Abstract
A 41-year-old woman presented with paresthesia and inability to walk for 7 days. She had history of fatigue, polyarthralgia and difficulty in swallowing food for the last 1 year. She became edentulous over the last 5 years and wore dentures for the same. She appeared pale, emaciated and had oral thrush. She had areflexic quadriparesis with weakness more in lower limbs compared with upper limbs. With the initial diagnosis of Guillian-Barre syndrome, she was given five cycles of plasmapheresis following which there was a significant improvement in power. Sjogren’s syndrome was suspected based on edentulous state in a middle-aged woman with multisystem involvement. Evaluation with Schirmer’s test, parotid scintigraphy and labial minor salivary gland biopsy confirmed the diagnosis. She was treated with steroids following which a dramatic improvement in haemoglobin and total leucocyte count was noted. We report a varied presentation of primary Sjogren’s syndrome.
Keywords: dentistry and oral medicine, peripheral nerve disease, Sjogren's syndrome
Background
Connective tissue disorders have myriad ways of presentation. Sjogren’s syndrome is one such disease known to present in a variety of ways. Often the symptoms are non-specific and there may be considerable delay in diagnosis usually due to lack of suspicion. Lack of knowledge about its potential presentations can lead to overlooking the diagnosis and has therapeutic implications. Sjogren’s syndrome is an autoimmune disease characterised by lymphocytic infiltration of the exocrine glands and it usually presents with dryness of mouth (xerostomia) and dry eyes. Extra-glandular manifestations especially neurological manifestations are very rare in patients with Sjogren’s syndrome. We report a case of Sjogren’s syndrome with Guillian-Barre syndrome-like presentation.
Case presentation
A 41-year-old woman presented with complaints of paresthesia below her knees for 7 days. On day 2 of her illness, she started developing progressive weakness of both lower limbs which she noticed initially when her knees buckled while walking. Within the next 5 days she was unable to walk without support and became bed bound. She had no complaints in her upper limbs. There were no symptoms suggestive of cranial nerves, bowel and bladder involvement. For the last 1 year she had been having fatigue and multiple joints pain involving both elbows, wrists, small joints of hands and knees. She also had history of recurrent oral ulcers in the last 1 year. She had trouble swallowing her food and often used to drink water to facilitate the same. She had been married for 15 years but had no children. She had been wearing dentures in her upper jaw for the last 5 years as she had lost most of her teeth by then.
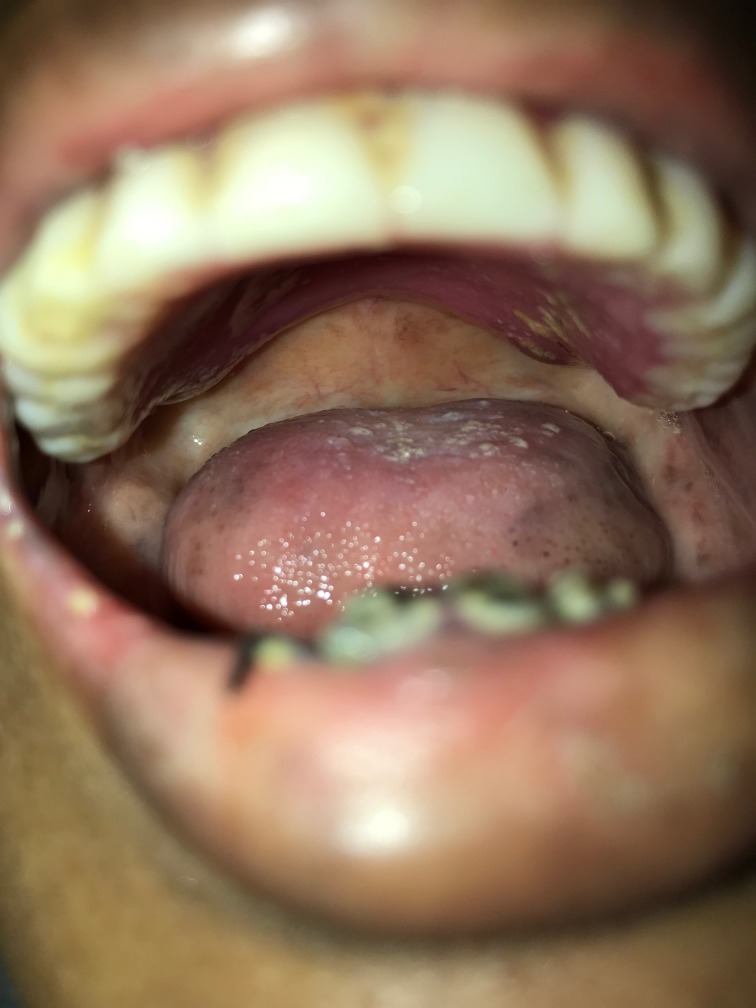
On examination, she was pale and emaciated. She had alopecia, oral thrush, was partially edentulous with caries in lower teeth and she wore a complete denture in her upper jaw (figure 1). There was no parotid enlargement and no significant lymphadenopathy. She had tachycardia at admission and was normotensive. She had weakness of upper limbs with a muscle power (assessed using Medical Research Council grading system) of 3/5 in shoulders, elbows and wrists, 2/5 in her hips and knees and 3/5 in her ankles with areflexia of all four limbs. She had impaired pallesthesia in her lower limbs upto anterior superior iliac spines and proprioception was impaired up to knees bilaterally. Babinski’s sign was negative.
Figure 1.
Clinical photograph of the patient with oral cavity revealing artificial denture in upper jaw and candidiasis.
Investigations
She had anaemia and severe leucopenia (haemoglobin (Hb) 73 g/L, total leucocyte count (TLC) 0.7×109/L). Direct Coomb’s test was negative and peripheral smear was suggestive of normocytic normochromic anaemia with mild anisopoikilocytosis. Nerve conduction study of recorded nerves showed features suggestive of demyelinating polyradiculoneuropathy with conduction block.
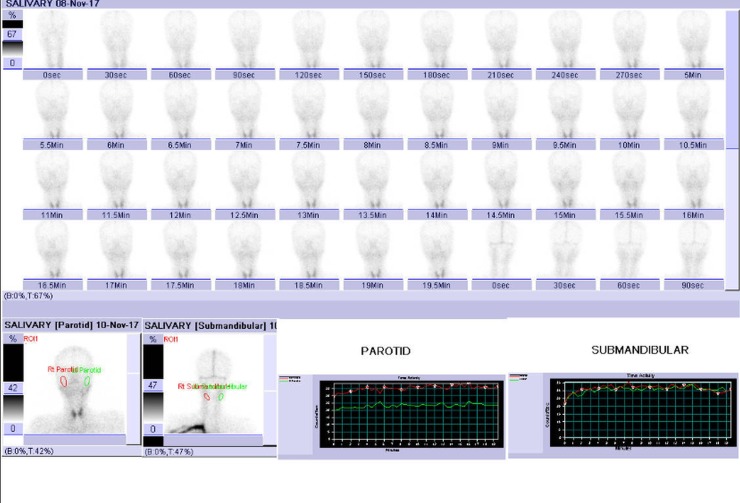
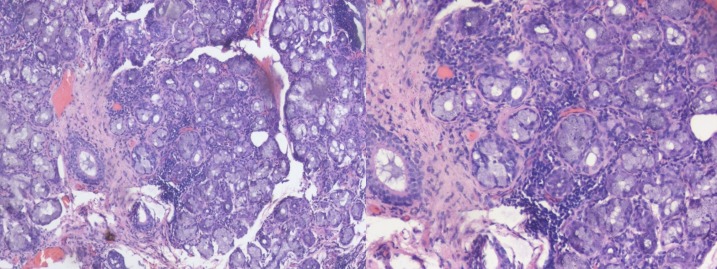
Her parotid scintigraphy revealed no uptake (figure 2), and Schirmer’s test was positive in both eyes. To confirm the diagnosis, a labial minor salivary gland biopsy was done. The biopsy showed lymphocytic and plasma cell infiltrates in salivary glands with focus score >2 (figure 3).
Figure 2.
Scintigraphy revealing no uptake in bilateral parotid and submandibular glands.
Figure 3.
Histopathology sections from minor salivary gland biopsy showing (left) seromucinous glands admixed with patchy aggregates of chronic inflammatory cells (H&E ×100); (right) high power view highlights lymphocytes and plasma cells (H&E ×200).
Her antinuclear antibody (ANA) was reported as 3+ positive with speckled pattern. However, extractable nuclear antigen profile including anti-Ro antibodies was negative (this was done after two cycles of plasmapheresis). Bone marrow aspiration was done in view of anaemia and profound neutropenia which showed 10% plasma cells with polyclonal increase; however, patient had no hypercalcemia, renal failure or lytic bone lesions suggestive of myeloma. Serum immunofixation, fluorescence in situ hybridisation and free light chain assay were negative for myeloma. IgG4 disease was considered in view of multisystem involvement with plasma cell infiltration in salivary gland and bone marrow. However, serum IgG4 levels were normal (1.84 g/L; normal range 0.03–2.0 g/L).
Differential diagnosis
Diagnosis of Sjogren’s syndrome was made in view of early loss of dentures along with no uptake in parotid scintigraphy, positive Schirmer’s test and lymphocytic infiltration of minor salivary glands. Since untreated patients with Sjogren’s syndrome can develop lymphoma and our patient had severe leucopenia, we had considered this possibility and it was ruled out based on minor salivary gland and bone marrow biopsy. Other differential diagnoses considered were multiple myeloma and IgG4-related disease based on plasmacytosis in bone marrow and plasma cell infiltration in salivary glands. Both diagnoses were ruled out due to lack of supporting evidence.
Treatment
In view of demyelinating polyradiculopathy, she received five cycles of plasmapheresis. We started her on 1 mg/kg prednisolone as she had haematological involvement in the form of anaemia and severe leucopenia. Hydroxychloroquine at a dosage of 200 mg/day was started for musculoskeletal symptoms but had to be stopped because of gastrointestinal side effects. Immunosuppressive agents were deferred due to severe leucopenia. She was given oral fluconazole for a week for oral thrush. Methylcellulose eye drops were prescribed for her dry eyes.
Outcome and follow-up
After five cycles of plasmapheresis there was a significant improvement in her power. Her lower limb power improved to 4/5 and she was able to walk without support. Patient had significant improvement in haemoglobin and her TLCs improved as well. After 2 months of steroid therapy her haemoglobin improved to 129 g/L and total count to 2.32×109/L. She has normal power of 5/5 in all four limbs on follow-up visits. She feels subjectively better, able to do household activities by herself and is under regular follow-up.
Discussion
Primary Sjogren’s syndrome has a high incidence of 9:1 in females compared with males.1 About 30%–40% of patients with primary Sjogren’s syndrome have systemic manifestations which often provide the first clue to the diagnosis.2 3 Neurological involvement of primary Sjogren’s syndrome are predominantly those of the peripheral nervous system among which the most common are distal axonal sensory and sensorimotor polyneuropathies.4–7 Polyradiculoneuropathy, a less common variant was found in only 4% of a cohort of 92 patients with primary Sjogren’s syndrome.4 Our patient had a demyelinating sensorimotor polyradiculoneuropathy which responded very well to five cycles of plasmapheresis.
Previous studies have documented appearance of neurological symptoms prior to those of sicca symptoms in 40%–93% of patients.5 7 Our patient had dental caries and loss of teeth about 5 years ago suggesting that sicca symptoms preceded her neurological manifestation much earlier. An European study found that loss of teeth correlated with histological grading of lip biopsy specimen.8 The study also found that on an average loss of teeth occurred about 9 years prior to the first symptom of xerostomia.
Various mechanisms have been accounted for the loss of teeth in Sjogren’s syndrome.9 Autoimmune destruction of salivary glands leads to decreased salivary flow which in turn leads to xerostomia. The IgA content of saliva decreases leading to decrease in antibacterial activity and increased susceptibility to dental caries.10 Moreover the loss of lubricating and buffering capacity of saliva predisposes to caries, despite good oral hygiene by patients.11 This in turn leads to loss of teeth. The same mechanisms predispose the patient to fungal infections especially of Candida species.10 12
Antibodies to Ro antigen is highly specific for Sjogren’s syndrome. Anti-Ro antibodies are less commonly found in those with neurological involvement compared with those without neurological involvement (40% vs 60%).13 Our patient was tested negative for anti-Ro antibodies. However, we did the assay for anti-Ro antibodies only after two cycles of plasmapheresis were completed when the suspicion of Sjogren’s syndrome became strong, whereas the ANA sample was processed on day 1 of hospital admission. One possible explanation for the negative anti-Ro antibody result could be due to significant reduction in the titer of antibodies by plasmapheresis which is described in literature.14 15
Severe anaemia was described in only 4% and severe leucopenia in 0.2% in a large series of patients with primary Sjogren syndrome.16 Our patient had anaemia (Hb 7.3 g/dL) and severe leucopenia (TLC 0.7×109/L) which is quite rare. She had normocytic normochromic anaemia and evidence of hypergammaglobulinemia which are the most common haematological manifestations of primary Sjogren’s syndrome.17
The diagnosis of primary Sjogren’s syndrome in our patient was made as per the recent American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for Sjogren’s syndrome.18 Her minor salivary gland biopsy showed lymphocytic infiltration with focus score >2 and she had positive Schirmer’s test, thereby getting a score of 4. In addition our patient had no uptake in parotid scintigraphy which was one of the tests included in the previous criteria.19
Treatment of Sjogren’s syndrome depends on initial symptoms and extra-glandular manifestations. Severe and acute systemic manifestations require treatment corticosteroids with or without immunosuppressive agents.20 21 Our patient responded dramatically to treatment with plasmapheresis for polyradiculoneuropathy and corticosteroids for haematological involvement. We could not start immunosuppressive agent in view of severe leucopenia.
Learning points.
Primary Sjogren’s syndrome should be considered in all females with symptoms of xerostomia. especially an edentulous jaw with no apparent cause.
All patients with Guillian-Barre syndrome-like paralysis should be investigated for possible Sjogren’s syndrome in the setting of an appropriate clinical picture.
Haematological involvement in Sjogren’s syndrome responds to treatment with steroids.
Ruling out other systemic autoimmune disorders and a watchful eye for non-Hodgkin’s lymphoma are important aspects of management.
Acknowledgments
The authors acknowledge the Department of Nuclear Medicine, JIPMER, Puducherry, 605006 for providing the scintigraphy images.
Footnotes
Contributors: NK was responsible for management of the patient and manuscript preparation. DS was involved in patient management and manuscript preparation. BHS was involved in patient management and manuscript preparation. CB was responsible for management of patient and manuscript preparation and is following up the patient currently.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:1983–9. 10.1136/annrheumdis-2014-205375 [DOI] [PubMed] [Google Scholar]
- 2. ACR Meeting Abstracts Epidemiologic Subsets Drive a Differentiated Clinical and Immunological Presentation of Primary Sjögren Syndrome: Analysis of 9302 Patients from the Big Data International Sjögren Cohort [Internet]. Available: https://acrabstracts.org/abstract/epidemiologic-subsets-drive-a-differentiated-clinical-and-immunological-presentation-of-primary-sjogren-syndrome-analysis-of-9302-patients-from-the-big-data-international-sjogren-cohort/ [Accessed 13 Sep 2018].
- 3. Fox RI. Sjögren's syndrome. Lancet 2005;366:321–31. 10.1016/S0140-6736(05)66990-5 [DOI] [PubMed] [Google Scholar]
- 4. Delalande S, de Seze J, Fauchais A-L, et al. Neurologic manifestations in primary Sjögren syndrome: a study of 82 patients. Medicine 2004;83:280–91. 10.1097/01.md.0000141099.53742.16 [DOI] [PubMed] [Google Scholar]
- 5. Mellgren SI, Conn DL, Stevens JC, et al. Peripheral neuropathy in primary Sjogren's syndrome. Neurology 1989;39:390–4. 10.1212/WNL.39.3.390 [DOI] [PubMed] [Google Scholar]
- 6. Mori K, Iijima M, Koike H, et al. The wide spectrum of clinical manifestations in Sjögren's syndrome-associated neuropathy. Brain 2005;128:2518–34. 10.1093/brain/awh605 [DOI] [PubMed] [Google Scholar]
- 7. Lafitte C, Amoura Z, Cacoub P, et al. Neurological complications of primary Sjögren's syndrome. J Neurol 2001;248:577–84. 10.1007/s004150170135 [DOI] [PubMed] [Google Scholar]
- 8. Baudet-Pommel M, Albuisson E, Kemeny JL, et al. Early dental loss in Sjögren's syndrome. histologic correlates. European community Study Group on diagnostic criteria for Sjögren's syndrome (EEC COMAC). Oral Surg Oral Med Oral Pathol 1994;78:181–6. 10.1016/0030-4220(94)90143-0 [DOI] [PubMed] [Google Scholar]
- 9. Mathews SA, Kurien BT, Scofield RH. Oral Manifestations of Sjögren’s Syndrome. J Dent Res 2008;87:308–18. 10.1177/154405910808700411 [DOI] [PubMed] [Google Scholar]
- 10. MacFarlane TW, Mason DK. Changes in the oral flora in Sjogren's syndrome. J Clin Pathol 1974;27:416–9. 10.1136/jcp.27.5.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pedersen AML, Bardow A, Nauntofte B. Salivary changes and dental caries as potential oral markers of autoimmune salivary gland dysfunction in primary Sjögren's syndrome. BMC Clin Pathol 2005;5:4 10.1186/1472-6890-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radfar L, Shea Y, Fischer SH, et al. Fungal load and candidiasis in Sjögren's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;96:283–7. 10.1016/S1079-2104(03)00224-5 [DOI] [PubMed] [Google Scholar]
- 13. Tobón GJ, Pers J-O, Devauchelle-Pensec V, et al. Neurological Disorders in Primary Sjögren’s Syndrome. Autoimmune Dis 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tonello M, Ruffatti A, Marson P, et al. Plasma exchange effectively removes 52- and 60-kDa anti-Ro/SSA and anti-La/SSB antibodies in pregnant women with congenital heart block. Transfusion 2015;55:1782–6. 10.1111/trf.13046 [DOI] [PubMed] [Google Scholar]
- 15. Van Der Leij JN, Visser GHA, Bink-Boelkens MTE, et al. Successful outcome of pregnancy after treatment of maternal anti-Ro (SSA) antibodies with immunosuppressive therapy and plasmapheresis. Prenat Diagn 1994;14:1003–7. 10.1002/pd.1970141019 [DOI] [PubMed] [Google Scholar]
- 16. Ramos-Casals M, Font J, Garcia-Carrasco M, et al. Primary Sjögren syndrome: hematologic patterns of disease expression. Medicine 2002;81:281–92. 10.1097/00005792-200207000-00004 [DOI] [PubMed] [Google Scholar]
- 17. Baimpa E, Dahabreh IJ, Voulgarelis M, et al. Hematologic manifestations and predictors of lymphoma development in primary Sjögren syndrome: clinical and pathophysiologic aspects. Medicine 2009;88:284–93. 10.1097/MD.0b013e3181b76ab5 [DOI] [PubMed] [Google Scholar]
- 18. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League against rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017;69:35–45. 10.1002/art.39859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vitali C, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis 2002;61:554–8. 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saraux A, Pers J-O, Devauchelle-Pensec V. Treatment of primary Sjögren syndrome. Nat Rev Rheumatol 2016;12:456–71. 10.1038/nrrheum.2016.100 [DOI] [PubMed] [Google Scholar]
- 21. Vivino FB, Carsons SE, Foulks G, et al. New treatment guidelines for Sjögren's disease. Rheum Dis Clin North Am 2016;42:531–51. 10.1016/j.rdc.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]