Significance
This study reveals the roles of PV interneurons surrounding perineuronal nets (PNNs) in both the hippocampus and the anterior cingulate cortex (ACC) in contextual fear memory. We show that increasing PNN expression in these 2 brain structures enhances the recall and reconsolidation of both recent and remote fear memory, corresponding to the enhancement of GABA-related theta oscillations. Conversely, removal of PNNs impairs consolidation and reconsolidation of both recent and remote fear memory via increased feedback inhibition and impairment of theta oscillations. Together, our results suggest that PNNs in the hippocampus and ACC affect behavioral plasticity during the consolidation and reconsolidation of contextual fear memory by regulating feedback inhibition of PV interneurons.
Keywords: perineuronal nets, presynaptic plasticity, long-term memory, parvalbumin interneurons, memory consolidation and reconsolidation
Abstract
Perineuronal nets (PNNs), a complex of extracellular matrix molecules that mostly surround GABAergic neurons in various brain regions, play a critical role in synaptic plasticity. The function and cellular mechanisms of PNNs in memory consolidation and reconsolidation processes are still not well understood. We hypothesized that PNNs protect long-term memory by limiting feedback inhibition from parvalbumin (PV) interneurons to projection neurons. Using behavioral, electrophysiological, and optogenetic approaches, we investigated the role of PNNs in fear memory consolidation and reconsolidation and GABAergic long-term potentiation (LTP). We made the discovery that the formation of PNNs was promoted by memory events in the hippocampus (HP), and we also demonstrated that PNN formation in both the HP and the anterior cingulate cortex (ACC) is essential for memory consolidation and reconsolidation of recent and remote memories. Removal of PNNs resulted in evident LTP impairments, which were rescued by acute application of picrotoxin, a GABAA receptor blocker, indicating that enhanced inhibition was the cause of the LTP impairments induced by PNN removal. Moreover, removal of PNNs switched GABAA receptor-mediated long-term depression to LTP through a presynaptic mechanism. Furthermore, the reduced activity of PV interneurons surrounded by PNNs regulated theta oscillations during fear memory consolidation. Finally, optogenetically suppressing PV interneurons rescued the memory impairment caused by removal of PNNs. Altogether, these results unveil the function of PV interneurons surrounding PNNs in protecting recent and remote contextual memory through the regulation of PV neuron GABA release.
Memory consists of formation, consolidation, and reconsolidation processes. The molecular pathways associated with memory consolidation and reconsolidation have been well studied. A number of the involved molecules have been reported, such as cytoplasmic polyadenylation element binding protein (1), CaMKII coupled with the NMDA receptor (2), protein kinase M zeta (3), and protein phosphatase 2A (4), most of which reside inside the postsynaptic spines and excitatory synapses (5). The cellular mechanisms of the extracellular matrix (ECM) surrounding parvalbumin interneurons take part in memory consolidation and reconsolidation processes; however, these have not been extensively studied.
Perineuronal nets (PNNs) are a complex of ECM molecules that mostly surround the soma and dendrites of GABAergic neurons in various brain regions, including the forebrain, midbrain, and cerebellum (6–8). The major component of PNNs consists of high-molecular-weight proteoglycans (6), which comprise a core protein attached by long chains of glycosaminoglycans, including heparan sulfates and chondroitin sulfates. PNNs are functionally involved in the stabilization of synapses by acting as a physical barrier and take part in the integration and generation of neuronal electrical activity by providing a continuous micromilieu facilitating the flow of cations across the membrane (7, 8). PNNs have also been reported to play a protection role on consolidation of cued fear conditioning (9), auditory cortex-dependent fear conditioning (10), reconsolidation of cocaine-induced conditioned place preference memory (11), and recognition memory in the perirhinal cortex (12).
In the CA1 region of the hippocampus, 24% of interneurons are parvalbumin-positive (PV) interneurons (13). These PV interneurons make basket-like connections with principal neurons and receive 94% of excitatory inputs from pyramidal cells (14). Thus, PV interneurons play an important role in feedforward and feedback inhibition in the hippocampal circuits and are also involved in the generation of network oscillations (15, 16). Recent evidence revealed a decisive role of hippocampal PV interneurons in contextual fear learning by perisomatic inhibition to hippocampal principle cells (17, 18). Meanwhile, numerous studies have shown that limiting GABAergic inhibitory tone on projection neurons impairs memory consolidation, retention, and reconsolidation (19–21). In addition, contextual fear conditioning (CFC) induced high-differentiation hippocampal PV network configurations with a high excitatory-to-inhibitory ratio in adult mice (22). However, few studies have investigated the functional role of PNNs surrounding the activity-dependent PV interneurons. We hypothesized that PNNs surrounding PV interneurons may protect recent contextual fear memory through feedback inhibition in the hippocampus.
Memory as a general category can be divided into recent and remote memory (23). Recent contextual fear memory is widely believed to be consolidated in the hippocampus (23), while several studies have suggested that the cortical area plays a critical role in remote, but not recent, memory (24, 25). The anterior cingulate cortex (ACC) has been identified as a storage site for remote memory. In animals, pharmacological inhibition and anatomical lesions of the ACC and prelimbic cortex preferentially impair remote spatial-discrimination memory and trace eye-blink conditioning, respectively (26). In humans, the ACC is thought to be critical in the recall of stored information (27). Similarly, pharmacological inhibition of ACC activity preferentially disrupts the retrieval of remote contextual fear memories (24). Meanwhile, removal of PNNs in the visual cortex disrupts recall of remote memory (28). However, little is known about the PNNs in ACC-dependent contextual remote fear memory. Although Tsien (5) has proposed that very long-term memory might be stored in the pattern of holes in PNNs, experimental evidence is still lacking. Therefore, we examined the PV cells surrounded by PNNs in the ACC after fear training and hypothesized that the formation of PNNs in the ACC would affect remote contextual fear memory.
Results
The Formation of PNNs Is Up-Regulated after Contextual Fear Conditioning in both the Hippocampus and the ACC.
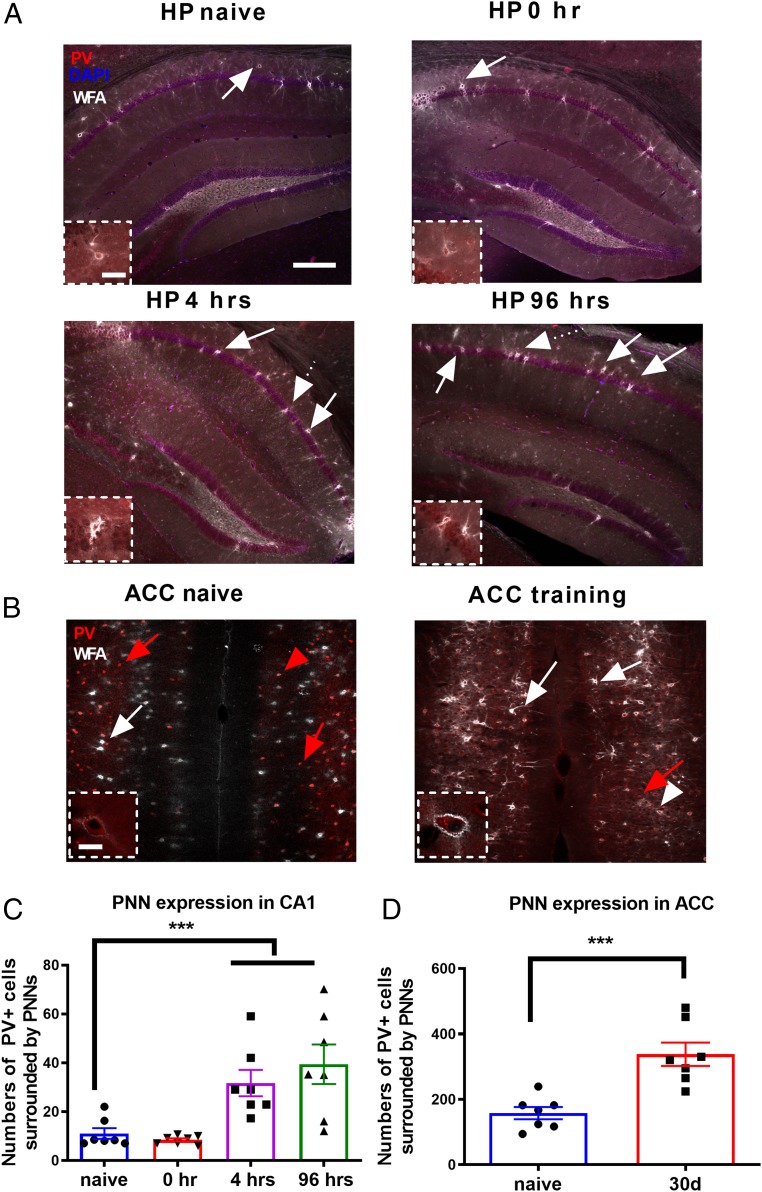
Since PNNs surrounding PV interneurons are essential for memory storage in the central nervous system, we first tested whether the formation of PNNs is regulated during memory events in both the hippocampus and the ACC using the fear conditioning model (10). Our results showed that, as detected by Wisteria floribunda agglutinin (WFA) lectin-histochemistry staining, the number of PV+ cells surrounded by PNNs in the hippocampus was significantly increased at 4 and 96 h after fear conditioning training compared with naïve unpaired controls [main effect of the treatment: F(3, 24) = 9.22, P < 0.0001, 1-way ANOVA followed by post hoc comparisons, n = 7 mice for each group, Fig. 1 A and C]. This result confirmed that the regulation of ECM proteins is long lasting in a contextual fear memory paradigm. In contrast, the number of PV neurons surrounded by PNNs showed no significant changes during the acquisition process (0 h) (n = 7 mice, Student’s t test, P > 0.05, Fig. 1 A and C). Similarly, the number of PV neurons ensheathed by PNNs in the ACC was also significantly increased 30 d after fear conditioning compared with naïve unpaired controls (Student’s t test, P < 0.001, n = 7 mice for each group, Fig. 1 B and D). These results suggest that the formation of PNNs was dynamically up-regulated by memory events in both the hippocampus and the ACC.
Fig. 1.
PNN/PV double-labeled neurons were specifically increased 4 h and 96 h after contextual fear conditioning. (A) Confocal laser scanning micrographs of double staining of PNN and parvalbumin in the native, 0 h, 4 h, and 96 h after fear conditioning training in the hippocampus, showing increased expression of PNN after training. White arrows indicate the WFA/PV double-labeled neurons. Dotted arrows indicate the cells in the higher magnification. (Scale bars: 200 µm.) Boxed areas are shown at a higher magnification. (Scale bars in Insets: 25 µm.) (B) Confocal laser scanning micrographs of PNN/PV double staining in the native and 30 d after fear conditioning in the ACC. Red arrows indicate the PV-labeled neurons. Boxed areas are shown at a higher magnification. (Scale bars in Insets: 20 µm.) (C) The number of PNN-enwrapped cells across the hippocampus was highest at 4 h after fear conditioning. (D) The number of PNN-expressing cells across the ACC was significantly higher 30 d after training than in the naïve state. n = 7 mice for each group. ***P < 0.001 by 1-way ANOVA followed by post hoc testing. All values are expressed as the mean ± SEM.
Effects of PNNs on the Consolidation and Retrieval of Contextual Fear Memory.
After the first work about the chondroitinase ABC (chABC) treatment for abolishing PNNs of the extracellular matrix (29), several studies have confirmed and clearly shown that ChABC treatment can remove PNN expression in various brain regions (9–11, 29). To confirm whether ChABC does remove PNN expression, we stained PNNs with biotinylated WFA, and as we expected, ChABC treatment 24 h before fear training significantly reduced the number of PV cells surrounded by PNNs (SI Appendix, Fig. S1), but not affecting PV neurons themselves in the CA1 of the hippocampus (SI Appendix, Fig. S1 A, Right). In contrast, ChABC treatment did not affect the expression of cartilage link protein (Hapln1; SI Appendix, Fig. S2). In addition, our data also demonstrated that injection of ChABC in the hippocampus didn’t affect the WFA signal in the posterior cingulate cortex (PCC), which localized relatively close to the hippocampus (SI Appendix, Fig. S1C), indicating that ChABC treatment in our current study was limited to the hippocampus where ChABC was injected.
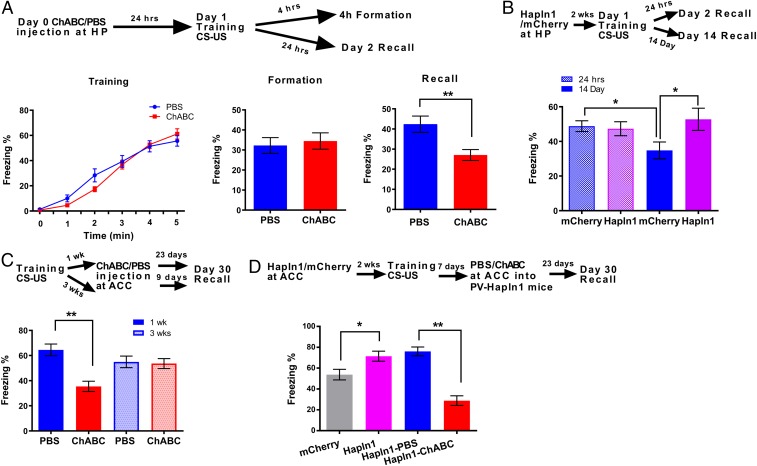
Thus, to study the effects of PNNs on contextual fear memory, mice received bilateral, intrahippocampal infusions of ChABC treatment or phosphate-buffered saline (PBS) as a control 24 h prior to fear conditioning training (9). Removal of the PNNs from the hippocampus by local ChABC treatment prior to the fear conditioning training did not influence the acquisition of fear memory [F(1, 37) = 0.17, P = 0.68, 2-way ANOVA; Fig. 2 A, Left]. However, in tests conducted either at 4 h (during memory formation) or 24 h later (for memory consolidation), our results showed that the formation process at 4 h was still intact (PBS: 32.08 + 6.20 [n = 13] vs. ChABC: 36.88 + 6.95% [n = 14], P > 0.05, Student’s t test; Fig. 2 A, Middle), but the contextual fear memory consolidation process after 24 h was significantly impaired (PBS: 42.37 ± 4.06% [n = 11] vs. ChABC: 27.07 ± 2.71% [n = 16], P < 0.01, Student’s t test; Fig. 2 A, Right). These results are consistent with previous findings in the cue-fear condition of the auditory cortex (10). To further investigate the specific role of PNNs surrounding PV interneurons, we decreased PNN expression by microinjection of pAAV-dio-shBCAN-GFP into the hippocampus in PV-cre mice before contextual fear conditioning training (30). The nets surrounding the PV neurons of shBCAN-treated mice appeared hazier than those of untreated mice and were less pronounced and less concentrated close to the plasma membrane (SI Appendix, Fig. S4). Interestingly, we found that reducing PNN expression surrounding PV+ cells by shBCAN treatment impaired the acquisition [F(1, 30) = 6.82, P < 0.05, 2-way ANOVA; n = 15 for GFP and n = 17 for shBCAN, SI Appendix, Fig. S3A]. Combined with ChABC-treated mice undergoing normal fear acquisition (13) and agonist of GABAA receptor impairing memory acquisition (24), these results suggest that PNNs surrounding PV+ cells, in contrast to non-PV+ neurons, may play an essential role in fear memory acquisition. Similar to the effects of ChABC treatment, shBCAN-treated mice also impaired memory consolidation (PBS: 57.08 ± 3.67% [n = 15] vs. ChABC: 38.18 ± 3.88% [n = 17], P < 0.05, Student’s t test, SI Appendix, Fig. S3B). Therefore, removal of PNNs by either ChABC or PV-shBCAN in the hippocampus before training impairs the memory consolidation processes.
Fig. 2.
Manipulations of PNNs in adult mice affected long-term contextual fear memory consolidation. (A, Top) Schematic drawing of the experimental design for contextual fear conditioning, indicating the timeline of the experimental manipulations. (A, Left) The percentage of time spent freezing during the presentation of the CS across 5 trials of fear conditioning training. (A, Middle) Four hours after training, the percentage of time mice spent freezing while exposed to the training context (n = 16 for PBS and n = 14 for ChABC). (A, Right) Tewnty-four hours after training, the percentage of time mice spent freezing while exposed to the training context (n = 11 for PBS and n = 16 for ChABC). (B, Top) Schematic drawing of the experimental design for contextual fear conditioning, indicating the timeline of the experimental manipulations. The figure shows the percentage of time mice spent freezing while exposed to the training context 24 h or 14 d after training (n = 13 for 24 h and n = 16 to 18 for 14 d). (C, Top) Schematic drawing of the experimental design for contextual fear conditioning, indicating the effect of removal of PNN by ChABC on the remote memory process. (C, Bottom) Thirty days after training, the percentage of time mice spent freezing while exposed to the training context. Significant differences were detected in ChABC treatment at ACC 1 wk after training (n = 18 to 22 for 3 wk and n = 10 to 18 for 1 wk). (D, Top) Schematic drawing of the experimental design for contextual fear conditioning, indicating the effect of overexpression of PNN on the remote memory process. (D, Bottom) Thirty days after training, the percentage of time mice spent freezing while exposed to the training context (n = 20 for mCherry and Hapln1; n = 14 for Hapln1-PBS and Hapln1-ChABC). **P < 0.01, *P < 0.05 by 1-way ANOVA followed by post hoc testing. All data are expressed as the mean ± SEM.
To validate the hypothesis that increase PNNs in PV interneurons could enhance the memory recall processes, we increased PNN expression by microinjection of the pAAV-dio-Hapln1-mCherry into the hippocampus of PV-cre mice (SI Appendix, Methods section and SI Appendix, Figs. S1, S4, and S5). PV-Hapln1 treatment significantly increased the number of PV cells surrounded by PNNs (SI Appendix, Fig. S1) and the WFA density of single PV cells (SI Appendix, Fig. S4). Our behavioral results showed that PV-Hapln1 treatments did not affect the memory consolidation process (mCherry control: 48.79 ± 2.94% vs. Hapln1: 47.33 ± 4.02%, n = 13 for both groups, P > 0.05, 1-way ANOVA followed by post hoc testing), but significantly affected the 14-d memory decay process (mCherry: 34.82 ± 4.89% [n = 18] vs. Hapln1: 52.76 ± 6.4% [n = 16], P < 0.05, 1-way ANOVA followed by post hoc testing, Fig. 2B). Interestingly, increasing PNN expression in the hippocampal PV interneurons significantly increased remote memory recall (SI Appendix, Fig. S6 B, Right). Taken together, these results indicate that increasing PNNs in the hippocampus can protect both recent and remote fear memory.
Since ACC is known as a particular storage site for remote contextual and spatial memory, we then examined the effects of removal of PNNs in ACC by local ACC ChABC infusion at 1 or 3 wk after fear conditioning training on remote contextual fear memory recall (Fig. 2C). We found that ACC infusion of ChABC 1 wk after fear conditioning training significantly impaired recall of remote fear memory (PBS: 64.53 ± 4.99% [n = 10] vs. ChABC: 35.45 ± 3.99% [n = 18], P < 0.01, 1-way ANOVA followed by post hoc testing, Fig. 2C), but did not affect recall of remote fear memory 3 wk after training (PBS: 54.97 ± 4.99% [n = 18] vs. ChABC: 53.60 ± 5.25% [n = 22], P > 0.05, 1-way ANOVA followed by post hoc testing, Fig. 2C). These results indicate that removal of PNNs from the ACC before the transfer of contextual memory to the cortex could impair remote memory recall (23); however, if the PNNs were removed after the memory had already been transferred to the ACC, the remote memory would remain intact (Fig. 2C). In addition, we also tested the effect of increasing PNN expression surrounding PV neurons in the ACC 30 d after training on recall of remote memory by injecting pAAV-Hapln1-mCherry virus into PV-cre mice 2 wk before CFC training. Our results showed that remote contextual fear memory was significantly enhanced by overexpression of Hapln1 of PV neurons (mCherry: 58.03 ± 4.79% vs. Hapln1: 71.86 ± 5.14%, n = 20 for both groups, P < 0.05, 1-way ANOVA followed by post hoc testing, Fig. 2D), and this effect was significantly reversed by sequential infusion of ChABC into the ACC 7 d after training (Hapln1-sal: 76.07 ± 5.99% vs. Hapln1-ChABC: 28.82 ± 5.06%, n = 14 for both groups, P < 0.01, 1-way ANOVA followed by post hoc testing, Fig. 2D). In addition, this sequential Hapln1-ChABC treatment significantly impaired normal remote memory in comparison with the mCherry group (mCherry: 58.03 ± 4.79% [n = 20] vs. Hapln1-ChABC: 28.82 ± 5.06% [n = 14], P < 0.05, 1-way ANOVA followed by post hoc testing, Fig. 2D). To examine whether manipulations of PNNs would influence recent memory in a similar manner, we injected either ChABC or pAAV-Hapln1 in ACC before training and found that manipulation of PNNs in ACC did not affect the recent memory consolidation (SI Appendix, Fig. S6 A, Left). Taken together, these results indicate that PNNs in the ACC are important for remote memory retrieval but not recent memories.
PNNs Are Essential for both Recent and Remote Memory Reconsolidation.
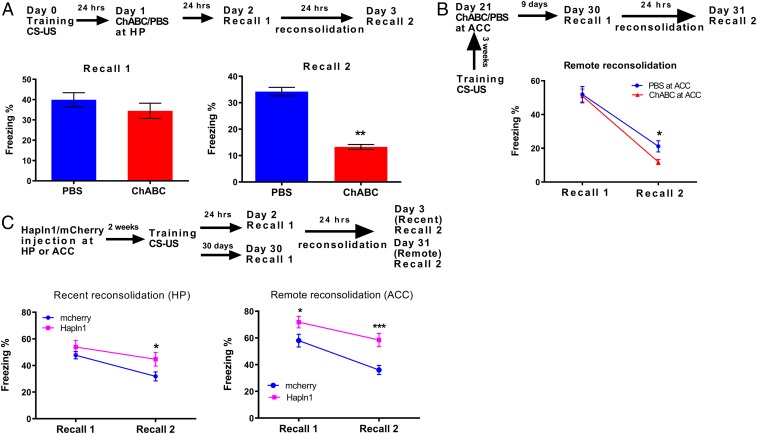
In order to test the effects of PNNs on the reconsolidation of contextual fear memory, mice received bilateral intrahippocampal infusions of ChABC or PBS as a control 24 h after fear conditioning consolidation. Our results showed that removal of PNNs by ChABC in the hippocampus after consolidation did not affect fear memory recall (PBS: 39.95 ± 3.45% [n = 16] vs. ChABC: 34.48 ± 3.75% [n = 19], P = 0.315, Student’s t test; Fig. 3 A, Left), but did impair the reconsolidation process (PBS: 23.73 ± 1.27% [n = 10]; ChABC: 13.26 ± 0.94% [n = 11]; P < 0.01, Student’s t test; Fig. 3 A, Right). Similarly, infusion of ChABC into the ACC 3 wk after fear condition training significantly affected the remote reconsolidation process (PBS: 22.25 ± 4.06% [n = 18] vs. ChABC: 9.60 ± 2.12% [n = 20], P < 0.05), but not the recall (PBS: 54.97 ± 4.99% vs. ChABC: 53.60 ± 5.25%, P > 0.05, 2-way repeated-measures ANOVA followed by post hoc testing, Fig. 3B). Similar to the effects of ChABC treatment, removal of PNNs in the hippocampus by shBCAN after fear training also impaired the reconsolidation process (P < 0.05, 2-way ANOVA followed by post hoc testing; n = 7 for GFP and n = 10 for shBCAN, SI Appendix, Fig. S3C). Taken together, the evidence suggests that removal of PNNs by either ChABC or shBCAN in both the hippocampus and ACC impairs the memory reconsolidation processes for both recent and remote memory.
Fig. 3.
Manipulations of PNNs in adult mice affected long-term contextual fear memory reconsolidation. (A, Top) Schematic drawing of the experimental design for contextual fear conditioning, indicating the effect of PNN on the reconsolidation process. (A, Left) No significant differences were detected in the recall process after ChABC treatment for 24 h after training. (A, Right) The percentage freezing during the 3-min context exposure demonstrated significantly less freezing for the ChABC-treated animals during the reconsolidation process. Animals were treated with ChABC 24 h after training (n = 16 for PBS and n = 19 for ChABC). (B) Significant differences were detected during the remote reconsolidation process in ChABC-treated mice in ACC, and ChABC-treated mice at ACC, treated 3 wk after training (n = 18 for PBS and n = 20 for ChABC). (C, Top) Schematic drawing of the experimental design for contextual fear conditioning, indicating the effect of PNN on the reconsolidation process. (C, Left) Significant differences were detected in the reconsolidation process of recent memory after increasing PNN expression by Hapln1 before training (n = 22 for each group). (C, Right) Significant differences were detected during the remote reconsolidation process in Hapln1-treated mice at ACC (n = 22 for mCherry and n = 27 for Hapln1). ***P < 0.001, **P < 0.01, *P < 0.05 by 2-way repeated-measures ANOVA. All data are expressed as the mean ± SEM.
To further test the effects of increasing the expression of PNNs in PV neurons on the reconsolidation of both recent and remote contextual fear memory, mice received bilateral intrahippocampal and intra-ACC injection of either Hapln1 or mCherry adeno-associated virus (AAV) into PV-cre mice before fear conditioning training (15). In the hippocampus, Hapln1 overexpression significantly enhanced the recent memory reconsolidation, but not the memory recall process (recall: 47.73 ± 2.73% vs. 53.89 ± 4.98%, P > 0.05; reconsolidation: 31.82 ± 2.94% vs. 44.69 ± 4.02%, n = 22 for each group, P < 0.05, 2-way repeated-measures ANOVA followed by post hoc testing; Fig. 3 C, Left). However, in ACC, Hapln1 overexpression significantly increased both remote contextual fear memory recall and the reconsolidation processes (recall: 58.03 ± 4.79% vs. 71.86 ± 5.14%, P < 0.05; reconsolidation: 36.02 ± 3.98% vs. 58.50 ± 5.88%, P < 0.001, n = 22 for mCherry and n = 27 for Hapln1, 2-way repeated-measures ANOVA followed by post hoc testing; Fig. 3 C, Right). In addition, although increasing the abundance of PNNs by increasing Hapln1 in the ACC could not affect the recent memory reconsolidation process, increasing PNNs in the hippocampus also significantly enhanced remote memory reconsolidation (SI Appendix, Fig. S6). Taken together, these results indicate that overexpression of PNNs can protect long-term memory during both recent and remote memory reconsolidation processes.
Manipulations of PNNs Affecting Synaptic Plasticity through a Presynaptic GABAergic Mechanism.
Since long-term potentiation (LTP) and long-term depression (LTD) constitute the cellular model for learning and memory (31), we used LTP as a cellular hallmark in the current study to detect the synaptic deficits of ChABC-treated mice. We thus evaluated the role of PNNs in synaptic plasticity of the field excitatory postsynaptic potentials (fEPSPs) of CA1 pyramidal neurons evoked by stimulation of the Schaffer collateral pathway (32).
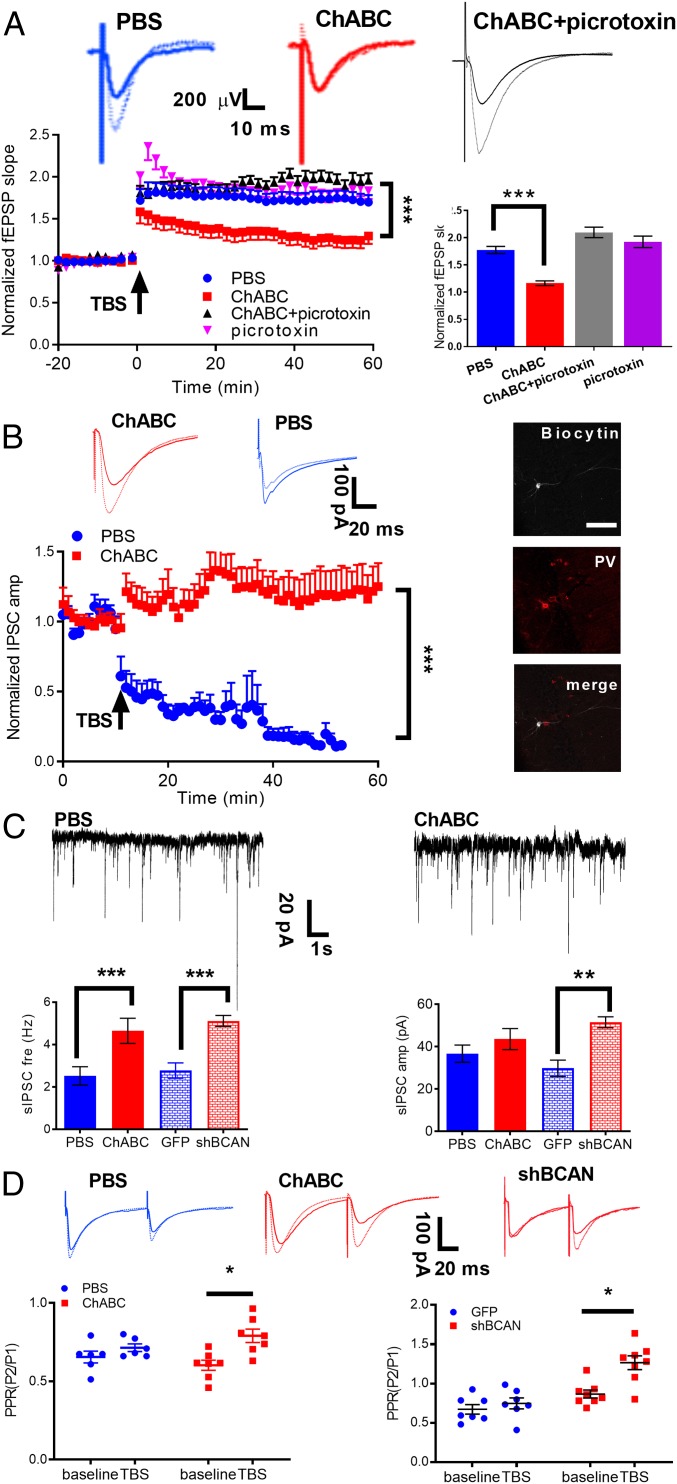
To test the relationship between PNNs and synaptic plasticity in the hippocampus, we intrahippocampally injected ChABC or PBS in vivo into 3-mo-old mice and performed acute recordings in the hippocampal CA1 slices 24 h after ChABC treatment (Fig. 4A). After 20 min of stable baseline recordings for fEPSPs, LTP, induced by 1 epoch of theta burst stimulation (TBS), was maintained for at least 60 min in the PBS control (blue circle, n = 10 slices, 5 mice, Fig. 4A); however, it was observed to decay over time in the ChABC-treated mice (red square, n = 12 slices, 5 mice, P < 0.001, 2-way repeated-measures ANOVA from 40 to 60 min after TBS compared with PBS control, Fig. 4A), demonstrating synaptic plasticity deficits. To further investigate the role of GABAergic inhibition in the synaptic plasticity of the ChABC-treated mice, we applied picrotoxin (100 μM), a GABAA receptor blocker, and it successfully prevented the LTP deficits in ChABC-treated mice (picrotoxin + ChABC: n = 8 slices, 4 mice, P < 0.01, 2-way repeated-measures ANOVA from 40 to 60 min after TBS compared between control and picrotoxin in ChABC-treated mice) in the picrotoxin group (purple triangle, n = 10 slices, 3 mice, Fig. 4A). Taken together, our data suggest that PNNs influencing synaptic plasticity may go through regulating GABAergic inhibition.
Fig. 4.
Manipulations of PNNs affected synaptic plasticity through presynaptic GABA release. (A, Top) Representative traces of fEPSPs before (solid line) and 50 min after (dotted line) TBS. (A, Bottom) Time course of relative changes of fEPSP slopes for PBS (blue circles) and ChABC (red triangles) pretreated mice (n = 10 to 12 cells for PBS and ChABC). These LTP deficits can be rescued by bath application of picrotoxin (black triangles) in ChABC pretreated mice (n = 8 cells), with normal LTP occurring after picrotoxin application in the PBS-pretreated mice (purple triangles, n = 10 cells). Picrotoxin was applied to the recording chamber 30 min before TBS and present throughout the experiments. One epoch of TBS was delivered at time 0. (B) Normalized amplitudes of IPSCs are plotted against the recording time for the PBS group (n = 8 cells, blue circle) and the ChABC pretreated group (n = 8 cells, red square). Averaged amplitude of IPSCs before (baseline, solid line) and at 30 min after TBS (dotted line) in the PBS (blue) and ChABC (red) treated groups. (B, Right) Intracellular labeling of the recorded CA1 pyramidal cells. (Scale bar: 50 μm.) (C, Top) Representative trace of sIPSCs in PBS control and ChABC treatment from hippocampal slices. Cells were voltage clamped at −70 mV. (C, Bottom) The frequency of sIPSCs increased significantly in ChABC- and shBCAN-treated mice compared with the control group. The amplitude of sIPSCs showed no change in ChABC-treated mice and increased in the shBCAN-treated mice compared with the control group (n = 16 cells for PBS and ChABC; n = 8 cells for GFP and shBCAN). (D) LTP is associated with an increase in the PPR (P2/P1) in the ChABC- and shBCAN-treated groups, indicating presynaptic plasticity (n = 16 cells for PBS and ChABC; n = 8 cells for GFP and shBCAN). (D, Right) Representative traces of sIPSCs in the PBS control (blue) and ChABC treatment groups (red) are shown. Significance was tested by 2-way repeated-measures ANOVA in A and B and Student’s t tests in C and D. ***P < 0.001, **P < 0.01, *P < 0.05. All data are expressed as the mean ± SEM.
To further investigate the cellular and molecular mechanisms underlying the effects of PNN expression on GABAergic plasticity, we performed whole-cell patch clamp recordings from the CA1 pyramidal cells in the presence of NMDA and AMPA receptor antagonists D-APV and DNQX, respectively (Fig. 4). The effect of PNNs on long-term plasticity in GABAergic transmission in the CA1 region of the hippocampus was then studied by comparing the TBS-evoked responses between the PBS control and ChABC-treated mice. Interestingly, a synaptic plasticity of the opposite directions was observed between the control and ChABC-treated mice, a TBS-induced GABA-LTD (normalized evoked inhibitory postsynaptic current (eIPSC) amplitude: 30.15 ± 8.15%, 30 min after TBS, n = 8 cells, 5 mice) in the PBS control mice and in the opposite way, a GABA-LTP in the ChABC-treated mice (normalized IPSC amplitude: 119.99 ± 15.66%, 2-way repeated-measures ANOVA from 20 to 45 min after TBS, n = 8 cells, 5 mice, P < 0.01, Fig. 4B).
To further understand whether ChABC treatment to dissolve PNNs would affect basal GABAergic transmission, we recorded spontaneous IPSCs (sIPSCs) in the pyramidal cells of hippocampal CA1. We found significantly increased frequency, but not amplitude, of sIPSCs in the ChABC-treated mice compared with the PBS control (PBS: 2.51 ± 0.43 Hz vs. ChABC: 4.65 ± 0.59 Hz; n = 16 cells from 5 mice for both the PBS and ChABC groups, P < 0.001, Student’s t test, Fig. 4C), indicating that reduced PNN expression by ChABC treatment would presynaptically increase GABA input to the hippocampal pyramidal neurons. Similarly, the reduction of PNN expression surrounding PV+ cells by AAV-shBCAN injection increased both the frequency and amplitude of sIPSCs and miniature IPSCs (mIPSCs) (SI Appendix, Fig. S7) (GFP: 2.77 ± 0.36 Hz vs. shBCAN: 5.11 ± 0.26 Hz; n = 8 cells, 3 mice, P < 0.001, Student’s t test, Fig. 4C). Furthermore, overexpression of Hapln1 significantly decreased both sIPSC and mIPSC frequency compared with the control (SI Appendix, Fig. S7). All these results indicate that manipulation of PNN expression surrounding PV interneurons in hippocampus alters GABA release and in turn affects pyramidal neurons.
In addition, the paired-pulse ratio (PPR) can be determined by 2 stimulations separated by an interval of several hundred milliseconds (100 ms). To investigate whether GABALTP in the ChABC-treated mice is mediated by pre- or postsynaptic mechanisms, we assessed changes in the PPR of eIPSC at 10 min before TBS (baseline recording) and between 30 and 40 min after TBS. After TBS, the PPR had significantly increased in the ChABC-treated mice (n = 16 cells, 5 mice, baseline: 0.64 ± 0.09; TBS: 0.86 ± 0.11, P < 0.05, Fig. 4D), suggesting that TBS-induced GABAergic plasticity changes were likely due to an decrease in the presynaptic release probability (33). Taken together, these results suggest that PNN expression in the hippocampus is an important regulatory mechanism for inhibitory neuron transmission and, in turn, regulation of hippocampal neuronal plasticity.
Effect of PNNs on Theta Oscillations after CFC Training.
In a previous study, it has been reported that theta oscillations are involved in learning and memory (34), particularly in the parvalbumin interneurons of the hippocampus (35). However, the role of PNNs in theta oscillations after CFC training remains unknown. To bridge this gap in the literature, we performed local field potential (LFPs) recordings in both hippocampus and ACC of freely behaving mice subjected to contextual fear conditioning in the home cage. In vivo multichannel recordings were used to measure LFPs in the hippocampus before and 22 h after the fear conditioning training in either control or ChABC- or Hapln1-treated mice (SI Appendix, Fig. S8). Our data showed that CFC training significantly increased the power of the theta waves (n = 11 mice, P < 0.05, 2-way repeated-measures ANOVA, SI Appendix, Fig. S8A), and ChABC treatment prevented this CFC training-induced theta power enhancement (n = 11 mice, P > 0.05, 2-way repeated-measures 2-way ANOVA, SI Appendix, Fig. S8). In contrast, the Hapln1 treatment significantly enhanced the CFC training-induced theta wave power increment compared to the control group (n = 10 mice, P < 0.05, 2-way repeated-measures ANOVA, SI Appendix, Fig. S8). For particular 4- to 8-Hz band power of recent memory recording, the Hapln1-treated mice significantly increased the enhancement of theta activity 1 d after training compared to the GFP control, which was normalized to the pretrained mice (n = 10 to 11 mice for each group, P < 0.05, 1-way ANOVA followed by post hoc testing, SI Appendix, Fig. S8). To further investigate the effect of PNNs on theta oscillations during remote memory, we similarly recorded LFPs in the ACC 30 d after CFC training. We found that increasing PNNs by Hapln1 significantly increased the enhancement of theta activity 30 d after fear training compared to the GFP control (n = 8 mice for each group, P < 0.05, 1-way ANOVA followed by post hoc testing, SI Appendix, Fig. S8). Taken together, these data suggest that PNNs can enhance theta oscillations in both the hippocampus and ACC which may contribute to the protection of recent and remote long-term memory.
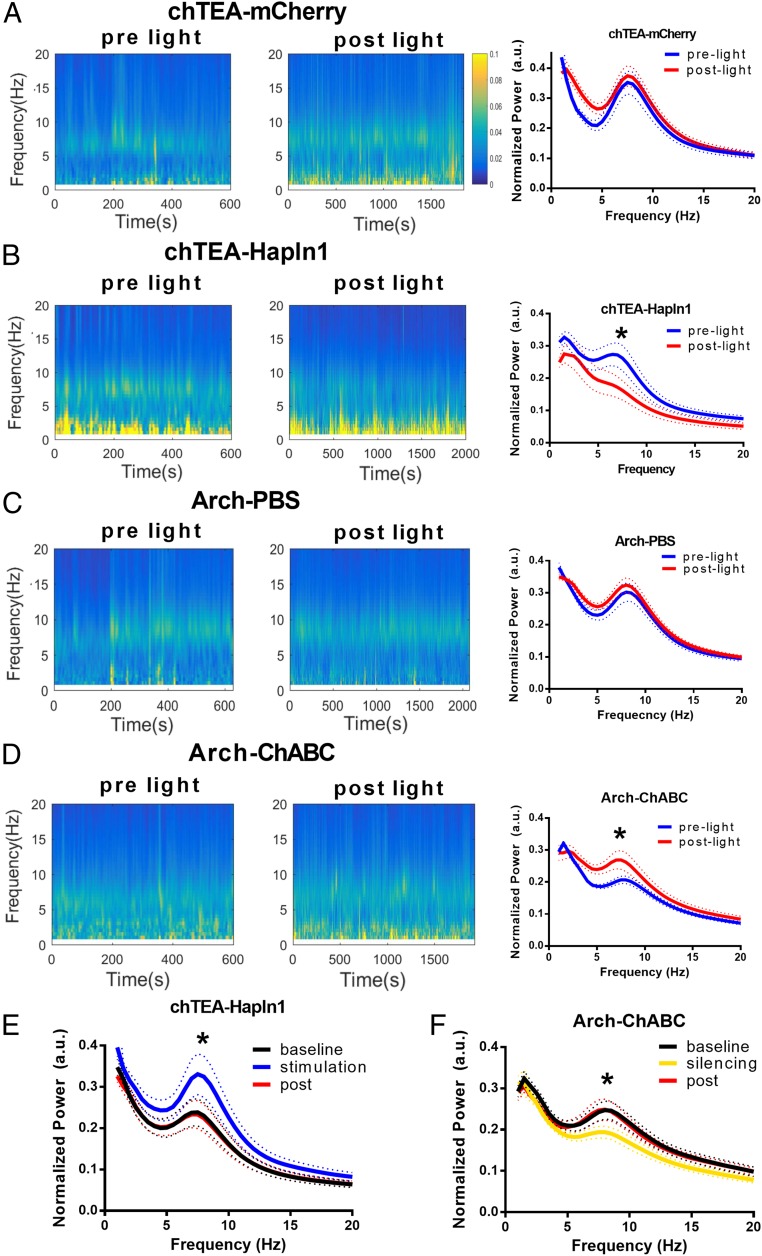
To study the specific role of PV interneurons and PNNs on theta oscillations after CFC training, we performed LFP recordings in the hippocampus of freely behaving mice 22 h after CFC training in the control, ChABC-treated, and Hapln1-treated mice while optogenetically stimulating or silencing PV interneurons. First, the expression of either the activating opsin chTEA or the silencing opsin Arch was driven by Cre-inducible AAVs in the hippocampus of PV-cre mice (35). A high degree of specificity of chTEA and Arch expression was obtained in the CA1 regions (see Fig. 6, Left). Next, we compared the theta oscillations before and after stimulation of the chTEA-expressing PV interneurons 22 h after CFC training. After light stimulation of the chTEA-expressing PV cells, the network theta frequency oscillations did not change in the control group (P > 0.05, 2-way repeated-measures ANOVA, Fig. 5A). However, theta wave power significantly decreased after light stimulation of the chTEA-expressing PV cells in Hapln1-treated mice (n = 5 mice, P < 0.05, 2-way repeated-measures ANOVA, Fig. 5 B and E). These results indicated that the overexpression of PNNs by Hapln1 limited the feedback inhibition, with high power of theta oscillations during the prelight baseline recording after the CFC training. After light stimulation of PV interneurons, feedback inhibition increased, which, in turn, decreased the power of theta oscillations afterward. On the other hand, LFP power showed no change at theta frequency after the transient light silencing of Arch-expressing PV cells compared to the baseline recording before light in the control group (n = 5 mice, P > 0.05, 2-way repeated-measures ANOVA, Fig. 5C). Meanwhile, we found that LFP power significantly increased at theta frequency after silencing of the arch-expressing PV cells compared to the baseline recording before light in the ChABC-treated mice (n = 5 mice, P < 0.05, 2-way repeated-measures ANOVA, Fig. 5D). These results indicated that removal of PNNs by ChABC increased feedback inhibition with low power of the theta wave during the baseline recording after the CFC training. After light silencing of PV interneurons, feedback inhibition decreased, which in turn increased the power of the theta wave afterward. Meanwhile, LFP power showed no change at theta frequency after the light stimulation of chTEA-expressing PV cells in the ChABC treatment group and after the light silencing of Arch-expressing PV cells in the hapln1 treatment group (SI Appendix, Fig. S9). Finally, we tested the effects of stimulation with chTEA-expressing or silencing Arch-expressing PV interneurons on theta oscillations, similar to a previous study (35). LFP theta power was increased or decreased in the Hapln1- or ChABC-treated mice, respectively (n = 5 mice, P < 0.05, 2-way repeated-measures ANOVA, Fig. 5 E and F).
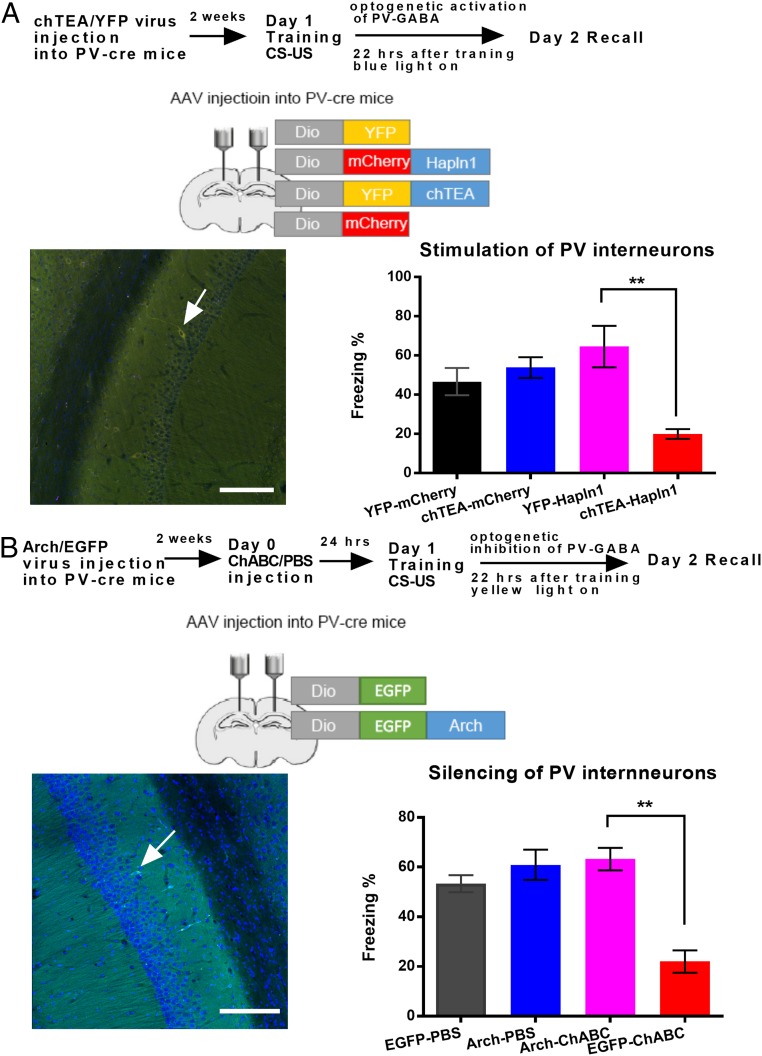
Fig. 6.
Optogenetic manipulation of PV interneurons and PNN on fear memory consolidation. (A, Top) Schematic drawing of the experimental design for contextual fear conditioning, indicating the effect of PNN on the optogenetic activation of PV interneurons. (A, Left) Schematic drawing of the virus injection on the hippocampus. (Scale bars: 100 µm.) (A, Right) Contextual fear memory test after optogenetic activation of PV interneurons in the mCherry- and Hapln1-treated groups. n = 8 for YFP-hapln1 and chTEA-hapln1; n = 11 to 12 for YFP and chTEA-mCherry. (B, Top) Schematic drawing of the experimental design for contextual fear conditioning, indicating the effect of PNN on the optogenetic inhibition of PV interneurons. (B, Left) Schematic drawing of the virus injection on the hippocampus. (Scale bars: 100 µm.) (B, Right) Contextual fear memory test after optogenetic inhibition of PV interneurons in the PBS- and ChABC-treated groups. n = 8 for Arch-ChABC and n = 11 for EGFP-ChABC; n = 10 for EGFP-PBS for Arch-PBS. All data are expressed as the mean ± SEM. Significance was tested by 1-way ANOVA followed by post hoc analysis. **P < 0.01.
Fig. 5.
Optogenetic manipulations of PV interneurons affected the theta oscillations after overexpression or removal of PNN. (A, Left) Representative spectrograms of hippocampal LFPs before and after light stimulation in the chTEA-YFP group. (A, Right) Normalized power spectrum of hippocampus LFPs recorded before and after light stimulation in the chTEA-YFP group. (B, Left) Representative spectrograms of hippocampal LFPs before and after light stimulation in the chTEA-Hapln1–treated group. (B, Right) Normalized power spectrum of hippocampus LFPs recorded before and after light stimulation in the chTEA-Hapln1 group. (C, Left) Representative spectrograms of hippocampal LFPs before and after light inhibition in the Arch-PBS group. (C, Right) Normalized power spectrum of hippocampal LFPs recorded before and after light inhibition in the control group. (D, Left) Representative spectrograms of hippocampal LFPs before and after light inhibition in the Arch-ChABC group. (D, Right) Normalized power spectra of hippocampal LFPs recorded before and after light inhibition in the Arch-ChABC group. (E) Normalized power spectra for chTEA-expressing PV interneurons activation in the chTEA-Hapln1 group, showing a significant increase in the frequency of the oscillations (dotted lines: SEM). (F) Normalized power spectra for Arch-expressing PV interneurons silenced in the Arch-ChABC group, showing a significant decrease in the power of the oscillations (dotted lines: SEM). a.u., arbitrary unit. All data are expressed as the mean ± SEM. Significance was tested by 2-way repeated-measures ANOVA, n = 5 mice for each group, *P < 0.05.
Optogenetic Manipulation of PV Interneurons and PNNs on Fear Memory Consolidation.
To further investigate the role of PV interneurons in hippocampal circuits after manipulation of PNNs, we optogenetically activated the chTEA-expressing PV interneurons 22 h after CFC training in either the mCherry control or Hapln1-transfected groups. Under control conditions, transient light activation of the chTEA PV interneurons during memory consolidation did not affect normal 24-h fear memory recall in comparison with the YFP-mCherry group (chTEA-mCherry: 53.85 ± 4.55%; YFP-mCherry: 46.67 ± 5.54%, P > 0.05, 1-way ANOVA followed by post hoc testing, n = 11 to 12 for each group; Fig. 6 A, Right). However, light activation of the PV interneurons in PV-Hapln1–overexpressing mice not only prevented hapln1 treatment-induced memory recall enhancement but also significantly impaired 24-h fear memory recall in comparison with Hapln1-treated mice without activation of PV interneurons (chTEA-Hapln1: 19.97 ± 3.55%; YFP-Hapln1: 71.17 ± 4.54%, P < 0.01, 1-way ANOVA followed by post hoc testing, n = 8 for each group; Fig. 6 A, Right). Taking into account the impairment of the theta wave in the PV-Hapln1 group after stimulation of chTEA-expressing PV interneurons in the LFP recording, these results demonstrated that rebound activity of PV terminals surrounding increasing PNNs after light stimulation could impair the theta wave and fear memory.
To further investigate the protective role of PNNs in the PV interneuron circuits, we optogenetically inhibited the Arch-expressing PV interneurons 22 h after CFC training in PBS and ChABC-treated mice. We found that removal of PNNs by ChABC still impaired 24-h fear memory in EGFP mice (EGFP-PBS: 53.97 ± 5.55% vs. EGFP-ChABC: 22.01 ± 4.48%, P < 0.01, 1-way ANOVA followed by post hoc testing, n = 10 to 11 for each group; Fig. 6 B, Right); however, the memory impairment induced by ChABC treatment could be rescued by optogenetically inhibiting PV interneurons (Arch-PBS: 64.73 ± 9.73% vs. Arch-ChABC: 66.29 ± 3.73%, 1-way ANOVA followed by post hoc testing, P > 0.05, n = 8 to 10 for each group; Fig. 6 B, Right). Taken together, these results demonstrated that PV basket cells surrounding PNNs could prevent memory loss by limiting the inhibition of pyramidal cells in the CA1 of the hippocampus.
Discussion
Multidisciplinary approaches were employed in the present study to address the contributions of PNNs to synaptic plasticity of GABAergic transmission and, subsequently, to contextual fear memory. We report the following findings. First, contextual fear conditioning training significantly increased the number of PV cells surrounded by PNNs in the hippocampus. Second, in the contextual fear condition, PNNs protected recent and remote memory during the memory consolidation and reconsolidation processes. Third, dissolving PNNs by ChABC treatment in mice not only impaired normal LTP, which could be rescued by blockade of GABAergic transmission, but also switched normal TBS-induced hippocampal CA1 GABAergic LTD to LTP. Furthermore, the PPR showed a presynaptic decrease in GABA release after TBS in ChABC-treated mice. Fourth, the activity level of PV interneurons surrounded by PNNs regulated theta oscillations during fear memory consolidation. Finally, optogenetically inhibiting PV interneurons rescued memory impairment caused by removal of PNNs. Taken together, the results unveil the functions of PNNs of PV neurons in the protection of long-term memory by regulating presynaptic GABA release and network oscillation.
Over the past 20 y, many hypotheses have been proposed for memory storage, including synaptic theory, memory engram cells (36) and the ECM (5). The most conspicuous form of ECM is the PNN (37). PNNs have certain physicochemical properties, including ion-buffering capacity (38), diffusion parameters (39), and binding properties for a variety of regulatory factors (40). Our current study suggests that removal of PNNs by ChABC may affect the above properties and further impair memory consolidation. However, the overexpression of cartilage-linked protein in the PV interneurons before training did not enhance 24-h memory consolidation. The cause may be a ceiling effect of PNNs expression or differential memory decay between the Hapln1 and mCherry control groups. We further tested contextual fear memory 14 d after training and found that fear memory resisted decay in the Hapln1 group. These results unveil the enhancing role of increased PNNs on the recent memory decay and reconsolidation processes.
Several proposals of underlying cellular mechanisms of the PNN effects (41), such as physical barrier to the formation of new synaptic connections and scaffold for binding other molecules, on neural plasticity and memory consolidation have been well studied (42–44). However, one of the proposals about the function of PNNs on the feedback inhibition to the pyramidal neuron on the synaptic plasticity has not been well studied (41). Although PNNs are highly involved in enhancing the excitatory input to regulate the excitatory–inhibitory balance of CA2 pyramidal neurons (45), which is important for inducing the critical period for experience-dependent neural plasticity (46), the modulation of inhibitory inputs from PV interneuron by PNNs was still under debate. Our studies demonstrated that manipulation of PNNs could regulate PV interneuron output and GABA release properties to CA1 pyramidal neurons. Fovuzzi et al. (30) reported the intrinsic properties of PV interneurons and inputs of these PV neurons after manipulation of PNNs. Recent studies reported in PNAS have investigated the structure maturation of cortical PNNs by superresolution images (44). All these recent exciting studies demonstrate the structural role of PNNs on PV interneuron inhibitory connections; however, few studies have investigated the feedback inhibition by PV interneurons and the network mechanism of pyramidal neurons. Given that CFC training can decrease the inhibitory tone of PV basket cells in the hippocampus (22), our results demonstrated that PNNs may protect long-term memory through limitation of feedback inhibition of PV interneurons. This increased inhibition in ChABC-treated mice can readily induce homosynaptic LTD, consistent with a previous study (47), in the adult CA1, which induces the depotentiation process at the cellular level (reverse LTP in the adult CA1) and continues to the memory process in vivo.
The current study has shown that the degradation of PNNs in the hippocampus increases the activity of PV interneurons. However, previous studies have shown that degradation of PNNs in the visual and prefrontal cortex decreases activity from PV interneurons (11, 46, 48). These conflicts may be due to different network mechanisms involved in the hippocampus and cortex. Since PNNs have been reported to enwrap the soma and dendrites of PV interneurons in the hippocampus (49), they may affect the intrinsic properties of these PV interneurons, further affecting their function in feedback inhibition and theta oscillations. A previous study has shown that deletion of the core protein of PNNs enhanced the excitability of PV interneurons by decreasing the action potential threshold and, in turn, increasing the feedback inhibition to the CA1 pyramidal neurons (30). Another study has also shown that removal of PNNs reduces the excitability of pyramidal neurons in CA1 of hippocampus (32). Our in vitro and in vivo electrophysiology recordings indeed showed that removal of PNNs increased the rate of sIPSCs in CA1 pyramidal neurons (Fig. 4) and induced the rebound theta activity after optogenetically silencing the arch terminal of PV interneurons, which resulted in a rescuing effect of memory impairment by ChABC treatment (Figs. 5D and 6B). On the other hand, overexpression of PNNs limited the feedback inhibition resulting in high theta oscillation after training. After the optogenetic stimulation of the PV interneurons, the sustained theta activity during the light induced a reduction in theta oscillations afterward, which resulted in memory impairment (Figs. 5B and 6A). These results indicated that PNNs exert a protective effect through limitation of GABAergic inhibition and plasticity, which is consistent with the LTP results that blockade of GABAA receptors by picrotoxin could rescue the impairment of LTP induced by ChABC (Fig. 4A). These results demonstrate that the PV interneuron surrounding PNNs may play an important role in feedback inhibition of hippocampal pyramidal neurons.
We demonstrated that PNNs in the ACC are also critically involved in the regulation of the storage and transfer of remote memory consolidation. Remote memory recall regulated by PNNs has been previously reported only in the visual cortex (28) and in the adult auditory cortex (10); however, few studies have investigated the functional role of PNNs in the ACC. The hippocampus and the cortex also have different functions and regulatory mechanisms based on their different structures and locations in brain networks and their involvement in learning and memory processes. Our findings that PNNs in the ACC also critically regulate remote memory, in addition to their function in the hippocampus in recent memory, further demonstrated and supported Roger Tsien’s early hypothesis (5) that the expression of PNNs surrounding PV interneurons is important in memory consolidation and storage.
In conclusion, this study reveals the important contribution of hippocampal and cortical PNNs to contextual fear memories. Here, we show that increasing PNNs in both the hippocampus and ACC enhances the consolidation and reconsolidation of recent and remote fear memory, respectively, with a concurrent enhancement of the theta oscillations. Furthermore, eliminating PNN expression was able to impair the consolidation and reconsolidation of recent and remote fear memory by increasing feedback inhibition and impairing theta oscillations. Together, our results suggest that PNNs of PV interneurons within both the hippocampus and ACC exert important modulatory effects on behavioral plasticity during the consolidation as well as the reconsolidation of contextual fear memory by regulating feedback inhibition.
Materials and Methods
Animal and Mouse Strains and Genotyping.
PV-Cre knockin mice express Cre recombinase in parvalbumin-expressing neurons without disrupting endogenous parvalbumin expression [strain name B6.129P2-Pvalbtm1(cre)Arbr/J] come from The Jackson Laboratory (JAX 017320). The laboratory animal facility was accredited by the Institutional Animal Care and Use Committee. Tsinghua University approved all animal protocols used in this study.
ChABC Treatment.
Protease-free chondroitinase ABC (ChABC, C3667, Sigma) was dissolved in filtered 0.1 M PBS to a final injection concentration of 200 U/mL. Detailed protocols are described in SI Appendix, Materials and Methods.
Immunohistochemistry, Electrophysiology, and Contextual Fear Conditioning Test.
Twenty-four hours after ChABC or PBS injection, adult mice were submitted to discriminative fear conditioning by pairing the conditional stimulation (CS) (conditioning box) with 5 unconditional stimulation (US) (a 2-s foot shock at 0.8 mA, intertrial interval: 60 s). Immunohistochemistry, electrophysiology, and contextual fear protocol are described in detail in SI Appendix, Materials and Methods.
Data Availability Statement.
All data are included in the manuscript and the supporting information.
Supplementary Material
Acknowledgments
We thank Lianzhang Wang and Zuolei Xie for their assistance with the behavioral experiment. We also thank Xinchen Chen, Dr. Yunlong Liu, Bo Lei, Hang Qi, Xiaoya Su, and Yikai Tang for their assistance on the immunohistochemistry and ChABC injection experiment. We are grateful to the Imaging Core Facility, Tsinghua University for providing Imaris software technical support and Shaoling Qi (Olympus, Beijing, China) for assistance with confocal microscopy and image processing. We thank Dr. Nashat Abumaria, Dr. Bing Huang, Dr. Yong Lu, and Dr. Yi Zhong for their critical comments and support on the manuscript. This work was funded by postdoctoral grant 2015M581073 from the Chinese National Postdoctoral Foundation (to W.S.), partially funded by National Science Foundation of China grants 61771282 and 31771188 to J.S. and Y.W., Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), and ZJLab.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902680116/-/DCSupplemental.
References
- 1.Kandel E. R., The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 5, 14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanhueza M., Lisman J., The CaMKII/NMDAR complex as a molecular memory. Mol. Brain 6, 10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacktor T. C., How does PKMζ maintain long-term memory? Nat. Rev. Neurosci. 12, 9–15 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Pi H. J., Lisman J. E., Coupled phosphatase and kinase switches produce the tristability required for long-term potentiation and long-term depression. J. Neurosci. 28, 13132–13138 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsien R. Y., Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc. Natl. Acad. Sci. U.S.A. 110, 12456–12461 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertolotto A., Manzardo E., Guglielmone R., Immunohistochemical mapping of perineuronal nets containing chondroitin unsulfated proteoglycan in the rat central nervous system. Cell Tissue Res. 283, 283–295 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Brückner G., Szeöke S., Pavlica S., Grosche J., Kacza J., Axon initial segment ensheathed by extracellular matrix in perineuronal nets. Neuroscience 138, 365–375 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Morris N. P., Henderson Z., Perineuronal nets ensheath fast spiking, parvalbumin-immunoreactive neurons in the medial septum/diagonal band complex. Eur. J. Neurosci. 12, 828–838 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Gogolla N., Caroni P., Lüthi A., Herry C., Perineuronal nets protect fear memories from erasure. Science 325, 1258–1261 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Banerjee S. B., et al. , Perineuronal nets in the adult sensory cortex are necessary for fear learning. Neuron 95, 169–179.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slaker M., et al. , Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J. Neurosci. 35, 4190–4202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romberg C., et al. , Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J. Neurosci. 33, 7057–7065 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezaire M. J., Soltesz I., Quantitative assessment of CA1 local circuits: Knowledge base for interneuron-pyramidal cell connectivity. Hippocampus 23, 751–785 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulyás A. I., Megías M., Emri Z., Freund T. F., Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J. Neurosci. 19, 10082–10097 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pouille F., Scanziani M., Routing of spike series by dynamic circuits in the hippocampus. Nature 429, 717–723 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Sohal V. S., Zhang F., Yizhar O., Deisseroth K., Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donato F., Chowdhury A., Lahr M., Caroni P., Early- and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning. Neuron 85, 770–786 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Schlingloff D., Káli S., Freund T. F., Hájos N., Gulyás A. I., Mechanisms of sharp wave initiation and ripple generation. J. Neurosci. 34, 11385–11398 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaral O. B., Luft T., Cammarota M., Izquierdo I., Roesler R., Temporary inactivation of the dorsal hippocampus induces a transient impairment in retrieval of aversive memory. Behav. Brain Res. 180, 113–118 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Bustos S. G., Maldonado H., Molina V. A., Midazolam disrupts fear memory reconsolidation. Neuroscience 139, 831–842 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Makkar S. R., Zhang S. Q., Cranney J., Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology 35, 1625–1652 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donato F., Rompani S. B., Caroni P., Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Wang H., Hu Y., Tsien J. Z., Molecular and systems mechanisms of memory consolidation and storage. Prog. Neurobiol. 79, 123–135 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Frankland P. W., Bontempi B., Talton L. E., Kaczmarek L., Silva A. J., The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304, 881–883 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Maviel T., Durkin T. P., Menzaghi F., Bontempi B., Sites of neocortical reorganization critical for remote spatial memory. Science 305, 96–99 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Takehara K., Kawahara S., Kirino Y., Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J. Neurosci. 23, 9897–9905 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridderinkhof K. R., Ullsperger M., Crone E. A., Nieuwenhuis S., The role of the medial frontal cortex in cognitive control. Science 306, 443–447 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Thompson E. H., et al. , Removal of perineuronal nets disrupts recall of a remote fear memory. Proc. Natl. Acad. Sci. U.S.A. 115, 607–612 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brückner G., et al. , Acute and long-lasting changes in extracellular-matrix chondroitin-sulphate proteoglycans induced by injection of chondroitinase ABC in the adult rat brain. Exp. Brain Res. 121, 300–310 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Favuzzi E., et al. , Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 95, 639–655.e10 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Squire L. R., Kandel E. R., Memory: From Mind to Molecules (Roberts &Co., ed. 2, 2009). [Google Scholar]
- 32.Minge D., et al. , Heparan sulfates support pyramidal cell excitability, synaptic plasticity, and context discrimination. Cereb. Cortex 27, 903–918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesuis S. L., Lucassen P. J., Krugers H. J., Early life stress impairs fear memory and synaptic plasticity; a potential role for GluN2B. Neuropharmacology 149, 195–203 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Backus A. R., Schoffelen J. M., Szebényi S., Hanslmayr S., Doeller C. F., Hippocampal-prefrontal theta oscillations support memory integration. Curr. Biol. 26, 450–457 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Amilhon B., et al. , Parvalbumin interneurons of Hippocampus tune population activity at theta frequency. Neuron 86, 1277–1289 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Liu X., et al. , Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brückner G., et al. , Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8, 183–200 (1993). [DOI] [PubMed] [Google Scholar]
- 38.Härtig W., et al. , Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 842, 15–29 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Morawski M., et al. , Ion exchanger in the brain: Quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci. Rep. 5, 16471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beurdeley M., et al. , Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J. Neurosci. 32, 9429–9437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galtrey C. M., Fawcett J. W., The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Brain Res. Rev. 54, 1–18 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Carstens K. E., Phillips M. L., Pozzo-Miller L., Weinberg R. J., Dudek S. M., Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J. Neurosci. 36, 6312–6320 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deepa S. S., Umehara Y., Higashiyama S., Itoh N., Sugahara K., Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors. Implications as a physiological binding partner in the brain and other tissues. J. Biol. Chem. 277, 43707–43716 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Sigal Y. M., Bae H., Bogart L. J., Hensch T. K., Zhuang X., Structural maturation of cortical perineuronal nets and their perforating synapses revealed by superresolution imaging. Proc. Natl. Acad. Sci. U.S.A. 116, 7071–7076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayani H., Song I., Dityatev A., Increased excitability and reduced excitatory synaptic input into fast-spiking CA2 interneurons after enzymatic attenuation of extracellular matrix. Front. Cell. Neurosci. 12, 149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lensjø K. K., Lepperød M. E., Dick G., Hafting T., Fyhn M., Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J. Neurosci. 37, 1269–1283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner J. J., Alger B. E., GABAergic and developmental influences on homosynaptic LTD and depotentiation in rat hippocampus. J. Neurosci. 15, 1577–1586 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tewari B. P., et al. , Perineuronal nets decrease membrane capacitance of peritumoral fast spiking interneurons in a model of epilepsy. Nat. Commun. 9, 4724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Celio M. R., Perineuronal nets of extracellular matrix around parvalbumin-containing neurons of the hippocampus. Hippocampus 3, 55–60 (1993). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and the supporting information.