Abstract
In most cases, neurological disorders that involve injuries of the cerebral white matter are accompanied by demyelination and oligodendrocyte damage. Promotion of remyelination process through the maturation of oligodendrocyte precursor cells (OPCs) is therefore proposed to contribute to the development of novel therapeutic approaches that could protect and restore the white matter from central nervous system diseases. However, efficient remyelination in the white matter could not be accomplished if various neighboring cell types are not involved to react with oligodendrocyte lineage cells in this process. Hence, profound understanding of cell-cell interaction between oligodendrocyte lineage cells and other cellular components is an essential step to achieve a breakthrough for the cure of white matter injury. In this mini-review, we provide recent updates on non-cell autonomous mechanisms of oligodendrocyte regeneration by introducing recent studies (e.g. published either in 2018 or 2019) that focus on crosstalk between oligodendrocyte lineage cells and the other constituents of the white matter.
Keywords: oligodendrocyte precursor cell, cell-cell interaction, oligodendrocyte, white matter injury, white matter repair
1. Introduction
Although remarkable progress in the field of medical science over the last few decades has enabled us to deal with some of the neurological disorders that have once been thought as incurable, majority of the diseases still remain unmanageable. Development of effective medications for the cerebral white matter damage caused by diseases such as stroke, small vessel disease, multiple sclerosis (MS), and traumatic brain injury, could be counted as one of those unmet needs. To achieve a therapeutic breakthrough towards the demyelinated white matter, it should be required to elucidate mechanisms by which facilitate remyelination. Assuming that interaction with other types of brain cells would be necessary for potentially myelin-producing oligodendrocytes to maintain their roles both under physiological and pathological conditions, many scientists have investigated into cell-cell interaction between oligodendrocyte lineage cells and other cellular components of the white matter. We previously summarized the non-cell autonomous mechanisms of oligodendrocyte (re)generation [29, 34, 52]. But recently, partly because of the development/advance of research tools in genetic manipulation and gene analysis, several profound studies using animal models of white matter-related diseases have demonstrated the importance of cell-cell interaction in regulating oligodendrocyte precursor cell (OPC) function under pathological conditions. In this mini-review, therefore, we provide recent updates on non-cell autonomous mechanisms of oligodendrocyte regeneration after white matter damage, by introducing recent studies (e.g. published either in 2018 or 2019) that featured the crosstalk between oligodendrocyte lineage cells and other types of brain cells. Below, we discuss how each white matter component regulates oligodendrocyte regeneration under the conditions of white matter diseases.
2. Neuronal axon
White matter consists primarily of axonal bundles ensheathed by mature-oligodendrocyte-derived myelin. Oligodendrocytes situate in close proximity to neuronal axons to from myelin sheath, which plays an important role in rapid and efficient signal propagation between the gray matter of different areas. Because of the close proximity of neuronal axons with myelinated oligodendrocytes, both cell types coordinately support mutual functions via exchanging soluble factors and/or through direct interactions. The underlying mechanisms have been extensively examined, but still, ongoing research is necessary in order to fully elucidate how oligodendrocytes support neuronal axons, and vice versa. Myelin sheath wraps neuronal axons, but under some conditions, oligodendrocytes may support axonal function in a myelin-independent manner. Indeed, a recent study using electrophysiological recordings of the corpus callosum demonstrated that oligodendrocytes directly support axonal function by supplying glucose through gap junction connexin-47 [48]. Conversely, as for the mechanisms as to how neuronal axons regulate oligodendrocyte lineage cells, molecules secreted from axons or axonal surface ligands have been reported to regulate differentiation and maturation processes of oligodendrocyte lineage cells. For example, neurons are known to be involved in glutamatergic synaptic signaling with OPCs. By modifying the properties of AMPA receptor at axon-OPC synapses in vivo by targeting GluA2 subunit, Chen et al. recently reported the importance of ionotropic and non-ionotropic properties of AMPA receptor in OPCs for the balancing of the OPC response to differentiation cues [14]. Upon knowledge that oligodendrocyte production and myelination is stimulated by neuronal activity in normal condition, another recent study established a mouse model to induce a moderate optogenetic stimulation of demyelinated axons in the corpus callosum at the level of the motor cortex to examine whether the activity of demyelinated axons restores their loss of function in a damaged environment [58]. The study demonstrated that moderate activation of demyelinated axons enhanced the differentiation of OPCs into mature oligodendrocytes [58], indicating the need of preserving an appropriate neuronal activity to promote remyelination within the injured lesion. Although recent studies have unveiled a complimentary relationship between the two components, mechanism by which oligodendrocytes myelinate axons still remains relatively unveiled. In addition, intrinsic mechanism of oligodendrocytes underlying the spatiotemporal specificity of central nervous system (CNS) myelination has also been undetermined and understudied. However, one study recently showed that transcription factor EB (TFEB), which is highly expressed by differentiating oligodendrocytes, may be a key molecular factor for the spatial and temporal regulation of myelination [66]. Furthermore, very recently, tight adhesion regulated by cell adhesion molecules such as Cadm4 was reported to be essential for an appropriate myelin targeting and membrane wrapping [21]. Taken together, recent research progress is now revealing the complex mechanisms of neuron-OPC interaction. Because proper axonal repair and/or regeneration after white matter injury may need to be accompanied with oligodendrocyte regeneration, deeper understanding of the compensative responses in the axon-OPC interface would be of help in developing an effective therapeutic strategy for white matter repair and remodeling.
3. Endothelial cells
The concept of neurovascular unit provides a conceptual framework that emphasizes the importance of cell-cell interactions between neuronal, glial, and vascular components [18, 31, 33, 42, 43, 73, 74]. One of the most examined aspects of the neurovascular unit in gray matter is observed in the cell-cell interaction between neuronal precursor cell and the vascular compartment in the so-called neurovascular niche. In the neurovascular niche, cell-cell signaling among these different cell types sustains pockets of ongoing neurogenesis and angiogenesis in the adult brain, which plays important roles in compensatory neurovascular remodeling after brain injury [5, 15, 26, 32, 54, 73, 74]. Similarly, in the white matter, endothelial cells (ECs) and OPCs may coordinately form an analogous oligovascular niche, wherein ECs support oligodendrocyte lineage cells by secreting trophic factors [1, 3]. Although whether or not mature oligodendrocytes physically contact with endothelial cells has not been confirmed, rodent immunohistological studies predict that some types of OPCs attach with endothelial cells in the peri-vascular region of the cerebral white matter [46, 64]. Another experiment using embryonic mouse brain supports this notion by revealing that perivascular migration and differentiation of OPCs are coordinated by endothelial-OPC interaction regulated by Wnt-chemokine receptor 4 (Cxcr4) signaling at the stage of development [68]. The same group also recently found that if this Wnt signaling is excessive, activated OPCs secrete Wnt inhibitory factor 1 which interferes with EC tight junction integrity leading to the disruption of the blood-brain barrier (BBB) [56]. More recently, another group proposed that mechanisms of vascular-OPC interaction may differ during neocortical development, depending on the three-dimensional vessel patterns [39]. All these studies introduced above mostly discussed the mechanisms of endothelium-OPC interaction during development or in cell-culture conditions, but the roles of oligovascular niche in compensatory oligodendrogenesis after white matter damage in adult brain are still unknown. However, a recent transcriptome profiling study of the perivascular area within the demyelinated lesion using a rat brain demonstrated that peri-vascular niche was enriched with secreted ligands of the bone morphogenic protein (BMP) and Wnt signaling pathways produced by activated OPCs and ECs, which would trigger the fate switch of neural progenitors to OPCs [70]. In the study, the authors proposed that these signals from OPCs/ECs became dominant in the remyelination process because of the low number of astrocytes within the site of injury and the low level of the astrocyte-derived dual BMP/Wnt inhibitor Sostdc1 in those areas. Nevertheless, we may need to be aware that function of BMPs have been described in a rather opposite way in other literatures, i.e. decreasing the proliferation of precursor cells and inhibiting the maturation of oligodendrocytes [25, 62]. Therefore, future studies are warranted to investigate how and why this identical factor BMP can show opposite effects on oligodendrocyte linage cells under diseased conditions. Aside from mature ECs, endothelial progenitor cells (EPCs) are also gathering attention to regulate OPC proliferation and differentiation. A recent study has demonstrated that a secretome from EPCs could boost OPC proliferation and maturation under diseased conditions both in vitro and in vivo [47]. In turn, a secretome from OPCs may also play some roles in the cerebral vascular system. Cultured OPCs maintained under hypoxic conditions secreted more pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), which then increased the viability and tube formation of ECs in vitro [38]. The same study also showed that when conditioned media from hypoxically cultured OPCs was introduced into the lesion caused by middle cerebral artery occlusion in mice, post-stroke angiogenesis was facilitated to alleviate the infarct volume [38]. Therefore, in adult CNS, progenitors for ECs and oligodendrocytes may not merely serve as a backup for regeneration processes, but rather, they may actively participate in the recovering mechanisms by promoting the differentiation (or proliferation) of neighboring progenitor cells. Because the disturbance of cerebrovascular system is one of the major characteristics of white mater-related diseases, examining the cell-cell interaction between oligodendrocyte lineage cells and endothelial lineage cells would be crucial for deeper understanding of the white matter pathology.
4. Astrocytes
Astrocytes, which constitute nearly half of brain cell population, also interact closely with oligodendrocyte lineage cells. Since early times, it has been reported that astrocytes directly interface with oligodendrocytes to support their function through gap junction in vitro [12, 57]. Other studies using in vitro cell culture system have demonstrated that astrocytes also affect OPC function indirectly, i.e. by secreting soluble factors that protect them from external stress such as hypoxia [2, 36]. Early animal studies which used a model of white matter injury showed that astrocytes regulate OPC function in vivo. For example, cultured astrocytes were shown to facilitate myelin restoration when transplanted into the demyelinated lesion by proliferating the endogenous OPCs [23], and transgenic mice with brain-derived neurotrophic factor (BDNF) downregulation in astrocytes exhibited lower levels of oligodendrocyte regeneration in a mouse model of subcortical ischemic vascular dementia (SIVD) [51]. Despite these knowledges, however, a key pathway to reinforce the remyelination process is still under investigation. Nevertheless, in regard to the direct interaction, relationship between oligodendrocyte gap junction connexin-47 (Cx47) and astrocyte gap junction connexin-43 (Cx43) was recently clarified [22]. Heterotypic channels formed by these 2 junctions were found to exhibit ionic and chemical rectification to barrier the movement of ions and larger negatively charged molecules from oligodendrocytes to astrocytes [22]. Further, the same study found that this barrier was abolished by a mutation (Cx47P90S) associated with leukodystrophy [22], implicating a novel disease mechanism. Importance of astrocyte for efficient remyelination was also demonstrated by recent high-throughput genome sequencing studies. Cell/region-specific gene expression analysis of astrocytes from optic nerve and spinal cord of experimental autoimmune encephalomyelitis (EAE) mouse model (a mouse model of MS) revealed that expression of cholesterol synthesis genes was decreased while immune pathway gene expression was increased in astrocytes, and more interestingly, gene pathways related to cholesterol-synthesis dominated as the top up-regulated pathways in oligodendrocyte lineage cells during remyelination process [35, 71]. From these results, insufficient supply of the cholesterol from astrocytes to myelin composition was proposed to be one of the important reasons why oligodendrocytes fail to remyelinate in MS. In regard to the astrocytic role in myelinating oligodendrocytes, another recent study demonstrated an importance of paranodal astrocytes in the regulation of myelin structure [19]. The study showed that inhibition of exocytosis leads to an increased nodal gap length and thinning of the myelin sheath in vivo [19]. Another important aspect of astrocyte-OPC interaction would be in a heterogeneity of reactive astrocytes (e.g. astrogliosis) after brain injury. Depending on the context, astrogliosis would be both harmful and beneficial for oligodendrocyte regeneration. For example, glial scar formation has been understood to inhibit the white matter regeneration [11]. On the other hand, recent study utilizing in situ hybridization/immunohistochemistry double staining of EAE mice spinal cord demonstrated that recruitment of OPCs into the scar lesion does not differ from that of healthy region, and that the astrocytes within the scar showed high expression of OPC guidance molecules such as Sema3A and Sema3F [10, 28]. Further studies are warranted to carefully determine how and why astrogliosis exhibits these opposite effects on oligodendrocyte lineage cells after white matter injury. Because astrocytes and oligodendrocytes constitute the two major types of glial cells in white matter, and because some subsets of OPCs tend to trans-differentiate into astrocytes under diseased conditions [20, 65], their interaction should play a pivotal role in compensatory gliogenesis, which contributes to white matter repair and regeneration.
5. Microglia
Similar to oligodendrocyte/OPCs and astrocytes, microglia belong to a type of glial cells. Microglia are responsible to immune responses in brain, and contribute to brain homeostasis by surveying their surrounding microenvironment for indicators [30, 37, 72]. Upon encountering indicators of injury or infection, microglia quickly become activated to clear cellular debris from dead/damaged cells by phagocytosis, as a response to maintain the microenvironment in the brain. In fact, microglia are known to affect OPC differentiation rate through the clearance of myelin debris or/and by the secretion of pro-regenerative factors [49, 50, 55]. However, under the acute phase of white matter damage, microglia also serve as a major source of pro-inflammatory molecules that are cytotoxic to white matter components, including neurons and oligodendrocytes, which results in disease initiation and progression [6, 9, 40, 60, 67]. This biphasic beneficial vs deleterious polarization/switching mechanism has been considered as a basic concept of microglial activation after CNS injury. However, accumulating evidence now suggests the existence of different microglial subsets and their functional diversity under diseased conditions. For example, a recent gene expression analysis revealed that a distinct amoeboid microglia population, formerly described as the “fountain of microglia”, which appears only postnatally in myelinated regions, such as corpus callosum, arise from a CNS endogenous microglia pool independent from circulating myeloid cells [27]. The same experiment demonstrated an essential role of postnatal microglia for the proper development and homeostasis of oligodendrocytes lineage cells by microglia depletion study in vivo [27]. Another recent finding shows that microglia-derived transglutaminase-2 (TG2) signals to adhesion G protein-coupled receptor ADGRG1 on OPCs in the presence of the extracellular matrix protein laminin, promoting OPC proliferation and improving remyelination in a mouse model of demyelination [24]. Diverse functional phenotypes of microglia range from pro-inflammatory classically activated phenotypes to pro-regenerative alternatively activated phenotypes. Past study has proposed that cell polarization of microglia to pro-regenerative phenotype is essential for efficient remyelination by demonstrating that oligodendrocyte differentiation was enhanced in vitro with alternatively activated cell conditioned media and impaired in vivo following intra-lesional depletion of alternatively activated microglia in MS model mice [49]. Difference in reaction towards the OPCs was also confirmed between the 2 phenotypes by recent experiments. When extracellular vesicles (EV) produced in vitro by either pro-inflammatory or pro-regenerative microglia were applied to lysolecithin-induced white matter lesion in mice, EV released from the former phenotype blocked remyelination while EV produced from the latter phenotype were found to promote recruitment of the OPCs and myelin repairment [44]. Another recent study also emphasized the importance of focusing on environment-induced cell polarization of microglia by revealing that classically activated microglia undergo necroptosis and repopulates to a regenerative state before the onset of remyelination in a mouse model of MS [41]. Notably, microglia play an essential role in white matter degeneration caused by aging as well. It is understood that age-related change in microglia lead to decline in the efficiency of remyelination [13, 24, 63]. Latest study shows that rising levels of circulating TGFβ in aged rats stimulate the aged microglia to release proteoglycan NG2, which diverts the differentiation of OPCs from oligodendrocytes into astrocytes [7]. As noted, microglia were traditionally considered as deleterious cells in the acute phase of CNS diseases, and in cerebral white matter excessive microglial activation damages neuronal axons and myelin sheaths. However, an emerging literature now suggest that a more complicated mechanism may involve in both damaging and beneficial microglia phenotypes. Therefore, understanding the molecular mechanisms by which regulate functional properties of microglial subsets may lead us to a novel and efficient therapy for various white matter diseases.
6. Pericytes
Pericytes are cells that are defined based on their anatomical location surrounding the blood vessel. They communicate with endothelial cells through physical contacts and paracrine signaling to maintain vascular homeostasis. Pericytes possess similar properties as the ones of smooth muscle cells, and traditionally, the major role of pericytes has been considered cerebral blood flow (CBF) regulation [8, 59]. However, pericytes would also play other multiple roles in the neurovascular unit, including regulation of BBB integrity [4, 8, 16], clearance of cellular debris and/or byproducts [61, 74], and serving as a cellular source of pluripotent stem cells [61]. In addition, an electron microscopic analysis suggested that pericytes are attached to OPCs via basal lamina [46], suggesting a potential anatomical and functional interaction between pericytes and OPCs. To date, precise mechanisms by which oligodendrocyte lineage cells interact with the pericytes remain mostly unknown. However, some of the recent studies have demonstrated that pericyte-derived factors could affect the fate of OPCs. A histopathological study of the white matter using a mouse model of chronic cerebral hypoperfusion showed high expression of BMP4 in pericytes accompanied by astrogliogenesis and reduced number of oligodendrocyte lineage cells. In the same study, recombinant BMP4 were shown to convert cultured OPCs into astrocytes [69]. A-kinase anchor protein (AKAP12) was also reported to regulate differentiation of OPCs into mature oligodendrocytes by controlling the secretion of growth factors from pericytes via PKA and PKC signaling [45]. In addition, a study using pericyte-deficient mice showed that lack of pericytes delayed remyelination of the damaged white matter, while remyelination proceeded to completion [17]. The same study identified that OPC differentiation was stimulated by Lama2 secreted by pericytes [17]. Another recent comprehensive study using pericyte-deficient mice also confirmed the crucial role of pericytes in oligodendrocyte homeostasis. By conducting magnetic resonance imaging (MRI), viral-based tract-tracing, behavior and tissue analysis, the study demonstrated that deprivation of pericytes resulted in a loss of myelin, axons and oligodendrocytes of the white matter [53]. This pathological change was attributed to an accumulation of toxic blood-derived fibrin (fibrinogen) deposits and CBF reduction triggered by disrupted white matter microcirculation [53], further supporting the importance of pericyte-oligodendrocyte interaction in white matter homeostasis. Compared to other white matter components, roles of pericytes in oligodendrocyte (re)generation are relatively understudied. However, as discussed in this section, pericytes are indeed an important cell type in regulating the function of oligodendrocyte lineage cells, especially in the vascular niche. Therefore, further studies are warranted to investigate how and when pericytes can promote OPC proliferation/differentiation after oligodendrocyte/myelin damage, for the purpose of deeper understanding the white matter pathology in demyelinating diseases.
7. Conclusion
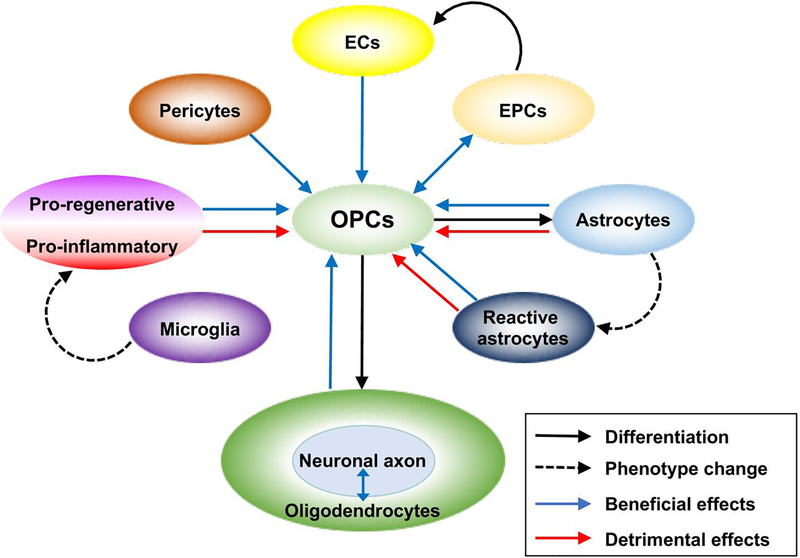
In most cases of CNS diseases, cerebral white matter injury involves demyelination and oligodendrocyte damage. Since it seems unrealistic to correspond simultaneously to multiple detrimental reactions that are predicted to cause death of oligodendrocytes, promoting remyelination process through the maturation of OPCs may be a reasonable approach to achieve an effective therapy for protecting and restoring the white matter from CNS diseases. As discussed in the text above, efficient oligodendrogenesis and remyelination may not be accomplished by oligodendrocyte lineage cells alone, and involvement of many other types of neighboring cells in the white matter should be essential for this process (Figure 1). Therefore, deeper understanding of cell-cell interaction between oligodendrocyte lineage cells and other cellular components in the white matter would be an important step to establish a breakthrough for the cure of white matter injury caused by various neurological diseases.
Figure 1.
Schematic drawing of cell-cell interaction between the oligodendrocyte lineage cells and other cellular components in the white matter. As the concept of NVU emphasizes, oligodendrocyte lineage cells collaborate with other cells to maintain homeostasis of the white matter under physiological conditions. Even under the pathophysiological conditions, OPC-related cell-cell interaction is maintained to promote the compensatory oligodendrogenesis (e.g. oligodendrocyte regeneration). On the other hand, after injury, some types of brain cells could be harmful for oligodendrocyte lineage cells, partly through releasing detrimental factors such as proinflammatory cytokines. Note that OPCs could trans-differentiate into astrocytes after white matter damage, but the underlying mechanisms still remain elucidated. Also, depending on the context (e.g. signals from neighboring cells and surrounding environmental conditions), each sub-type of brain cells could be both beneficial and detrimental under the conditions of white matter diseases. Profound understanding of these cell-cell interactions is essential for the discovery of therapies that would effectively cure the degeneration of the white matter in CNS diseases. Black arrow: differentiation, black dotted arrow: phenotype change, blue arrow: beneficial effects, red arrow:
Highlights.
Promotion of remyelination process can be a novel therapeutic approach for CNS diseases.
Oligodendrocyte lineage cells receive trophic support from neighboring cells.
We provide recent updates on non-cell autonomous mechanisms of oligodendrocyte regeneration.
Acknowledgements
Supported in part by National Institutes of Health. The authors thank Drs. Eng H. Lo, Gen Hamanaka, and Hajime Takase for many helpful discussions.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Arai K, Lo E, Oligovascular signaling in white matter stroke, Biological & pharmaceutical bulletin 32 (2009) 1639–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arai K, Lo EH, Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling, J Neurosci Res 88 (2010) 758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arai K, Lo EH, An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells, The Journal of neuroscience : the official journal of the Society for Neuroscience 29 (2009) 4351–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C, Pericytes regulate the blood-brain barrier, Nature 468 (2010) 557–561. [DOI] [PubMed] [Google Scholar]
- [5].Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O, Neuronal replacement from endogenous precursors in the adult brain after stroke, Nat Med 8 (2002) 963–970. [DOI] [PubMed] [Google Scholar]
- [6].Banati RB, Gehrmann J, Schubert P, Kreutzberg GW, Cytotoxicity of microglia, Glia 7 (1993) 111–118. [DOI] [PubMed] [Google Scholar]
- [7].Baror R, Neumann B, Segel M, Chalut KJ, Fancy SPJ, Schafer DP, Franklin RJM, Transforming growth factor-beta renders ageing microglia inhibitory to oligodendrocyte generation by CNS progenitors, Glia 67 (2019) 1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV, Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging, Neuron 68 (2010) 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Block ML, Zecca L, Hong JS, Microglia-mediated neurotoxicity: uncovering the molecular mechanisms, Nat Rev Neurosci 8 (2007) 57–69. [DOI] [PubMed] [Google Scholar]
- [10].Boyd A, Zhang H, Williams A, Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models, Acta neuropathologica 125 (2013) 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR, Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination, Glia 62 (2014) 452–467. [DOI] [PubMed] [Google Scholar]
- [12].Butt AM, Ibrahim M, Ruge FM, Berry M, Biochemical subtypes of oligodendrocyte in the anterior medullary velum of the rat as revealed by the monoclonal antibody Rip, Glia 14 (1995) 185–197. [DOI] [PubMed] [Google Scholar]
- [13].Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lutjohann D, Mobius W, Simons M, Defective cholesterol clearance limits remyelination in the aged central nervous system, Science 359 (2018) 684–688. [DOI] [PubMed] [Google Scholar]
- [14].Chen TJ, Kula B, Nagy B, Barzan R, Gall A, Ehrlich I, Kukley M, In Vivo Regulation of Oligodendrocyte Precursor Cell Proliferation and Differentiation by the AMPA-Receptor Subunit GluA2, Cell Rep 25 (2018) 852–861.e857. [DOI] [PubMed] [Google Scholar]
- [15].Chopp M, Zhang ZG, Jiang Q, Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke, Stroke 38 (2007) 827–831. [DOI] [PubMed] [Google Scholar]
- [16].Daneman R, Zhou L, Kebede AA, Barres BA, Pericytes are required for blood-brain barrier integrity during embryogenesis, Nature 468 (2010) 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].De La Fuente AG, Lange S, Silva ME, Gonzalez GA, Tempfer H, van Wijngaarden P, Zhao C, Di Canio L, Trost A, Bieler L, Zaunmair P, Rotheneichner P, O’Sullivan A, Couillard-Despres S, Errea O, Mae MA, Andrae J, He L, Keller A, Batiz LF, Betsholtz C, Aigner L, Franklin RJM, Rivera FJ, Pericytes Stimulate Oligodendrocyte Progenitor Cell Differentiation during CNS Remyelination, Cell Rep 20 (2017) 1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].del Zoppo GJ, Inflammation and the neurovascular unit in the setting of focal cerebral ischemia, Neuroscience 158 (2009) 972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dutta DJ, Woo DH, Lee PR, Pajevic S, Bukalo O, Huffman WC, Wake H, Basser PJ, SheikhBahaei S, Lazarevic V, Smith JC, Fields RD, Regulation of myelin structure and conduction velocity by perinodal astrocytes, Proc Natl Acad Sci U S A 115 (2018) 11832–11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eg S, Rosenzweig S, DiTullio D, Hinman JD, Bridges SP, Marin MA, Kawaguchi R, Coppola G, Carmichael ST, White matter stroke induces a unique oligo-astrocyte niche that inhibits recovery, J Neurosci (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Elazar N, Vainshtein A, Golan N, Vijayaragavan B, Schaeren-Wiemers N, Eshed-Eisenbach Y, Peles E, Axoglial Adhesion by Cadm4 Regulates CNS Myelination, Neuron 101 (2019) 224–231.e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fasciani I, Pluta P, Gonzalez-Nieto D, Martinez-Montero P, Molano J, Paino CL, Millet O, Barrio LC, Directional coupling of oligodendrocyte connexin-47 and astrocyte connexin-43 gap junctions, Glia 66 (2018) 2340–2352. [DOI] [PubMed] [Google Scholar]
- [23].Franklin RJ, Crang AJ, Blakemore WF, Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord, Journal of neurocytology 20 (1991) 420–430. [DOI] [PubMed] [Google Scholar]
- [24].Giera S, Luo R, Ying Y, Ackerman SD, Jeong SJ, Stoveken HM, Folts CJ, Welsh CA, Tall GG, Stevens B, Monk KR, Piao X, Microglial transglutaminase-2 drives myelination and myelin repair via GPR56/ADGRG1 in oligodendrocyte precursor cells, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Govier-Cole AE, Wood RJ, Fletcher JL, Gonsalvez DG, Merlo D, Cate HS, Murray SS, Xiao J, Inhibiting Bone Morphogenetic Protein 4 Type I Receptor Signaling Promotes Remyelination by Potentiating Oligodendrocyte Differentiation, eNeuro 6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Greenberg DA, Jin K, From angiogenesis to neuropathology, Nature 438 (2005) 954–959. [DOI] [PubMed] [Google Scholar]
- [27].Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L, Prinz M, Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood, Acta neuropathologica 134 (2017) 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Haindl MT, Kock U, Zeitelhofer-Adzemovic M, Fazekas F, Hochmeister S, The formation of a glial scar does not prohibit remyelination in an animal model of multiple sclerosis, Glia 67 (2019) 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hamanaka G, Ohtomo R, Takase H, Lok J, Arai K, Role of oligodendrocyte-neurovascular unit in white matter repair, Neurosci Lett 684 (2018) 175–180. [DOI] [PubMed] [Google Scholar]
- [30].Hanisch UK, Kettenmann H, Microglia: active sensor and versatile effector cells in the normal and pathologic brain, Nat Neurosci 10 (2007) 1387–1394. [DOI] [PubMed] [Google Scholar]
- [31].Hawkins BT, Davis TP, The blood-brain barrier/neurovascular unit in health and disease, Pharmacol Rev 57 (2005) 173–185. [DOI] [PubMed] [Google Scholar]
- [32].Iadecola C, Neurovascular regulation in the normal brain and in Alzheimer’s disease, Nat Rev Neurosci 5 (2004) 347–360. [DOI] [PubMed] [Google Scholar]
- [33].Iadecola C, Neurovascular regulation in the normal brain and in Alzheimer’s disease, Nat Rev Neurosci 5 (2004) 347–360. [DOI] [PubMed] [Google Scholar]
- [34].Itoh K, Maki T, Lok J, Arai K, Mechanisms of cell-cell interaction in oligodendrogenesis and remyelination after stroke, Brain Res 1623 (2015) 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Itoh N, Itoh Y, Tassoni A, Ren E, Kaito M, Ohno A, Ao Y, Farkhondeh V, Johnsonbaugh H, Burda J, Sofroniew MV, Voskuhl RR, Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes, Proc Natl Acad Sci U S A 115 (2018) E302–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kato S, Aoyama M, Kakita H, Hida H, Kato I, Ito T, Goto T, Hussein MH, Sawamoto K, Togari H, Asai K, Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury, J Neurosci Res 89 (2011) 1566–1574. [DOI] [PubMed] [Google Scholar]
- [37].Kettenmann H, Hanisch UK, Noda M, Verkhratsky A, Physiology of microglia, Physiol Rev 91 (2011) 461–553. [DOI] [PubMed] [Google Scholar]
- [38].Kishida N, Maki T, Takagi Y, Yasuda K, Kinoshita H, Ayaki T, Noro T, Kinoshita Y, Ono Y, Kataoka H, Yoshida K, Lo EH, Arai K, Miyamoto S, Takahashi R, Role of Perivascular Oligodendrocyte Precursor Cells in Angiogenesis After Brain Ischemia, Journal of the American Heart Association 8 (2019) e011824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Komabayashi-Suzuki M, Yamanishi E, Watanabe C, Okamura M, Tabata H, Iwai R, Ajioka I, Matsushita J, Kidoya H, Takakura N, Okamoto T, Kinoshita K, Ichihashi M, Nagata KI, Ema M, Mizutani KI, Spatiotemporally Dependent Vascularization Is Differently Utilized among Neural Progenitor Subtypes during Neocortical Development, Cell Rep 29 (2019) 1113–1129 e1115. [DOI] [PubMed] [Google Scholar]
- [40].Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS, Role of nitric oxide in inflammation-mediated neurodegeneration, Ann N Y Acad Sci 962 (2002) 318–331. [DOI] [PubMed] [Google Scholar]
- [41].Lloyd AF, Davies CL, Holloway RK, Labrak Y, Ireland G, Carradori D, Dillenburg A, Borger E, Soong D, Richardson JC, Kuhlmann T, Williams A, Pollard JW, des Rieux A, Priller J, Miron VE, Central nervous system regeneration is driven by microglia necroptosis and repopulation, Nat Neurosci (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lo EH, Broderick JP, Moskowitz MA, tPA and proteolysis in the neurovascular unit, Stroke 35 (2004) 354–356. [DOI] [PubMed] [Google Scholar]
- [43].Lo EH, Dalkara T, Moskowitz MA, Mechanisms, challenges and opportunities in stroke, Nat Rev Neurosci 4 (2003) 399–415. [DOI] [PubMed] [Google Scholar]
- [44].Lombardi M, Parolisi R, Scaroni F, Bonfanti E, Gualerzi A, Gabrielli M, Kerlero de Rosbo N, Uccelli A, Giussani P, Viani P, Garlanda C, Abbracchio MP, Chaabane L, Buffo A, Fumagalli M, Verderio C, Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure, Acta neuropathologica (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Maki T, Choi YK, Miyamoto N, Shindo A, Liang AC, Ahn BJ, Mandeville ET, Kaji S, Itoh K, Seo JH, Gelman IH, Lok J, Takahashi R, Kim KW, Lo EH, Arai K, A-Kinase Anchor Protein 12 Is Required for Oligodendrocyte Differentiation in Adult White Matter, Stem cells (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Maki T, Maeda M, Uemura M, Lo EK, Terasaki Y, Liang AC, Shindo A, Choi YK, Taguchi A, Matsuyama T, Takahashi R, Ihara M, Arai K, Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter, Neurosci Lett 597 (2015) 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Maki T, Morancho A, Martinez-San Segundo P, Hayakawa K, Takase H, Liang AC, Gabriel-Salazar M, Medina-Gutierrez E, Washida K, Montaner J, Lok J, Lo EH, Arai K, Rosell A, Endothelial Progenitor Cell Secretome and Oligovascular Repair in a Mouse Model of Prolonged Cerebral Hypoperfusion, Stroke 49 (2018) 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Meyer N, Richter N, Fan Z, Siemonsmeier G, Pivneva T, Jordan P, Steinhauser C, Semtner M, Nolte C, Kettenmann H, Oligodendrocytes in the Mouse Corpus Callosum Maintain Axonal Function by Delivery of Glucose, Cell Rep 22 (2018) 2383–2394. [DOI] [PubMed] [Google Scholar]
- [49].Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, Ffrench-Constant C, M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination, Nat Neurosci 16 (2013) 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Miron VE, Franklin RJ, Macrophages and CNS remyelination, Journal of neurochemistry 130 (2014) 165–171. [DOI] [PubMed] [Google Scholar]
- [51].Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M, Arai K, Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor, J Neurosci 35 (2015) 14002–14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Miyamoto N, Pham LD, Seo JH, Kim KW, Lo EH, Arai K, Crosstalk between cerebral endothelium and oligodendrocyte, Cell Mol Life Sci 71 (2014) 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, Barnes SR, Daianu M, Ramanathan A, Go A, Lawson EJ, Wang Y, Mack WJ, Thompson PM, Schneider JA, Varkey J, Langen R, Mullins E, Jacobs RE, Zlokovic BV, Pericyte degeneration causes white matter dysfunction in the mouse central nervous system, Nat Med 24 (2018) 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [54].Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M, Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors, Cell 110 (2002) 429–441. [DOI] [PubMed] [Google Scholar]
- [55].Natrajan MS, de la Fuente AG, Crawford AH, Linehan E, Nunez V, Johnson KR, Wu T, Fitzgerald DC, Ricote M, Bielekova B, Franklin RJ, Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination, Brain : a journal of neurology 138 (2015) 3581–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Niu J, Tsai HH, Hoi KK, Huang N, Yu G, Kim K, Baranzini SE, Xiao L, Chan JR, Fancy SPJ, Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation, Nat Neurosci 22 (2019) 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Orthmann-Murphy JL, Abrams CK, Scherer SS, Gap junctions couple astrocytes and oligodendrocytes, Journal of molecular neuroscience : MN 35 (2008) 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ortiz FC, Habermacher C, Graciarena M, Houry PY, Nishiyama A, Oumesmar BN, Angulo MC, Neuronal activity in vivo enhances functional myelin repair, JCI insight 5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Peppiatt CM, Howarth C, Mobbs P, Attwell D, Bidirectional control of CNS capillary diameter by pericytes, Nature 443 (2006) 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Perry VH, Nicoll JA, Holmes C, Microglia in neurodegenerative disease, Nat Rev Neurol 6 (2010) 193–201. [DOI] [PubMed] [Google Scholar]
- [61].Sa-Pereira I, Brites D, Brito MA, Neurovascular unit: a focus on pericytes, Mol Neurobiol 45 (2012) 327–347. [DOI] [PubMed] [Google Scholar]
- [62].Sabo JK, Aumann TD, Merlo D, Kilpatrick TJ, Cate HS, Remyelination is altered by bone morphogenic protein signaling in demyelinated lesions, The Journal of neuroscience : the official journal of the Society for Neuroscience 31 (2011) 4504–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, Simons M, Age-related myelin degradation burdens the clearance function of microglia during aging, Nat Neurosci 19 (2016) 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Seo JH, Maki T, Maeda M, Miyamoto N, Liang AC, Hayakawa K, Pham LD, Suwa F, Taguchi A, Matsuyama T, Ihara M, Kim KW, Lo EH, Arai K, Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling, PLoS One 9 (2014) e103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sozmen EG, Rosenzweig S, Llorente IL, DiTullio DJ, Machnicki M, Vinters HV, Havton LA, Giger RJ, Hinman JD, Carmichael ST, Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice, Proc Natl Acad Sci U S A 113 (2016) E8453–E8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sun LO, Mulinyawe SB, Collins HY, Ibrahim A, Li Q, Simon DJ, Tessier-Lavigne M, Barres BA, Spatiotemporal Control of CNS Myelination by Oligodendrocyte Programmed Cell Death through the TFEB-PUMA Axis, Cell 175 (2018) 1811–1826.e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Torreilles F, Salman-Tabcheh S, Guerin M, Torreilles J, Neurodegenerative disorders: the role of peroxynitrite, Brain Res Brain Res Rev 30 (1999) 153–163. [DOI] [PubMed] [Google Scholar]
- [68].Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ, Chan JR, Daneman R, Fancy SP, Oligodendrocyte precursors migrate along vasculature in the developing nervous system, Science 351 (2016) 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Uemura MT, Ihara M, Maki T, Nakagomi T, Kaji S, Uemura K, Matsuyama T, Kalaria RN, Kinoshita A, Takahashi R, Pericyte-derived bone morphogenetic protein 4 underlies white matter damage after chronic hypoperfusion, Brain pathology (Zurich, Switzerland) 28 (2018) 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ulanska-Poutanen J, Mieczkowski J, Zhao C, Konarzewska K, Kaza B, Pohl HB, Bugajski L, Kaminska B, Franklin RJ, Zawadzka M, Injury-induced perivascular niche supports alternative differentiation of adult rodent CNS progenitor cells, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Voskuhl RR, Itoh N, Tassoni A, Matsukawa MA, Ren E, Tse V, Jang E, Suen TT, Itoh Y, Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis, Proc Natl Acad Sci U S A 116 (2019) 10130–10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wake H, Moorhouse AJ, Miyamoto A, Nabekura J, Microglia: actively surveying and shaping neuronal circuit structure and function, Trends Neurosci 36 (2013) 209–217. [DOI] [PubMed] [Google Scholar]
- [73].Zacchigna S, Lambrechts D, Carmeliet P, Neurovascular signalling defects in neurodegeneration, Nat Rev Neurosci 9 (2008) 169–181. [DOI] [PubMed] [Google Scholar]
- [74].Zlokovic BV, The blood-brain barrier in health and chronic neurodegenerative disorders, Neuron 57 (2008) 178–201. [DOI] [PubMed] [Google Scholar]