Abstract
Retinal degenerative diseases caused by photoreceptor cell death are major causes of irreversible vision loss. As only primates have a macula, the nonhuman primate (NHP) models have a crucial role not only in revealing biological mechanisms underlying high-acuity vision but also in the development of therapies. Successful translation of basic research findings into clinical trials and, moreover, approval of the first therapies for blinding inherited and age-related retinal dystrophies has been reported in recent years. This article explores the value of the NHP models in understanding human vision and reviews their contribution to the development of innovative therapeutic strategies to save and restore vision.
Keywords: vision, retinal diseases, vision restoration, nonhuman primate, macaque
As part of human multisensorial interaction with the world, vision is often perceived as the dominant sensory modality. Eyes provide most of our information about the environment, and efficient vision is considered to be vital to our overall health. Its loss has potentially greater effects on daily life than loss of limbs, memory, hearing, or speech (1). The reduced independence associated with blindness, leading to social isolation, unemployment, and depression, ranks it among the major health concerns, on a level with cancer and Alzheimer’s (1). In addition to the personal impact of visual disability, the economic burden on health and social systems is significant (2).
Vision: A Vital Sense
Retinal degenerative diseases caused by photoreceptor cell death are major causes of irreversible blindness in industrialized countries. Photoreceptor cell death leading eventually to impairment of central vision occurs in monogenic diseases such as retinitis pigmentosa, Stargardt disease, and Leber congenital amaurosis, as well as in complex age-related diseases such as age-related macular degeneration (AMD). For these blinding conditions, retinal implants and gene and cell therapy may allow therapeutic intervention at various stages of the disease (3, 4). This article emphasizes advancements in therapies for retinal degenerative diseases and the contribution of the nonhuman primate (NHP) model to progress in the clinical translation of emerging retinal therapies.
Understanding Vision
Since most visual diseases are caused by eye pathologies, the preservation of sight and the curing of blindness are intrinsically linked to understanding vision mechanisms within the eye. Fortunately, the eye has several features that facilitate investigation and experimentation, including direct optical and mechanical access, small volume, internal compartmentalization, stable cell populations, and relative immune privilege. The visual system is one of the best understood sensory systems, due at least in part to the optical transparency of the eye. This allows direct visualization and precise morphological evaluation via noninvasive high-resolution imaging. The straightforward in vivo and ex vivo functional and anatomical analyses of eye cells and tissues provide highly responsive and reliable models for in-depth functional studies. Furthermore, brain-imaging studies describing visual cortex activity aid our understanding of the visual system and its functions and also serve as important tools for studying the brain (5–8).
Uniqueness of the Primate Model for Human Vision
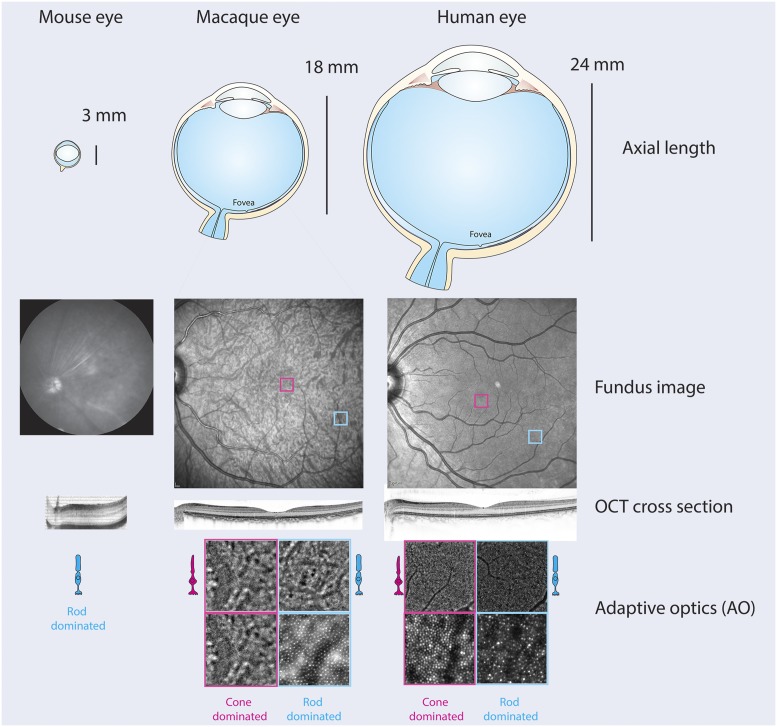
An important feature of human vision, shared only by primates, is the central visual pathway starting at the macula in the center of the retina, which is an area dominated by cone photoreceptor cells (Fig. 1). The distribution of intermediate neurons and of ganglion cells, as well as the large cortical representation of the central visual field, account for the important high-resolution central vision. This has strong implications for normal vision and many disease conditions.
Fig. 1.
Anatomy of the rodent and primate eye. (Upper) Gross anatomy of the mouse, macaque, and human eyes with their corresponding axial lengths. (Middle) Fundus and OCT images of the back of the eye in each species. (Lower) Adaptive optics images of rod and cone-dominated regions of the primate retina. Image courtesy of D.D., Kate Grieve (artist), and Céline Jaillard (artist).
The genus Macaca, as the most widespread NHP, diverged from its ape and human ancestors 25 million years ago. It has become a valuable animal model for investigating human biology and validating biomedical research. The genus diversified ∼5 million years ago and includes over 20 species that share >90% DNA sequence similarity and highly conserved protein sequences with humans. Moreover, humans and macaques share susceptibility genes for AMD and genotype–phenotype correlations for rare forms of inherited retinal diseases like achromatopsia (9) and retinitis pigmentosa (10). Old World monkeys such as rhesus macaques (Macaca mulatta) and cynomolgus macaques (Macaca fascicularis) are probably the best models for the study of ocular diseases (11). However, due to the low prevalence of spontaneous disease, only a few animals are affected in even the largest research colonies, and their disease phenotypes have been long ignored (9). Reduction in the cost of DNA sequencing has revealed the amount of functional genetic variation in rhesus macaque research colonies and allowed broad surveys of genetic mutations that display a disease phenotype (12). Other NHP species, such as the squirrel monkey (Saimiri sciureus), have also been useful for testing experimental therapies. For example, gene therapy was tested on adult squirrel monkeys defective in the L-opsin gene (13). Although such rare inheritable genetic defects in monkeys make them an invaluable model for the development and testing of mutation-specific gene therapies, a current limitation of NHPs is the lack of specific models that fully replicate most human conditions. Most cutting-edge therapies today are directed toward late-stage rod-cone dystrophy and atrophic or wet AMD, where loss of photoreceptors has already occurred. For this, the lack of severe inherited models displaying outer retinal loss has been compensated by the generation of acute models via laser ablation (14–16), chemically induced photoreceptor ablation (16), or the separation of the outer retina in reaction to a subretinally implanted retinal prosthesis (17). Other promising vision restoration strategies such as optogenetics have been tested extensively in normal NHPs, including marmosets (18) and macaques (19, 20).
Another important advantage of NHPs for therapy development is the availability and similarity of ocular phenotyping instruments that reveal extraordinary insight into the state of the retina in living NHPs (21). Routine use is now made of optical coherence tomography (OCT) and its full-field version, as these provide transverse optical imaging of the retina and identification of the different neuronal layers (Fig. 1). Directional OCT adds optical contrast to directionally reflective retinal structures and provides information about the orientation of retinal cell components in vivo, e.g., in the outer nuclear layer. Pivotal to these technological advances is adaptive optics (AO) that makes neuroretina structures directly observable in vivo in a noninvasive, safe, and easily tolerated manner. Different multimodal imaging systems are under development to enhance the potential for studying individual cells in the living eye (Fig. 1). These revolutionary imaging tools offer unprecedented insight into the human retina in health and disease (reviewed in refs. 22 and 23). Many psychophysical (e.g., microperimetry), electrophysiological (e.g., multifocal and full-field electroretinogram), real-life performance-based tests, and computer models can be combined with eye movement tests to understand cognition and behavior. OCT and adaptive optics scanning laser ophthalmoscopy can be performed in vivo, allowing the identification of slight structural changes in the retina, exploration of interactions between rods and cones, and measurement of responses to therapies in targeted cells (24, 25).
The Primate Model for Understanding Vision
Vision is initiated in the retina, and images collected by the eye are interpreted in the brain. The functional anatomy of the visual system is the key to interpreting the complex process of “seeing” as a target for vision restoration therapies. Shared essential anatomical features and organization of the visual pathways make NHPs an ideal model to reproduce the human visual system and human eye diseases and to study the principles of brain function. A significant body of evidence reveals features of the primate eye and brain that are distinctly different from those of other mammals, and many behavioral adaptations are consequences of high retinal acuity (reviewed in ref. 26). Notably, a macula located temporal to the optic nerve is a distinctive attribute of the primate retina (Fig. 1). Primate visual perception with the macula and the fovea, located within the macula at the optical center of the retina, is unique among mammals. Moreover, photoreceptor cell organization within the macula is similar in humans and NHPs (27), with densely packed cones in the fovea of up to 160,000 to 200,000 cones/mm2; cone photoreceptor packing density decreases faster with increasing eccentricity from the foveola. This photoreceptor distribution profile directly reflects the specialized functions of the macula and macular cones that ensure sharp central vision in bright light conditions and color vision. As the foveal region covers about 2° of visual angle, fast and precise saccadic eye movements in primates are essential for visual perception bringing new visual information to the foveal region. Further common features of Old World primates include trichromatic color vision and forward-facing eyes with overlapping visual fields and a high degree of binocular convergence. As in other mammals, the primate retina has 3 nuclear layers: the photoreceptor layer, the inner nuclear layer, and the ganglion cell layer. Axons of ganglion cells connect to the brain via the optic nerve and reach 2 main targets: the lateral geniculate nucleus, which projects to the primary visual cortex (V1), and the superior colliculus.
The visual system is part of the central nervous system and is most developed in primates, where ∼55% of the cortex is specialized for visual processing, compared to 3% for auditory processing and 11% for somatosensory processing (28). The NHP brain closely resembles the human brain in developmental trajectory and anatomy, including encephalization, the number and density of cortical neurons, a large prefrontal cortex, and greater myelination. The evolutionary proximity of NHPs and humans is also reflected in their similar perceptual capacities and ability to perform complex tasks. Alone or in combination with other sensory stimuli, visual signals are an important component of NHP behavior. Primates share other important aspects of their lifestyles (such as diurnality, terrestriality, omnivory) and sensory/perceptual abilities (such as color vision, greater reliance on vision than olfaction and other senses, the use of hands and thumbs; reviewed in ref. 29). Because primates learn through observation and perform tasks in constrained experimental conditions, investigation of brain responses to visual stimuli provides unique insight into high-level perceptual skills and distinct primate behavioral adaptations such as precise manual motor behavior (reaching and grasping) and complex social interactions. For instance, optical imaging has enabled the definition of ocular dominance maps (5). Calcium imaging has revealed the orthogonal microorganization of orientation and spatial frequency maps in layer II/III of the visual cortex (6). Recently, we defined retinotopic maps and presented functional evidence for ocular dominance columns within the depth of the visual cortex using ultrafast ultrasound imaging in behaving NHPs (8). Furthermore, anatomical tracing and histological techniques have provided access to the connectome between higher visual centers (7, 30) and structural correlates to functional maps such as ocular dominance columns (31–34). Primates also present opportunities to study visual attention. One of the most critical functions of sensory and motor systems is preparation for upcoming events. In sensory systems, this preparation confers perceptual and behavioral advantage. Given the location of an upcoming event, subjects respond with shorter reaction times and greater accuracy, an internal state often referred to as spatial attention. Attention has been studied in humans for over a century (35), based on behavioral tests as well as noninvasive methods such as electroencephalography and magnetic resonance imaging. The neural circuits that give rise to the perceptual and behavioral advantages conferred by attention have been studied in NHPs for decades, focused at first on single neurons in the visual cortex (36) that change their responsiveness or gain (37, 38). With advances in technology, investigation of attention in NHPs has revealed how populations of neurons may shift the neural code and improve perception (39–42).
Translation of Therapies Targeting the Fovea (Gene Therapy, Optogenetics)
High-acuity vision is dependent on the cone photoreceptor visual pathway in all species with a macular function. In human retinal disease, visual acuity loss is commonly the result of cone photoreceptor dysfunction observed in age-related macular degeneration. In addition, mutations that disrupt cone function have been identified in more than 40 genes (43). NHP models are vital for the development of therapeutics targeting the cones that restore and maintain high-acuity vision in humans. Unfortunately, there are only a few NHP models of macular disease. Examples of individual NHPs with validated inherited retinal disease have been described (44–46), but the genetic origin is known for only one rare form of achromatopsia (9). These animals, with all of the characteristic signs, symptoms, and functional deficits of phosphodiesterase 6C (PDE6C)-related achromatopsia, will be decisive in the development of gene or cell therapies targeting the fovea.
Finally, the identification of further NHPs with spontaneously occurring inherited retinal dystrophies (IRDs) will generate new resources for further preclinical validation of the stream of future therapeutics expected to enter clinical testing. We anticipate that NHP models of retinal disorders will reduce the time required for translation of gene- or cell-based therapies to human clinical trials, such as the ones described below, and dramatically improve the success rate.
NHP: Models and Therapies for Complex Diseases
NHPs have also been used for modeling and developing treatments for complex diseases such as diabetic retinopathy, choroidal neovascularization, wet AMD (47, 48), and glaucoma (49, 50). Since neovascularization is a common symptom in several ocular diseases, including diabetic retinopathy, age-related macular degeneration, and retinopathy of prematurity, an NHP model presenting this feature was created using intraocular administration of adeno-associated virus (AAV) AAV2 vectors encoding human vascular endothelial growth factor (VEGF) (47). Overexpression of VEGF was achieved and led rapidly to features of diabetic retinopathy or macular edema, depending on the targeted cell type/mode of production of VEGF and diffusion of VEGF. NHP models were also useful in testing long-term safety and efficacy of AAV-mediated treatments for blinding neovascular diseases such as wet AMD, as mentioned previously (48). With glaucoma being an important retinal neurodegenerative disease affecting over 70 million people worldwide, NHP models recapitulating symptoms of this condition were also sought. Intraocular pressure, a common risk factor for glaucoma, is tightly regulated by aqueous humor production and outflow pathways. One of the tissues that contribute significantly to this complex regulation is the trabecular meshwork, which is fully developed only in humans and NHPs (51). Laser- and bead-induced ocular hypertension models in NHPs have been proposed to test and develop treatments for glaucoma (49, 50). These models are similar to the human disease, presenting with reduced outflow facility, nerve fiber layer defects, and progressive enlargement of the cup-to-disk ratio, and, thus, they are highly predictive for drug efficacy in humans. The trabecular meshwork also represents a potential target for gene therapy in glaucoma, as genes delivered into the anterior chamber of the NHP eye preferentially reach this tissue, allowing permanent secretion of therapeutic factors (52). Early studies used Ad recombinant vectors to transduce the trabecular meshwork (53). Later studies used AAV vectors (54) and lentiviral vectors (55) to deliver potentially therapeutic genes targeting the Rho/ROCK and prostaglandin pathways in NHPs. For example, single intracameral injection of AAVs ensured a stable and long-term expression (>2 y) of a reporter gene in the trabecular meshwork of living Cynomolgus monkeys with an early onset and a safe clinical profile (54). Intracameral delivery of exoenzyme C3 transferase (C3) vector to monkey eyes led to a safe and persistently lowered intraocular pressure for 112 d as a result of inactivated RhoA in trabecular meshwork, disruption of the actin cytoskeleton, and altered cell morphology (56). Lentiviral-mediated delivery of the prostaglandin F synthase (PHFS) gene to the anterior segment in NHP has been shown to lower intraocular pressure for a period of 5 mo (57). Furthermore, experimental studies in NHPs provided strong evidence that the optic nerve head is the primary site for disturbances in axonal transport (reviewed in refs. 58 and 59), contributing to our understanding of basic mechanisms of disease. High-resolution in vivo imaging techniques, along with histological studies, were able to precisely quantify the effects of changes in the intraocular pressure on the optic nerve head and surrounding tissues, identifying the peripapillary connective tissue, lamina cribrosa, and neural tissue as structures susceptible to intraocular pressure (59–61). The NHP model revealed distinct structural differences of optic nerve head in primates when compared to rodents. Specifically, in the primate eye, retinal ganglion cell axons pass through a meshwork of astrocyte-covered, capillary-containing, connective tissue beams known as the lamina cribrosa (reviewed in ref. 58), similar to human optic nerve head structure.
Surrogate Models on the Development Path
As the step from rodents to humans appears too distant to ensure the success of therapies in patients with retinal degenerative disorders (62), therapeutic strategies must be validated in NHP models after proof-of-concept studies in rodents (63). However, prior to in vivo studies in NHPs, different ex vivo/in vitro primate models are advantageous for screening promoters, genes, viral vectors, and molecular targets on NHP or even human cells. For instance, retinal cells have been cultured from various species, including NHPs or postmortem human retina (64, 65). The survival of human retinal cells in culture has been exploited to characterize their electrophysiological properties (33). The morphology of dissociated retinal cell changes and the lack of synaptic contact may also alter cell physiology and gene expression profiles. Retinal explant cultures that retain tissue architecture with cell synaptic and neurocrine interactions were developed to assess retinal cell neuroprotection in different animal models of retinal disease (66–69). Furthermore, retinal explant cultures have been produced from primate and postmortem human retina (62, 70–72). These in vitro preparations allowed us to demonstrate functional expression of genes such as microbial opsins in cone photoreceptors (70) and retinal ganglion cells (73) in the development of optogenetic therapy for restoring vision. We also used ex vivo NHP retinal explants to assess the efficacy of the PRIMA photovoltaic retinal prosthesis (17). The recent development of retinal organoids from induced pluripotent stem cells (iPSCs) has extended the interest in in vitro studies by providing an unlimited source of human retinal cells, with the additional possibility of generating tissues from patients affected by specific gene mutations (72, 74–77). Such in vitro/ex vivo retinal models will allow an essential validation step prior to in vivo NHP studies and thus reduce the number of animals in preclinical studies.
Despite their value for drug development, viral vector selection, and neuroscience research, there are major limits to modeling in vivo conditions using retinal explants and self-forming retinal organoids derived from human embryonic stem cells (hESCs) or human iPSCs. First, the heterogeneity in production, composition, and maturation of organoids is problematic, with differentiation and maturation efficiencies varying not only between iPSC lines but even between organoids originated at the same time. Second, these in vitro tissues lack vascularization and will thus obscure how metabolic and oxygen supplies may affect the degenerative process and therapeutic intervention. Third, such preparations are often separated into neural retina and retinal pigment epithelium (RPE) at the expense of physiological interactions between photoreceptors and RPE. A recent report suggests that these shortcomings can be overcome using a new microphysiological model of the human retina enhanced with a vasculature-like perfusion system in addition to a support system with RPE (78). These favor the establishment of in vivo-like physiological processes such as outer segment phagocytosis and calcium dynamics in retinal cells, significantly expanding the relevance of such a system. However, even such sophisticated retinal organoids have limitations, for example, for investigations that probe vision, which is a result of interplay between the visual cortex and the retina. They are also not appropriate for studies in which pharmacodynamics and immunology are important factors, as these in vitro tissues do not represent the whole eye connection to the body and the immune system.
Therapy Development Involving Nonhuman Primates In Vivo
Gene Therapy.
Gene therapy approaches today fall into 4 broad categories. The first is gene replacement therapy, also referred to as gene addition or gene supplementation. This employs cDNA encoding a healthy copy of a recessive mutant gene. After an initial success in the treatment of type 2 Leber congenital amaurosis, a dozen further clinical trials used this strategy against well-characterized monogenic recessive diseases of the retina, including choroideremia, Leber hereditary optic neuropathy, retinoschisis, Stargardt disease, Usher 1B, and achromatopsia (79). In the absence of representative genetic models of these conditions in NHPs, most of the clinical trials followed regulatory safety and toxicity studies on healthy NHPs. However, it is perhaps in inherited cone dystrophies that an NHP model will provide the most accurate readouts, with the emergence of a model for testing gene therapies directed toward PDE6C supplementation (9). Such work may allow comparison of outcomes using a model with identical genotypes/phenotypes to humans and also lead to gene replacement studies that have already restored cone cell function in mouse models with CNGB3 achromatopsia (80–82), as well as canine and sheep models of achromatopsia with mutations in CNGA3 (83, 84).
As well as for gene replacement, PDE6 mutant NHPs may be a good model for testing the second form of gene therapy based on CRISPR-Cas9 modification of disease-causing mutations. CRISPR-Cas9–mediated base editing or homology-directed repair (CRISPR-HDR) can be used to produce a functional protein. Since no other genetically characterized, spontaneously occurring IRD models exist in NHPs, PDE6C-mutant macaques may be a unique model to test CRISPR targeting of this specific mutation, with the possibility of functional readouts. As for other gene-editing attempts to target various disease-causing mutations, a productive rate of editing of the target gene can be tested with a comparable surrogate NHP vector (85) to determine whether a therapeutic threshold is met or not under any given circumstances. This approach has demonstrated the ability of CRISPR-Cas9 to edit somatic primate cells in vivo in a study aimed at restoring vision loss in Leber congenital amaurosis type 10 (86).
The third approach to gene therapy uses secretable factors to hamper adverse symptoms of retinal disease. These factors typically include soluble anti-VEGF molecules for the treatment of wet AMD (48, 87, 88) and trophic factors like RdCVF (25, 89), GDNF (90, 91), and Nrf2 (92). Secretable factors such as soluble fms-like tyrosine kinase (sFLT) have been proposed together with therapeutic options, alleviating the need to readminister anti-VEGF antibodies in some AMD patients. Preclinical studies in macaques have tested both the safety and the dosage thresholds required for the follow-up clinical trial (48). There is an ever-increasing list of known disease-causing mutations in monogenic inherited retinal diseases. Thus, the identification of a generic gene-based treatment that can alleviate photoreceptor loss is a priority. Secretable factors widely applicable in inherited retinal diseases such as rod-cone dystrophy have been intensively researched in rodent models of rod-cone dystrophy. Several promising candidate survival factors are now in the preclinical stages of testing, with regulatory studies foreseen in NHPs (24).
The fourth gene therapy category is based on optogenetics, i.e., the use of light-sensitive proteins to transform any neuron of the retina into an artificial photoreceptor (93), and restore vision in rod-cone dystrophy following the loss of cones in outer segments (94). Optogenetics holds promise for biomedical applications particularly in the retina, and research over the past decade, first in rodents and then in NHPs, has led to translation into 2 clinical trials using this strategy (93). Most of the studies to date used microbial opsins from algae or bacteria, which have specific wavelength and light-intensity requirements and the ability to respond to light in the absence of chromophore replenishment by the RPE. The activity of these microbial opsins can thus be evaluated in normal primate retinas ex vivo, where fading of the endogenous light responses allows specific recording of the optogenetic activity (19, 20, 70, 95). These strategies have been tested in normal primates including marmosets (18), which have a fovea but a very small eye, making dose-related extrapolations difficult. For this reason, macaques have been used for most mandatory dose-extrapolation and functional studies of AAV-mediated delivery of optogenetic sensors prior to clinical translation (19, 20). In fact, the use of viral vectors, particularly AAV, to deliver therapeutic genes is the common denominator of the majority of human gene therapy studies. The efficacy of therapeutic DNA as a prodrug depends largely on efficient targeting of the cells of interest and induction of adequate expression levels; each case is a unique scenario. This challenge is discussed below.
AAV screen.
NHPs are a potential model not only to evaluate treatment of affected NHPs but also to optimize viral-mediated treatment modalities by subretinal and intravitreal injections. Intraocular injection of AAV vectors has been an evident route for delivering genes to retinal cells. However, gaps in our understanding of AAV transduction patterns within the anatomically unique environments of the subretinal and intravitreal space of the NHP eye, which differ from small animal models and unfoveated large animals, has slowed the establishment of noninvasive and efficient gene delivery important for the clinical application of gene therapy. Intraocular (subretinal or intravitreal) injections of AAVs encoding fluorescent proteins and the resulting expression can be followed using noninvasive employment of a retinal fundus camera to visualize neurons expressing the transgene (96–100). The similarity to the ocular anatomical barriers of humans is a major advantage of working with NHPs (101). Surgical methods, pharmacological parameters such as dosage, and the access of AAV vectors to various retinal neurons can be rigorously evaluated before going to clinical trials. Testing of new vector–promoter combinations for cone transduction, particularly in the fovea, can only be conducted in NHPs (20, 98, 102, 103). Based on these studies, AAV9 and its variants have been shown to lead to efficient foveal cone transduction when injected into the subretinal space (20, 98). An AAV9 variant (AAV9-7m8) injected several millimeters away from the fovea, without detaching this delicate region, induced significant gene expression in this region. An engineered AAV2 variant (AAV2-7m8) delivers genes to foveal cones with a well-tolerated dose when administered intravitreally. Although a healthy NHP is an excellent model to determine the adequacy of AAV capsids and doses in combination with appropriate injection routes (96, 101, 104, 105), studies in mouse models, human iPSC-derived organoids (20, 106), and postmortem human retinal explants (70, 71, 95) are required to complement findings in normal macaques. Although use of macaques can efficiently predict the distribution of AAV in a normal retina, viral transduction patterns change dramatically in retinal disease (107–109), making it necessary to add further model systems. Tests in several model systems provide the most accurate insight into the behavior of AAV vectors across species in order to validate the safety and efficacy required for gene therapy in neurodegenerative disorders.
Promoter screen.
Specific promoters can be used to restrict expression of genes to defined cell types. A large-scale promoter screen recently performed in mouse, NHP, and human retinas (62) showed that particular promoters rarely targeted the same cell types in mice and humans; however, the correspondence between NHPs and humans was much better. Since a promoter screen in humans can only be performed in vitro, use of NHPs can determine whether the selected promoter is also active and selective in vivo.
Implants.
Retinal prostheses aim at restoring vision in blind patients lacking photoreceptors by activating the residual retina with electrical currents delivered by a multielectrode array. The current state of the art is represented by the Argus II implant, which received the CE (Conformité Européenne) mark and FDA approval (humanitarian exemption) for commercialization in Europe and the United States, respectively (110), and was discontinued in 2019. After the clear demonstration that electrical currents can activate the retina (111), an initial prototype with 16 electrodes chronically implanted in blind patients showed that electrical stimulation can provide visual perception with maintained activity over time (112). Patients could already perform simple shape detection and movement perception with this 4 × 4 array. The current Argus II device with a 60-electrode array allowed some patients to recognize letters and even read simple words (113). Prior to their clinical use, these implants were mainly tested in normal and blind dogs (114, 115). However, the first photovoltaic implants introduced into the subretinal space at the original position of the photoreceptors were instead tested in cats (116). Another photovoltaic subretinal implant with a wire connection to a battery located behind the ear has obtained the CE mark following positive results in blind patients (117). It was discontinued in 2019.
We recently tested the new photovoltaic prosthesis PRIMA invented by Daniel Palanker on NHPs to demonstrate the efficiency and high resolution that would allow transfer to clinical trials. PRIMA was found to provide high visual acuity in blind rats (118). Prior to transfer of these implants into clinical trials, PRIMA devices were first fabricated with a clinical-grade process. To demonstrate their potential superiority to the existing devices, we assessed their activity on the primate retina. First, we showed that postmortem human retina can be activated by these implants. We then removed photoreceptors by sectioning the NHP retina with a vibratome and demonstrated that individual retinal ganglion cells but not implant neighbors were activated by a single electrode. Finally, to mimic the patient situation, PRIMA implants were introduced into the subretinal space, eliciting photoreceptor degeneration and creating a blind spot at the implant position, as previously reported (116). Taking advantage of this blind spot, we showed that NHP eyes generated visual saccades in response to activation of a single implant electrode by infrared activation of the photovoltaic implant. PRIMA implants are now in clinical trials in patients with advanced atrophic age-related macular degeneration in France and the United States.
Stem Cells.
Transplantation studies.
Cell therapy is an alternative method to repair degenerated retina. Transplantation of retinal cells is a potential vision restoration strategy for retinal degenerative diseases, particularly in disease stages associated with significant cell damage. This therapeutic approach aims at replacing lost retinal cells using progenitor cells or stem cell-derived retinal cells (76, 77, 119–121). The advantage of cell therapies is that they are mutation-independent and can be used in a wide range of conditions. Furthermore, culture systems for the production of 3D tissue organoids from pluripotent stem cells (PSCs), which reflect a developmental environment more closely related to the in vivo situation, now allow the generation of specific cell types with high precision and amounts. This was demonstrated in self-organizing organoids (74, 122) to address critical issues for intraocular cell transplantation. However, determination of the most appropriate surgical approach; the application of treatment in eyes of size and structure similar to the human eye, including the presence of a macula; and a thorough understanding of the immunological considerations for graft survival and the consequences of grafted rejection can only be assessed in transplantation studies conducted with NHPs. Promising therapies, such as those based on transplantation of iPSC-derived retinal sheets (15, 16) or RPE (123, 124), conducted in acute outer retinal degeneration models in NHPs, contributed greatly to their clinical translation. Indeed, the first-in-human safety and tolerability clinical trial to evaluate subretinal injection of human ESC-derived RPE cells into patients with dry AMD and Stargardt disease is ongoing (125). Cell transplantation experiments in NHPs are expected to address several outstanding questions, such as which type of cells are best to transplant and whether transplanted precursors differentiate in vivo into functional photoreceptors and form functional synaptic connections with downstream neurons. NHP models of photoreceptor loss will also be useful for evaluating cell transplantation in an allogenic context based on the specific use of macaque iPSCs. Indeed, it is very difficult to know whether xenograft models—generally used to validate cell transplantation benefits in specific diseases—are truly predictive of transplantation efficacy in humans. Photoreceptor transplantation studies focusing on cones can now be tested in the genetic model of achromatopsia, taking advantage of the cone-silent state and relatively stable architecture of the macula in these NHPs (9). Cone replacement has suffered from challenges in the production of cone photoreceptors in vitro from human iPSC-derived retinal organoids, although some strategies have been reported to bias cells to adopt this fate (126, 127). There is now renewed interest in investigating human cell transplantation to the retina (128), as advances in the past decade using mouse models appear to be in part secondary to material transfer between transplanted cells and endogenous cells (129, 130).
Discussion
NHPs are used in biomedical research as a highly relevant model because of their similarities to humans in physiology, neuroanatomy, reproduction, development, cognition, and social complexity. Indeed, over the past decades, research involving NHPs has played a vital role in key medical and scientific advances in the fields of neurodegenerative diseases (e.g., Alzheimer’s and Parkinson’s), neurologic and cognitive conditions (e.g., stroke and anxiety), HIV/AIDS, development of vaccines, and studies of the reproductive system and in vitro fertilization. Being the species closest to humans, NHPs also have a high degree of sentience; thus, ethical principles require NHP use in research to be strictly justified. The laws regulating research with animals aim at securing laboratory animal welfare while allowing research to continue. In the United States, research with animals follows a complex set of federal and state laws, regulations, guidelines, and institutional policies. In Europe, the principle of the 3Rs—replacement (avoid/replace the use of animals), reduction (minimize the number of animals used), and refinement (minimize animal suffering and improve welfare)—must be considered when selecting approaches to the regulatory testing of human and veterinary medicinal products (131). Wherever possible, at an early stage in potentially translational research, a significant reduction in animal studies should be achieved by alternative cellular and organotypic in vitro, in vivo, and in silico models and methods. The proper design of studies and the implementation of mathematical models for outcome prediction could also contribute significantly to a marked reduction of animals in research. However, in various areas of medicine, including neurosciences and vision research, translation to the clinic often needs the use of primates in order to achieve results that unequivocally confirm benefits for human health. Given that only primates have a fovea, there is no realistic alternative to NHPs for translational studies in genetic and age-related blinding disorders associated with macular pathology that are a major health and social burden. NHP research in the emerging visual prosthetics field is also essential. In these cases, NHP research alone can provide precise and conclusive data that either facilitate the pathway “from bench to bedside” or stop further steps in research and/or translation to the clinic of inadequate treatment candidates, thus saving animals, effort, and money. In other words, NHP research secures a greater success rate in clinical studies.
Data Availability.
No new data were generated for this paper.
Acknowledgments
The authors thank Olivier Marre for valuable comments; Philippe Hantraye, Céline Jaillard, and Pierre Pouget for their contributions to primate studies; and Kate Grieve and Céline Jaillard for contributing the images in Fig. 1. This work was supported by grants from Programme Investissements d’Avenir IHU FOReSIGHT (ANR-18-IAHU-01), Programme Investissements d’Avenir LabEx LIFESENSES (ANR-10-LABX-65), European Research Council (ERC) Synergy “HELMHOLTZ,” Banque publique d’Investissement, Foundation Fighting Blindness, ERC StG (REGENETHER, 639888).
Footnotes
Competing interest statement: K.M. and O.G. declare no competing interests. D.D. has received grants and personal fees from GenSight Biologics and is coinventor on patent 9193956 (Adeno-associated virus virions with variant capsid and methods of use thereof), with royalties paid to Avalanche Biotechnologies. J.-A.S. is a consultant (with no consulting fee) for Pixium Vision, GenSight Biologics, and SparingVision and has personal financial interests in Pixium Vision, GenSight Biologics, Prophesee, and Chronolife. B.R. is chair of the Scientific Advisory Board of GenSight Biologics. S.P. is a consultant for Pixium Vision and GenSight Biologics and has personal financial interests in Pixium Vision, GenSight Biologics, and ICONEUS.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Using Monkey Models to Understand and Develop Treatments for Human Brain Disorders,” held January 7–8, 2019, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. NAS colloquia began in 1991 and have been published in PNAS since 1995. From February 2001 through May 2019 colloquia were supported by a generous gift from The Dame Jillian and Dr. Arthur M. Sackler Foundation for the Arts, Sciences, & Humanities, in memory of Dame Sackler’s husband, Arthur M. Sackler. The complete program and video recordings of most presentations are available on the NAS website at http://www.nasonline.org/using-monkey-models.
This article is a PNAS Direct Submission.
References
- 1.Scott A. W., Bressler N. M., Ffolkes S., Wittenborn J. S., Jorkasky J., Public attitudes about eye and vision health. JAMA Ophthalmol. 134, 1111–1118 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Chakravarthy U., et al. , The economic impact of blindness in Europe. Ophthalmic Epidemiol. 24, 239–247 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Scholl H. P. N., et al. , Emerging therapies for inherited retinal degeneration. Sci. Transl. Med. 8, 368rv6 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Roska B., Sahel J.-A., Restoring vision. Nature 557, 359–367 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Blasdel G., Campbell D., Functional retinotopy of monkey visual cortex. J. Neurosci. 21, 8286–8301 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauhaus I., Nielsen K. J., Disney A. A., Callaway E. M., Orthogonal micro-organization of orientation and spatial frequency in primate primary visual cortex. Nat. Neurosci. 15, 1683–1690 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donahue C. J., et al. , Using diffusion tractography to predict cortical connection strength and distance: A quantitative comparison with tracers in the monkey. J. Neurosci. 36, 6758–6770 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaize K., et al. Functional ultrasound imaging of deep visual cortex in awake non-human primates. bioRxiv:10.1101/551663 (21 February 2019).
- 9.Moshiri A., et al. , A nonhuman primate model of inherited retinal disease. J. Clin. Invest. 129, 863–874 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda Y., et al. , Discovery of a cynomolgus monkey family with retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 59, 826–830 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Ribka E. P., Dubielzig R. R., Multiple ophthalmic abnormalities in an infant rhesus macaque (Macaca mulatta). J. Med. Primatol. 37 (suppl. 1), 16–19 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue C., et al. , The population genomics of rhesus macaques (Macaca mulatta) based on whole-genome sequences. Genome Res. 26, 1651–1662 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancuso K., et al. , Gene therapy for red-green colour blindness in adult primates. Nature 461, 784–787 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merigan W. H., Pasternak T., Zehl D., Spatial and temporal vision of macaques after central retinal lesions. Invest. Ophthalmol. Vis. Sci. 21, 17–26 (1981). [PubMed] [Google Scholar]
- 15.Tu H.-Y., et al. , Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine 39, 562–574 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirai H., et al. , Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc. Natl. Acad. Sci. U.S.A. 113, E81–E90 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prévot P., et al. , Functional efficacy of stimulations with a photovoltaic sub-retinal prosthesis in non-human primates. Nat. Biomed. Eng. (2019), in press. [Google Scholar]
- 18.Ivanova E., Hwang G.-S., Pan Z.-H., Troilo D., Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Invest. Ophthalmol. Vis. Sci. 51, 5288–5296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaffiol A., et al. , A new promoter allows optogenetic vision restoration with enhanced sensitivity in macaque retina. Mol. Ther. 25, 2546–2560 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khabou H., et al. , Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight 3, 96029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi E. A., et al. , Imaging individual neurons in the retinal ganglion cell layer of the living eye. Proc. Natl. Acad. Sci. U.S.A. 114, 586–591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burns S. A., Elsner A. E., Sapoznik K. A., Warner R. L., Gast T. J., Adaptive optics imaging of the human retina. Prog. Retin. Eye Res. 68, 1–30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paques M., et al. , Adaptive optics ophthalmoscopy: Application to age-related macular degeneration and vascular diseases. Prog. Retin. Eye Res. 66, 1–16 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Sahel J.-A., et al. , Functional rescue of cone photoreceptors in retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 251, 1669–1677 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Aït-Ali N., et al. , Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 161, 817–832 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Mitchell J. F., Leopold D. A., The marmoset monkey as a model for visual neuroscience. Neurosci. Res. 93, 20–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volland S., Esteve-Rudd J., Hoo J., Yee C., Williams D. S., A comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS One 10, e0125631 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felleman D. J., Van Essen D. C., Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991). [DOI] [PubMed] [Google Scholar]
- 29.Phillips K. A., et al. , Why primate models matter. Am. J. Primatol. 76, 801–827 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nassi J. J., Lyon D. C., Callaway E. M., The parvocellular LGN provides a robust disynaptic input to the visual motion area MT. Neuron 50, 319–327 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy C., et al. , Metabolic mapping of the primary visual system of the monkey by means of the autoradiographic [14C]deoxyglucose technique. Proc. Natl. Acad. Sci. U.S.A. 73, 4230–4234 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubel D. H., Wiesel T. N., Stryker M. P., Anatomical demonstration of orientation columns in macaque monkey. J. Comp. Neurol. 177, 361–380 (1978). [DOI] [PubMed] [Google Scholar]
- 33.Picaud S., Hicks D., Forster V., Sahel J., Dreyfus H., Adult human retinal neurons in culture: Physiology of horizontal cells. Invest. Ophthalmol. Vis. Sci. 39, 2637–2648 (1998). [PubMed] [Google Scholar]
- 34.Takahata T., Miyashita M., Tanaka S., Kaas J. H., Identification of ocular dominance domains in New World owl monkeys by immediate-early gene expression. Proc. Natl. Acad. Sci. U.S.A. 111, 4297–4302 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrasco M., Visual attention: The past 25 years. Vision Res. 51, 1484–1525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran J., Desimone R., Selective attention gates visual processing in the extrastriate cortex. Science 229, 782–784 (1985). [DOI] [PubMed] [Google Scholar]
- 37.Reynolds J. H., Pasternak T., Desimone R., Attention increases sensitivity of V4 neurons. Neuron 26, 703–714 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Williford T., Maunsell J. H. R., Effects of spatial attention on contrast response functions in macaque area V4. J. Neurophysiol. 96, 40–54 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Cohen M. R., Maunsell J. H. R., Attention improves performance primarily by reducing interneuronal correlations. Nat. Neurosci. 12, 1594–1600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell J. F., Sundberg K. A., Reynolds J. H., Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63, 879–888 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyder A. C., Morais M. J., Smith M. A., Dynamics of excitatory and inhibitory networks are differentially altered by selective attention. J. Neurophysiol. 116, 1807–1820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder A. C., Yu B. M., Smith M. A., Distinct population codes for attention in the absence and presence of visual stimulation. Nat. Commun. 9, 4382 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roosing S., et al. , Causes and consequences of inherited cone disorders. Prog. Retin. Eye Res. 42, 1–26 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Hummel M., Li Z. G., Pfaffinger D., Neven L., Scanu A. M., Familial hypercholesterolemia in a rhesus monkey pedigree: Molecular basis of low density lipoprotein receptor deficiency. Proc. Natl. Acad. Sci. U.S.A. 87, 3122–3126 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dray B. K., et al. , Mismatch repair gene mutations lead to lynch syndrome colorectal cancer in rhesus macaques. Genes Cancer 9, 142–152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominik Fischer M., et al. , Detailed functional and structural characterization of a macular lesion in a rhesus macaque. Doc. Ophthalmol. 125, 179–194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lebherz C., et al. , Nonhuman primate models for diabetic ocular neovascularization using AAV2-mediated overexpression of vascular endothelial growth factor. Diabetes 54, 1141–1149 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Maclachlan T. K., et al. , Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol. Ther. 19, 326–334 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgoyne C. F., The non-human primate experimental glaucoma model. Exp. Eye Res. 141, 57–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambert W. S., et al. , Towards a microbead occlusion model of glaucoma for a non-human primate. Sci. Rep. 9, 11572 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohen J. W., Morphology of the uveal tract. Int. Ophthalmol. Clin. 5, 581–667 (1965). [DOI] [PubMed] [Google Scholar]
- 52.Nilsson S. F. E., The uveoscleral outflow routes. Eye (Lond.) 11, 149–154 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Borrás T., Gabelt B. T., Klintworth G. K., Peterson J. C., Kaufman P. L., Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J. Gene Med. 3, 437–449 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Buie L. K., et al. , Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest. Ophthalmol. Vis. Sci. 51, 236–248 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barraza R. A., et al. , Prolonged transgene expression with lentiviral vectors in the aqueous humor outflow pathway of nonhuman primates. Hum. Gene Ther. 20, 191–200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan J., et al. , Lentiviral vector-mediated expression of exoenzyme C3 transferase lowers intraocular pressure in monkeys. Mol. Ther. 27, 1327–1338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee E. S., et al. , Prospects for lentiviral vector mediated prostaglandin F synthase gene delivery in monkey eyes in vivo. Curr. Eye Res. 39, 859–870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamm E. R., Ethier C. R.; Lasker/IRRF Initiative on Astrocytes and Glaucomatous Neurodegeneration Participants , Biological aspects of axonal damage in glaucoma: A brief review. Exp. Eye Res. 157, 5–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel N., McAllister F., Pardon L., Harwerth R., The effects of graded intraocular pressure challenge on the optic nerve head. Exp. Eye Res. 169, 79–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H., et al. , Deformation of the normal monkey optic nerve head connective tissue after acute IOP elevation within 3-D histomorphometric reconstructions. Invest. Ophthalmol. Vis. Sci. 50, 5785–5799 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strouthidis N. G., Fortune B., Yang H., Sigal I. A., Burgoyne C. F., Effect of acute intraocular pressure elevation on the monkey optic nerve head as detected by spectral domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 52, 9431–9437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jüttner J., et al. , Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat. Neurosci. 22, 1345–1356 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Danesh-Meyer H. V., Levin L. A., Neuroprotection: Extrapolating from neurologic diseases to the eye. Am. J. Ophthalmol. 148, 186–191.e2 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Hicks D., Forster V., Dreyfus H., Sahel J., Survival and regeneration of adult human photoreceptors in vitro. Brain Res. 643, 302–305 (1994). [DOI] [PubMed] [Google Scholar]
- 65.Gaudin C., Forster V., Sahel J., Dreyfus H., Hicks D., Survival and regeneration of adult human and other mammalian photoreceptors in culture. Invest. Ophthalmol. Vis. Sci. 37, 2258–2268 (1996). [PubMed] [Google Scholar]
- 66.Caffé A. R., Söderpalm A., van Veen T., Photoreceptor-specific protein expression of mouse retina in organ culture and retardation of rd degeneration in vitro by a combination of basic fibroblast and nerve growth factors. Curr. Eye Res. 12, 719–726 (1993). [DOI] [PubMed] [Google Scholar]
- 67.Mohand-Said S., et al. , Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc. Natl. Acad. Sci. U.S.A. 95, 8357–8362 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vallazza-Deschamps G., et al. , Excessive activation of cyclic nucleotide-gated channels contributes to neuronal degeneration of photoreceptors. Eur. J. Neurosci. 22, 1013–1022 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Froger N., et al. , Taurine provides neuroprotection against retinal ganglion cell degeneration. PLoS One 7, e42017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Busskamp V., et al. , Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 329, 413–417 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Fradot M., et al. , Gene therapy in ophthalmology: Validation on cultured retinal cells and explants from postmortem human eyes. Hum. Gene Ther. 22, 587–593 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Cowan C. S., et al. , Cell types of the human retina and its organoids at single-cell resolution: Developmental convergence, transcriptomic identity, and disease map. bioRxiv:10.1101/703348 (16 July 2019).
- 73.Sengupta A., et al. , Red-shifted channelrhodopsin stimulation restores light responses in blind mice, macaque retina, and human retina. EMBO Mol. Med. 8, 1248–1264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Llonch S., Carido M., Ader M., Organoid technology for retinal repair. Dev. Biol. 433, 132–143 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Foltz L. P., Clegg D. O., Patient-derived induced pluripotent stem cells for modelling genetic retinal dystrophies. Prog. Retin. Eye Res. 68, 54–66 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Gagliardi G., Ben M’Barek K., Goureau O., Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: A pluripotent stem cell-based approach. Prog. Retin. Eye Res. 71, 1–25 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Jin Z.-B., et al. , Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 69, 38–56 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Achberger K., et al. , Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife 8, e46188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bennett J., Taking stock of retinal gene therapy: Looking back and moving forward. Mol. Ther. 25, 1076–1094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Michalakis S., et al. , Restoration of cone vision in the CNGA3-/- mouse model of congenital complete lack of cone photoreceptor function. Mol. Ther. 18, 2057–2063 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai X., et al. , Long-term retinal cone rescue using a capsid mutant AAV8 vector in a mouse model of CNGA3-achromatopsia. PLoS One 12, e0188032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pang J.-J., et al. , Achromatopsia as a potential candidate for gene therapy. Adv. Exp. Med. Biol. 664, 639–646 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ezra-Elia R., et al. , Flicker cone function in normal and day blind sheep: A large animal model for human achromatopsia caused by CNGA3 mutation. Doc. Ophthalmol. 129, 141–150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson D. A., et al. ; Monaciano Consortium , Advancing therapeutic strategies for inherited retinal degeneration: Recommendations from the monaciano symposium. Invest. Ophthalmol. Vis. Sci. 56, 918–931 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahel J. A., Dalkara D., Gene therapy for retinal dystrophy. Nat. Med. 25, 198–199 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Maeder M. L., et al. , Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 25, 229–233 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Rakoczy E. P., et al. , Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet 386, 2395–2403 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Heier J. S., et al. , Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase 1, open-label trial. Lancet 390, 50–61 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Byrne L. C., et al. , Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J. Clin. Invest. 125, 105–116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frasson M., et al. , Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest. Ophthalmol. Vis. Sci. 40, 2724–2734 (1999). [PubMed] [Google Scholar]
- 91.McGee Sanftner L. H., Abel H., Hauswirth W. W., Flannery J. G., Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol. Ther. 4, 622–629 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Xiong W., MacColl Garfinkel A. E., Li Y., Benowitz L. I., Cepko C. L., NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Invest. 125, 1433–1445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Busskamp V., Roska B., Optogenetic approaches to restoring visual function in retinitis pigmentosa. Curr. Opin. Neurobiol. 21, 942–946 (2011). [DOI] [PubMed] [Google Scholar]
- 94.Sahel J.-A., Roska B., Gene therapy for blindness. Annu. Rev. Neurosci. 36, 467–488 (2013). [DOI] [PubMed] [Google Scholar]
- 95.Lin J. Y., Knutsen P. M., Muller A., Kleinfeld D., Tsien R. Y., ReaChR: A red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin L., et al. , Intravitreal injection of AAV2 transduces macaque inner retina. Invest. Ophthalmol. Vis. Sci. 52, 2775–2783 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dalkara D., et al. , In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 5, 189ra76 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Vandenberghe L. H., et al. , AAV9 targets cone photoreceptors in the nonhuman primate retina. PLoS One 8, e53463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vandenberghe L. H., et al. , Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 3, 88ra54 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kay C. N., et al. , Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One 8, e62097 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boye S. E., et al. , Highly efficient delivery of adeno-associated viral vectors to the primate retina. Hum. Gene Ther. 27, 580–597 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ye G.-J., et al. , Cone-specific promoters for gene therapy of achromatopsia and other retinal diseases. Hum. Gene Ther. 27, 72–82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boye S. E., et al. , The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum. Gene Ther. 23, 1101–1115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tshilenge K.-T., et al. , Vitrectomy before intravitreal injection of AAV2/2 vector promotes efficient transduction of retinal ganglion cells in dogs and nonhuman primates. Hum. Gene Ther. Methods 27, 122–134 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Kotterman M. A., et al. , Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 22, 116–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garita-Hernandez M., et al. , Optogenetic light sensors in human retinal organoids. Front. Neurosci. 12, 789 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kolstad K. D., et al. , Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum. Gene Ther. 21, 571–578 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vacca O., et al. , Using adeno-associated virus as a tool to study retinal barriers in disease. J. Vis. Exp. 98,e52451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vacca O., et al. , AAV-mediated gene delivery in Dp71-null mouse model with compromised barriers. Glia 62, 468–476 (2014). [DOI] [PubMed] [Google Scholar]
- 110.da Cruz L., et al. ; Argus II Study Group , Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology 123, 2248–2254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weiland J. D., et al. , Understanding the origin of visual percepts elicited by electrical stimulation of the human retina. Graefes Arch. Clin. Exp. Ophthalmol. 237, 1007–1013 (1999). [DOI] [PubMed] [Google Scholar]
- 112.Humayun M. S., et al. , Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 43, 2573–2581 (2003). [DOI] [PubMed] [Google Scholar]
- 113.da Cruz L., et al. , The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br J. Ophthalmol. 97, 632–636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Majji A. B., et al. , Long-term histological and electrophysiological results of an inactive epiretinal electrode array implantation in dogs. Invest. Ophthalmol. Vis. Sci. 40, 2073–2081 (1999). [PubMed] [Google Scholar]
- 115.Güven D., et al. , Long-term stimulation by active epiretinal implants in normal and RCD1 dogs. J. Neural Eng. 2, S65–S73 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Chow A. Y., et al. , Implantation of silicon chip microphotodiode arrays into the cat subretinal space. IEEE Trans. Neural Syst. Rehabil. Eng. 9, 86–95 (2001). [DOI] [PubMed] [Google Scholar]
- 117.Stingl K., et al. , Interim results of a multicenter trial with the new electronic subretinal implant alpha AMS in 15 patients blind from inherited retinal degenerations. Front. Neurosci. 11, 445 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lorach H., et al. , Photovoltaic restoration of sight with high visual acuity. Nat. Med. 21, 476–482 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gasparini S. J., Llonch S., Borsch O., Ader M., Transplantation of photoreceptors into the degenerative retina: Current state and future perspectives. Prog. Retin. Eye Res. 69, 1–37 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Dalkara D., Goureau O., Marazova K., Sahel J.-A., Let there be light: Gene and cell therapy for blindness. Hum. Gene Ther. 27, 134–147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reichman S., et al. , From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc. Natl. Acad. Sci. U.S.A. 111, 8518–8523 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gagliardi G., et al. , Characterization and transplantation of CD73-positive photoreceptors isolated from human iPSC-derived retinal organoids. Stem Cell Rep. 11, 665–680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kamao H., et al. , Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2, 205–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ben M’Barek K., Monville C., Cell therapy for retinal dystrophies: From cell suspension formulation to complex retinal tissue bioengineering. Stem Cells Int. 2019, 4568979 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zarbin M., Sugino I., Townes-Anderson E., Concise review: Update on retinal pigment epithelium transplantation for age-related macular degeneration. Stem Cells Transl. Med. 8, 466–477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eldred K. C., et al. , Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science 362, eaau6348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reichman S., et al. , Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno-free and feeder-free conditions. Stem Cells 35, 1176–1188 (2017). [DOI] [PubMed] [Google Scholar]
- 128.Garita-Hernandez M., et al. , Restoration of visual function by transplantation of optogenetically engineered photoreceptors. Nat. Commun. 10, 4524 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santos-Ferreira T., et al. , Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat. Commun. 7, 13028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pearson R. A., et al. , Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 7, 13029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union.https://eur-lex.europa.eu/eli/dir/2010/63/oj. Accessed 18 April 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated for this paper.