Abstract
Which regions of the cerebral cortex are the origin of descending commands that influence internal organs? We used transneuronal transport of rabies virus in monkeys and rats to identify regions of cerebral cortex that have multisynaptic connections with a major sympathetic effector, the adrenal medulla. In rats, we also examined multisynaptic connections with the kidney. In monkeys, the cortical influence over the adrenal medulla originates from 3 distinct networks that are involved in movement, cognition, and affect. Each of these networks has a human equivalent. The largest influence originates from a motor network that includes all 7 motor areas in the frontal lobe. These motor areas are involved in all aspects of skeletomotor control, from response selection to motor preparation and movement execution. The motor areas provide a link between body movement and the modulation of stress. The cognitive and affective networks are located in regions of cingulate cortex. They provide a link between how we think and feel and the function of the adrenal medulla. Together, the 3 networks can mediate the effects of stress and depression on organ function and provide a concrete neural substrate for some psychosomatic illnesses. In rats, cortical influences over the adrenal medulla and the kidney originate mainly from 2 motor areas and adjacent somatosensory cortex. The cognitive and affective networks, present in monkeys, are largely absent in rats. Thus, nonhuman primate research is essential to understand the neural substrate that links cognition and affect to the function of internal organs.
Keywords: cerebral, cortex, adrenal, mind, body
How does the mind (conceptually associated with the cerebral cortex) influence autonomic and endocrine systems that control internal organs? And, which regions of the cerebral cortex are the origin of descending commands to direct organ function? The popular press as well as the scientific literature are replete with examples of how the mind or mental processes influence our health and well-being. There is abundant evidence to support the positive impact of exercise and the “placebo effect” and the negative impact of emotional stress on the gastrointestinal, cardiovascular, metabolic, and immune systems. This “mind–body connection” is essential for normal organ function and also is viewed as the basis for psychosomatic disorders. Although the concept that mental operations can influence the function of a variety of organ systems has been popularized, it is often viewed with some skepticism, in part, because it has lacked a firm biological basis.
The connection between the central nervous system and internal organs is mediated by sympathetic and parasympathetic subdivisions of the autonomic nervous system. We know a great deal about the neural connections that link autonomic output from centers in the brainstem and spinal cord to specific organs (1). However, the neural circuits that link higher brain function and central sites (e.g., the cerebral cortex) to autonomic output and organ function have not been clearly defined (2, 3). The multisynaptic nature of these circuits has made them difficult to study. This is because most conventional tracers are capable of defining only the direct inputs to and outputs from an organ. This shortcoming has been overcome by the introduction of neurotropic viruses as transneuronal tracers (4–6).
Here, we review some of our results using the N2c strain of rabies virus (RV) to reveal the areas of the cerebral cortex that influence the adrenal medulla of the monkey and rat. We will also review the results of RV transport from the kidney in the rat. The adrenal medulla and kidney are controlled exclusively by sympathetic efferents and are therefore, ideal for defining the cortical areas that influence this division of autonomic circuitry. Our results using retrograde transneuronal transport of RV emphasize 2 fundamental points. First, in nonhuman primates, descending influences over the adrenal medulla originate from cortical areas involved in movement, cognition, and affect. These cortical areas represent key nodes in a “stress and depression connectome.” Second, in the rat, descending influences over the adrenal medulla, as well as the kidney, originate largely from cortical motor areas. In fact, the cortical areas that are the major source of cognitive control in the monkey appear to be absent in the rat. Thus, the mind–body connection in primates is more widespread and complex than in rats.
The general experimental paradigm we employ is one that can be applied to reveal multisynaptic circuits in a wide variety of networks. For example, injections of RV into limb muscles can reveal the networks involved in the voluntary control of movement (7, 8). Transport of RV from laryngeal muscles can reveal the central circuits responsible for vocalization;*,† RV injections into the heart and stomach can reveal circuits responsible for the central control over the cardiovascular and gastrointestinal systems; and RV transport from the spleen can reveal the central neural circuits that influence immune function.
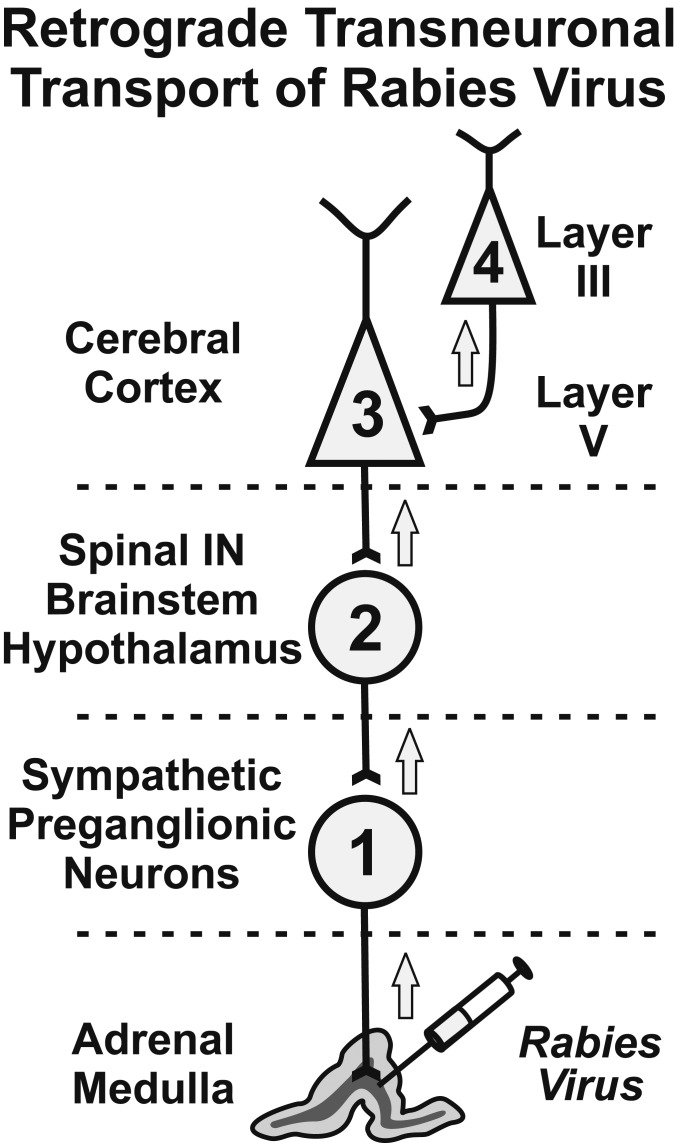
Here, we injected RV into the adrenal medulla. It is taken up and transported in the retrograde direction to label 1st-order neurons in the intermediolateral column of the thoracic spinal cord (Fig. 1). Functionally, these neurons are sympathetic preganglionic neurons (SPNs), i.e., the motoneurons of the sympathetic nervous system. The virus then replicates and moves transneuronally in the retrograde direction to label all of the inputs to SPNs. The major inputs to SPNs are 2nd-order neurons that originate in specific regions of the spinal cord, brainstem, and hypothalamus (9, 10). By 2nd order, we mean that the virus has been transported in the retrograde direction through a chain of 2 synaptically linked neurons. The virus then undergoes another cycle of transneuronal transport to label 3rd-order neurons in layer V of the cerebral cortex and at other central sites. At still-longer survival times, the virus undergoes an additional cycle of transneuronal transport to label 4th-order neurons at multiple sites, including cortical layers II–IV and VI. Extending the survival time further results in additional stages of transneuronal transport to label 5th- and 6th-order neurons. By systematically adjusting the survival time, it is possible to identify chains of as many as 6 synaptically linked neurons (i.e., 6th-order neurons) (11).
Fig. 1.
Schematic diagram of the experimental paradigm. We have used retrograde transneuronal transport of RV to identify the cortical neurons that influence a specific organ, the adrenal medulla. RV is transported transneuronally in the retrograde direction in a time-dependent fashion. By varying of the survival time, the extent of transport can be limited to 1st-order (1), 2nd-order (2), 3rd-order (3), or 4th-order (4) neurons. A more complete description of the various circuits linking the cerebral cortex to the adrenal medulla may be found in ref. 11. Spinal IN, spinal interneurons.
After RV injections into the adrenal medulla, we first observed substantial numbers of infected neurons in the cerebral cortex of monkeys with 4th-order labeling (n = 4) (11). In these monkeys, we determined that RV had progressed through a chain of 4 synaptically linked neurons (hence 4th order), based on the presence of a small number of labeled neurons in layer III of the cerebral cortex (Fig. 1). However, most of the infected neurons in these 4th-order monkeys were located in layer V, the source of descending cortical outputs to subcortical targets. To identify cortical areas that may be less directly connected to the adrenal medulla (but perhaps no less important), we extended the survival time to allow transneuronal transport of virus across 1 (5th order; n = 2) or 2 (6th order; n = 2) additional synapses (6, 11). This resulted in a dramatic increase (20- to 100-fold) in the numbers of labeled neurons in the cerebral cortex. In these animals, large numbers of labeled neurons were located not only in layer V, but also in supragranular and infragranular layers of cortex. Nevertheless, the cortical areas with dense labeling in 6th-order animals were the same as those that were densely labeled in 4th-order animals (compare figures 2 and 3 in ref. 11). As a consequence, we will display the results from a 6th-order animal to emphasize the cortical areas with the greatest influence over the adrenal medulla (Fig. 2).
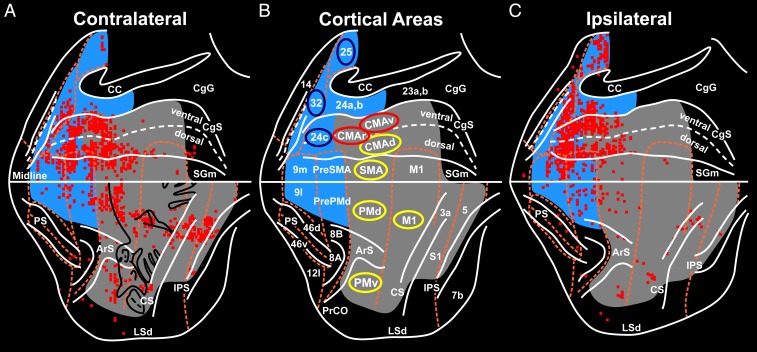
Fig. 2.
Origin of cortical inputs to the primate adrenal medulla. The survival time in this animal allowed retrograde transneuronal transport of rabies to label 6th-order neurons. The red squares indicate 200-μm bins with the highest numbers of labeled neurons (top 15%). (A) Flat map of the hemisphere contralateral to the injected adrenal medulla. The medial wall of the hemisphere is reflected upward and aligned on the midline. (B) Relevant areas of the cerebral cortex. Motor and somatosensory regions are shaded gray. Medial prefrontal regions are shaded blue. The cortical motor areas are indicated by yellow ellipses; cingulate motor areas that are involved in cognitive control are indicated by red ellipses; selected areas of the affective network in the medial prefrontal cortex are indicated by blue ellipses. (C) Flat map of the ipsilateral hemisphere. ArS, arcuate sulcus; CC, corpus callosum; CgG, cingulate gyrus; CgS, cingulate sulcus; CS, central sulcus; IPS, intraparietal sulcus; LSd, dorsal lip of the lateral sulcus; midline, junction between medial and lateral surfaces; mPF, medial prefrontal cortex; PrCO, precentral opercular cortex; PS, principal sulcus; RS, rostral sulcus; SGm, medial superior frontal gyrus; S1, primary somatosensory cortex. Numbers designate cytoarchitectonic regions. Adapted from ref. 11.
Monkey—Origin of Cortical Projections to the Adrenal Medulla
The cortical influence over the adrenal medulla in monkeys (Cebus apella) originates from 3 distinct networks (Fig. 2). These networks include cortical areas involved in movement, cognition, and affect. We illustrate later that each network has a human equivalent (Fig. 3).
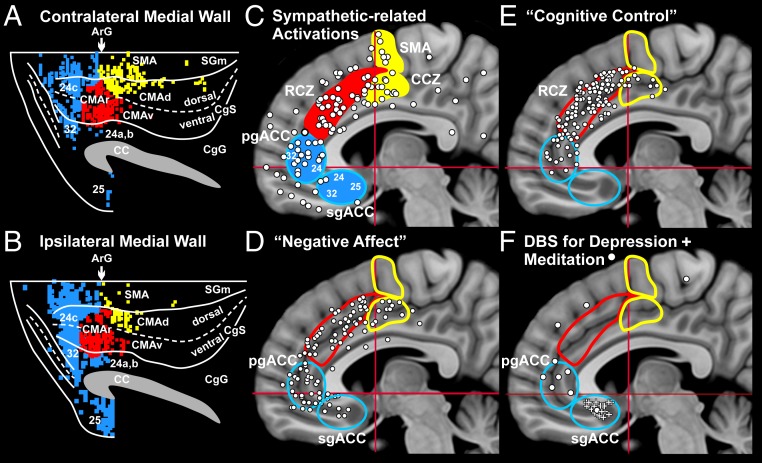
Fig. 3.
Comparison of monkey and human results on the medial wall of the hemisphere. (A and B) Contralateral (A) and ipsilateral (B) hemisphere of the monkey. The dense labeling is color-coded: motor areas, yellow; motor areas involved in “cognitive control,” red; affective network areas, blue. Dorsal is at the top, and anterior is to the left for all of the diagrams. Abbreviations are as in Fig. 2. Each of the cortical motor areas in the monkey has a human equivalent. (A–C) In monkeys, the SMA is located on the superior frontal gyrus at levels caudal to the arcuate sulcus (A and B), and in humans, the SMA is located on the same gyrus at levels caudal to the Vca line (C) (33). Together, the CMAr and CMAv of the monkey (A and B) correspond to the RCZ of humans, which is located rostral to the Vca line (C). The CMAd (A and B) corresponds to the caudal cingulate zone (CCZ) of humans, which is posterior to the Vca line (C) (33, 34). (C–F) Sites of activation on the medial wall reported in human studies. Each diagram shows the location of the SMA, CCZ, RCZ, pgACC, and sgACC. Motor areas are in yellow; cognitive motor areas are in red; affective areas are in blue. White circles indicate sites of activation. (C) Sympathetic-related activations from 36 studies (11). (D) Negative affect (from ref. 36). (E) Cognitive control (from ref. 36). (F) Sites of deep-brain stimulation (DBS) for treatment-resistant depression (white pluses) (from ref. 75). Sites of activation associated with meditation are shown (11). CA-CP, red horizontal lines; Vca, red vertical lines. Adapted from ref. 11.
The Motor Network.
The largest descending influence originates from a motor network that includes all 7 of the cortical motor areas in the frontal lobe. With some exceptions (see below), the influence from the motor areas originates mainly from the contralateral hemisphere (Fig. 2 A and C). The cortical areas that contribute to this network lie on the lateral surface and the medial wall of the hemisphere. Those on the lateral surface include the primary motor cortex (M1) and the dorsal premotor (PMd) and ventral premotor (PMv) areas (Fig. 2); and those on the medial wall include the supplementary motor area (SMA), as well as the rostral cingulate motor (CMAr), dorsal cingulate motor (CMAd), and ventral cingulate motor (CMAv) areas (Figs. 2 and 3 A and B). These motor areas are densely interconnected and form an integrated motor network at the cortical level (12, 13). Specific regions of somatosensory cortex (areas 3a, 1, and 2) and posterior parietal cortex (area 5) also project to the adrenal medulla and are therefore included in this network (Fig. 2).
All of these cortical motor areas project directly to the spinal cord (12, 14) and to regions of the reticular formation (15). Thus, it is likely that the influence of the motor network on the adrenal medulla is mediated by corticospinal and corticobulbo-spinal pathways. This conclusion is supported by classic studies in which surface stimulation of sites within M1, the primary somatic sensory cortex (S1), and the PMd evoked changes in blood pressure. These effects were abolished by lesions of the pyramidal tract (16, 17).
We found that output to the adrenal medulla originates largely from specific sites within the cortical motor areas (Fig. 2). For example, based on Woolsey’s classic motor map (18), output to the adrenal medulla originates mainly from the trunk and axial representation of M1 and the PMd (Fig. 2A). Because of this arrangement, we speculate that there is a link between the cortical control of “core” muscles and the regulation of sympathetic output. This association could provide a neural explanation for the use of core exercises, such as yoga and Pilates, to ameliorate stress (19). On the other hand, several lines of evidence suggest that poor control of core muscles, as in a slumped body posture, is associated with altered stress responses, negative affect, and poorer cognitive processing (20–22).
How important is the use of core muscles of the trunk and the engagement of the cortical motor areas in ameliorating stress and the symptoms of depression? It is well known that exercise, particularly aerobic exercise, has a positive impact on the symptoms of depression (23–25). Surprisingly, a recent study (26) found that a regular program of voluntary active stretching was as effective as aerobic exercise in relieving symptoms. Although the mechanisms by which exercise influences depression are undoubtedly complex and remain to be fully elucidated, a common feature of aerobic exercise and stretching is the volitional engagement of core muscles of the trunk. Our results raise the possibility that the coordinated activation of these muscles and sympathetic output by the cortical motor areas may contribute to the effects of exercise on the symptoms of stress and depression.
A second, small focus of output to the adrenal medulla originates bilaterally from the orofacial representation of M1 (Fig. 2 A and C). This output may provide a link between the activation of facial muscles, as in a “standard” or “genuine” smile, and a reduction in the response to stress (27). A third, larger region of output from the motor network is located in postcentral cortex and corresponds to the sensory representation of the trunk and viscera in primary somatosensory cortex (Fig. 2) (28–31). This output may provide a neural substrate for the reduction of anxiety and stress that follows passive stimulation of back muscles during a massage (32).
The Cognitive Network.
The primate adrenal medulla receives a sizable multisynaptic input from the CMAr and CMAv. These 2 cortical areas are considered to be the main components of the cognitive network. In humans, these areas correspond to the rostral cingulate zone (RCZ) (Fig. 3 A–C) (33, 34). The CMAr and CMAv are included within the definition of premotor areas in the frontal lobe because their outputs project to both M1 and to the spinal cord (12–14, 35). However, we also consider these cortical areas to be major components of a cognitive network because of their specific involvement in cognitive control tasks in monkeys and humans (Fig. 3E) (36, 37).
The CMAr and CMAv are unique among the motor areas in that they project bilaterally to the adrenal medulla. These cortical areas also have strong interconnections with regions of lateral prefrontal cortex and portions of the anterior cingulate gyrus (13, 38). These connections give the CMAr and CMAv access to the mnemonic and executive functions that are mediated by the lateral prefrontal cortex (39–41), as well as to the processing of cognitive and affective information that takes places in anterior cingulate cortex (42, 43).
Physiological studies in monkeys confirm that neurons in the CMAr and CMAv are active during simple arm movements (44–50), but the relationship between this neuronal activity and movement parameters is more complex than that observed in the other cortical motor areas. CMAr neurons display changes in activity that reflect the preparation and selection of a motor response (51, 52) and the detection of a response error (37, 53). The activity of some CMAr neurons differentiates between rewarding and nonrewarding stimuli and varies in relation to the value of an expected reward for performing a specific task (45, 54, 55). Overall, the activity of many neurons in the CMAr is better linked to a variety of higher-order cognitive operations than it is to specific parameters of movement.
Imaging studies in humans confirm that the RCZ (the human equivalent of the CMAr and CMAv) displays functional activation during the performance of simple motor tasks (34). The RCZ also is activated in association with sympathetic arousal (Fig. 3C) (11) and behavioral tasks that induce negative affect and pain (Fig. 3D) (36). However, the most robust activation of the RCZ is seen during the performance of tasks that require “cognitive control,” such as selection between competing responses, awareness of errors, and conflict resolution (Fig. 3E) (refs. 56–61; see refs. 36 and 62 for reviews). Not surprisingly, all of these cognitive tasks reliably initiate an adrenal response (63). Carter et al. (57) concluded that RCZ “ . . . serves an evaluative function, detecting cognitive states such as response competition . . . , and representing the knowledge that strategic processes need to be engaged.” Thus, the RCZ may provide the link that enables cognitive processes to induce the appropriate sympathetic output.
The Affective Network.
An affective network originates from multiple regions of medial prefrontal cortex (Figs. 2 and 3 A–D). Cortical areas in both hemispheres contribute to the affective network, but twice as many neurons originate from the ipsilateral hemisphere as from the contralateral hemisphere (Figs. 2 A and C and 3 A and B). The core of this network is located in the pregenual anterior cingulate cortex (pgACC) that includes portions of areas 32 and 24, and in the subgenual anterior cingulate cortex (sgACC) that consists primarily of area 25 (Figs. 2 and 3 A–D). Comparable cytoarchitectonic regions exist in humans (Fig. 3 A–D) (42, 64, 65). The affective network is the 2nd largest of the 3 networks and comprises nearly 25% of cortical neurons that influence the adrenal medulla (11).
The sgACC and pgACC are densely interconnected and have well-established connections with other limbic regions, including the amygdala, nucleus accumbens, entorhinal cortex, parahippocampal cortex, and subiculum (66–68). The sgACC and pgACC also are connected to regions of orbitofrontal cortex that have been included within a “medial visceromotor network” (66). In nonhuman primates, the sgACC and pgACC do not project directly to the spinal cord (14, 15, 69). Instead, these cortical areas must influence sympathetic output via multisynaptic connections with descending circuits from the hypothalamus, periaqueductal gray, and the medullary reticular formation (15, 67, 70–74).
In humans, the sgACC and pgACC display activation during tasks that are associated with negative affect (Fig. 3D). The sgACC is generally included within the cortical regions considered to be part of the “depression connectome” (64, 65). For instance, patients with bipolar familial depression exhibit histological and metabolic changes in the sgACC (65). Deep-brain stimulation in or near the sgACC mitigates some of the symptoms of treatment-resistant depression (Fig. 3F) (64, 75).
The pgACC is a site of activation during mindful meditation (Fig. 3F), a behavioral technique utilized to treat anxiety and reduce stress. The region comparable to the pgACC in monkeys appears to be uniquely linked with reward-related systems in the basal ganglia (76). This region may be involved in regulating anxiety and adjusting emotional valence while deciding on a course of action (42, 77–79).
The affective network, together with the cognitive network, may provide the neural circuitry that links negative affect (e.g., sadness) and cognitive control processes (e.g., awareness of errors) to immediate responses in stressful situations. The same substrate may mediate comparable stress responses when a sad situation or an error/conflict is recalled (80, 81). Furthermore, abnormal activation of this circuitry may be fundamental to conditions such as posttraumatic stress disorder. It may be useful to consider all 3 cortical networks that influence the adrenal medulla as key nodes of a “stress and depression connectome” (64). Perhaps some of these nodes represent additional targets for therapeutic intervention in affective disorders. In this context, the size of the motor network may hint at its importance for the reduction of stress and the treatment of depression. In fact, the engagement of the cortical motor areas may be key to the ameliorating effects of exercise on stress and depression (23–25).
Rat—Origin of Cortical Projections to the Adrenal Medulla
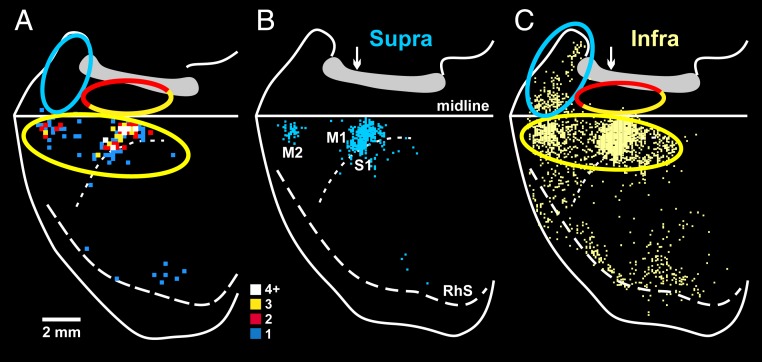
Rodents have been a major experimental model for exploring the organization and function of the autonomic nervous system and for examining the central modulation of stress responses. Therefore, for comparative purposes, we examined the origin of cortical inputs to the adrenal medulla of the rat using techniques identical to those that we employed in the monkey (Fig. 4). We injected RV into the adrenal medulla of several rats and first observed substantial numbers of infected neurons in layer V of cerebral cortex of 3rd-order animals (n = 3). Thus, the minimal neural circuit from output neurons in layer V to the adrenal medulla in the rat is the same as in the monkey, i.e., a series of 3 synaptically linked neurons. Almost all of the labeled neurons in layer V are located in the hemisphere contralateral to the injected adrenal medulla (>95%). The vast majority (93%) of the output neurons that influence the adrenal medulla are located in 3 cortical areas: M1 (74%), S1 (13%), and the secondary motor cortex (M2; 6%) (Fig. 4A, yellow ellipse).
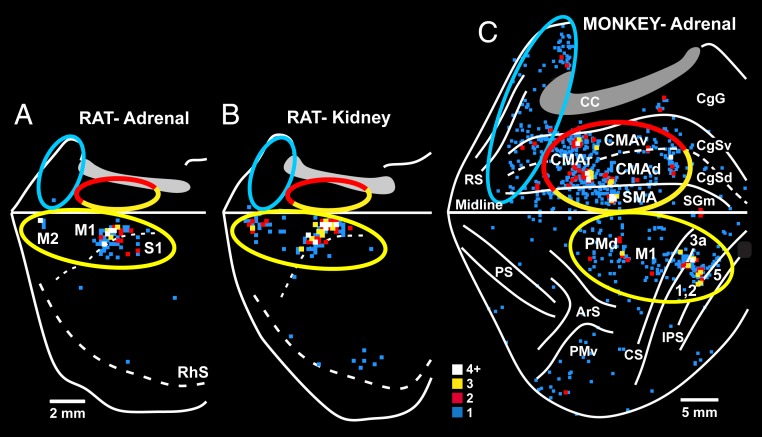
Fig. 4.
Origin of sympathetic outputs from the cerebral cortex: rat–monkey comparison. Reconstructed maps display the distribution of cortical neurons in the contralateral hemisphere that were labeled by retrograde transneuronal transport of RV from the adrenal medulla (rat or monkey) or kidney (rat). (A) Rat. Rabies transport from the adrenal medulla was confined to layer V neurons. (B) Rat. Rabies transport from the kidney was confined to layer V neurons. (C) Monkey. Rabies transport from the adrenal medulla was mostly confined to layer V neurons, but also included a few 4th-order neurons in layers above and below layer V. The ratio of nonlayer-V to layer-V labeling in this animal was below 1:4. The major components of the motor (yellow), cognitive (red), and affective (blue) networks are enclosed with ellipses. The yellow–red ellipse encloses components of the motor and cognitive networks. Similar ellipses were placed at comparable locations on the rat brain. The medial wall of the hemisphere is reflected upwards in both maps. The colored squares indicate the number of labeled neurons located in 200-μm bins (color key). Abbreviations are as in Fig. 2. RhS, rhinal sulcus.
We compared the results in the rat to those of a monkey in which transport was largely limited to 3rd-order neurons in layer V (>80%), but also extended to label a few 4th-order neurons in layers above and below layer V (Fig. 4C). The labeled neurons in this monkey are located within the same motor, cognitive, and affective networks we described above for monkeys with transport to 6th-order neurons (Fig. 2).
For illustrative purposes, we have encircled the rat and monkey networks with colored ellipses (Fig. 4). The rat and monkey maps both display a motor network on the lateral surface of the hemisphere (yellow ellipses in Fig. 4). However, the rat lacks the portion of the motor network that is clearly present on the medial wall of the hemisphere in the monkey (Fig. 4, half yellow ellipses). Furthermore, the affective and cognitive networks that are present on the medial wall of the hemisphere in the monkey (blue and half red ellipses, Fig. 4C) are absent in the rat (Fig. 4 A and B).
Clearly, major differences exist between the rat and the monkey in the origin of cortical influences on the adrenal medulla. In the rat, the descending control originates almost exclusively from a motor network comprising M1, S1, and M2. In contrast, in the monkey, the descending control originates from all 7 of the cortical motor areas and from cortical areas involved in cognition and affect.
We have also examined the origin of cortical influences over the rat kidney (82). The kidney, like the adrenal medulla, is innervated only by sympathetic efferents. However, the minimal neural circuit from output neurons in layer V of the cerebral cortex to the kidney is a series of 4 synaptically linked neurons (transport to 4th-order neurons; n = 5). The extra link in the kidney circuit is due to the insertion of postganglionic neurons between preganglionic neurons in the spinal cord and the kidney. The vast majority (92%) of the 4th-order neurons in layer V that innervate the rat kidney are located in 3 cortical areas: M1 (68%), S1 (9%), and M2 (15%) (Fig. 5A, yellow ellipse). Labeled neurons have been reported in similar locations after transneuronal transport of pseudorabies virus from the kidney of the rat (83, 84). Clearly, the origin of descending influences over the rat kidney is comparable to the rat adrenal medulla and originates almost entirely from a motor network on the lateral surface of the hemisphere.
Fig. 5.
Origin of cortical inputs to the rat kidney. (A) These results were obtained after a survival time that allowed retrograde transneuronal transport of RV that was limited to cortical neurons in layer V (Fig. 1). The results are displayed as the number of labeled neurons in a 200-μm bin (color key). (B and C) These results were obtained after a survival time that allowed retrograde transneuronal transport of RV to label cortical neurons in supragranular (B) and infragranular (C) layers (Fig. 1). Each square represents a labeled neuron. White arrow, bregma. Rostral is to the left. Conventions and abbreviations are as in Figs. 2 and 4. Adapted from ref. 82.
In a number of experiments on the rat kidney (n = 7), we extended the survival time to infect 5th-order neurons (Fig. 5 B and C). We used this approach to examine whether other cortical areas have less direct, but perhaps no less important, control over the rat kidney. In these 5th-order animals, labeled neurons are found in both supragranular (Fig. 5B) and infragranular (Fig. 5C) cortical layers. The location of labeled neurons in the supragranular layers closely matches the location of labeled neurons in layer V with 4th-order labeling (compare Fig. 5B with Fig. 5A, yellow ellipse). In other words, the motor network in M1, S1, and M2 is labeled in both instances. These same cortical areas contain large numbers of labeled neurons in the infragranular layers (yellow ellipse in Fig. 5C). Less dense populations of labeled neurons are located in the infragranular layers at 2 sites: laterally in cortical areas near the rhinal sulcus and rostrally in areas of medial prefrontal cortex on the medial wall of the hemisphere (blue ellipse in Fig. 5C). This latter region of the medial prefrontal cortex is likely to be comparable to the affective network seen in the monkey (blue ellipse in Fig. 4C). On the other hand, almost no labeled neurons are found in the regions of the medial wall of the hemisphere that contain motor areas and the cognitive network in the monkey (compare red–yellow ellipse in Fig. 5C with red–yellow ellipse in Fig. 4C).
In summary, extending the survival time reveals that the rat kidney is influenced not only by a motor network, but also less directly by a small affective network. On the other hand, the rat kidney, like the rat adrenal medulla, lacks input from the cognitive and motor networks on the medial wall of the hemisphere that are an important source of input to the adrenal medulla in the monkey.
Discussion
For well over a century, sympathetic responses were known to be linked with everyday behaviors such as exercise and emotional expression (2, 3, 85–87). For instance, the anticipation and initiation of exercise results in a simultaneous increase in cardiovascular activity that is correlated to the motor effort and the metabolic demands of the exercise (2, 3, 87). The coordinated activation of motor and cardiovascular systems was attributed to “central commands” originating in separate motor and cardiovascular centers in the cerebral cortex (3). Nevertheless, the cortical origin of this and similar descending control over sympathetic output has been uncertain. Indeed, Williamson (88) concluded, “ . . . We are still left without a definitive neuroanatomy for a central command.”
Our observations provide a network perspective on the neuroanatomical organization of the cortical influences over the sympathetic nervous system. The power of transneuronal tracing with RV is that it reveals the entire extent of the cortical influence over this system. In this way, it identifies the potential origins of the elusive “central commands” from the cerebral cortex.
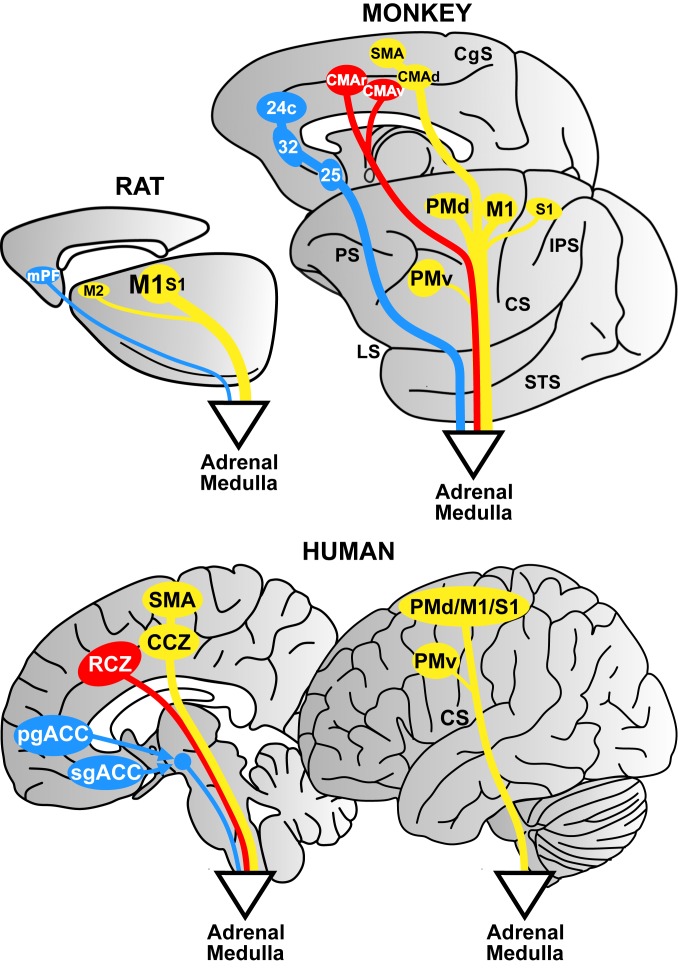
One of our major findings is that descending commands to the adrenal medulla originate from distinct motor, cognitive, and affective networks in the primate cerebral cortex (Fig. 6). The broad origin of the cortical output to the adrenal medulla argues against the concept of an isolated cortical center for sympathetic control (3, 42). Instead, we show that at least 11 cortical areas, each part of larger cortical networks, have independent and parallel access to the adrenal medulla. One clear implication of this organization is that the sympathetic responses which occur during activities such as exercise, the performance of demanding cognitive tasks, and the experience of emotions are generated by neural activity from the same cortical areas that are responsible for these behaviors.
Fig. 6.
Cortical origin of top-down influences over the adrenal medulla: rat–nonhuman primate–human comparison. Motor networks are in yellow, cognitive networks are in red, and affective networks are in blue. Rat: Cortical output to the adrenal medulla originates largely from M1 on the lateral surface of the hemisphere. Monkey and human: Cortical output to the adrenal medulla originates from a motor network (M1, PMd, PMv, and S1 on the lateral surface and the SMA and CMAd on the medial wall [mirror image]). The medial wall motor areas are absent in the rat. Output from a cognitive network arises from the CMAr and CMAv on the medial wall (comparable to the RCZ of humans). This cognitive network is absent in the rat. Both the motor and cognitive influences are mediated, at least in part, by the corticospinal system. An affective network consists of areas 24c, 32, and 25 on the rostral medial wall in the monkey and corresponds to the pgACC and the sgACC in humans. The affective influence is mediated by various subcortical routes. Abbreviations are as in Figs. 2 and 3.
The link between sympathetic output and motor behavior is especially clear. There are 7 cortical motor areas in the frontal lobe that are involved in diverse aspects of motor control, such as preparing for action, guiding movement based on external cues, generating sequences of movement, and specifying patterns of muscle activity and movement parameters. Each of these motor areas is a source of descending commands to the adrenal medulla. We speculate that colocalizing skeletomotor and sympathetic control within the same cortical areas enhances coordination between the 2 systems and essentially ensures that the adjustment of sympathetic output is appropriate to meet the demands of the skeletomotor system. Furthermore, placing some aspects of sympathetic control in motor areas that are concerned with the preparation for movement may provide a basis for the predictive or anticipatory control of sympathetic output that is associated with some motor behaviors (3, 87, 89, 90).
The existence of body maps in the primary motor and somatosensory cortex provides an additional framework for interpreting the significance of cortical output to the adrenal medulla. For example, it is noteworthy that output to the adrenal medulla originates from localized regions of the primary motor and somatosensory cortex rather than the entire body map. Sites within the axial body and face representation of M1 as well as the back representation in somatosensory cortex have a preferential access to adrenal output. We have no way of determining whether the descending signals from these cortical neurons enhances or depresses adrenal responses. However, the presence of these connections provides a concrete neural substrate to support suggestions that activation of core muscles and the muscles of facial expression as well as sensory stimulation of the shoulders and back have an impact on our response to stress.
The adrenal medulla can be considered as our “first responder” in situations requiring fight or flight. Thus, one might expect the input to it to be highly conserved across species. In fact, the cortical motor areas are a major source of input to the adrenal medulla in both the rat and the monkey. However, here, the similarities end (Fig. 6). M1, primary somatosensory cortex, and a single secondary motor area account for ∼93% of the cortical input to the adrenal medulla in the rat. In contrast, the monkey adrenal medulla receives input not only from cortical motor areas (∼53%), but also from cortical areas involved in cognition and affect (∼35%). Furthermore, the monkey adrenal medulla receives substantial input from motor areas on the medial wall of the hemisphere (SMA, CMAd, CMAv, and CMAr) that don’t exist in the rat. Thus, the monkey adrenal medulla is the target of output from a broader set of cortical areas and is influenced by a more diverse set of behaviors. Importantly, each network found in the monkey has a human equivalent (Fig. 6). Taken together, these observations suggest that nonhuman primate models are essential for examining the influences of higher-order aspects of movement, cognition, and effect on sympathetic function.
Finally, our observations are relevant to concepts about “psychosomatic” disorders in which mental operations are thought to have a negative impact on normal physiology and result in organ dysfunction. Modern medicine has generally viewed the concept of psychosomatic disease with suspicion. This is partly because of a lack of information about the neural networks that connect the “mind,” conceptually associated with the cerebral cortex, with autonomic and endocrine systems that regulate internal organs. As a consequence, some definitions of psychosomatic disorders include dismissive descriptions, such as “all in the mind,” “irrational,” or “subconscious.” Our findings should correct this perspective because they provide a concrete neural substrate for cortical areas involved in movement, cognition, and affect to influence a major sympathetic effector, the adrenal medulla. We suggest the adoption of the view reflected in the exchange between Harry Potter and Professor Dumbledore at the end of Harry Potter and the Deathly Hallows (91), where Harry says, “Tell me one last thing, is this real? Or has this been happening inside my head?” Professor Dumbledore replies, “Of course it is happening inside your head, Harry, but why on earth should that mean that it is not real?”
Data Availability
Data are available from the corresponding author upon request.
Acknowledgments
We thank M. Schnell (Thomas Jefferson University) for supplying the N2c strain of RV; A. Wandeler (Animal Disease Research Institute) for supplying the antibody to the RV; M. Page for the development of computer programs; and L. Chedwick and M. Pemberton for their technical assistance. This work was supported in part by NIH Grants P40 OD010996 (to P.L.S.) and R01 AT010414 (to P.L.S.); US Army Research Office Multidisciplinary University Research Initiative Grant W911NF-16-1-0474 (to P.L.S.); and a DSF Charitable Foundation grant (to P.L.S.).
Footnotes
The authors declare no competing interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Using Monkey Models to Understand and Develop Treatments for Human Brain Disorders,” held January 7–8, 2019, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. NAS colloquia began in 1991 and have been published in PNAS since 1995. From February 2001 through May 2019 colloquia were supported by a generous gift from The Dame Jillian and Dr. Arthur M. Sackler Foundation for the Arts, Sciences, & Humanities, in memory of Dame Sackler’s husband, Arthur M. Sackler. The complete program and video recordings of most presentations are available on the NAS website at http://www.nasonline.org/using-monkey-models.
This article is a PNAS Direct Submission.
*C. M. Cerkevich, P. L. Strick, “How primary is primary motor cortex for the control of vocalization?” in 2017 Neuroscience Meeting Planner (Society for Neuroscience, Washington, DC, 2017), Program 408.12.
†C. M. Cerkevich, P. L. Strick, “Cortical adaptations to enable enhanced vocalization” in 2018 Neuroscience Meeting Planner (Society for Neuroscience, San Diego, CA, 2018), Program 588.21.
References
- 1.Ulrich-Lai Y. M., Herman J. P., Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cechetto D. F., Saper C. B., “Role of the cerebral cortex in autonomic function” in Central Regulation of Autonomic Function, Lowey A. D., Spyer K. M., Eds. (Oxford University Press, Oxford, 1990), pp. 208–223. [Google Scholar]
- 3.Williamson J. W., Fadel P. J., Mitchell J. H., New insights into central cardiovascular control during exercise in humans: A central command update. Exp. Physiol. 91, 51–58 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Kelly R. M., Strick P. L., Rabies as a transneuronal tracer of circuits in the central nervous system. J. Neurosci. Methods 103, 63–71 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Ugolini G., Advances in viral transneuronal tracing. J. Neurosci. Methods 194, 2–20 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Dum R. P., Strick P. L., Transneuronal tracing with neurotropic viruses reveals network macroarchitecture. Curr. Opin. Neurobiol. 23, 245–249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathelot J. A., Strick P. L., Muscle representation in the macaque motor cortex: An anatomical perspective. Proc. Natl. Acad. Sci. U.S.A. 103, 8257–8262 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rathelot J. A., Strick P. L., Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc. Natl. Acad. Sci. U.S.A. 106, 918–923 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buijs R. M., et al. , Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 11, 1535–1544 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Strack A. M., Sawyer W. B., Platt K. B., Loewy A. D., CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 491, 274–296 (1989). [DOI] [PubMed] [Google Scholar]
- 11.Dum R. P., Levinthal D. J., Strick P. L., Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc. Natl. Acad. Sci. U.S.A. 113, 9922–9927 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dum R. P., Strick P. L., Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J. Neurosci. 25, 1375–1386 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dum R. P., Strick P. L., Motor Cortex in Voluntary Movements, Riehle A., Vaadia E., Eds. (CRC Press, Boca Raton, FL, 2005), pp. 3–47. [Google Scholar]
- 14.Dum R. P., Strick P. L., The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 11, 667–689 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keizer K., Kuypers H. G., Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp. Brain Res. 74, 311–318 (1989). [DOI] [PubMed] [Google Scholar]
- 16.Wall P. D., Pribram K. H., Trigeminal neurotomy and blood pressure responses from stimulation of lateral cerebral cortex of Macaca mulatta. J. Neurophysiol. 13, 409–412 (1950). [DOI] [PubMed] [Google Scholar]
- 17.Wall P. D., Davis G. D., Three cerebral cortical systems affecting autonomic function. J. Neurophysiol. 14, 507–517 (1951). [DOI] [PubMed] [Google Scholar]
- 18.Woolsey C. N., et al. , Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 30, 238–264 (1952). [PubMed] [Google Scholar]
- 19.Innes K. E., Bourguignon C., Taylor A. G., Risk indices associated with the insulin resistance syndrome, cardiovascular disease, and possible protection with yoga: A systematic review. J. Am. Board Fam. Pract. 18, 491–519 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Nair S., Sagar M., Sollers J. 3rd, Consedine N., Broadbent E., Do slumped and upright postures affect stress responses? A randomized trial. Health Psychol. 34, 632–641 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Bergström I., Kilteni K., Slater M., First-person perspective virtual body posture influences stress: A virtual reality body ownership study. PLoS One 11, e0148060 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkes C., Kydd R., Sagar M., Broadbent E., Upright posture improves affect and fatigue in people with depressive symptoms. J. Behav. Ther. Exp. Psychiatry 54, 143–149 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Bernstein E. E., McNally R. J., Acute aerobic exercise helps overcome emotion regulation deficits. Cogn. Emotion 31, 834–843 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Kvam S., Kleppe C. L., Nordhus I. H., Hovland A., Exercise as a treatment for depression: A meta-analysis. J. Affect. Disord. 202, 67–86 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Schuch F. B., et al. , Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 77, 42–51 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Krogh J., Videbech P., Thomsen C., Gluud C., Nordentoft M., DEMO-II trial. Aerobic exercise versus stretching exercise in patients with major depression-a randomised clinical trial. PLoS One 7, e48316 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraft T. L., Pressman S. D., Grin and bear it: The influence of manipulated facial expression on the stress response. Psychol. Sci. 23, 1372–1378 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Amassian V. E., Cortical representation of visceral afferents. J. Neurophysiol. 14, 433–444 (1951). [DOI] [PubMed] [Google Scholar]
- 29.Nelson R. J., Sur M., Felleman D. J., Kaas J. H., Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J. Comp. Neurol. 192, 611–643 (1980). [DOI] [PubMed] [Google Scholar]
- 30.Felleman D. J., Nelson R. J., Sur M., Kaas J. H., Representations of the body surface in areas 3b and 1 of postcentral parietal cortex of Cebus monkeys. Brain Res. 268, 15–26 (1983). [DOI] [PubMed] [Google Scholar]
- 31.Pons T. P., Garraghty P. E., Cusick C. G., Kaas J. H., The somatotopic organization of area 2 in macaque monkeys. J. Comp. Neurol. 241, 445–466 (1985). [DOI] [PubMed] [Google Scholar]
- 32.Li Y. H., Wang F. Y., Feng C. Q., Yang X. F., Sun Y. H., Massage therapy for fibromyalgia: A systematic review and meta-analysis of randomized controlled trials. PLoS One 9, e89304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picard N., Strick P. L., Motor areas of the medial wall: A review of their location and functional activation. Cereb. Cortex 6, 342–353 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Amiez C., Petrides M., Neuroimaging evidence of the anatomo-functional organization of the human cingulate motor areas. Cereb. Cortex 24, 563–578 (2014). [DOI] [PubMed] [Google Scholar]
- 35.He S. Q., Dum R. P., Strick P. L., Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the medial surface of the hemisphere. J. Neurosci. 15, 3284–3306 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shackman A. J., et al. , The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 12, 154–167 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen C., et al. , Anterior cingulate cortex cells identify process-specific errors of attentional control prior to transient prefrontal-cingulate inhibition. Cereb. Cortex 25, 2213–2228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu M. T., Preston J. B., Strick P. L., Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J. Comp. Neurol. 341, 375–392 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Petrides M., Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 1455–1461, discussion 1461–1462 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Levy R., Goldman-Rakic P. S., Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp. Brain Res. 133, 23–32 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Christophel T. B., Klink P. C., Spitzer B., Roelfsema P. R., Haynes J. D., The distributed nature of working memory. Trends Cogn. Sci. 21, 111–124 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Vogt B. A., Submodalities of emotion in the context of cingulate subregions. Cortex 59, 197–202 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Rolls E. T., Limbic systems for emotion and for memory, but no single limbic system. Cortex 62, 119–157 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Shima K., et al. , Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J. Neurophysiol. 65, 188–202 (1991). [DOI] [PubMed] [Google Scholar]
- 45.Shima K., Tanji J., Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282, 1335–1338 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Cadoret G., Smith A. M., Input-output properties of hand-related cells in the ventral cingulate cortex in the monkey. J. Neurophysiol. 73, 2584–2590 (1995). [DOI] [PubMed] [Google Scholar]
- 47.Cadoret G., Smith A. M., Comparison of the neuronal activity in the SMA and the ventral cingulate cortex during prehension in the monkey. J. Neurophysiol. 77, 153–166 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Procyk E., Tanaka Y. L., Joseph J. P., Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat. Neurosci. 3, 502–508 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Akkal D., Bioulac B., Audin J., Burbaud P., Comparison of neuronal activity in the rostral supplementary and cingulate motor areas during a task with cognitive and motor demands. Eur. J. Neurosci. 15, 887–904 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Russo G. S., Backus D. A., Ye S., Crutcher M. D., Neural activity in monkey dorsal and ventral cingulate motor areas: Comparison with the supplementary motor area. J. Neurophysiol. 88, 2612–2629 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Isomura Y., Ito Y., Akazawa T., Nambu A., Takada M., Neural coding of “attention for action” and “response selection” in primate anterior cingulate cortex. J. Neurosci. 23, 8002–8012 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merten K., Nieder A., Comparison of abstract decision encoding in the monkey prefrontal cortex, the presupplementary, and cingulate motor areas. J. Neurophysiol. 110, 19–32 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Ito S., Stuphorn V., Brown J. W., Schall J. D., Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science 302, 120–122 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Nishijo H., et al. , Single neuron responses in the monkey anterior cingulate cortex during visual discrimination. Neurosci. Lett. 227, 79–82 (1997). [DOI] [PubMed] [Google Scholar]
- 55.Shidara M., Richmond B. J., Anterior cingulate: Single neuronal signals related to degree of reward expectancy. Science 296, 1709–1711 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Carter C. S., et al. , Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280, 747–749 (1998). [DOI] [PubMed] [Google Scholar]
- 57.Carter C. S., et al. , Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U.S.A. 97, 1944–1948 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubia K., et al. , Mapping motor inhibition: Conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage 13, 250–261 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Pardo J. V., Pardo P. J., Janer K. W., Raichle M. E., The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acad. Sci. U.S.A. 87, 256–259 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lane R. D., Fink G. R., Chau P. M., Dolan R. J., Neural activation during selective attention to subjective emotional responses. Neuroreport 8, 3969–3972 (1997). [DOI] [PubMed] [Google Scholar]
- 61.Critchley H. D., Mathias C. J., Dolan R. J., Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 29, 537–545 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Bush G., Luu P., Posner M. I., Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Gerra G., et al. , Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology 26, 91–107 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Mayberg H. S., Targeted electrode-based modulation of neural circuits for depression. J. Clin. Invest. 119, 717–725 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Price J. L., Drevets W. C., Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 16, 61–71 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Ongür D., Price J. L., The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10, 206–219 (2000). [DOI] [PubMed] [Google Scholar]
- 67.Price J. L., Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann. N. Y. Acad. Sci. 1121, 54–71 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Heilbronner S. R., Rodriguez-Romaguera J., Quirk G. J., Groenewegen H. J., Haber S. N., Circuit-based corticostriatal homologies between rat and primate. Biol. Psychiatry 80, 509–521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toyoshima K., Sakai H., Exact cortical extent of the origin of the corticospinal tract (CST) and the quantitative contribution to the CST in different cytoarchitectonic areas. A study with horseradish peroxidase in the monkey. J. Hirnforsch. 23, 257–269 (1982). [PubMed] [Google Scholar]
- 70.Veazey R. B., Amaral D. G., Cowan W. M., The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). II. Efferent connections. J. Comp. Neurol. 207, 135–156 (1982). [DOI] [PubMed] [Google Scholar]
- 71.Holstege G., “Subcortical limbic system projections to caudal brainstem and spinal Cord” in The Human Nervous System, Paxinos G., Ed. (Academic Press, San Diego, 1990), pp. 261–286. [Google Scholar]
- 72.An X., Bandler R., Ongür D., Price J. L., Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J. Comp. Neurol. 401, 455–479 (1998). [PubMed] [Google Scholar]
- 73.Ongür D., An X., Price J. L., Prefrontal cortical projections to the hypothalamus in macaque monkeys. J. Comp. Neurol. 401, 480–505 (1998). [PubMed] [Google Scholar]
- 74.Barbas H., Saha S., Rempel-Clower N., Ghashghaei T., Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 4, 25 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamani C., et al. , The subcallosal cingulate gyrus in the context of major depression. Biol. Psychiatry 69, 301–308 (2011). [DOI] [PubMed] [Google Scholar]
- 76.Eblen F., Graybiel A. M., Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J. Neurosci. 15, 5999–6013 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsumoto K., Suzuki W., Tanaka K., Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science 301, 229–232 (2003). [DOI] [PubMed] [Google Scholar]
- 78.Kennerley S. W., Walton M. E., Behrens T. E., Buckley M. J., Rushworth M. F., Optimal decision making and the anterior cingulate cortex. Nat. Neurosci. 9, 940–947 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Amemori K., Graybiel A. M., Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat. Neurosci. 15, 776–785 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phan K. L., Wager T., Taylor S. F., Liberzon I., Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16, 331–348 (2002). [DOI] [PubMed] [Google Scholar]
- 81.Euston D. R., Gruber A. J., McNaughton B. L., The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levinthal D. J., Strick P. L., The motor cortex communicates with the kidney. J. Neurosci. 32, 6726–6731 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sly D. J., Colvill L., McKinley M. J., Oldfield B. J., Identification of neural projections from the forebrain to the kidney, using the virus pseudorabies. J. Auton. Nerv. Syst. 77, 73–82 (1999). [PubMed] [Google Scholar]
- 84.Cano G., Card J. P., Sved A. F., Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J. Comp. Neurol. 471, 462–481 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Neafsey E. J., Prefrontal cortical control of the autonomic nervous system: Anatomical and physiological observations. Prog. Brain Res. 85, 147–165, discussion 165–166 (1990). [DOI] [PubMed] [Google Scholar]
- 86.Saper C. B., The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 25, 433–469 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Shoemaker J. K., Wong S. W., Cechetto D. F., Cortical circuitry associated with reflex cardiovascular control in humans: Does the cortical autonomic network “speak” or “listen” during cardiovascular arousal. Anat. Rec. (Hoboken) 295, 1375–1384 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Williamson J. W., Autonomic responses to exercise: Where is central command? Auton. Neurosci. 188, 3–4 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Tsuchimochi H., Matsukawa K., Komine H., Murata J., Direct measurement of cardiac sympathetic efferent nerve activity during dynamic exercise. Am. J. Physiol. Heart Circ. Physiol. 283, H1896–H1906 (2002). [DOI] [PubMed] [Google Scholar]
- 90.Sterling P., Allostasis: A model of predictive regulation. Physiol. Behav. 106, 5–15 (2012). [DOI] [PubMed] [Google Scholar]
- 91.Rowling J. K., Harry Potter and the Deathly Hallows (Scholastic Inc., New York, 2007), p. 784. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon request.