Significance
Natural killer (NK) cells provide essential host protection against cancer and infection. Inhibitory self-receptors for ubiquitously expressed MHC class I (MHC I) molecules are needed to educate NK cells and help prevent NK cells from attacking autologous cells in the host. NK cells also utilize activation self-receptors that can augment NK cell responsiveness to viral infections. We show herein that a distinct pair of functionally discordant self-receptors work in tandem to identify and eliminate infected targets, which coincides with host survival, primarily because they effectively increase NK cell activation and proliferation during infection.
Keywords: exome sequencing, gene editing, Ly49 receptor allotypes, NK cell education, viral immunity
Abstract
Natural killer (NK) cells mediate vital control of cancer and viral infection. They rely on MHC class I (MHC I)-specific self-receptors to identify and lyse diseased cells without harming self-MHC I-bearing host cells. NK cells bearing inhibitory self-receptors for host MHC I also undergo education, referred to as licensing, which causes them to become more responsive to stimulation via activation receptor signaling. Previous work has shown that licensed NK cells selectively expand during virus infections and they are associated with improved clinical response in human patients experiencing certain chronic virus infections, including HIV and hepatitis C virus. However, the importance of inhibitory self-receptors in NK-mediated virus immunity is debated as they also limit signals in NK cells emanating from virus-specific activation receptors. Using a mouse model of MHC I-dependent (H-2Dk) virus immunity, we discovered that NK cells depend on the Ly49G2 inhibitory self-receptor to mediate virus control, which coincided with host survival during murine cytomegalovirus infection. This antiviral effect further requires active signaling in NK cells via the Ly49R activation receptor that also binds H-2Dk. In tandem, these functionally discordant Ly49 self-receptors increase NK cell proliferation and effector activity during infection, resulting in selective up-regulation of CD25 and KLRG1 in virus-specific Ly49R+ Ly49G2+ NK cells. Our findings establish that paired self-receptors act as major determinants of NK cell-mediated virus sensing and immunity.
Natural killer (NK) cells are innate lymphocytes that play a nonredundant role in sustaining host immunity to virus infections (1, 2). They respond to environmental cues by integrating signals from diverse arrays of activation and inhibitory receptors, including structurally unrelated killer Ig-like receptor (KIR) or Ly49 receptors expressed in different species. Both human KIR and rodent Ly49 families include germline-encoded inhibitory and activation receptors that bind highly polymorphic host (self) MHC I molecules and control NK effector functions. Adaptive selection for binding self MHC I in the different species presumably underlies convergent diversification of clustered KIR or Ly49 receptor genes, which aids in pathogen protection and reproductive functions (3, 4).
Both KIR and Ly49 inhibitory self-receptors help tune NK cells during interaction with host MHC I (5, 6). “Self-aware” NK cells that have been tuned are said to be educated or licensed, as evidenced by enhanced effector function following activation receptor stimulation, and the ability to kill missing-self (MHC I−) target cells (7–9). Licensed NK cells may improve clinical outcomes in human patients with chronic virus infections, including hepatitis C virus (HCV) or HIV (10–12). Indeed, licensed NK cells have been found to respond and accumulate during several different human virus infections, including Hantavirus, Chikungunya virus, hepatitis B virus, HCV, HIV, and cytomegalovirus (CMV) (13–17).
The H-2Dk class I molecule promotes NK cell-mediated control of murine CMV (MCMV) infection in different mouse strains, including MA/My and C57L.Dk (18–21). Host resistance in these strains was found to correspond with the Ly49G2 inhibitory receptor encoded in most Ly49 haplotypes so far studied (18, 22, 23). Importantly, Ly49G2 self-receptor allotypes expressed in MA/My and C57L.Dk mice can license NK cells via H-2Dk, whereas others (e.g., Ly49G2b6) cannot (18, 24, 25). This licensing effect correlates with H-2Dk–dependent virus control and is abolished by specifically depleting Ly49G2+ NK cells prior to MCMV infection (18, 24, 26–28).
Although the importance of inhibitory self-receptors for MHC I in virus immunity is still debated, Ly49 activation receptors have been shown to specifically recognize and target NK cell lysis of virus-infected host cells. For example, Ly49H, which binds MCMV m157, directs virus-specific NK cell lysis of infected target cells in B6 mice (29, 30). Similarly, Ly49L recognition of MCMV gp34–H-2Dk complexes was shown to mediate MHC I-dependent MCMV resistance in BALB.K mice (19, 20, 31). Activation receptors thus might also contribute in Ly49G2+ NK cell responses during MCMV infection, as predicted (26). The Ly49R activation receptor, encoded in MA/My-related Ly49 haplotypes, is interesting in this regard as it was shown to modestly bind soluble H-2Dk tetramers (32, 33). Moreover, the Ly49DB6 activation receptor, an allele variant of Ly49R, was shown to augment virus-specific NK responses during MCMV infection. Thus, we interrogated Ly49R’s role in H-2Dk-dependent resistance to MCMV infection. We discovered that the discordant Ly49G2 and Ly49R self-receptors enable NK cells to mediate MHC I-dependent virus control and overall host survival. Our findings highlight a vital role for such paired self-receptor systems that rely on licensing to increase activation receptor-driven antiviral NK cell effector activities.
Results
Generation of Ly49g2-Deficient Mice.
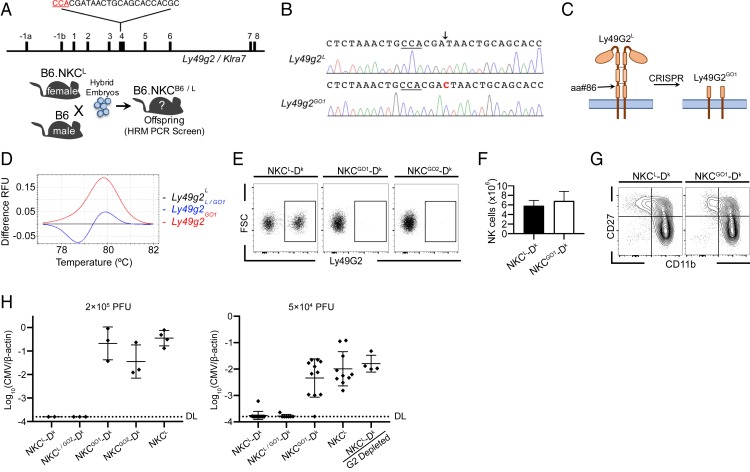
Ly49G2+ NK cells were previously shown to mediate H-2Dk-dependent MCMV resistance in MA/My, C57L.Dk, and B6.NKCL-Dk mice (18, 28). A specific role of Ly49G2 in virus control, however, remained poorly defined. Thus, we used CRISPR/Cas9 genome editing to initially generate B6.NKCL (NKCL) mice deficient in Ly49G2 expression. The C57L allele of Ly49g2L exon 4 was selectively targeted in NKCB6/L heterozygous embryos, which aided in genotypic and allotypic screening for mutant founders (Fig. 1A and SI Appendix, Fig. S1). Two NKCB6/L founders carrying exon 4 indels were identified using Ly49g2-specific high-resolution melting (HRM) PCR and the resultant mutant alleles were termed G Out1 and G Out2 (GO1 and GO2) (SI Appendix, Fig. S1B).
Fig. 1.
NK cells develop normally in Ly49g2-deficient GO mice but fail to control MCMV infection. (A) Diagram of CRISPR/Cas9-mediated editing of Ly49g2L genomic DNA (gDNA) and the breeding scheme used to generate Ly49g2-mutant founders. The protospacer adjacent motif (PAM) sequence is indicated in red. (B) Sequence flanking the CRISPR sgRNA/Cas9 target site of WT Ly49g2L and mutant Ly49g2GO1 alleles. The PAM sequence is underlined and a single cytosine insertion is shown in red. (C) Schematic of putative truncation site. (D) Ly49g2 exon 4-specific HRM PCR was performed with tail gDNA from WT (Ly49g2L), heterozygous (Ly49g2L/GO1), and GO1 (Ly49g2GO1) mice. (E) Representative flow plots show Ly49G2 staining of NK cells from the spleens of uninfected NKCL-Dk, NKCGO1-Dk, and NKCGO2-Dk mice. (F) Spleen NK cell numbers in uninfected NKCL-Dk and NKCGO1-Dk mice. (G) CD27 and CD11b profiles of spleen NK cells from uninfected NKCL-Dk and NKCGO1-Dk mice. (H) Mice were infected intraperitoneally with 2 × 105 PFU (Left) or 5 × 104 PFU (Right) MCMV and evaluated for spleen virus levels 90 h postinfection. Each symbol represents an individual mouse and error bars indicate mean ± SD. DL, detection limit. Data in D are representative of >20 independent experiments. Data in E–G are representative of 3 independent experiments with 3 to 4 mice per group. (H, Left) Two to 4 mice per group. (Right) Combined data from 3 separate experiments with 3 to 5 mice per group. Error bars indicate mean ± SD.
Ly49G2 allotype-specific staining showed that NK cells from GO founder offspring had reduced cell surface Ly49G2L expression (SI Appendix, Fig. S1C). Direct sequencing revealed identical cytosine insertions in GO1 and GO2 Ly49g2 alleles at the anticipated CRISPR/Cas9 target site, resulting in Ly49G2 truncation within the stalk region prior to a critical dimerization domain (Fig. 1 B and C). Both GO founders transmitted their mutations through the germline to establish homozygous Ly49g2GO1- and Ly49g2GO2-null mice, which can be identified from littermates carrying Ly49g2L alleles using HRM PCR (Fig. 1D).
GO mice were further crossed with NKCL-Dk to establish NKCGO1-Dk and NKCGO2-Dk strains for virus resistance studies. We found NK cells from both strains lack Ly49G2L NK cell surface expression (Fig. 1E). Whole-genome exome sequencing confirmed Ly49g2 cytosine insertions in both GO strains. Moreover, only WT exome sequences (i.e., no mutations) were detected in highly related Ly49 genes for the regions spanning the CRISPR target site in Ly49g2 (SI Appendix, Tables S1–S3). Highly specific Ly49 gene-editing thus selectively abolished Ly49G2 surface expression on GO NK cells.
NK Cells Develop Normally in Ly49g2-Deficient GO Mice but Fail to Control MCMV Infection.
Homozygous GO mice breed well and develop normally, without obvious health defects. Additionally, NK cell numbers and CD27 and CD11b expression profiles are similar in NKCGO1-Dk and NKCL-Dk mice (Fig. 1 F and G). Thus, Ly49G2 deficiency did not appreciably alter NK cell development at baseline.
We next assessed Ly49G2’s effect on host resistance by comparing spleen virus levels several days after MCMV infection. In comparison to WT Ly49G2 (NKCL-Dk), both GO strains displayed higher MCMV levels as in NKCL or NKCL-Dk mice depleted of Ly49G2+ NK cells (Fig. 1H). Thus, the Ly49G2 inhibitory self-receptor is required for NK cells to mediate MHC I-dependent MCMV control.
MHC I Dk-Dependent MCMV Control Is Comparable to That Mediated via Ly49H and Is Abolished by Ly49R Neutralization.
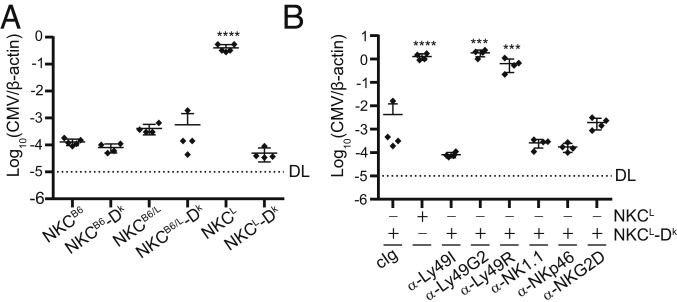
MHC I-independent MCMV resistance has been well characterized in B6 mice; however, less is known about the extent of viral control conferred by MHC I-dependent mechanisms in comparison. As expected, NKCL NK cells that lack m157-specific Ly49H receptors failed to control MCMV in the absence of H-2Dk (Fig. 2A). In contrast, NKCL-Dk Ly49G2+ NK cells controlled MCMV as effectively as virus-specific NK cells in B6 mice (Fig. 2A). These data demonstrate H-2Dk–dependent MCMV resistance is as robust as that provided by Ly49H.
Fig. 2.
MHC I Dk-dependent MCMV control is abolished by Ly49R neutralization. (A) Quantification of viral genomes in the spleens of the indicated NKC congenic ± Dk-transgene expression. (B) Quantification of viral genomes in the spleens of mice that were treated with depleting or neutralizing antibodies against NK cell surface receptors prior to infection. Mice in A and B were infected intraperitoneally with 2 × 105 PFU MCMV and evaluated for spleen virus levels 90 h postinfection. All data are representative of 2 to 5 independent experiments with 4 to 5 mice per group. Error bars indicate mean ± SD. ***P < 0.001, ****P < 0.0001.
We then interrogated a role for activation receptors in NKCL-Dk mice. Strikingly, the Ly49R-specific mAb 12A8 selectively abolished MCMV resistance in comparison to NKp46- or NKG2D-blocking mAbs (Fig. 2B). The extent of mAb 12A8’s effect was comparable to selective depletion of Ly49G2+ NK cells, which suggested both receptors may be important in MCMV control. In contrast to Ly49G2+ NK cells depletion, however, total NK cell numbers were unaffected by the α-Ly49R mAb. Rather, a comparable subset of Ly49R+ NK cells remained after treatment, which was readily detected using another Ly49R-reactive mAb, clone 4E5 (SI Appendix, Fig. S2A). Moreover, prolonged 12A8 treatment in NKCL-Dk mice did not interfere with IFN-γ production during NK cell stimulation via the Nkp46 activation receptor, nor did it alter the ability of Ly49G2+ NK cells to reject non-Dk bone marrow cell targets in vivo (SI Appendix, Fig. S2 B–D). These data indicate mAb 12A8 treatment functionally neutralizes Ly49R signaling without depleting or broadly impairing NK effector cells. We infer that Ly49R signaling in Ly49G2+ NK cells is required to mediate MHC I Dk-dependent MCMV resistance.
Ly49G2 and Ly49R Receptors Engage MHC I Dk.
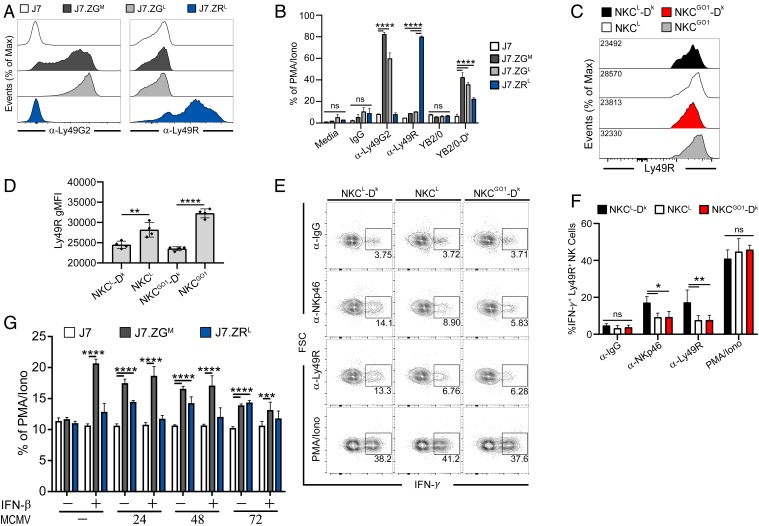
Prior work showed that both Ly49G2 and Ly49R receptors can bind soluble H-2Dk tetramers (32). We hypothesized that these discordant self-receptors for a shared MHC I ligand may be an important element for specific virus control. To pursue this question, we established a reporter cell system (34) to examine whether MA/My- or C57L-derived self-receptor allotypes bind Dk. In comparison to control J7 cells, J7.ZGM, J7.ZGL, and J7.ZRL reporter cells were selectively stained and stimulated with Ly49-specific mAbs 4D11 or 12A8 (Fig. 3 A and B). Moreover, each of these reporter cell lines specifically responded when cocultured with YB2/0-Dk cells, but not YB2/0 cells (Fig. 3B). Thus, Ly49G2 and Ly49R self-receptors both bind H-2Dk.
Fig. 3.
Ly49G2 and Ly49R receptors specifically bind MHC I Dk. (A) Surface expression of chimeric Ly49 receptors on J7 reporter cells, as determined by anti-Ly49G2 (4D11) and anti-Ly49R (12A8) mAb staining of J7.ZGM, J7.ZGL, or J7.ZRL reporter cells. (B) Reporter cells were stimulated with plate-bound mAbs or target cells. (C) Representative histograms of Ly49R expression by splenic NK cells. (D) Quantification of Ly49R gMFI from C. (E) Representative intracellular IFN-γ staining of spleen NK cells from uninfected NKCL-Dk, NKCL, and NKCGO1-Dk mice following stimulation with the indicated plate-bound mAbs or PMA/ionomycin. (F) Percentages of splenic Ly49R+ NK cells that express IFN-γ from E. (G) Reporter cells were cocultured for 12 h with infected M2-10B4 cells pretreated with IFN-β 16 h before coculture, as indicated. Target cells were infected for the indicated times prior to coculture with reporter cells. Data in A and B are representative of 3 to 5 independent experiments with 2 to 5 samples per group. Data in C and D are representative of 3 experiments with 3 to 4 mice per group. Data in E and F are representative of 2 independent experiments with 4 mice per group. Data in G is representative of 2 experiments with 3 to 6 samples per group. Error bars indicate mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Considering that Ly49 activation receptor expression on NK cells is sensitive to the presence of its cognate ligand in the host (35–37), we further examined Ly49R expression on NK cells in H-2Dk–disparate mice. Consistent with the results obtained using reporter cells, we found that Ly49R expression varied in direct relation to host H-2Dk (Fig. 3 C and D), confirming that Dk is a cognate ligand for both self-receptors. Since licensed NK cells display greater responsiveness to activation receptor stimulation than their unlicensed counterparts (7–9), we next assessed whether Ly49G2 is a primary licensing receptor in NKCL-Dk mice. Following stimulation with plate-bound mAbs specific for Nkp46 or Ly49R, we observed a significantly higher percentage of responsive Ly49R+ NK cells from mice that express the licensing receptor and its cognate ligand (Fig. 3 E and F). A small fraction of responsive NK cells (2 to 4%) from each strain were also observed to respond during control stimulation, which suggests immobilized IgG may elicit low-level CD16 signaling. However, background stimulation via control Ig was inadequate to elicit disparate responses in NK cells from the different strains. The Ly49G2 licensing self-receptor thus enhances Ly49R+ NK cell responsiveness in NKCL-Dk mice.
We next tested whether Ly49G2 and Ly49R self-receptors can recognize MCMV-infected M2-10B4 targets bearing H-2Dk ligands. Despite IFN-β–induced H-2Dk surface expression on M2-10B4 cells (SI Appendix, Fig. S3), only the Ly49G2 reporters significantly responded. Additionally, despite both Ly49G2 and Ly49R reporters responding to targets infected with MCMV for 24 to 72 h (Fig. 3G), Ly49G2 signals diminished as MCMV infection progressed and H-2Dk cell surface expression declined (SI Appendix, Fig. S3), whereas Ly49R signaling was maintained throughout. Intriguingly, IFN-β treatment of MCMV-infected targets prevented Ly49R-specific recognition, whereas Ly49G2 reporters were undeterred. Together, these data demonstrate that while both self-receptors recognize H-2Dk, their binding affinities and MCMV response patterns differ.
Ly49R+Ly49G2+ NK Cells Are Specifically Activated during MCMV Infection.
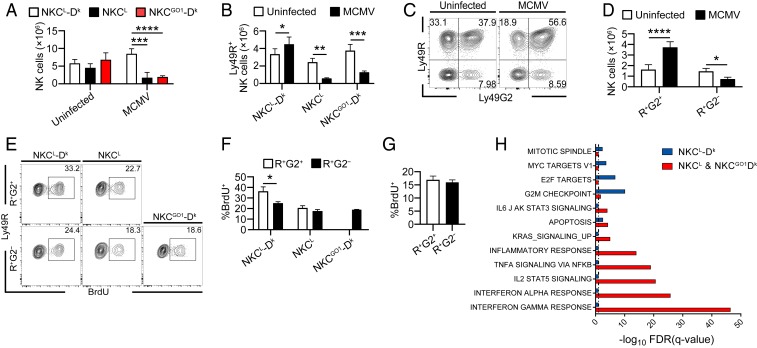
Because NK cells differentiate in response to virus-induced inflammation (38, 39), we examined the impact of Ly49G2-coexpression on Ly49R+ NK cell responses during MCMV infection. First, we assessed differentiation profiles for Ly49R+Ly49G2+ (R+G2+) and Ly49R+Ly49G2– (R+G2–) NK cells. Since the proportions of NK cells in immature (CD27+ CD11b–), transitional (CD27+ CD11b+), and more mature (CD27– CD11b+) differentiation stages (40, 41) were similar among R+G2+ and R+G2– cells at 4 d postinfection (dpi) (SI Appendix, Fig. S4 A and B), these data suggest that the different NK subsets undergo similar maturation during infection. We also measured KLRG1 since virus-specific Ly49H+ NK cells were shown to acquire and maintain KLRG1hi expression during MCMV infection (38, 42). We observed that a significantly higher fraction of NKCL-Dk R+G2+ NK cells exhibited KLRG1 terminal differentiation than their counterpart R+G2– NK cells, and this difference was Dk-dependent as both subsets displayed similar KLRG1hi frequencies in NKCL mice (Fig. 4 A and B).
Fig. 4.
Ly49R+ Ly49G2+ NK cells are specifically activated during MCMV infection. (A and B) Flow plots and quantification of KLRG1hi splenic NK cell subsets from uninfected or mice 4 dpi. (C and D) Flow plots and quantification of CD25+ splenic NK cell subsets from mice 4 dpi. (E) Histograms of CD62L expression by splenic NK cell subsets from mice uninfected or 4 dpi. (F) Frequency of NK cells that remain CD62L+ at day 4 postinfection. Mice were infected with 5 × 104 PFU MCMV. All Data are representative of 2 to 3 independent experiments with 3 to 4 mice per group. Error bars indicate mean ± SD. *P < 0.05, **P < 0.01.
CD25 up-regulation on NK cells also occurs during MCMV infection (SI Appendix, Fig. S4C), largely due to virus-induced IL-12 (43, 44). In mice lacking Ly49G2 or Dk, CD25 increased on Ly49R+ NK cells to a greater extent than what was seen in NKCL-Dk mice (Fig. 4 C and D). This is likely due to the sustained inflammatory environment in mice lacking Ly49G2 or Dk caused by unfettered viral spread (Fig. 1G) (45). In NKCL-Dk mice, however, CD25 selectively increased on R+G2+ cells. We also observed lower CD62L on R+G2+ NK cells in comparison to R+G2– NK cells only in NKCL-Dk mice (Fig. 4 E and F), similar to virus-specific NK cells in MCMV-infected B6 mice (46). Together, these data suggest that Dk-licensed R+G2+ NK cells experienced virus-specific activation, as opposed to more general, cytokine-mediated stimulation resulting from virus-induced inflammation.
Ly49G2 Promotes Ly49R+ NK Cell Accumulation and Proliferation during MCMV Infection.
We showed in prior work that Dk-licensed Ly49G2+ NK cells selectively accumulate in response to MCMV (27, 47). We thus examined whether Ly49G2 governs this expansion. Whereas R+G2+ NK cells significantly increased in NKCL-Dk spleens by 4 dpi, there was a notable decrease in mice lacking either the self-receptor or its cognate ligand (Fig. 5 A and B). This finding is consistent with previous work showing that high viral burden induces splenic lymphopenia and lymphoid architecture collapse (47). Additionally, there was a greater representation R+G2+ NK cells within the NK cell compartment in NKCL-Dk mice (Fig. 5 C and D). This skewing is also seen near the peak of NK cell expansion 6 dpi (SI Appendix, Fig. S4 D and E). These results suggest Dk-licensed R+G2+ NK cells are the dominant responding subset during MCMV infection.
Fig. 5.
Ly49G2 promotes Ly49R+ NK cell accumulation, proliferation, and differential gene expression during MCMV infection. (A and B) Total NK cells and Ly49R+ NK cells from the spleens of uninfected mice and mice 4 dpi. (C and D) Distribution profiles of Ly49R and Ly49G2 on NK cells in naïve mice or mice 4 dpi. NKCL-Dk mice. Representative flow plots are shown in C. Quantified numbers of R+G2− and R+G2+ subsets are shown in D. (E and F) BrdU incorporation after 3-h pulse BrdU treatment 4 dpi. (G) BrdU incorporation after 3-h pulse BrdU treatment 3 d after pIC injection. In A–F, mice were infected with 5 × 104 PFU MCMV. (H) Selected hallmark genes and corresponding gene enrichment analysis of NKCL-Dk splenic NK cells. Data are representative of 3 to 6 independent experiments with 3 to 4 mice per group. (G) Data are representative of 2 independent experiments with 3 to 4 mice per group. Error bars indicate mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We assessed whether cell survival differences might explain subset variation during infection. R+G2+ and R+G2– NK cells from infected NKCL-Dk mice exhibited similar caspase activation, which indicated that apoptosis does not explain differential subset accumulation (SI Appendix, Fig. S5). We next measured NK cell incorporation of BrdU to gauge whether increased proliferation accounts for selectively expanded R+G2+ NK cells. We observed that a greater proportion of NKCL-Dk R+G2+ NK cells incorporated BrdU during infection than their R+G2– counterparts (Fig. 5 E and F). Variation in subset proliferation was not seen in NKCL or GO1-Dk mice, suggesting that Dk-licensed R+G2+ NK cells selectively increased proliferation during infection.
To test whether subset skewing was due to an intrinsic defect in R+G2– proliferation, we injected mice with PolyI:C (pIC) to mimic virus-induced inflammation and again measured NK cell uptake of BrdU. In contrast to the results obtained during MCMV infection, both subsets responded equivalently following pIC treatment, which indicated that R+G2– NK cells are competent to undergo rapid proliferation (Fig. 5G). Taken together, these data demonstrate that the selective accumulation of R+G2+ NK cells resulted from enhanced proliferation in response to MCMV infection.
High-Dimensional Transcriptomic Profiling of Mouse Splenic NK Cells during MCMV Infection.
Single-cell RNA sequencing (scRNA-seq) was used to evaluate transcriptomic differences in NK cells responding to MCMV. An unbiased t-distributed stochastic neighbor embedding (t-SNE) approach was applied to analyze scRNA-seq data (48). We observed NK cells from MHC I- or Ly49G2-disparate mouse strains clustered on the basis of sample, such that NKCL-Dk clusters differed from those in NKCL and NKCGO1 (SI Appendix, Fig. S6A). This suggests that NKCL and NKCGO1 NK cells are transcriptionally similar during infection, in contrast to NK cells from infected NKCL-Dk mice (SI Appendix, Fig. S6B). Indeed, examination of gene differences in NKCL and NKCGO1, aside from H-2D or Klra7, revealed little substantive variation (SI Appendix, Fig. S6C).
Gene set enrichment analysis (GSEA) using published hallmark gene sets revealed that NKCL and NKCGO1 NK gene expression is significantly skewed toward up-regulation of an inflammatory response, including signaling via IL-2/STAT5, and strong TNF-α and IFN signatures (Fig. 5H). On the other hand, NKCL-Dk mice with licensed R+G2+ NK cells up-regulated genes associated with cell cycle control and proliferation. A net effect of self-receptor–dependent virus control thus resulted in profoundly altered gene expression to enable NK cell expansion.
To ascertain whether strain-specific differences in NK cell gene expression were simply due to different extrinsic signals based on host environment, we performed a similar analysis using NK cells from infected NKCL-Dk mice only. Three distinctive t-SNE clusters were identified for comparison (SI Appendix, Fig. S7A). GSEA of hallmark genes showed that cluster 1 (C1) was highest in genes associated with up-regulation of cell cycle control, DNA repair, and metabolic activity (SI Appendix, Fig. S7 B–D), similar to data obtained for all NKCL-Dk NK cells in Fig. 5H. As a dominant responding subset with enhanced proliferative and metabolic function, C1 NK cells likely contributed significant MCMV control in NKCL-Dk mice. In contrast, analysis of clusters 2 and 3 (C2 and C3) NK cells revealed significant up-regulation of complement and inflammatory response pathways as was observed for NKCL and NKCGO1 NK cells (Fig. 5H and SI Appendix, Fig. S7D). Together, these data indicate C2- and C3-type NK cell responses were not limited only to a highly inflammatory environment as in infected NKCL or NKCGO1 mice. Rather, they represented a significant NK cell gene-expression signature, possibly underpinning key antiviral activities. We infer C2- and C3-type NK responses may be overactivated in the absence of highly specific antiviral NK cells. More importantly, these data demonstrate that the Ly49G2 self-receptor itself drives an intrinsic difference in NK cells specifically responding to infection, whereas extrinsic factors were also involved.
Ly49G2 Receptor Licensing Enables Ly49R+ NK Cell Activation, Virus Control, and Host Survival during MCMV Infection.
To verify Ly49G2’s role in the activation and expansion of Ly49R+ NK cells, we cotransferred differentially labeled NKCL-Dk and NKCGO1-Dk NK cells into NKCL-Dk or NKCGO1-Dk recipients prior to MCMV infection (Fig. 6A). Remarkably, R+G2+ NK cells rapidly responded and displayed enhanced proliferation and expansion in NKCGO1-Dk recipients (Fig. 6 B and C). Moreover, R+G2+ NK cells displayed selective up-regulation of CD25 and KLRG1 expression, especially in NKCGO1-Dk recipients, in comparison to either R+G2– or R+G2null NK cells (Fig. 6D). Together, these data demonstrate a cell-intrinsic role for Ly49G2 in promoting specific Ly49R+ NK cell responses to MCMV infection.
Fig. 6.
Ly49G2 receptor licensing enables Ly49R+ NK cell activation, virus control, and host survival during MCMV infection. (A) Diagram illustrating the adoptive transfer of NKCL-Dk (WT, CFSE-labeled) and NKCGO1-Dk (GO1-Dk, CTV-labeled) donor splenic NK cells (mixed 1:1) into NKCL-Dk or NKCGO1-Dk recipients 24 h prior to MCMV infection. (B) CFSE and CTV dilution profiles of enriched donor splenic NK cells from NKCL-Dk or NKCGO1-Dk mice. (C) Quantification of expansion indices of data in B. (D) Frequency of CD25+ or KLRG1hi donor NK cells in B on day 4 postinfection (E) Diagram illustrating NKCL-Dk NK enrichment and flow sorting into 2 major subpopulations. Sorted cells (∼3 × 105) were adoptively transferred into NKCB6-Dk-CD45.1 mice pretreated with α-Ly49H (3D10) and α-NK1.1(PK136) mAbs. (F) Quantification of viral genomes in the spleens of recipient mice 4 dpi. (G) Accumulation of transferred NK cells in the spleen day 4 postinfection. (H) Ly49G2 positivity 4 dpi after cell sorting. (I) Host survival curves for NKCL-Dk or NKCGO1-Dk mice following infection with 1 × 106 PFU MCMV. In A–G, mice were infected with 2.5 × 104 PFU MCMV. Data are representative of 3 to 4 independent experiments with 3 to 4 mice per group. Error bars indicate mean ± SD. In F, statistical significance determined by post hoc Dunn’s test. *P < 0.05, **P < 0.01, ****P < 0.0001. In I, data are from a single experiment with 9 to 10 mice per group. Log-ranked Mantle–Cox test was used to determine statistical significance P = 0.0068.
To confirm that R+G2+ NK cells are responsible for enhanced virus control, we enriched R+G2– and R+G2+ NK subsets and separately transferred them into B6.Dk (i.e., NKCB6) recipients. Since NKCL-derived NK cells are resistant to PK136 (anti-NK1.1) depletion (18, 21), this system allowed us to ablate endogenous NKCB6 NK cells in recipients prior to transfer. Thus, any effects on virus control stem from the transferred NK cells (Fig. 6E). While R+G2– NK cells had no impact on virus control, recipients of R+G2+ NK cells exhibited lower viral burden and greater accumulation of NK cells in spleen than recipients of R+G2– NK cells (Fig. 6 F and G), thus confirming that licensed R+G2+ NK cells provide essential MCMV control.
Ly49g2129 gene activation has been shown to occur in mature NK cells in vitro in the presence of IL-2 (49). Additionally, Ly49G2+ NK cells in B6 mice expand nonspecifically following bone marrow transplantation and MCMV infection (50). Whether this is due to clonal expansion or de novo Ly49g2 expression in Ly49G2– NK cells remains uncertain, but it may be up-regulated in activated NK cells. Whereas most adoptively transferred R+G2– NK cells remained so during infection, a minor fraction clearly expressed Ly49G2 receptors (Fig. 6H). This could be the result of Ly49g2 gene activation, or possibly clonal expansion of a rare population of residual R+G2+ cells remaining following flow sorting prior to adoptive transfer. Nonetheless, G2– NK cells are not a significant precursor population to G2+ NK cells and R+G2+ NK cells undergo dramatic clonal expansion during MCMV infection.
Having verified the importance of the Ly49G2 receptor on Ly49R+ NK cell-mediated MCMV control in the spleen, we assessed their role in host survival by administering a sublethal dose of MCMV to Ly49G2 WT and GO mice. All mice with WT Ly49G2+ NK cells survived the infection, whereas >50% of GO mice succumbed (Fig. 6I). Thus, the Ly49G2 inhibitory self-receptor is essential in MHC I-dependent virus immunity and host survival when coexpressed on NK cells with its functionally discordant Ly49R self-receptor counterpart.
Discussion
While a widely held paradigm suggests licensed NK cells primarily thwart NK-mediated virus control, here we demonstrate that the inhibitory Ly49G2 NK cell receptor is required to specifically augment host defenses, including NK cell differentiation and proliferation, and limit virus spread during MCMV infection. A role for an inhibitory receptor in virus control may seem paradoxical since several studies show NK-mediated antiviral activities are diminished in their presence (51–54). Moreover, NK cells can mediate MCMV control in MHC I-deficient animals lacking self-receptor ligands (51, 55, 56). However, MCMV m157-specific Ly49H+ NK cells display MHC I-independent MCMV control (29, 30). Hence, licensed NK cells may be dispensable if virus-specific recognition by NK activation receptors is adequate to overcome tolerance. Nonetheless, we found that licensed R+G2+ NK cells are essential to mediate vigorous MHC I-dependent host immunity during WT MCMV infection. Moreover, licensed NK cells disarmed by exposure to MHC Ilo host cells can regain the capacity to mediate missing-self responses after MCMV-induced activation (57, 58). Licensed NK cells thus may be uniquely poised to overcome self-tolerance during MCMV infection.
Although seemingly counterintuitive, inhibitory receptors have been shown to augment lymphocyte effector functions. In T cells, the inhibitory NKG2A receptor was shown to increase control of ectromelia virus infection by promoting NKG2A+ CD8 T cell survival (59). Related to this, human inhibitory KIRs enhanced murine CD8 T cell proliferation ex vivo in response to stimulation by dendritic cells bearing transgenic cognate HLA molecules (60). Additionally, expression of self-specific inhibitory KIRs was found to coincide with increased CD8 T cell survival and better overall virus control in patients infected with HIV, HCV, or human T cell leukemia virus type 1 (61).
Self-MHC I-specific inhibitory receptors that license developing NK cells also increase the extent of activation receptor stimulation (7–9). Licensed NK cells that are educated on self-MHC I undergo expansion and differentiation in response to MCMV infection (24, 27, 62, 63), and in HCMV-infected individuals (17). Memory NK cells expressing self-specific inhibitory Ly49 receptors in hapten-sensitized mice likewise display enhanced recall responses (64, 65). Despite that licensed NK cells expand in these varied contexts, a basis for this response is poorly understood. We envision several possibilities may account for selective expansion in response to viral infection. 1) Licensing could increase activation receptor signals in response to virus or virus-induced antigens via altering activation signal transduction cascades. This explanation predicts that both licensing and activation receptors can specifically recognize and respond to virus-infected target cells. 2) Sustained binding of the inhibitory receptor could promote NK cell synapse formation and conjugation to infected target cells. 3) The licensing receptor could modify the activation receptor ligand so that activation signals are increased. Ongoing studies are focused on determining how the Ly49G2 receptor enables NK cells to specifically recognize and respond to MCMV infection.
Although the Ly49G2 receptor was found to be essential, virus control was abolished when the Ly49R self-receptor was neutralized. Two additional activation receptors implicated in H-2k–dependent MCMV resistance include Ly49L and Ly49P, which are potential allele variants that both bind MCMV gp34–Dk complexes (19, 20, 66). Indeed, adult Ly49L+ NK cells protected BALB.K neonates upon transfer and subsequent MCMV challenge. A role for Ly49P+ NK cells in vivo remains elusive since there is no available serologic or genetic tool to selectively ablate this subset. Still, we found that MCMV resistance in NKCL-Dk mice is abolished either by serologic or genetic depletion of Ly49G2+ NK cells. Moreover, the Ly49R-monospecific mAb sufficed to abrogate MCMV control to a similar extent as immunodepletion of Ly49G2+ NK cells. A role for Ly49P in MHC I-dependent MCMV resistance thus is unclear.
Our data instead demonstrated expression of both Ly49G2 and Ly49R receptors in individual NK cells is required to elicit MCMV control. Although H-2Dk tetramers were previously shown to bind Ly49R, they were folded with human β2-microglobulin, which could have affected the interaction (32). Consistent with the prior study, we found Ly49R reporter cells were specifically stimulated by Dk-bearing YB20-Dk rat lymphoma cells and MCMV-infected M2-10B4 bone marrow stromal cells. Curiously, Ly49R reporters did not respond to uninfected or IFN-β–stimulated M2-10B4 cells with high Dk. We speculate that Dk conformational differences in the different cell lines may underlie disparate Ly49R responses. Nonetheless, MCMV infected M2-10B4 cells consistently triggered Ly49R signaling, which was abrogated by IFN-β treatment. We additionally found that Ly49R expression on mouse NK cells is regulated by host cell Dk expression, similar to Ly49D down-regulation in the presence of its ligand, Dd (37). Altogether, these data suggest the Ly49R self-receptor is sensitive to variations in Dk expression, especially during MCMV infection.
Since both Ly49R and Ly49G2 self-receptors bind the same ligand, a qualitative change in H-2Dk on infected target cells might result in a loss of Ly49G2-dependent self-control, increased Ly49R-mediated recognition, or a combination of these effects leading to increased NK cell activity, proliferation, and virus control. This might occur through NK self-receptor–dependent recognition of viral peptide ligands or virus-induced modification of host MHC I. In human, select peptides can nullify stimulation of KIR inhibitory receptors by their cognate MHC I ligands (67, 68). In contrast, KIR2DS2, a human NK cell-activating receptor, exhibits a strong affinity for highly conserved flavivirus peptide motifs presented by HLA-C*0102 (69), which suggests MHC I-specific NK activation receptors can specifically recognize viral antigens presented by MHC molecules. Although Ly49 receptors interface with MHC I molecules beneath the peptide binding groove, they can also display peptide selectivity (70, 71). It is possible that Ly49 activation receptors might display similar specificity for virus peptide-modified host MHC I (72).
Specific proliferation is a salient feature of antigen-dependent effector NK cell responses during MCMV infection. Splenic NK cells generally expand and become activated in an antigen-independent manner via cytokine stimulation (73), whereas Ly49H+ NK cells exhibit DAP12-dependent proliferation in B6 mice (74). Selective expansion of the R+G2+ subset in H-2Dk mice is reminiscent of that seen with MCMV m157-specific Ly49H+ NK cells. In addition to increased proliferation, these NK cells also become KLRG1hi CD62Llo CD25+ (38, 39, 75). Our data are thus consistent with increased CD25 observed for NK cells responding to MCMV (44). We additionally observed nonselective CD25 up-regulation on NK cells in infected mice lacking Ly49G2 or Dk, consistent with the hypothesis that CD25 is regulated independent of Ly49 activation receptors (44). It is possible that licensed virus-specific NK cells are more sensitive, or have better access to IL-12 during MCMV infection. Altogether, our data suggest R+G2+ NK cells undergo antigen-specific stimulation, which promotes their differentiation and effector functions.
Ly49R signals in isolation are inadequate since Ly49G2 coexpression is required for optimal NK effector function. scRNA-seq analysis revealed that extrinsic and intrinsic factors affected NK cells expressing discordant self-receptors to increase genes for cell cycle regulation and proliferation during MCMV infection. In comparison, NK cells from mice lacking the Ly49G2 self-receptor or its cognate ligand skewed gene expression toward inflammatory response pathways. We infer that NK proliferation and differentiation is dependent on a balance of inhibitory and activation receptor signaling pathways in R+G2+ NK cells, which shifts to overcome self-tolerance upon recognition of infected targets. We further posit that R+G2– NK cells are unable to overcome disarming in the absence of licensing receptor-enhanced recognition of target cells.
In conclusion, our data uncover an underappreciated role for inhibitory self-receptors in promoting activation and expansion of NK cells in response to viral infection. This mechanism of NK cell detection of viral infection is reliant upon a receptor pair with discordant functions. We predict these self-receptors working in tandem may be much more sensitive to subtle variations in MHC I ligands (i.e., altered-self) so as to trigger highly aggressive NK cell effector activities and increased proliferation. This intricate host–pathogen interaction may be an important immune strategy in nature, which underscores the need for further research to determine if similar receptor pairings are present in humans. A better understanding of such inhibitory and activation receptor pairs will further the development of new strategies to augment host immunity and improve clinical outcomes in the context of viral infections, tissue transplant, and cancer.
Materials and Methods
Ethics Statement.
Mouse experiments were performed in accordance with the Animal Welfare Act and approved by the University of Virginia Institutional Animal Care and Use Committee.
Mice.
B6.NKCC57L-Dk (NKCL-Dk), B6.NKCC57L (NKCC57L), and B6.Dk mice (28), as well as B6.NKCGO1 (GO1) and B6.NKCGO1-Dk (GO1-Dk) mice, were generated and maintained at the University of Virginia under specific pathogen-free conditions. B6.SJL-PtprcaPepcb/BoyJ mice (Jackson Laboratory) were crossed with B6.Dk mice (28) to generate B6.Dk-CD45.1 mice.
MCMV.
Salivary gland passaged MCMV (Smith Strain; ATCC) was titered on NIH 3T3 or M2-10B4 and intraperitoneally injected at stated doses, as described previously (76, 77). Mice were injected intraperitoneally with 200 μg PK136, 4D11 or AT8 48 h before infection to deplete NK cells. Ly49R was neutralized using 200 μg mAb 12A8 (a gift from John Ortaldo, National Cancer Institute, National Institutes of Health, Frederick, MD) given intraperitoneally 72 and 24 h before infection (32, 78, 79). Ly49H, NKp46, or NKG2D were, respectively, neutralized using 200 μg mAbs 3D10, 29A1.4, or C7 given intraperitoneally 24 h before infection (80–82). Infected mouse spleen DNA was measured for MCMV genomes via quantitative PCR, as described previously (83).
Antibodies and Flow Cytometry.
Flow cytometry (FC) was performed using BD FACS Canto II, CytoFLEX, or Aurora Northern Lights flow cytometers. Data were respectively collected using FACSDiva, CytExpert, or Spectroflo software and analyzed using FlowJo (versions 9.7.2 and 10.1 to 10.4). Fluorescent mAbs were purchased from BioLegend, BD Biosciences, and eBioscience. The 2.4G2, PK136, 3D10, AT8, and 4D11 mAbs were purified from spent supernatants by the University of Virginia Lymphocyte Culture Center. The 12A8 mAb was kindly provided by John Ortaldo.
Fluorescent mAbs from BioLegend, BD Biosciences, and eBioscience were titrated for optimal resolution and used to stain CD3 (145-2C11), CD19 (6D5), NK1.1 (PK136), CD49b (DX5), NKp46 (29A1.4), Ly49G2 (4D11 and Cwy-3), Ly49R (12A8), Ly49ROV (4E5), CD27 (LG.7F9), CD11b (M1/70), DNAM1 (10E5), KLRG1 (2F1), Ki67 (16A8), BrdU (BU20a), IFN-γ (XMG1.2), GZMB (NGZB), or CD62L (MEL-14). LIVE/DEAD fixable dyes (Thermo Fisher Scientific) were used to assess cell viability.
Adoptive Transfers.
B6.Dk-CD45.1 mice were pretreated with NK depleting (PK136) and Ly49H neutralizing (3D10) mAbs 48 h before and on the day of adoptive transfer. CFSE [5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester]-labeled spleen NK cells (bulk transfer), or flow-sorted (Influx, University of Virginia FC Core Facility) Ly49+ NK subsets (≥99% purity) were intravenously injected into host mice 24 h before infection. For CFSE staining, 500 μL of 10 μM CFSE (made fresh in complete RPMI) was added dropwise to enriched NK cells in 500 μL with vortexing and then incubated for 5 min at room temperature before quenching in 10 mL complete RPMI. Next, 3 × 105 donor NKCL-Dk NK cells were intravenously injected into host mice 24 h prior to infection. The expansion index is a measure of the fold-expansion of the original population (total divided cells/estimated original starting population).
In Vitro Stimulation and Intracellular Cytokine Staining.
Mouse splenocytes cultured in complete RPMI plus IL-2 (200 U/mL; Peprotech) were used in ex vivo stimulations. Splenocytes (1 to 2 million) were stimulated with immobilized mAbs 12A8, NKp46, or control IgG (plates coated with 20 μg/mL mAb overnight at 4 °C) or PMA (100 ng/mL) and ionomycin (1 μg/mL) for 1 h prior to brefeldin A (BFA) addition, and an additional 4 h with BFA. Stimulated cells were fixed and permeabilized using a kit (Cytofix/Cytoperm; BD Biosciences) followed by staining for intracellular cytokines at 4 °C.
BrdU Incorporation Assay.
Mice were intraperitoneally injected with BrdU (1 mg/ 200 μL PBS) 3 h prior to being killed. BrdU staining was performed using a kit (BD Biosciences) per the manufacturer’s instructions.
In Vivo Cytotoxicity Assay.
In vivo cytotoxicity was performed essentially as described previously (24, 84). Briefly, a 1:1 mix of 2 × 106 Dk and no-Dk bone marrow cells (in 200 μL RPMI), respectively labeled with 5 μM CFSE or CellTrace Violet (CTV), were intravenously injected into host mice. Spleens were harvested 20 h posttransfer and analyzed for residual donor cells by FC.
Statistical Analysis.
Statistical analysis was performed using Graphpad Prism (v7.04). Significance was assessed using 1- or 2-way ANOVA in conjunction with Tukey or Holms–Sidak post hoc tests unless otherwise stated (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Student’s t-test or Mann–Whitney U rank test was used when comparing the means of 2 independent groups.
Design and In Vitro Transcription of Single-Guide RNA.
An allele-specific single-guide RNA (sgRNA) (5′-GCG UGG UGC UGC AGU UAU CG-3′) was used to edit Ly49g2L exon 4 based on available 129 and C57L allele sequences using https://zlab.bio/guide-design-resources, as previously described (85). The sgRNA was selected to maximize the likelihood of specific Ly49g2L exon4 editing while minimizing the potential to edit highly related Ly49 genes. Notably, a 5′ G was appended to the sgRNA to ensure efficient in vitro transcription with T7 polymerase. Ly49g2L allele-specific oligonucleotides (Integrated DNA Technologies) cloned in pX330-U6-Chimeric_BB-CBh-hSpCas9 (kindly provided by Feng Zhang, Broad Institute of MIT, Cambridge, MA; Addgene plasmid #42230) were used to validate gene-editing efficiency in stem cells prior to work with mouse embryos.
The in vitro transcription template was amplified using a high-fidelity DNA polymerase (Phusion, New England Biolabs), pX330-Ly49g2-exon4 vector, and a primer designed to append the T7 promoter to the Ly49g2-sgRNA encoding oligonuceltide (86). The template was purified using a kit (Qiagen QIAQuick PCR purification) followed by dialysis against 1× TE. The template was then transcribed and its product purified using Ambion, MEGAshortscript, and MEGAclear T7 kits.
HRM PCR Genotyping for Edited Ly49g2 Alleles.
Ly49g2L exon4-specific primers (For 5′-GAC TAA CTT AGT TTT TCA GC-3′ and Rev 5′-GCA GTT CAT CCT TCA AGT TGA-3′) spanning the sgRNA target site were designed essentially as described previously (87). Primers (Integrated DNA Technologies) were optimized and used in HRM PCR as described previously (33, 87, 88).
Generation and Validation of Ly49g2 Deficient GO Mice.
B6 (NKCB6) (Jackson Labs) males were bred to superovulated B6.NKCC57L (NKCL) females to generate B6.NKCB6/L embryos that were microinjected with Cas9 protein (Integrated DNA Technologies) and Ly49g2 exon4-specific sgRNA prior to implantation into foster mothers. Offspring tail DNA was prepared using a kit (Gentraprep) and screened in HRM PCR using Ly49g2L exon4-specific primers. Five viable offspring carried Ly49g2L exon 4 indels. Two founders transmitted exon4 indels through the germline and were separately crossed back to NKCL to generate homozygous GO mice, before further crossing to NKCL-Dk.
Ly49g2 GO alleles were validated using whole-genome exome sequencing of liver DNA, which was performed by the Genomic Services Lab at Hudson Alpha essentially as described previously (89). Briefly, GO1 and GO2 FastQ files were separately aligned to the Ly49g2L reference sequence using BWA-MEM in Sequencher (Gene Codes Corporation). A Burrows–Wheeler Aligner (BWA)-MEM–generated BAM file was opened in Tablet (James Hutton Institute) to visualize and identify CRISPR-modified Ly49 sequences overlapping the target sequence. WT and CRISPR-modified Ly49 sequences from this alignment were exported and realigned using high-stringency parameters (minimum overlap 25 nucleotides, minimum match 97%) in Sequencher. Individual GO Ly49 contig alignments were reviewed for nucleotide discrepancies and consensus sequences overlapping the Ly49g2 CRISPR target site are reported in SI Appendix, Tables S1–S3.
scRNA-Seq Analysis of NK Cells.
Single-cell cDNA libraries were prepared from negatively enriched (3 rounds NK isolation kit, Miltenyi Biotec) NKCL-Dk, NKCL, or NKCGO1-Dk spleen NK cells (>80% viability, 90 to 95% purity) and sequenced by the University of Virginia Genome Analysis and Technology Core using a Chromium Controller instrument (10X Genomics) and the Chromium Single Cell 3′ Reagent Kit V3 (10X Genomics) following the manufacturer’s protocol. The indexed libraries were sized using the Agilent 4200 TapeStation and pooled into equimolar concentration. Samples were pooled and sequenced in a single run to avoid batch effects. High-throughput sequencing was performed using a NextSeq 500 Sequencer (Illumina) and the High Output Kit V2.5 (150 cycles) using the following settings for Read 1 (26 cycles), Read 2 (98 cycles), and Read Index (8 cycles). Collected data (.bcl files) were exported for data processing and quality assessment prior to further analysis using Cell Ranger (10X Genomics) data analysis pipelines. Reads were aligned to the transcriptome using the “Count” function in Cell Ranger so that expression of selected genes were assigned to single cells via attached barcodes.
Cell Ranger was used to perform t-SNE clustering of single-cell data, which was further analyzed using the Loupe Cell Browser (10X Genomics). Differentially expressed genes were identified and ranked by statistical significance. Statistically significant genes were further ranked by log2 FC expression differences between clusters and visualized in heatmaps generated in Prism (Graphpad v7.05). Heatmap rank = up-regulated genes per cluster (most to least) with a log2 FC of at least 1.
Chimeric CD3ζ-Ly49 Reporter Cells.
Cd3ζ-Ly49g2MAMY, Cd3ζ-Ly49g2C57L, and Cd3ζ-Ly49r chimeric receptor gene cassettes were generated using PCR essentially as described for Ly49daz (90), although we fused Cd3z cytoplasmic tail and Ly49g transmembrane domain coding sequences, followed by coding sequences for Ly49g or Ly49r ectodomains. Sequence-verified constructs were separately subcloned into pMXs-IRES-PURO (kindly provided by T. Kitamura, University of Tokyo, Tokyo, Japan). Expression constructs were transfected into 293T cells together with pMD2.G (kindly provided by Didier Trono, Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland) (Addgene plasmid #12259; http://www.addgene.org/12259/; RRID:Addgene_12259) and pHIT60 (kindly provided by Alan Kingsman, Oxford University, Oxford, United Kingdom) (91) using lipofectamine. Retroviral supernatants were used to transduce J7 cells (a gift from K. Iizuka) and Ly49 receptor-expressing reporter cells were selected in puromycyin-containing media essentially as described previously (92). CD3ζ-Ly49G2C57L (also known as J7.ZGL) reporter cells were flow-sorted for high expression, comparable to CD3ζ-Ly49G2MAMY (akaalso known as J7.ZGM) and CD3ζ-Ly49R (also known as J7.ZRL). Ly49 reporter cells (2 × 105) were stimulated for 8–12 h with plate-bound mAbs, YB20, YB20-Dk, or M2-10B4 (±IFN-β or MCMV infection) target cells, or PMA + ionomycin. LacZ activity was determined using the substrate chlorophenol red-d-galactoside (CPRG), as described previously (93).
Data Availability.
All scRNA-seq data are accessible from the National Center for Biotechnology Information Gene Expression Omnibus depository using GEO accession no. GSE132394.
Supplementary Material
Acknowledgments
We thank Jeff Teoh, Jessica Prince, Karolina Dziewulska, Jeremy Gatesmen, and Lucas Turner for technical assistance; and Oscar Aguilar, Stephen Anderson, Jon Heusel, Koho Iizuka, Kevin Kane, Lewis Lanier, John Ortaldo, Andrew Makrigiannis, and Aimen Shaaban for kindly providing reagents. We are grateful for services provided by the Fold Change, Genetically Engineered Murine Model, and Genome Analysis and Technology Cores at the University of Virginia. This work was supported by Public Health Service Grant R01 AI050072 (to M.G.B.), the Department of Medicine, Division of Nephrology and the Beirne Carter Center for Immunology Research, University of Virginia, and an American Association of Immunologists Career award (to M.G.B. and A. Gamache). A. Gamache and J.M.C. received support on Public Health Service Training Grant T32 AI07496. W.T.N. received support on Public Health Service Training Grant T32 DK072922.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database (accession nos. GSE132394 and GSM3861587–GSM3861589).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913064117/-/DCSupplemental.
References
- 1.Biron C. A., Byron K. S., Sullivan J. L., Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320, 1731–1735 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Mace E. M., Orange J. S., Genetic causes of human NK cell deficiency and their effect on NK cell subsets. Front. Immunol. 7, 545 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abi-Rached L., Parham P., Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues. J. Exp. Med. 201, 1319–1332 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley J., Walter L., Trowsdale J., Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet. 1, 129–139 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long E. O., Kim H. S., Liu D., Peterson M. E., Rajagopalan S., Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 31, 227–258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodin P., Lakshmikanth T., Johansson S., Kärre K., Höglund P., The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood 113, 2434–2441 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Fernandez N. C., et al. , A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 105, 4416–4423 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S., et al. , Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436, 709–713 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Anfossi N., et al. , Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Khakoo S. I., et al. , HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305, 872–874 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Romero V., et al. , Interaction of NK inhibitory receptor genes with HLA-C and MHC class II alleles in Hepatitis C virus infection outcome. Mol. Immunol. 45, 2429–2436 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin M. P., et al. , Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39, 733–740 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Björkström N. K., et al. , Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 208, 13–21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petitdemange C., et al. , Unconventional repertoire profile is imprinted during acute Chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 7, e1002268 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Béziat V., et al. , CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur. J. Immunol. 42, 447–457 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Eller M. A., et al. , Human immunodeficiency virus type 1 infection is associated with increased NK cell polyfunctionality and higher levels of KIR3DL1+ NK cells in ugandans carrying the HLA-B Bw4 motif. J. Virol. 85, 4802–4811 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Béziat V., et al. , NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 121, 2678–2688 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie X., Stadnisky M. D., Brown M. G., MHC class I Dk locus and Ly49G2+ NK cells confer H-2k resistance to murine cytomegalovirus. J. Immunol. 182, 7163–7171 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyzik M., et al. , Distinct MHC class I-dependent NK cell-activating receptors control cytomegalovirus infection in different mouse strains. J. Exp. Med. 208, 1105–1117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fodil-Cornu N., Loredo-Osti J. C., Vidal S. M., NK cell receptor/H2-Dk-dependent host resistance to viral infection is quantitatively modulated by H2q inhibitory signals. PLoS Genet. 7, e1001368 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dighe A., et al. , Requisite H2k role in NK cell-mediated resistance in acute murine cytomegalovirus-infected MA/My mice. J. Immunol. 175, 6820–6828 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Brown M. G., Scalzo A. A., NK gene complex dynamics and selection for NK cell receptors. Semin. Immunol. 20, 361–368 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abolins S., et al. , The comparative immunology of wild and laboratory mice, Mus musculus domesticus. Nat. Commun. 8, 14811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei H., Nash W. T., Makrigiannis A. P., Brown M. G., Impaired NK-cell education diminishes resistance to murine CMV infection. Eur. J. Immunol. 44, 3273–3282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silver E. T., Lavender K. J., Gong D.-E., Hazes B., Kane K. P., Allelic variation in the ectodomain of the inhibitory Ly-49G2 receptor alters its specificity for allogeneic and xenogeneic ligands. J. Immunol. 169, 4752–4760 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Xie X., et al. , MHC class I D(k) expression in hematopoietic and nonhematopoietic cells confers natural killer cell resistance to murine cytomegalovirus. Proc. Natl. Acad. Sci. U.S.A. 107, 8754–8759 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince J., et al. , Multiparametric analysis of host response to murine cytomegalovirus in MHC class I-disparate mice reveals primacy of Dk-licensed Ly49G2+ NK cells in viral control. J. Immunol. 191, 4709–4719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teoh J. J., et al. , Acute virus control mediated by licensed NK cells sets primary CD8+ T cell dependence on CD27 costimulation. J. Immunol. 197, 4360–4370 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arase H., Mocarski E. S., Campbell A. E., Hill A. B., Lanier L. L., Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Smith H. R. C., et al. , Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. U.S.A. 99, 8826–8831 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kielczewska A., et al. , Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J. Exp. Med. 206, 515–523 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makrigiannis A. P., et al. , Class I MHC-binding characteristics of the 129/J Ly49 repertoire. J. Immunol. 166, 5034–5043 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Brown M. G., et al. , Natural killer gene complex (Nkc) allelic variability in inbred mice: Evidence for Nkc haplotypes. Immunogenetics 53, 584–591 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Iizuka Y.-M., Somia N. V., Iizuka K., Identification of NK cell receptor ligands using a signaling reporter system. Methods Mol. Biol. 612, 285–297 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Tripathy S. K., et al. , Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J. Exp. Med. 205, 1829–1841 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J. C., Lanier L. L., Tolerance of NK cells encountering their viral ligand during development. J. Exp. Med. 205, 1819–1828 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.George T. C., Ortaldo J. R., Lemieux S., Kumar V., Bennett M., Tolerance and alloreactivity of the Ly49D subset of murine NK cells. J. Immunol. 163, 1859–1867 (1999). [PubMed] [Google Scholar]
- 38.Fogel L. A., Sun M. M., Geurs T. L., Carayannopoulos L. N., French A. R., Markers of nonselective and specific NK cell activation. J. Immunol. 190, 6269–6276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dokun A. O., et al. , Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2, 951–956 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Chiossone L., et al. , Maturation of mouse NK cells is a 4-stage developmental program. Blood 113, 5488–5496 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Hayakawa Y., Smyth M. J., CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 176, 1517–1524 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Nabekura T., Lanier L. L., Tracking the fate of antigen-specific versus cytokine-activated natural killer cells after cytomegalovirus infection. J. Exp. Med. 213, 2745–2758 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman B. E., Raué H.-P., Hill A. B., Slifka M. K., Cytokine-mediated activation of NK cells during viral infection. J. Virol. 89, 7922–7931 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S.-H., Fragoso M. F., Biron C. A., Cutting edge: A novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells. J. Immunol. 189, 2712–2716 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nash W. T., Gillespie A. L., Brown M. G., Murine cytomegalovirus disrupts splenic dendritic cell subsets via type I interferon-dependent and -independent mechanisms. Front. Immunol. 8, 251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francois S., et al. , NK cells improve control of friend virus infection in mice persistently infected with murine cytomegalovirus. Retrovirology 10, 58 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillespie A. L., et al. , Genomic modifiers of natural killer cells, immune responsiveness and lymphoid tissue remodeling together increase host resistance to viral infection. PLoS Pathog. 12, e1005419 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gamache A., Brown M.G., sc-RNA-seq data set. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE132394. Deposited 7 June 2019.
- 49.Makrigiannis A. P., Rousselle E., Anderson S. K., Independent control of Ly49g alleles: Implications for NK cell repertoire selection and tumor cell killing. J. Immunol. 172, 1414–1425 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Barao I., et al. , Mouse Ly49G2+ NK cells dominate early responses during both immune reconstitution and activation independently of MHC. Blood 117, 7032–7041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orr M. T., Murphy W. J., Lanier L. L., ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat. Immunol. 11, 321–327 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmoud A. B., et al. , Influenza virus targets class I MHC-educated NK cells for immunoevasion. PLoS Pathog. 12, e1005446 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahim M. M. A., et al. , The mouse NKR-P1B:Clr-b recognition system is a negative regulator of innate immune responses. Blood 125, 2217–2227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahim M. M. A., et al. , Expansion and protection by a virus-specific NK cell subset lacking expression of the inhibitory NKR-P1B receptor during murine cytomegalovirus infection. J. Immunol. 197, 2325–2337 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Tay C. H., Welsh R. M., Brutkiewicz R. R., NK cell response to viral infections in beta 2-microglobulin-deficient mice. J. Immunol. 154, 780–789 (1995). [PubMed] [Google Scholar]
- 56.Polić B., et al. , Lack of MHC class I complex expression has no effect on spread and control of cytomegalovirus infection in vivo. J. Gen. Virol. 77, 217–225 (1996). [DOI] [PubMed] [Google Scholar]
- 57.Sun J. C., Lanier L. L., Cutting edge: Viral infection breaks NK cell tolerance to “missing self”. J. Immunol. 181, 7453–7457 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bern M. D., et al. , Inducible down-regulation of MHC class I results in natural killer cell tolerance. J. Exp. Med. 216, 99–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rapaport A. S., et al. , The inhibitory receptor NKG2A sustains virus-specific CD8+ T cells in response to a lethal poxvirus infection. Immunity 43, 1112–1124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ugolini S., et al. , Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+ T cells. Nat. Immunol. 2, 430–435 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Boelen L., et al. , Inhibitory killer cell immunoglobulin-like receptors strengthen CD8+ T cell-mediated control of HIV-1, HCV, and HTLV-1. Sci. Immunol. 3, eaao2892 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sungur C. M., et al. , Murine natural killer cell licensing and regulation by T regulatory cells in viral responses. Proc. Natl. Acad. Sci. U.S.A. 110, 7401–7406 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zamora A. E., et al. , Licensing delineates helper and effector NK cell subsets during viral infection. JCI Insight 2, 87032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wight A., et al. , Critical role for the Ly49 family of class I MHC receptors in adaptive natural killer cell responses. Proc. Natl. Acad. Sci. U.S.A. 115, 11579–11584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Leary J. G., Goodarzi M., Drayton D. L., von Andrian U. H., T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Desrosiers M.-P., et al. , Epistasis between mouse Klra and major histocompatibility complex class I loci is associated with a new mechanism of natural killer cell-mediated innate resistance to cytomegalovirus infection. Nat. Genet. 37, 593–599 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fadda L., et al. , Peptide antagonism as a mechanism for NK cell activation. Proc. Natl. Acad. Sci. U.S.A. 107, 10160–10165 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borhis G., et al. , A peptide antagonist disrupts NK cell inhibitory synapse formation. J. Immunol. 190, 2924–2930 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naiyer M. M., et al. , KIR2DS2 recognizes conserved peptides derived from viral helicases in the context of HLA-C. Sci. Immunol. 2, eaal5296 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Su R. C., et al. , Ly-49CB6 NK inhibitory receptor recognizes peptide-receptive H-2Kb. J. Immunol. 163, 5319–5330 (1999). [PubMed] [Google Scholar]
- 71.Deng L., et al. , Molecular architecture of the major histocompatibility complex class I-binding site of Ly49 natural killer cell receptors. J. Biol. Chem. 283, 16840–16849 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown M. G., Gamache A., Nash W. T., Cronk J., Natural selection for killer receptors and their MHC class I ligands: In pursuit of gene pairs that fit well in tandem. J. Leukoc. Biol. 105, 489–495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biron C. A., Tarrio M. L., Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus. Med. Microbiol. Immunol. 204, 345–354 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.French A. R., et al. , DAP12 signaling directly augments proproliferative cytokine stimulation of NK cells during viral infections. J. Immunol. 177, 4981–4990 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Sun J. C., Beilke J. N., Lanier L. L., Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodriguez M., Sabastian P., Clark P., Brown M. G., Cmv1-independent antiviral role of NK cells revealed in murine cytomegalovirus-infected New Zealand White mice. J. Immunol. 173, 6312–6318 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Xie X., et al. , Deficient major histocompatibility complex-linked innate murine cytomegalovirus immunity in MA/My.L-H2b mice and viral downregulation of H-2k class I proteins. J. Virol. 81, 229–236 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mason L. H., et al. , The Ly-49D receptor activates murine natural killer cells. J. Exp. Med. 184, 2119–2128 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.George T. C., Mason L. H., Ortaldo J. R., Kumar V., Bennett M., Positive recognition of MHC class I molecules by the Ly49D receptor of murine NK cells. J. Immunol. 162, 2035–2043 (1999). [PubMed] [Google Scholar]
- 80.Brown M. G., et al. , Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292, 934–937 (2001). [DOI] [PubMed] [Google Scholar]
- 81.Narni-Mancinelli E., et al. , Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 335, 344–348 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Ho E. L., et al. , Costimulation of multiple NK cell activation receptors by NKG2D. J. Immunol. 169, 3667–3675 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Wheat R. L., Clark P. Y., Brown M. G., Quantitative measurement of infectious murine cytomegalovirus genomes in real-time PCR. J. Virol. Methods 112, 107–113 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Oberg L., et al. , Loss or mismatch of MHC class I is sufficient to trigger NK cell-mediated rejection of resting lymphocytes in vivo—Role of KARAP/DAP12-dependent and -independent pathways. Eur. J. Immunol. 34, 1646–1653 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Ran F. A., et al. , Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang H., Wang H., Jaenisch R., Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 9, 1956–1968 (2014). [DOI] [PubMed] [Google Scholar]
- 87.Lundgren A., Kim S., Stadnisky M. D., Brown M. G., Rapid discrimination of MHC class I and killer cell lectin-like receptor allele variants by high-resolution melt analysis. Immunogenetics 64, 633–640 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown M. G., et al. , A 2-Mb YAC contig and physical map of the natural killer gene complex on mouse chromosome 6. Genomics 42, 16–25 (1997). [DOI] [PubMed] [Google Scholar]
- 89.Gillespie A., Lee H., Robertson C., Cabot M., Brown M. G., Genome-wide exome analysis of Cmv5-disparate mouse strains that differ in host resistance to murine cytomegalovirus infection. G3 (Bethesda) 7, 1979–1984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furukawa H., Iizuka K., Poursine-Laurent J., Shastri N., Yokoyama W. M., A ligand for the murine NK activation receptor ly-49D: Activation of tolerized NK cells from beta 2-microglobulin-deficient mice. J. Immunol. 169, 126–136 (2002). [DOI] [PubMed] [Google Scholar]
- 91.Soneoka Y., et al. , A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23, 628–633 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ito D., Iizuka Y.-M., Katepalli M. P., Iizuka K., Essential role of the Ly49A stalk region for immunological synapse formation and signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 11264–11269 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iizuka K., Naidenko O. V., Plougastel B. F. M., Fremont D. H., Yokoyama W. M., Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nat. Immunol. 4, 801–807 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All scRNA-seq data are accessible from the National Center for Biotechnology Information Gene Expression Omnibus depository using GEO accession no. GSE132394.