu M., He F., Estimating regional species richness: The case of China’s vascular plant species. Glob. Ecol. Biogeogr. 26, 835–845 (2017). [Google Scholar]
- 27.Qin H. N., et al. , Threatened species list of China’s higher plants. Biodivers. Sci. 25, 696–744 (2017). [Google Scholar]
- 28.Venter O., et al. , Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7, 12558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu C., et al. , Macroecological factors explain large-scale spatial population patterns of ancient agriculturalists. Glob. Ecol. Biogeogr. 24, 1030–1039 (2015). [Google Scholar]
- 30.Chen M. X., Gong Y. H., Li Y., Lu D. D., Zhang H., Population distribution and urbanization on both sides of the Hu Huanyong Line: Answering the Premier’s question. J. Geogr. Sci. 26, 1593–1610 (2016). [Google Scholar]
- 31.Newbold T., et al. , Widespread winners and narrow-ranged losers: Land use homogenizes biodiversity in local assemblages worldwide. PLoS Biol. 16, e2006841 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Köster N., Kreft H., Nieder J., Barthlott W., Range size and climatic niche correlate with the vulnerability of epiphytes to human land use in the tropics. J. Biogeogr. 40, 963–976 (2013). [Google Scholar]
- 33.Cámara-Leret R., et al. , Fundamental species traits explain provisioning services of tropical American palms. Nat. Plants 3, 16220 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Dullinger S., et al. , Post-glacial migration lag restricts range filling of plants in the European Alps. Glob. Ecol. Biogeogr. 21, 829–840 (2012). [Google Scholar]
- 35.Normand S., et al. , Postglacial migration supplements climate in determining plant species ranges in Europe. Proc. Biol. Sci. 278, 3644–3653 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z., Fang J., Tang Z., Lin X., Patterns, determinants and models of woody plant diversity in China. Proc. Biol. Sci. 278, 2122–2132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.IUCN , Guidelines for Using the IUCN Red List Categories and Criteria (Version 11.0, IUCN, Gland, Switzerland, 2014).
- 38.Murray N. J., et al. , The use of range size to assess risks to biodiversity from stochastic threats. Divers. Distrib. 23, 474–483 (2017). [Google Scholar]
- 39.Jetz W., Rahbek C., Geographic range size and determinants of avian species richness. Science 297, 1548–1551 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Franklin J., et al. , Modeling plant species distributions under future climates: How fine scale do climate projections need to be? Glob. Change Biol. 19, 473–483 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Xu W. B., et al. , Plant geographical range size and climate stability in China: Growth form matters. Glob. Ecol. Biogeogr. 27, 506–517 (2018). [Google Scholar]
- 42.Jetz W., Sekercioglu C. H., Watson J. E. M., Ecological correlates and conservation implications of overestimating species geographic ranges. Conserv. Biol. 22, 110–119 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Gaston K. J., Species-range-size distributions: Patterns, mechanisms and implications. Trends Ecol. Evol. 11, 197–201 (1996). [DOI] [PubMed] [Google Scholar]
- 44.Yang W. J., Ma K. P., Kreft H., Geographical sampling bias in a large distributional database and its effects on species richness-environment models. J. Biogeogr. 40, 1415–1426 (2013). [Google Scholar]
- 45.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 46.Gent P. R., et al. , The Community Climate System Model version 4. J. Clim. 24, 4973–4991 (2011). [Google Scholar]
- 47.Watanabe S., et al. , MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model Dev. 4, 845–872 (2011). [Google Scholar]
- 48.Araújo M. B., New M., Ensemble forecasting of species distributions. Trends Ecol. Evol. 22, 42–47 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Barbet-Massin M., Jiguet F., Albert C. H., Thuiller W., Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 3, 327–338 (2012). [Google Scholar]
- 50.Phillips S. J., et al. , Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 19, 181–197 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Liu C. R., Berry P. M., Dawson T. P., Pearson R. G., Selecting thresholds of occurrence in the prediction of species distributions. Ecography 28, 385–393 (2005). [Google Scholar]
- 52.Thuiller W., Lafourcade B., Engler R., Araújo M. B., BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 32, 369–373 (2009). [Google Scholar]
- 53.Huang J. H., Chen J. H., Ying J. S., Ma K. P., Features and distribution patterns of Chinese endemic seed plant species. J. Syst. Evol. 49, 81–94 (2011). [Google Scholar]
- 54.Cribari-Neto F., Zeileis A., Beta regression in R. J. Stat. Softw. 34, 1–24 (2010). [Google Scholar]
- 55.Diniz-Filho J. A. F., Bini L. M., Modelling geographical patterns in species richness using eigenvector-based spatial filters. Glob. Ecol. Biogeogr. 14, 177–185 (2005). [Google Scholar]
- 56.R Core Team , R: A Language and Environment for Statistical Computing (Version 3.4.3, R Foundation for Statistical Computing, Vienna, Austria, 2017).
- 57.Oksanen J., et al. , Vegan: Community Ecology Package (R Package Version 2.5-2, 2011). https://cran.r-project.org/web/packages/vegan/index.html. Accessed 17 May 2018.
- 58.Dutilleul P., Clifford P., Richardson S., Hemon D., Modifying the t test for assessing the correlation between two spatial processes. Biometrics 49, 305–314 (1993). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The main source of the species distribution data used in this study, the specimen information, is accessible through the Chinese Virtual Herbarium (http://www.cvh.ac.cn). The products that are based on the species distribution data (species observed and potential range sizes and range filling) are indicated in Dataset S1.