Significance
Itch signal processing is dynamically modulated. However, the neural mechanism underlying the modulation of itch processing at the spinal level remains elusive. Here, we studied the inhibitory control of spinal itch circuitry. We demonstrated that, in addition to local inhibitory neurons, long-range inhibitory neurons also play a critical role in modulating the spinal itch circuit by forming inhibitory synapses with spinal GRPR+ neurons, which are known to be essential for itch processing in the spinal cord. Our study revealed the mechanism underlying the inhibitory control of the spinal itch circuit.
Keywords: spinal GRPR+ neurons, inhibitory control, galanin+ GABAergic neurons, rostral ventromedial medulla, gating itch processing
Abstract
Spinal gastrin-releasing peptide receptor-expressing (GRPR+) neurons play an essential role in itch signal processing. However, the circuit mechanisms underlying the modulation of spinal GRPR+ neurons by direct local and long-range inhibitory inputs remain elusive. Using viral tracing and electrophysiological approaches, we dissected the neural circuits underlying the inhibitory control of spinal GRPR+ neurons. We found that spinal galanin+ GABAergic neurons form inhibitory synapses with GRPR+ neurons in the spinal cord and play an important role in gating the GRPR+ neuron-dependent itch signaling pathway. Spinal GRPR+ neurons also receive inhibitory inputs from local neurons expressing neuronal nitric oxide synthase (nNOS). Moreover, spinal GRPR+ neurons are gated by strong inhibitory inputs from the rostral ventromedial medulla. Thus, both local and long-range inhibitory inputs could play important roles in gating itch processing in the spinal cord by directly modulating the activity of spinal GRPR+ neurons.
Itch is an unpleasant sensation that evokes a desire for scratching, which in turn causes severe skin or tissue damage in patients with chronic itch (1–3). The spinal cord plays an important role in relaying itch information from peripheral tissue to the brain (2, 4, 5). Early electrophysiological studies showed that spinal neurons are multimodal (6, 7). Recently, several key components of the spinal circuits for chemical and mechanical itch have been identified in molecular and cellular studies (8–13), some of which have indicated that there are itch-selective neurons in the spinal cord. It has been shown that gastrin-releasing peptide receptor-expressing (GRPR+) neurons in the dorsal spinal cord are critical for chemical itch but not nociceptive signal processing (8, 9). However, little is known about the circuit mechanism underlying the regulation of spinal GRPR+ neurons.
Spinal inhibitory interneurons play an important role in gating sensory information processing (5, 14, 15). Spinal inhibitory interneurons can be classified into 4 largely nonoverlapping subpopulations; namely, neurons that express galanin, those that express neuronal nitric oxide synthase (nNOS), those that express parvalbumin (PV), and those that express neuropeptide Y (NPY) (16, 17). Recent studies have started to reveal the functional role of spinal inhibitory neurons in sensory processing (15, 18–22). It has been shown that spinal NPY-expressing (NPY+) neurons modulate the mechanical itch pathway by gating spinal urocortin 3+ and NPY1R+ neurons (12, 13, 20). In addition, genetic studies suggest a tonic inhibition of the spinal itch pathway by Bhlhb5-expressing interneurons (22, 23). These Bhlhb5-expressing interneurons modulate itch transmission through both the fast inhibitory neurotransmitter gamma-aminobutyric acid (GABA)/glycine and the slow inhibitory neuromodulator dynorphin (22). The dynorphin-expressing neurons likely modulate itch processing via suppressing spinal GRPR+ neurons (21). It remains unknown how the different subtypes of spinal inhibitory neurons directly modulate spinal GRPR+ neurons.
In addition, descending pathways can dynamically regulate the spinal circuitry underlying itch processing. It has been shown that the periaqueductal gray is involved in modulating spinal pruritic processing by descending pathways via the rostral ventromedial medulla (RVM) (24, 25). Moreover, serotonergic neurons in the RVM facilitate spinal itch transmission by activating spinal GRPR+ neurons via 5-HT1A receptors (26). Given the diversity of cell types in the RVM and the complexity of the GABAergic modulation of spinal circuits (27–29), it remains to be determined how excitatory or inhibitory neurons in the RVM modulate the activity of spinal GRPR+ neurons.
Here, we investigated the mechanisms underlying the modulation of spinal GRPR+ neurons. We identified the subtype of local inhibitory neurons that is critical for modulating itch signal processing by gating the activity of spinal GRPR+ neurons, and demonstrated the direct inhibitory control of spinal GRPR+ neurons by the RVM.
Results
Functional Role of Different Subtypes of Spinal GABAergic Neurons in Itch Processing.
Previous studies have indicated that spinal inhibitory neurons play an important role in regulating itch processing (5). We first confirmed the functional role of spinal GABAergic neurons in itch modulation with a pharmacogenetic approach. We injected an adeno-associated virus (AAV) expressing hM3Dq, a designer receptor exclusively activated by designer drug (DREADD) (30), in a Cre-dependent manner into the right side of the cervical dorsal spinal cord of Vgat-Cre mice (SI Appendix, Fig. S1 A and B). The control group was injected with AAV-DIO-EYFP. We found that pharmacogenetic activation of the spinal GABAergic neurons almost completely abolished scratching behavior evoked by intradermal injection of histamine, chloroquine (CQ), and endothelin-1 (ET-1) (SI Appendix, Fig. S1 C–F). By contrast, activation of the GABAergic neurons in the dorsal spinal cord did not significantly affect locomotion, nor affect the scratching behavior evoked by pruritogens injected into the contralateral side (SI Appendix, Fig. S1 G–J). Furthermore, the activation of the GABAergic neurons in the cervical dorsal spinal cord also increased the withdrawal threshold in response to mechanical stimulation (SI Appendix, Fig. S1K). These results confirmed that spinal inhibitory neurons play a critical role in modulating itch processing.
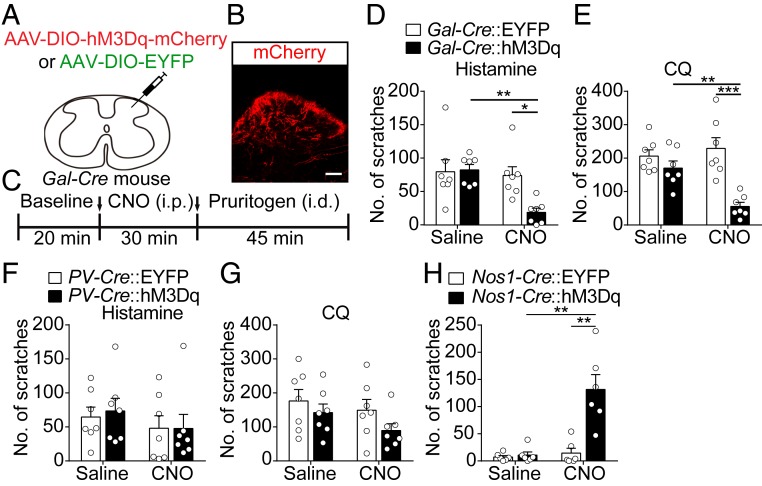
Four subtypes of GABAergic neurons have been identified in the dorsal spinal cord (16). Among these neurons, NPY+ neurons have been shown to gate GRPR+ neuron-independent mechanical itch (20). However, the functional role of other subtypes of inhibitory interneurons, which are labeled with galanin, nNOS, or PV, in itch signal processing remains unclear. We thus examined the functional role of these 3 groups of spinal GABAergic neurons in modulating itch. We injected AAV-DIO-hM3Dq-mCherry or AAV-DIO-EYFP into the right cervical dorsal spinal cord of galanin-Cre (Gal-Cre) mice (Fig. 1A). The hM3Dq-mCherry was expressed in the cervical dorsal spinal cord, but not in the dorsal root ganglion (DRG) (Fig. 1B and SI Appendix, Fig. S2A). In the behavioral test, the pharmacogenetic activation of spinal galanin+ neurons significantly suppressed scratching behavior evoked by both histamine and CQ (Fig. 1 C–E). In contrast, this manipulation did not significantly affect motor function or locomotion (SI Appendix, Fig. S2 B and C). We further determined whether the activation of spinal galanin+ neurons also affects other somatosensations, especially nociception. We found that the pharmacogenetic activation of galanin+ neurons in the lumbar dorsal spinal cord did not significantly affect nociceptive responses to mechanical, thermal, or chemical stimuli (SI Appendix, Fig. S2 D–H), indicating that spinal galanin+ neurons play a negligible role in modulating nociception.
Fig. 1.
Modulation of itch processing by different subtypes of spinal GABAergic neurons. (A) A schematic showing the unilateral injection of AAV-DIO-hM3Dq-mCherry or AAV-DIO-EYFP virus into the dorsal spinal cord of Gal-Cre mice. (B) Image showing mCherry-expressing neurons (red) in the dorsal spinal cord with post hoc immunohistochemistry for mCherry. (Scale bar, 100 μm.) (C) A timeline of the behavioral experiments. (D and E) The effects of the pharmacogenetic activation of galanin+ neurons in the dorsal spinal cord by the injection of CNO (1 mg/kg, i.p.) on scratching behavior induced by histamine (D) or chloroquine (E), n = 7 mice, 2-way ANOVA. (F and G) The effects of the pharmacogenetic activation of PV+ neurons in the dorsal spinal cord by the injection of CNO (1 mg/kg, i.p.) on scratching behavior induced by histamine (F) or chloroquine (G), n = 7 mice, 2-way ANOVA. (H) Behavioral responses after the pharmacogenetic activation of nNOS+ neurons in the dorsal spinal cord, n = 6 mice, 2-way ANOVA. All error bars represent the SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
By the same approach, we examined the role of spinal PV+ neurons in itch processing. We found that activation of the PV+ neurons in the cervical dorsal spinal cord did not significantly affect scratching behavior in response to either histamine or chloroquine (Fig. 1 F and G). Next, we examined the role of spinal nNOS+ neurons. Surprisingly, the pharmacogenetic activation of the nNOS+ neurons in the cervical dorsal spinal cord evoked spontaneous scratching behavior in the absence of pruritogens (Fig. 1H). We found that nNOS and GRPR were coexpressed in a small fraction of neurons in the dorsal spinal cord (SI Appendix, Fig. S3). Thus, the pharmacogenetic activation of nNOS+ neurons could have activated a small proportion of spinal GRPR+ neurons, leading to robust scratching behavior. Taken together, these results indicate that spinal galanin+ GABAergic neurons play a critical role in modulating itch processing.
Ablation of Spinal Galanin+ Neurons Enhanced Itch Processing.
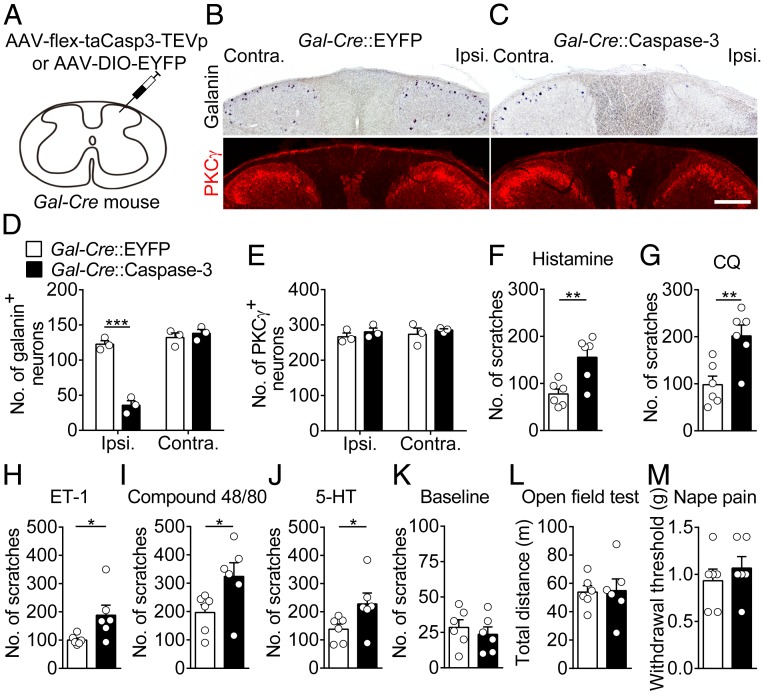
We next asked whether spinal galanin+ neurons are necessary for gating itch processing at the spinal level. To address this question, we examined the effect of ablating the spinal galanin+ neurons on itch processing by a caspase-3-based method (31). We injected AAV-flex-taCasp3-TEVp or AAV-DIO-EYFP into the right cervical dorsal spinal cord of Gal-Cre mice (Fig. 2A). The number of galanin+ neurons in the cervical dorsal spinal cord decreased significantly in mice injected with AAV-flex-taCasp3-TEVp compared with the control group (Fig. 2 B–D). Consistent with the partial overlap between galanin+ and dynorphin+ neurons (17), the number of dynorphin+ neurons also decreased (SI Appendix, Fig. S4 A and B). In contrast, the number of PKCγ+, GRPR+ neurons, and the density of primary sensory fibers did not change significantly (Fig. 2 B, C, and E and SI Appendix, Fig. S4 A, C, and D). The galanin+ neurons in the DRG were not affected (SI Appendix, Fig. S5 A and B).
Fig. 2.
Effects of the ablation of spinal galanin+ neurons on itch signal processing. (A) A schematic showing the unilateral injection of AAV-flex-taCasp3-TEVP or AAV-DIO-EYFP virus into the dorsal spinal cord of Gal-Cre mice. (B and C) A graph showing the distribution of galanin+ and PKCγ+ neurons in the spinal cord of Gal-Cre::EYFP (B) and Gal-Cre::Caspase-3 (C) mice. (Scale bar, 200 μm.) (D and E) A summary of the number of galanin+ (D) and PKCγ+ (E) neurons in the dorsal horn. n = 3 mice, unpaired t test. (F–J) The effects of the ablation of spinal galanin+ neurons on scratching behavior induced by histamine (F), chloroquine (G), endothelin-1 (H), compound 48/80 (I), or serotonin (J). (K) The effects of the ablation of spinal galanin+ neurons on basal scratching behavior without any pruritic agents. (L) The effects of the ablation of spinal galanin+ neurons on locomotor activity. (M) The ablation of galanin+ neurons in the spinal cord did not affect the mechanical threshold in the nape pain test. All error bars represent the SEM. n = 6 mice in each group, Mann–Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001. Contra., contralateral; Ipsi., ipsilateral.
Next, we tested the behavioral effect of ablating spinal galanin+ neurons. We found that the number of scratching bouts evoked by histamine increased significantly after ablation of spinal galanin+ neurons (Fig. 2F), supporting that galanin+ neurons are necessary for gating itch processing at the spinal level. Consistently, this manipulation also significantly increased the scratching behavior induced by CQ, ET-1, compound 48/80, or serotonin (5-HT; Fig. 2 G–J). In contrast, the ablation of spinal galanin+ neurons did not affect the scratching behavior on the contralateral side of virus injection, spontaneous scratching behavior, locomotor activity, or nociceptive responses (Fig. 2 K–M, and SI Appendix, Fig. S5 C and D). These results further support the idea that spinal galanin+ neurons play a critical role in dynamically modulating spinal itch signal transmission.
Verification of GRPR-Flpo Mice.
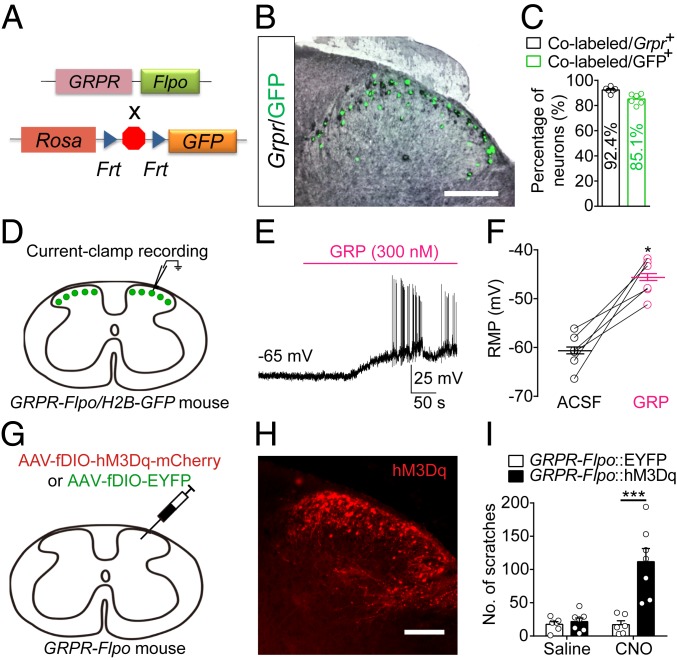
We next aimed to determine the functional synaptic connection between different subtypes of spinal GABAergic neurons and GRPR+ neurons. To achieve this goal, we planned to record from spinal GRPR+ neurons by patch-clamp recording while activating distinct subpopulations of spinal GABAergic neurons with optogenetics. We generated a GRPR-Flpo mouse line, which allowed us to label GRPR+ neurons and GABAergic neurons when crossed with Cre mouse lines specific for different subtypes of GABAergic neurons. We first verified the specificity of the GRPR-Flpo mouse line. By crossing GRPR-Flpo mice with the Flpo-dependent reporter mouse line (32) (Fig. 3A), we found that the distribution of the green fluorescent protein (GFP)-positive neurons in the brain and spinal cord was similar to that of Grpr+ neurons (Fig. 3B and SI Appendix, Fig. S6). Next, we further examined whether these GFP+ neurons in the spinal cord of GRPR-Flpo/H2B-GFP mice were GRPR positive by double staining. We found that 92.4% of Grpr+ neurons in the spinal cord were positive for GFP, and 85.1% of GFP+ neurons in the spinal cord were positive for Grpr (Fig. 3 B and C), indicating the high specificity and efficiency of the GRPR-Flpo mouse line. Consistently, bath application of GRP (300 nM) induced the progressive depolarization of all of the recorded GFP+ neurons in the dorsal spinal cord of GRPR-Flpo/H2B-GFP mice (Fig. 3 D–F).
Fig. 3.
Verification of the GRPR-Flpo mouse line. (A) GRPR-Flpo mice were crossed with H2B-GFP mice. The stop cassette (octagon) was flanked by 2 Frt sites (triangles) and was excised in GRPR+ neurons by Flpo recombinase. (B) The expression of Grpr and GFP in the dorsal spinal cord. (Scale bar, 200 μm.) (C) The percentage of neurons expressing both Grpr mRNA and GFP, n = 6 mice. (D) A schematic showing the experimental configuration for recordings in cervical spinal cord slices of GRPR-Flpo/H2B-GFP mice. (E) Response elicited by bath application of GRP (300 nM) were recorded from a GFP+ neuron in ACSF containing NBQX (10 μM), CPP (5 μM), PTX (50 μM), and STR (2 μM), n = 6 from 2 mice. (F) A summary graph showing resting membrane potential before (black) and after GRP application (magenta), n = 6 from 2 mice, Wilcoxon signed-rank test. (G) A schematic showing the unilateral injection of AAV-fDIO-hM3Dq-mCherry or AAV-fDIO-EYFP virus into the dorsal spinal cord of GRPR-Flpo mice. (H) The expression of hM3Dq-mCherry in the dorsal spinal cord. (Scale bar, 200 μm.) (I) The effects of the pharmacogenetic activation of GRPR+ neurons in the dorsal spinal cord by the injection of CNO (1 mg/kg, i.p.) on scratching behavior, n = 6 to 7 mice in each group, 2-way ANOVA. All error bars represent the SEM. *P < 0.05, ***P < 0.001. RMP, resting membrane potential; GRP, gastrin-releasing peptide.
Previous studies showed that the activation of spinal GRPR evokes scratching behavior (8, 33). We injected the AAV-fDIO-hM3Dq-mCherry into the cervical spinal cord of GRPR-Flpo mice and found that pharmacogenetic activation of the spinal GRPR+ neurons induced robust scratching behavior (Fig. 3 G–I). Moreover, we injected the AAV-fDIO-hM4Di-mCherry into the lumbar spinal cord of GRPR-Flpo mice and found that pharmacogenetic inhibition of the spinal GRPR+ neurons did not significantly affect nociceptive responses to mechanical, thermal, or chemical stimuli (SI Appendix, Fig. S7). These results are consistent with the results from previous studies (8, 9). Together, these results indicate that spinal GRPR+ neurons are specifically labeled with the GRPR-Flpo mouse line.
Local Inhibitory Control of Spinal GRPR+ Neurons.
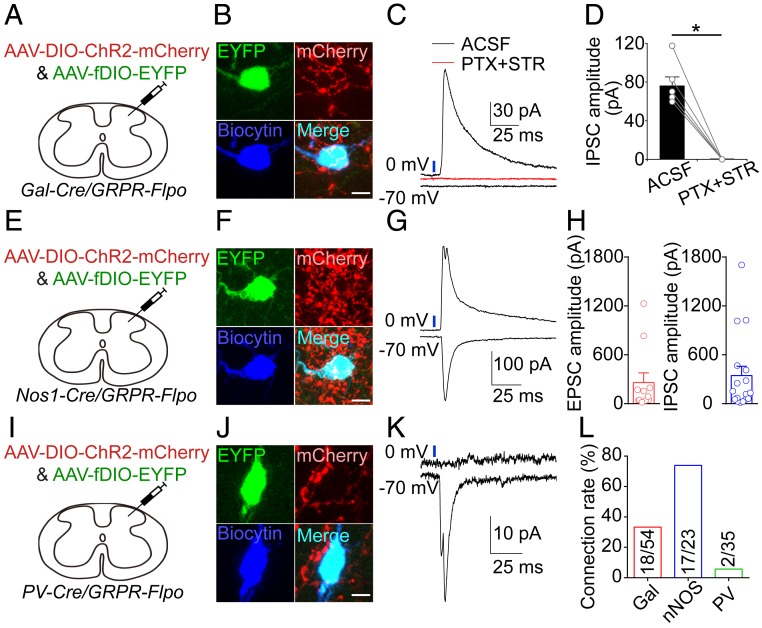
With the GRPR-Flpo mouse line, we next examined the functional synaptic connection between spinal GABAergic neurons and GRPR+ neurons. Given that spinal galanin+ neurons are important for modulating itch processing, we first examined the synaptic connection between spinal galanin+ neurons and GRPR+ neurons. By crossing the GRPR-Flpo mouse line with the Gal-Cre mouse line, we obtained a Gal-Cre/GRPR-Flpo double transgenic mouse line. We injected AAV-DIO-ChR2-mCherry and AAV-fDIO-EYFP virus into the cervical dorsal spinal cord of these mice to selectively express ChR2 in galanin+ neurons and enhanced yellow fluorescent protein (EYFP) in GRPR+ neurons (Fig. 4A). In cervical spinal cord slices, we recorded GRPR+ neurons labeled with EYFP by whole-cell patch-clamp recording. The optogenetic activation of spinal galanin+ neurons evoked inhibitory postsynaptic currents (IPSCs) in GRPR+ neurons, but light-evoked excitatory postsynaptic currents (EPSCs) were not detected (Fig. 4 B and C). The light-evoked IPSCs were blocked by bath application of the GABAA receptor antagonist picrotoxin (PTX) and the glycine receptor antagonist strychnine (STR; Fig. 4 C and D). As GABA and glycine are often coreleased from spinal GABAergic neurons (34), the 2 antagonists were applied together. These results indicate that spinal galanin+ neurons form inhibitory synapses with GRPR+ neurons.
Fig. 4.
Synaptic inputs from local GABAergic neurons to spinal GRPR+ neurons. (A) A schematic showing the unilateral injection of AAV-DIO-ChR2-mCherry and AAV-fDIO-EYFP into the dorsal spinal cord of Gal-Cre/GRPR-Flpo mice. (B) Post hoc staining of a recorded EYFP+ neuron for biocytin (blue) and mCherry+ fibers. (Scale bar, 10 μm.) (C) Responses evoked by the photostimulation of galanin+ neurons or fibers in a spinal GRPR+ neuron in ACSF and in the presence of picrotoxin and strychnine. Blue bar, LED stimulation (475 nm, 1 ms). (D) Summary data showing the amplitude of IPSCs recorded in GRPR+ neurons evoked by the photostimulation of galanin+ neurons or fibers before and after the application of picrotoxin and strychnine, n = 6 neurons from 3 mice, Wilcoxon signed-rank test. *P < 0.05. (E) A schematic showing the injection of AAV-DIO-ChR2-mCherry and AAV-fDIO-EYFP into the spinal cord of Nos1-Cre/GRPR-Flpo mice. (F) Post hoc staining of a recorded EYFP+ neuron for biocytin (blue) and mCherry+ fibers. (Scale bar, 10 μm.) (G) EPSC and IPSC recorded from a GRPR+ neuron after the photostimulation of spinal nNOS+ neurons. Blue bar, LED stimulation (475 nm, 1 ms). (H) A summary of the amplitudes of EPSCs (11/23 neurons) and IPSCs (17/23 neurons) in GRPR+ neurons evoked by the photostimulation of spinal nNOS+ neurons. (I) A schematic showing the injection of AAV-DIO-ChR2-mCherry and AAV-fDIO-EYFP into the spinal cord of PV-Cre/GRPR-Flpo mice. (J) Post hoc staining of a recorded EYFP+ neuron for biocytin (blue) and mCherry+ fibers. (Scale bar, 10 μm.) (K) EPSC recorded from a GRPR+ neuron after the photostimulation of spinal PV+ neurons. Blue bar, LED stimulation (475 nm, 1 ms). (L) A summary of the connection rate from spinal galanin+, nNOS+ or PV+ neurons to GRPR+ neurons. All error bars represent the SEM.
By the same approach, we next examined the synaptic connection between spinal nNOS+ neurons and GRPR+ neurons. The optogenetic activation of spinal nNOS+ neurons evoked both IPSCs and EPSCs in GRPR+ neurons (Fig. 4 E–H), which is consistent with the observation that spinal nNOS+ neurons consist of both inhibitory and excitatory neurons (35). In a small subset of GRPR+ neurons, we also observed direct light-evoked currents, which is consistent with the colocalization of nNOS and GRPR in the dorsal spinal cord, and these neurons were excluded from further analysis. Next, using PV-Cre/GRPR-Flpo double transgenic mice, we examined the synaptic connection between spinal PV+ neurons and GRPR+ neurons. We found that the optogenetic activation of spinal PV+ neurons evoked EPSCs in a small subset of GRPR+ neurons (2 out of 35 neurons, Fig. 4 I–L), and we did not detect light-evoked IPSCs in spinal GRPR+ neurons.
Long-Range Inhibitory Inputs to Spinal GRPR+ Neurons Originated from the RVM.
Descending pathways have been implicated in itch modulation (4, 25, 26). We thus determined whether spinal GRPR+ neurons also receive direct long-range descending inputs from supraspinal regions by using rabies virus tracing (36). We injected helper AAV virus encoding Cre-dependent avian sarcoma leucosis virus glycoprotein EnvA receptor (AAV-flex-TC66T) mixed with AAV virus encoding rabies virus envelope glycoprotein (AAV-flex-RG), followed by injecting RV-EnVA-EGFP in the cervical spinal cord of GRPR-iCreERT2 mice. To induce the Cre recombinase, mice were treated with tamoxifen for 5 consecutive days after injection of AAV-flex-TC66T and AAV-flex-RG. We collected brain and spinal tissue 10 d after the injection of RV-EnVA-EGFP (SI Appendix, Fig. S8 A and B). Neurons coexpressing mCherry and EGFP, identified as starter cells, were restricted to the superficial dorsal horn. We detected some nearby neurons expressing only EGFP, most of which represented the presynaptic partners of GRPR+ neurons in the local circuits (SI Appendix, Fig. S8 C–E), as this modified method has less leakage (36). In the brain, EGFP+ neurons were detected in the RVM, suggesting that RVM neurons form direct synapses with spinal GRPR+ neurons (SI Appendix, Fig. S8G). Consistent with previous observations (26), a small percentage of the presynaptic neurons of spinal GRPR+ neurons in the RVM are serotoninergic (SI Appendix, Fig. S8 F and H–J).
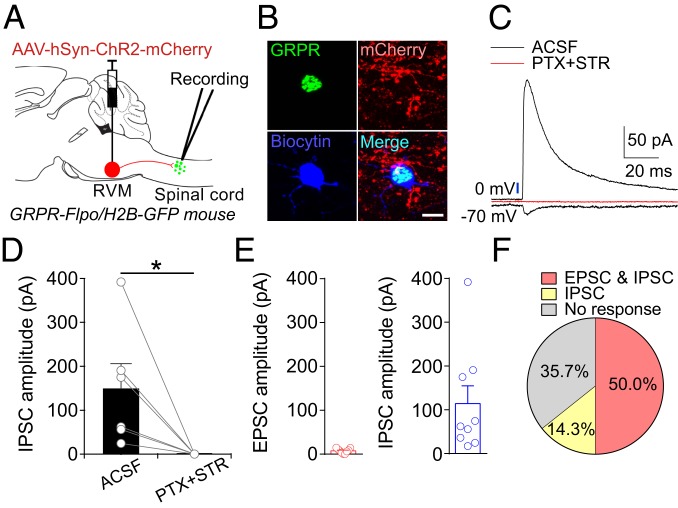
We further examined direct synaptic inputs from the RVM to spinal GRPR+ neurons by slice electrophysiology. We injected AAV-hSyn-ChR2-mCherry into the RVM of GRPR-Flpo/H2B-GFP mice (Fig. 5A) and recorded GRPR+ neurons labeled with GFP in cervical spinal cord slices by whole-cell patch-clamp recording (Fig. 5B). We found that the photostimulation of ChR2+ fibers originating from the RVM evoked predominately IPSCs with small EPSC components in spinal GRPR+ neurons (Fig. 5 C, E, and F). The light-evoked IPSCs were blocked by bath application of the GABAA receptor antagonist picrotoxin and the glycine receptor antagonist strychnine (Fig. 5 C and D). These results support the idea that the RVM can also modulate the activity of spinal GRPR+ neurons via direct inhibitory synaptic connections.
Fig. 5.
Synaptic inputs from the RVM to spinal GRPR+ neurons. (A) A schematic showing the injection of AAV-hSyn-ChR2-mCherry into the RVM of GRPR-Flpo/H2B-GFP mice followed by patch clamp recording of GFP+ neurons in the dorsal spinal cord. (B) Post hoc staining of a recorded GRPR+ neuron for biocytin (blue) and mCherry+ fibers. (Scale bar, 10 μm.) (C) Responses evoked by the photostimulation of ChR2-mCherry+ fibers in a spinal GRPR+ neuron in ACSF and in the presence of picrotoxin and strychnine. Blue bar, LED stimulation (475 nm, 1 ms). (D) Summary data showing the amplitude of IPSCs recorded in GRPR+ neurons evoked by the photostimulation of ChR2-mCherry+ fibers before and after the application of picrotoxin and strychnine, n = 6 neurons from 3 mice, Wilcoxon signed-rank test. *P < 0.05. (E) A summary showing the amplitudes of EPSCs (7/14 neurons) and IPSCs (9/14 neurons) recorded in spinal GRPR+ neurons induced by the photoactivation of ChR2+ fibers from the RVM. (F) The connectivity of spinal GRPR+ neurons receiving different inputs from the RVM is shown. All error bars represent the SEM.
Discussion
In this study, we examined local and long-range inhibitory inputs to the spinal GRPR+ neurons and determined the subtype of spinal inhibitory neurons essential for gating itch processing at the spinal level. Our results revealed that 3 subtypes of spinal GABAergic neurons exhibit diverse inhibitory and excitatory control of GRPR+ neurons. We found that spinal galanin+ neurons mainly form inhibitory synapses with GRPR+ neurons and play an important role in gating the itch pathway. In addition, spinal GRPR+ neurons are also modulated by descending inhibitory inputs from the RVM.
Spinal inhibitory neurons play important roles in dynamically modulating itch signal processing (2, 4, 5). Our results demonstrated that spinal galanin+ neurons play a critical role in suppressing itch processing at the spinal level. This was supported by both electrophysiological and behavioral results. We showed that spinal galanin+ neurons form functional inhibitory synapses with spinal GRPR+ neurons, as evidenced by the observation that the optogenetic activation of spinal galanin+ neurons evoked IPSCs but not EPSCs in spinal GRPR+ neurons. As spinal GRPR+ neurons play a critical role in itch processing (9), galanin+ neurons can dynamically modulate itch processing by modulating the activity of spinal GRPR+ neurons. The important role of spinal galanin+ neurons in itch modulation is further supported by the behavioral results showing that the pharmacogenetic activation of spinal galanin+ neurons suppressed pruritogen-evoked scratching behaviors. Consistently, the ablation of spinal galanin+ neurons amplified the itch signal. This is in line with previous observation that the number of galanin+ neurons is reduced in the spinal cord in Bhlhb5 mutant mice, which exhibit spontaneous scratching behavior (23). However, we did not observe spontaneous scratching behavior after the ablation of spinal galanin+ neurons, although spinal galanin+ neurons account for a large proportion of Bhlhb5-expressing neurons (22). Several reasons may explain this difference. The loss of Bhlhb5 causes a decrease in the number of not only galanin+ neurons but also other subtypes of neurons (22, 23); thus, the spontaneous scratching behavior observed in Bhlhb5 mutant mice may be due to the loss of other types of neurons. Moreover, we ablated galanin+ neurons at the adult stage, while Bhlhb5 mutant mice lack Bhlhb5 congenitally. Furthermore, the region of ablation in the spinal cord was much more restricted in our study. Nevertheless, our results suggest that galanin+ neurons play a key role in dampening strong itch signals, but do not tonically suppress the itch pathway. Although we emphasize the important role of spinal galanin+ neurons in controlling the activity of spinal GRPR+ neurons, we do not exclude the possibility that spinal galanin+ neurons influence itch transmission by targeting other types of neurons in the spinal cord; for example, spinal GRP+ neurons that could also be important for itch processing (37).
Galanin+ neurons in the dorsal spinal cord largely overlap with dynorphin+ neurons (16, 38). This is in line with our observation that the number of spinal dynorphin+ neurons was reduced significantly after the ablation of spinal galanin+ neurons. Moreover, the behavioral results obtained by manipulating galanin+ neurons are consistent with the observation that the pharmacogenetic activation of spinal dynorphin+ neurons also suppresses pruritogen-evoked scratching behaviors (21). Interestingly, the activation of spinal dynorphin+ neurons also enhances mechanical pain (21), whereas we observed no significant effect on pain sensitivity after the pharmacogenetic activation of spinal galanin+ neurons. Given that dynorphin+ neurons consist of both excitatory and inhibitory interneurons, it is likely that excitatory dynorphin+ interneurons play a critical role in modulating mechanical pain (21). However, our study is inconsistent with a study by Duan et al. (39), which showed that itch processing was not affected by the ablation of spinal dynorphin+ neurons. This discrepancy may be due to the difference in strategies used for manipulating the spinal dynorphin+ neurons. The strategy used in the study by Duan et al. might have captured cells that transiently express dynorphin during development.
Spinal interneurons play diverse roles in gating sensory information processing (5, 40, 41). Among the 4 major subtypes of spinal inhibitory neurons, NPY+ neurons have been shown to be important for gating mechanical itch, likely in a GRPR+ neuron-independent manner (12, 13, 20). In our study, we examined the potential role of the other 2 subtypes of spinal inhibitory neurons in addition to galanin+ neurons. We found that spinal PV+ neurons play a negligible role in modulating itch processing, as evidenced by the result showing that the activation of spinal PV+ neurons did not significantly affect pruritogen-evoked scratching behaviors. Consistently, we showed that spinal PV+ neurons only form functional excitatory synapses with spinal GRPR+ neurons with very low probability. Spinal PV+ neurons are likely involved in gating touch-evoked pain (18). In addition, we found that the activation of spinal nNOS+ neurons evoked spontaneous scratching behavior, although a previous study showed that the activation of spinal nNOS+ neurons do not affect itch-related behavior induced by chloroquine (21). This may be due to different virus injection sites and differential labeling efficiency of nNOS+ neurons in the 2 different mouse lines. The spontaneous scratching behavior induced by the activation of the nNOS+ neurons is likely due to the coexpression of nNOS and GRPR. It is worth noting that neurons labeled by a single molecular marker may be functionally heterogeneous, as exemplified by the fact that spinal nNOS+ neurons can be both excitatory and inhibitory. Our results showed that both excitatory and inhibitory nNOS+ interneurons synapse with spinal GRPR+ neurons. It is possible that inhibitory nNOS+ interneurons might also be involved in modulating itch signal processing. However, given the overlap between nNOS+ and GRPR+ neurons at the spinal level, the effect of inhibitory nNOS+ interneurons may have been masked by excitatory nNOS+ interneurons in the behavioral tests. Therefore, the involvement of different subsets of spinal nNOS+ neurons in itch signal processing remains to be further characterized.
The long-range descending pathway also plays an important role in modulating the spinal itch signal processing (25). Our results showed that RVM neurons form functional inhibitory synapses with spinal GRPR+ neurons. As synaptic inputs from the RVM to the spinal GRPR+ neurons are largely inhibitory, the RVM can directly suppress spinal itch processing. However, our previous study suggested that the RVM plays a critical role in the descending facilitation of spinal itch processing initiated by the periaqueductal gray (PAG) (25). Thus, it is likely that the RVM can modulate spinal itch processing via multiple parallel pathways. One possible scenario is that RVM neurons can facilitate spinal itch processing via disinhibition by recruiting local inhibitory neurons. A similar mechanism has been demonstrated for pain modulation by the RVM (29). In addition, serotonergic neurons in the RVM also form synapses with spinal GRPR+ neurons, as demonstrated by our viral tracing experiments. This is consistent with a previous study showing that serotonergic neurons in the RVM can facilitate spinal itch processing via a direct interaction between 5-HT1A and GRPR receptors expressed in GRPR+ neurons (26). Thus, the RVM can bidirectionally control spinal itch processing via different descending projections.
In summary, our study revealed the local and long-range inhibitory circuits that gate itch signal processing, probably via the direct regulation of the spinal GRPR+ neurons (SI Appendix, Fig. S9). These results expand our knowledge of the neural circuits underlying itch modulation at the spinal level and pave the way for further dissecting the circuit mechanism of itch signal processing.
Materials and Methods
Full detailed information about the materials and methods used in our study can be found in SI Appendix, Materials and Methods. All relevant data are available in the main text and SI Appendix. There is no restriction on data availability in this manuscript. All code for analysis can be made available by the corresponding author upon reasonable request.
Animals.
All procedures were approved by the Animal Care and Use Committee of the Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, China.
Behavior Tests.
Animals were habituated to the testing room for at least 2 d before all behavioral tests.
Electrophysiological Slice Recording.
Slice electrophysiology was performed as described previously (42). Cervical segments of the spinal cord were excised and transverse spinal cord slices (290 µm) were sectioned at slicing speed of 0.07 mm/s. Forty-five minutes after recovery, the slices were then transferred into a recording chamber and perfused with oxygenated artificial cerebrospinal fluid (ACSF) at 3 mL/min at 30 to 32 °C
Statistical Analysis.
All statistical analysis was 2-tailed comparisons. All data met the assumptions of the statistical tests used.
Supplementary Material
Acknowledgments
We thank Yan-Jing Zhu, Zheng-Run Gao, and Yu-Ting Xu for technical support. We thank Dr. Miao He at Fudan University for providing the H2B-GFP mice. This work is supported by the National Natural Science Foundation of China (No. 31825013), the Shanghai Municipal Science and Technology Major Project (Grant No. 2018SHZDZX05), Program of Shanghai Academic/Technology Research Leader (19XD1424200), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB32010200).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905658116/-/DCSupplemental.
References
- 1.Ständer S., et al. , Clinical classification of itch: A position paper of the international forum for the study of itch. Acta Derm. Venereol. 87, 291–294 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Bautista D. M., Wilson S. R., Hoon M. A., Why we scratch an itch: The molecules, cells and circuits of itch. Nat. Neurosci. 17, 175–182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikoma A., Steinhoff M., Ständer S., Yosipovitch G., Schmelz M., The neurobiology of itch. Nat. Rev. Neurosci. 7, 535–547 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Dong X., Dong X., Peripheral and central mechanisms of itch. Neuron 98, 482–494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch S. C., Acton D., Goulding M., Spinal circuits for touch, pain, and itch. Annu. Rev. Physiol. 80, 189–217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carstens E., Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J. Neurophysiol. 77, 2499–2514 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Davidson S., et al. , The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J. Neurosci. 27, 10007–10014 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y. G., Chen Z. F., A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Sun Y. G., et al. , Cellular basis of itch sensation. Science 325, 1531–1534 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra S. K., Hoon M. A., The cells and circuitry for itch responses in mice. Science 340, 968–971 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albisetti G. W., et al. , Dorsal horn gastrin-releasing peptide expressing neurons transmit spinal itch but not pain signals. J. Neurosci. 39, 2238–2250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan H., et al. , Identification of a spinal circuit for mechanical and persistent spontaneous itch. Neuron 103, 1135–1149.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acton D., et al. , Spinal neuropeptide Y1 receptor-expressing neurons form an essential excitatory pathway for mechanical itch. Cell Rep. 28, 625–639.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandkühler J., Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 89, 707–758 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Foster E., et al. , Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 85, 1289–1304 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyle K. A., et al. , A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 363, 120–133 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiong S. Y., Polgár E., van Kralingen J. C., Watanabe M., Todd A. J., Galanin-immunoreactivity identifies a distinct population of inhibitory interneurons in laminae I-III of the rat spinal cord. Mol. Pain 7, 36 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petitjean H., et al. , Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep. 13, 1246–1257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui L., et al. , Identification of early RET+ deep dorsal spinal cord interneurons in gating pain. Neuron 91, 1137–1153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourane S., et al. , Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350, 550–554 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J., et al. , Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci. 21, 707–716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kardon A. P., et al. , Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82, 573–586 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross S. E., et al. , Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65, 886–898 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samineni V. K., Grajales-Reyes J. G., Sundaram S. S., Yoo J. J., Gereau R. W. 4th, Cell type-specific modulation of sensory and affective components of itch in the periaqueductal gray. Nat. Commun. 10, 4356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z. R., et al. , Tac1-Expressing neurons in the periaqueductal gray facilitate the itch-scratching cycle via descending regulation. Neuron 101, 45–59.e9 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z. Q., et al. , Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron 84, 821–834 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marinelli S., Vaughan C. W., Schnell S. A., Wessendorf M. W., Christie M. J., Rostral ventromedial medulla neurons that project to the spinal cord express multiple opioid receptor phenotypes. J. Neurosci. 22, 10847–10855 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones B. E., Holmes C. J., Rodriguez-Veiga E., Mainville L., GABA-synthesizing neurons in the medulla: Their relationship to serotonin-containing and spinally projecting neurons in the rat. J. Comp. Neurol. 313, 349–367 (1991). [DOI] [PubMed] [Google Scholar]
- 29.Francois A., et al. , A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 93, 822–839.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban D. J., Roth B. L., DREADDs (designer receptors exclusively activated by designer drugs): Chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 55, 399–417 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Yang C. F., et al. , Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He M., et al. , Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron 91, 1228–1243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowan A., Khunawat P., Zhu X. Z., Gmerek D. E., Effects of bombesin on behavior. Life Sci. 37, 135–145 (1985). [DOI] [PubMed] [Google Scholar]
- 34.Zeilhofer H. U., Wildner H., Yévenes G. E., Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 92, 193–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sardella T. C., Polgár E., Watanabe M., Todd A. J., A quantitative study of neuronal nitric oxide synthase expression in laminae I-III of the rat spinal dorsal horn. Neuroscience 192, 708–720 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamichi K., et al. , Dissecting local circuits: Parvalbumin interneurons underlie broad feedback control of olfactory bulb output. Neuron 80, 1232–1245 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ralvenius W. T., et al. , Itch suppression in mice and dogs by modulation of spinal α2 and α3GABAA receptors. Nat. Commun. 9, 3230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sardella T. C., et al. , Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol. Pain 7, 76 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan B., et al. , Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braz J., Solorzano C., Wang X., Basbaum A. I., Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron 82, 522–536 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan B., Cheng L., Ma Q., Spinal circuits transmitting mechanical pain and itch. Neurosci. Bull. 34, 186–193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y. G., Wu C. S., Lu H. C., Beierlein M., Target-dependent control of synaptic inhibition by endocannabinoids in the thalamus. J. Neurosci. 31, 9222–9230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.