Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that is the sixth leading cause of death and the most common cause of dementia worldwide. Over the last few decades, significant advancements have been made in our understanding of AD by investigating the molecular mechanisms underlying amyloid-β and tau pathology. Despite this progress, no disease-modifying treatments exist for AD, an issue that will exacerbated by the rising costs and prevalence of the disorder. Moreover, effective therapies to address the devastating cognitive and behavioral symptoms are also urgently needed. This perspective focuses on the value of nonhuman primate (NHP) models in bridging the molecular, circuit, and behavioral levels of analysis to better understand the complex genetic and environmental/lifestyle factors that contribute to AD pathogenesis. These investigations could provide an opportunity for translating our understanding of the pathogenesis and physiological mechanisms underlying AD and related disorders into new diagnostic approaches and disease-modifying therapies to prevent disease or restore brain function for symptomatic individuals.
Keywords: Alzheimer’s disease, nonhuman primates, dementia
Dementia is a clinical syndrome characterized by impairment in several cognitive domains that prevents an individual from living a fully functional and autonomous life (1). The most common cause of dementia is Alzheimer’s disease (AD), accounting for nearly 60 to 80% of all cases (2). AD is the sixth leading cause of death, with an estimated prevalence of nearly 30 million people worldwide. Age is the most important risk factor for AD, with an exponential increase in prevalence from 3 to 32% from the ages of 65 to 85 y old (3). An estimated 5.6 million individuals over the age of 65 suffer from AD in the United States, a number projected to nearly triple to 13.8 million by 2050 due to increases in population and lifespan. Caring for an individual with AD often results in multiple hospital admissions over extended periods of time, and behavioral problems, such as agitation and psychosis, often result in the need for long-term care in facilities. Due to this insidious nature of the disease, Medicare and Medicaid spending in 2019 caring for those with AD will reach an estimated $195 billion, and it is projected to rise to $1 trillion by 2050 (3). These issues will only be exacerbated by inadequate symptomatic treatments and the lack of disease-modifying treatments.
AD is a chronic neurodegenerative disorder characterized histopathologically by the presence of amyloid-β (Aβ) peptides in extracellular senile plaques and the formation of intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated, microtubule-associated protein tau (4, 5). Because dementia can also arise from a number of etiologies that masquerade as or coexist with AD, pathological confirmation at autopsy (or rarely, biopsy in living individuals) has traditionally been necessary for definitive diagnosis. However, the advent of positron emission tomography (PET) imaging and fluid biomarkers of plaques and tangles has enabled detection of these pathologies in living individuals. For example, in individuals with mild cognitive impairment, a positive amyloid PET scan or characteristic cerebrospinal fluid levels of Aβ, tau, and phospho-tau are sensitive and specific biomarkers of AD that often, but not always, predict the likelihood of progressing to dementia (6, 7). These biomarkers have proven useful in tracking AD progression in humans, and our understanding of core AD pathologies has improved dramatically due to new experimental approaches, such as cell culture and transgenic mouse models. Unfortunately, these biomarkers have not yet translated into treatments for AD and therefore, offer an opportunity to use nonhuman primate (NHP) models in order to mirror the early stages of neurodegeneration and identify new therapeutic options. In this perspective, we briefly review the current state of knowledge about the pathogenesis and treatment of AD and highlight gaps where there are important opportunities for NHP research to advance the field.
NHP Models for Identifying the Molecular Pathogenesis and Physiological Mechanisms of AD
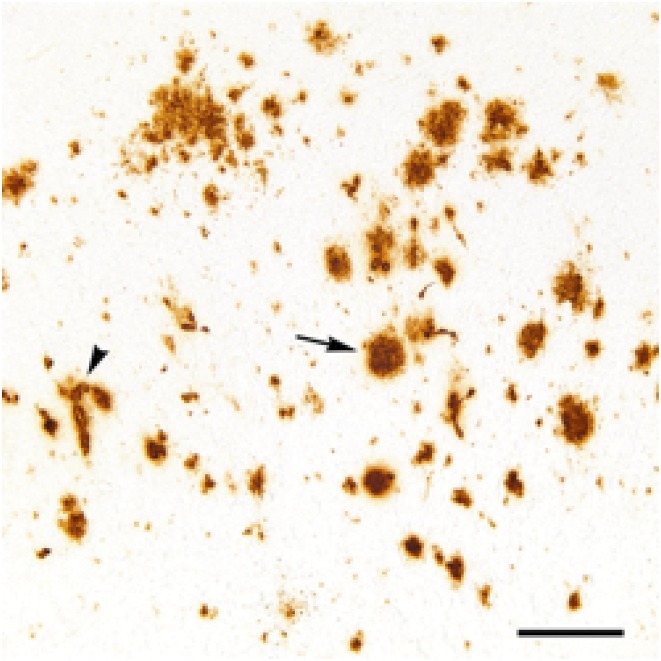
Insights into the pathogenesis of AD arose from the pioneering neuropathological observations of senile plaques and NFTs by Alois Alzheimer (8) and the discovery 80 y later that these plaques consist of a 39- to 43-amino acid peptide now known as Aβ (9). Aβ is derived from the sequential cleavage of the amyloid-β precursor protein (APP) by β-secretase and γ-secretase to primarily yield 2 major isoforms, Aβ40 and Aβ42, along with various C- and N-terminally truncated and/or modified isoforms. In the disease state, Aβ acquires a β strand-rich molecular conformation, a state prone to self-assemble into oligomers, diffuse plaques, and dense-core (amyloid) plaques. Many dense-core plaques, which are thought to represent a late stage of development, are surrounded by degenerating neurites as well as activated microglia and astrocytes (5). Soluble amyloid-β oligomers (AβOs), rather than dense-core plaques, are now thought to be linked to early synaptic loss and reflect the earliest stages of AD (10). Aβ also frequently accumulates in the walls of brain blood vessels (cerebral amyloid angiopathy [CAA]) (11), although the amount of CAA varies considerably among cases (12) (Fig. 1).
Fig. 1.
Aβ-immunoreactive deposits (brown) in the neocortex of a 35-y-old female rhesus monkey. One of a morphologic variety of Aβ plaques is indicated by the arrow, and an Aβ-positive blood vessel (CAA) is indicated by the arrowhead. (Scale bar: 200 μm.)
The role of Aβ in AD pathogenesis is further supported by genetic mutations in rare, autosomal-dominant, familial cases of AD. These mutations in APP and the presenilin 1 and 2 components of the γ-secretase complex are associated with the overproduction and/or accumulation of Aβ as well as a more aggressive disease course, including an early age of onset compared with sporadic cases of AD (13–16). These studies implicate the Aβ peptide as a causative agent in triggering a sequence of events that ultimately leads to the core pathologies, neuronal dysfunction, synaptic loss, cerebral atrophy, and dementia (10, 17)—at least in these rare families with autosomal-dominant mutations.
NFTs are also a core lesion that, along with Aβ plaques, defines all cases of AD (4, 5, 18–20). NFTs are intracellular deposits of hyperphosphorylated, microtubule-associated protein tau. Tau typically binds tubulin to control the stability of microtubules and regulate axonal transport. In its hyperphosphorylated form, tau self-assembles to form paired helical filaments at the ultrastructural level (21, 22) and NFTs at the light microscopic level (18–20). The emergence of NFTs follows a characteristic spatiotemporal progression across brain regions known as the Braak stages (18). Cortical NFTs initially appear in layers II and III of the transentorhinal region (stage I) and progress to the hippocampal formation (stage II) (20). NFTs then deposit in basal areas of the temporal lobe (stage III) and neocortical association areas (stage IV) followed by deposition in motor and sensory areas in stages V and VI. NFTs often correlate with neuronal loss and symptom progression in AD (20), beginning with subtle memory deficits followed by multidomain cognitive impairment (6, 7, 23, 24). Neurofibrillary changes have been found in the locus coeruleus of young people, and these are thought by some to be the first pathology in the AD brain, even predating the cortical development of NFTs (20). The mechanisms by which NFTs and early locus coeruleus pathology contribute to the emergence and progression of AD are exciting areas of research (25–28).
Although our knowledge of the molecular pathogenesis and physiology of AD derives largely from understanding the hallmark neuropathologies, recent evidence has suggested that AD is a complex disorder involving both genetic (70 to 80%) and environmental factors (29–31), with our understanding of each far from complete. For example, genome-wide association studies have now identified 29 loci associated with AD risk, with the majority of the genetic variance still unexplained (32). Despite the complex nature of the disease, most experimental studies of AD pathogenesis have used mouse models with mutant forms of the APP and PSEN genes to recapitulate autosomal-dominant familial AD (33), which accounts for less than 1% of human AD cases. These aggressive mutations, when introduced into rodents, cause age-dependent accumulation of Aβ plaques. However, cognitive impairment is generally mild and/or variable, with minor neurodegeneration, and the mice do not develop human-like NFTs, all core clinicopathologic features of the human condition. Mice also have been developed that express tau that bears mutations associated with human primary tauopathies (34). Although these mice exhibit substantial abnormal tau in the brain, it does not fully recapitulate the tauopathy of AD, even when the mice are crossed with APP-transgenic mice (34, 35).
Reduced clearance rather than overproduction of Aβ seems to be the driver of Aβ accumulation in the commonly occurring late-onset form of AD (36). Moreover, clinical trials of β-secretase and γ-secretase inhibitors attempting to reduce amyloid have been ineffective or even exacerbated AD in humans (37). Hence, AD as it occurs in humans is more complex than the pathology modeled in rodents that have been genetically engineered to overproduce human-sequence Aβ. Some of these limitations may be due to the fact that these animals only model a portion of AD pathology, and they do not include frequent co-occurring disorders, such as vascular disease and other proteopathies (tauopathy, synucleinopathy, TDP-43 proteopathy, etc.). Another possibility is that more highly evolved brain systems are essential to manifest the full spectrum of AD (38).
Owing to their relatively long lives and biological propinquity to humans, NHPs are an important source of insights into brain aging and vulnerability to neurodegenerative diseases. Despite the profoundly important role of aging as the major risk factor for AD, there is very limited understanding of the underlying mechanisms. All NHPs studied to date develop Aβ pathology with advancing age, although the onset, distribution, and appearance of the lesions vary depending on the species and lifespan (39, 40) (Fig. 1). Even within species, the timing and pattern of Aβ deposition vary among individuals (34). Similar to humans, mature Aβ plaques in NHPs are often surrounded by significant gliosis and swollen neurites. Interestingly, CAA (which is highly variable in humans with AD) is common in all NHPs analyzed to date, including species ranging from prosimians to great apes (39, 40) (Fig. 1).
In contrast to Aβ pathology, NFTs and associated neuronal loss are relatively rare in the aged NHP brain (39, 40). Some studies have reported significant hyperphosphorylated tau in the brains of aged monkeys (41–44), and one recent study reported early tau pathology in the form of paired helical filaments and NFTs in layer II of the entorhinal cortex in macaques of extreme old age (41). However, the full clinicopathologic spectrum that defines AD, including multidomain cognitive impairment along with profuse Aβ plaques and NFTs, has not yet been demonstrated in a nonhuman species (38). Even so, it is possible that aged monkeys represent an early stage of AD pathogenesis and thus, could be useful for investigating the critical, but still obscure, mechanisms linking Aβ proteopathy to tauopathy, specifically in the absence of autosomal-dominant mutations. Aged macaque models have begun to identify molecular mechanisms that may explain the presence of hyperphosphorylated tau observed in AD (41). For example, as discussed by Arnsten in this issue (45), these phenomena associated with AD may arise from a feedforward cycle involving calcium and cAMP-PKA-K+ signaling that ultimately leads to neurodegeneration via calcium-induced excitotoxicity. Alterations in calcium are proposed to occur before the development of AD pathology and therefore, may provide a number of therapeutic targets to prevent excitotoxicity in AD models.
To address the long time course involved in generating such models, an NHP model of AD has been developed that relies on intraventricular injections of soluble AβOs, an Aβ species currently thought to be involved in the early stages of AD and to induce synaptic loss and dementia (10). Discussed by Beckman et al. in detail in this issue (46), soluble AβOs accumulate in the hippocampus and dorsolateral prefrontal cortex, where they trigger synaptic loss and the activation of microglia, features associated with the early stages of AD. Such biologically proximal models may help to clarify the pathogenic mechanisms underlying AD, including the key roles of aging, genetics, and the environment.
NHPs for Characterizing Clinical Symptom Progression and Identifying Novel Treatment Options for AD
Although the development of treatments for disease modification and prevention of AD are key goals, tens of millions of individuals are currently affected worldwide with very limited treatment options to improve clinical symptoms. Memory loss is a core clinical feature of AD, typically with insidious onset of a progressive anterograde long-term episodic amnesia. Early stages of AD can also be characterized by executive dysfunction, which manifests as a reduction in the ability to multitask, solve problems, or maintain attention. As the disease progresses, individuals develop other cognitive impairments, including apraxia, visuospatial impairments, and language dysfunction. In its most severe stages, individuals lose the ability to speak and move, and they rely solely on their caregivers to carry out simple tasks. Behavioral symptoms are also common in AD, including anxiety and depression, even in early stages. Other behavioral symptoms, such as apathy and social disengagement, agitation, and psychosis, are huge treatment challenges. As these symptoms progress, care becomes overwhelmingly difficult and often necessitates placement in long-term care facilities. In the terminal stages, complications, such as dehydration, malnutrition, and infection, frequently culminate in death.
In the United States, several acetylcholinesterase inhibitors (AChEIs) are approved for treatment of cognitive symptoms in mild to moderate AD. AChEIs increase acetylcholine at the synaptic cleft in order to counteract the significant loss of cholinergic neurons in the nucleus basalis of Meynert in AD (47). Based on a metaanalysis of 3,000 individuals with mild to moderate AD, AChEIs provide a modest benefit in performance on general measures of cognition (increase of 1.37 points on the Mini-Mental State Examination) (48). Whether AChEIs reduce nursing home admissions and improve the ability to perform daily activities remains controversial (49–53). The efficacy of AChEIs is also quite variable, with ∼30 to 50% of individuals showing no observable benefit (54, 55). Memantine is a second symptomatic treatment option and is approved for more advanced-stage disease. Memantine weakly inhibits N-methyl-d-aspartate receptors in order to prevent excitotoxicity from excessive glutamatergic stimulation, and it is thought to provide neuroprotective effects (56, 57). Memantine provides symptomatic relief that is similar to the AChEI donepezil (Aricept), with no improvement in long-term outcomes when the 2 are used in combination (58, 59). Of note, no treatment for AD has been approved by the Food and Drug Administration (FDA) since 2003, including Aβ immunization therapies and γ-secretase and β-secretase inhibitors, all of which have been shown to reduce Aβ but without improving cognitive status (37, 60). There also are no FDA-approved treatments for mild cognitive impairment or for behavioral symptoms other than anxiety and depression.
NHP research is well positioned to link AD pathophysiology to the clinical presentation of the disease and to inform the development of urgently needed new therapies for both the cognitive and behavioral symptoms. Symptom-based treatments are guided by understanding the relevant neural circuits and systems affected in disease. Early lesion studies in NHPs identified the medial temporal lobe (MTL), which includes the hippocampus, entorhinal cortex, perirhinal cortex, and parahippocampal cortices, as the locus of long-term episodic memory formation (61–63). These fundamental studies on the neuroanatomical foundations of memory were critical for defining the role of the MTL and early vulnerability to memory loss in AD. NHP research continues to play crucial roles in fundamental brain research, with promise to illuminate how circuit dysfunction contributes to cognitive and behavioral impairments. Neuropsychological tools with relevance for humans are also emerging from NHP research. For example, highly sensitive assessments of nonverbal memory in NHP have been developed by using eye movements to quantify the amount of time that an animal views novel parts of an image (64–69), a technology that holds promise for predicting the risk of progression to AD in humans (64). Unlike other clinical measures of memory, these paradigms allow for the rapid assessment of memory with little instruction and minimal user input while predicting memory loss in AD with high sensitivity and specificity (64, 65, 67). The lack of ceiling effects and their recent translation to mobile devices make these paradigms promising for sensitive and efficient remote tracking of memory trajectories in population studies and in the clinic.
NHP research also offers an important opportunity to link alterations in the molecular networks involved in AD to the progression of clinical symptoms. Recent advances in molecular profiling technology have enabled identification of the genetic, transcriptomic, epigenetic, proteomic, and metabolic landscape in order to develop unbiased, data-driven network models of AD. Molecular networks for synaptic injury, neuroinflammation, and other pathophysiological mechanisms have been strongly linked to cognitive trajectory and the hallmark pathologies, even in preclinical stages of disease (70, 71). Because of their close phylogenetic relatedness to humans, NHPs exhibit a greater degree of genetic, molecular, and anatomical convergence with humans than do rodents. NHPs also exhibit similarities in complex behaviors, such as sleep, memory, and executive function, which are known to be disrupted early in AD. Furthermore, they are susceptible to age-related diseases, such as atherosclerosis, diabetes, and stroke (72, 73), which are established risk factors for dementia in humans. This biological correspondence will likely enable the identification of molecular networks and behavioral trajectories of AD that are conserved across both species (74).
Unlike in humans, the evolution of age-related changes in the brain can be examined—with temporal precision—in the context of controlled changes in lifestyle, diet, exercise, and cognitive enrichment, which may reduce the risk of dementia and induce cognitive resilience (75–79). The molecular drivers of these networks can also be pharmacologically or genetically targeted in NHPs (for example, with emerging methods, such as CRISPR-Cas9 or antisense therapy) in order to slow disease progression or provide symptomatic relief. Postmortem histopathological examination and in vivo electrophysiological recordings could also be used to understand the manifestations of these molecular changes and manipulations at the circuit level. Such efforts may provide a critical link in identifying how underlying molecular alterations relate to cognitive decline, and they can serve as an important preclinical testbed for proof-of-concept studies and rapid prototyping of potential treatment options prior to conducting human clinical trials.
Conclusion
Substantial advancements have been made over the last few decades in our understanding of AD from investigations of the physiological mechanisms and pathogenesis underlying Aβ proteopathy, tauopathy, and their neurodegenerative sequelae. Despite this progress, no disease-modifying treatments exist for addressing the increasing prevalence and high social and economic costs of AD. NHPs are close to humans phylogenetically, they develop Aβ amyloidosis and incipient tauopathy as they grow old, and they are naturally susceptible to many other age-related disorders that increase the risk of dementia. NHPs can, therefore, serve as an important model for studying the molecular and behavioral alterations that occur in the aging brain, and they can serve as an intermediary between rodent and human studies for rapidly testing therapeutic candidates identified from unbiased, data-driven network models of AD. Such efforts may prove instrumental in bridging the gap between our understanding of the pathogenesis and physiological mechanisms underlying AD and their therapeutic relevance.
Data Availability.
There are no new data in this manuscript.
Acknowledgments
We thank Lary C. Walker for helpful discussions.
Footnotes
The authors declare no competing interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Using Monkey Models to Understand and Develop Treatments for Human Brain Disorders,” held January 7–8, 2019, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. NAS colloquia began in 1991 and have been published in PNAS since 1995. From February 2001 through May 2019 colloquia were supported by a generous gift from The Dame Jillian and Dr. Arthur M. Sackler Foundation for the Arts, Sciences, & Humanities, in memory of Dame Sackler’s husband, Arthur M. Sackler. The complete program and video recordings of most presentations are available on the NAS website at http://www.nasonline.org/using-monkey-models.
This article is a PNAS Direct Submission. E.A.B. is a guest editor invited by the Editorial Board.
References
- 1.McKhann G. M., et al. , The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker W. W., et al. , Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord. 16, 203–212 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association , 2019. Alzheimer’s disease facts and figures. Alzheimers Dement. 15, 321–387 (2019). [Google Scholar]
- 4.Jack C. R., Jr, et al. ; Contributors , NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serrano-Pozo A., Frosch M. P., Masliah E., Hyman B. T., Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1, a006189 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson P. T., et al. , Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 71, 362–381 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack C. R., Jr, et al. , Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hippius H., Neundörfer G., The discovery of Alzheimer’s disease. Dialogues Clin. Neurosci. 5, 101–108 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glenner G. G., Wong C. W., Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890 (1984). [DOI] [PubMed] [Google Scholar]
- 10.Selkoe D. J., Hardy J., The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biffi A., Greenberg S. M., Cerebral amyloid angiopathy: A systematic review. J. Clin. Neurol. 7, 1–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamara D. M., et al. , Cerebral amyloid angiopathy: Similarity in African-Americans and caucasians with Alzheimer’s disease. J. Alzheimers Dis. 62, 1815–1826 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherrington R., et al. , Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375, 754–760 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Scheuner D., et al. , Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 2, 864–870 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Goate A., et al. , Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349, 704–706 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Levy-Lahad E., et al. , Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269, 973–977 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Hardy J. A., Higgins G. A., Alzheimer’s disease: The amyloid cascade hypothesis. Science 256, 184–185 (1992). [DOI] [PubMed] [Google Scholar]
- 18.Braak H., Braak E., Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–278 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del Tredici K., Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braak H., Thal D. R., Ghebremedhin E., Del Tredici K., Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 70, 960–969 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Kidd M., Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature 197, 192–193 (1963). [DOI] [PubMed] [Google Scholar]
- 22.Lee V. M. Y., Balin B. J., Otvos L., Trojanowski J. Q., A68: A major subunit of paired helical filaments and derivatized forms of normal tau. Science 251, 675–678 (1991). [DOI] [PubMed] [Google Scholar]
- 23.Nelson P. T., et al. , Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 121, 571–587 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack C. R., Jr, et al. , Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA 321, 2316–2325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heneka M. T., et al. , Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. U.S.A. 107, 6058–6063 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalermpalanupap T., et al. , Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res. Ther. 5, 21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalermpalanupap T., et al. , Locus coeruleus ablation exacerbates cognitive deficits, neuropathology, and lethality in P301S tau transgenic mice. J. Neurosci. 38, 74–92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perea J. R., Llorens-Martín M., Ávila J., Bolós M., The role of microglia in the spread of Tau: Relevance for tauopathies. Front. Cell. Neurosci. 12, 172 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gatz M., et al. , Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 63, 168–174 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Wingo T. S., Lah J. J., Levey A. I., Cutler D. J., Autosomal recessive causes likely in early-onset Alzheimer disease. Arch. Neurol. 69, 59–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertram L., Lill C. M., Tanzi R. E., The genetics of Alzheimer disease: Back to the future. Neuron 68, 270–281 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Jansen W. J., et al. ; Amyloid Biomarker Study Group , Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 313, 1924–1938 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaFerla F. M., Green K. N., Animal models of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a006320 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jankowsky J. L., Zheng H., Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol. Neurodegener. 12, 89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitazawa M., Medeiros R., Laferla F. M., Transgenic mouse models of alzheimer disease: Developing a better model as a tool for therapeutic interventions. Curr. Pharm. Des. 18, 1131–1147 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mawuenyega K. G., et al. , Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330, 1774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karran E., Mercken M., De Strooper B., The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10, 698–712 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Walker L. C., Jucker M., The exceptional vulnerability of humans to Alzheimer’s disease. Trends Mol. Med. 23, 534–545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heuer E., Rosen R. F., Cintron A., Walker L. C., Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr. Pharm. Des. 18, 1159–1169 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Dam D., De Deyn P. P., Non human primate models for Alzheimer’s disease-related research and drug discovery. Expert Opin. Drug Discov. 12, 187–200 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Paspalas C. D., et al. , The aged rhesus macaque manifests Braak stage III/IV Alzheimer’s-like pathology. Alzheimers Dement. 14, 680–691 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oikawa N., Kimura N., Yanagisawa K., Alzheimer-type tau pathology in advanced aged nonhuman primate brains harboring substantial amyloid deposition. Brain Res. 1315, 137–149 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Uchihara T., et al. , Tau pathology in aged cynomolgus monkeys is progressive supranuclear palsy/corticobasal degeneration- but not Alzheimer disease-like -Ultrastructural mapping of tau by EDX. Acta Neuropathol. Commun. 4, 118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultz C., et al. , Filamentous tau pathology in nerve cells, astrocytes, and oligodendrocytes of aged baboons. J. Neuropathol. Exp. Neurol. 59, 39–52 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Arnsten A. F. T., et al. , Alzheimer’s-like pathology in aging rhesus macaques: Unique opportunity to study the etiology and treatment of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 116, 26230–26238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beckman D., et al. , Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc. Natl. Acad. Sci. U.S.A. 116, 26239–26246 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitehouse P. J., et al. , Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 215, 1237–1239 (1982). [DOI] [PubMed] [Google Scholar]
- 48.Birks J., Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, CD005593 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trinh N. H., Hoblyn J., Mohanty S., Yaffe K., Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in alzheimer disease: A meta-analysis. JAMA 289, 210–216 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Courtney C., et al. ; AD2000 Collaborative Group , Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): Randomised double-blind trial. Lancet 363, 2105–2115 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Lopez O. L., et al. , Cholinesterase inhibitor treatment alters the natural history of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 72, 310–314 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howard R., et al. , Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 366, 893–903 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Howard R., et al. , Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: Secondary and post-hoc analyses. Lancet Neurol. 14, 1171–1181 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Cummings J. L., Use of cholinesterase inhibitors in clinical practice: Evidence-based recommendations. Am. J. Geriatr. Psychiatry 11, 131–145 (2003). [PubMed] [Google Scholar]
- 55.Clark C. M., Karlawish J. H., Alzheimer disease: Current concepts and emerging diagnostic and therapeutic strategies. Ann. Intern. Med. 138, 400–410 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Lancelot E., Beal M. F., “Glutamate toxicity in chronic neurodegenerative disease”in Progress in Brain Research, Ottersen O. P., Langmoen I. A., Gjerstad L., Eds. (Elsevier, 1998), pp. 331–347. [DOI] [PubMed] [Google Scholar]
- 57.Kornhuber J., Weller M., Schoppmeyer K., Riederer P., Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J. Neural Transm. Suppl. 43, 91–104 (1994). [PubMed] [Google Scholar]
- 58.Schneider L. S., Dagerman K. S., Higgins J. P. T., McShane R., Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch. Neurol. 68, 991–998 (2011). [DOI] [PubMed] [Google Scholar]
- 59.McShane R., et al. , Memantine for dementia. Cochrane Database Syst. Rev. 3, CD003154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panza F., Lozupone M., Logroscino G., Imbimbo B. P., A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 15, 73–88 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Zola S. M., Squire L. R., Relationship between magnitude of damage to the hippocampus and impaired recognition memory in monkeys. Hippocampus 11, 92–98 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Mishkin M., Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature 273, 297–298 (1978). [DOI] [PubMed] [Google Scholar]
- 63.Squire L. R., Stark C. E. L., Clark R. E., The medial temporal lobe. Annu. Rev. Neurosci. 27, 279–306 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Crutcher M. D., et al. , Eye tracking during a visual paired comparison task as a predictor of early dementia. Am. J. Alzheimers Dis. Other Demen. 24, 258–266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lagun D., Manzanares C., Zola S. M., Buffalo E. A., Agichtein E., Detecting cognitive impairment by eye movement analysis using automatic classification algorithms. J. Neurosci. Methods 201, 196–203 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bott N. T., et al. , Web camera based eye tracking to assess visual memory on a visual paired comparison task. Front. Neurosci. 11, 370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haque R. U., et al. , VisMET: A passive, efficient, and sensitive assessment of visuospatial memory in healthy aging, mild cognitive impairment, and Alzheimer’s disease. Learn. Mem. 26, 93–100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryan J. D., Althoff R. R., Whitlow S., Cohen N. J., Amnesia is a deficit in relational memory. Psychol. Sci. 11, 454–461 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Smith C. N., Hopkins R. O., Squire L. R., Experience-dependent eye movements, awareness, and hippocampus-dependent memory. J. Neurosci. 26, 11304–11312 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seyfried N. T., et al. , A multi-network Approach identifies protein-specific Co-expression in Asymptomatic and symptomatic Alzheimer’s disease. Cell Syst. 4, 60–72.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wingo A. P., et al. , Large-scale proteomic analysis of human brain identifies proteins associated with cognitive trajectory in advanced age. Nat. Commun. 10, 1619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mattison J. A., Vaughan K. L., An overview of nonhuman primates in aging research. Exp. Gerontol. 94, 41–45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Didier E. S., et al. , Contributions of nonhuman primates to research on aging. Vet. Pathol. 53, 277–290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swarup V., et al. ; International Frontotemporal Dementia Genomics Consortium , Identification of evolutionarily conserved gene networks mediating neurodegenerative dementia. Nat. Med. 25, 152–164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brasure M., et al. , Physical activity interventions in preventing cognitive decline and alzheimer-type dementia: A systematic review. Ann. Intern. Med. 168, 30–38 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Lourida I., et al. , Association of lifestyle and genetic risk with incidence of dementia. JAMA, 10.1001/jama.2019.9879 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ngandu T., et al. , A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385, 2255–2263 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Pentikäinen H., et al. , Cardiorespiratory fitness and brain volumes in men and women in the FINGER study. Age Ageing 46, 310–313 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Kivipelto M., Mangialasche F., Ngandu T., Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 14, 653–666 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no new data in this manuscript.