Significance
Identifying new targets for deep brain stimulation in epilepsy requires a deeper understanding of the brain networks engaged by seizure activity. For over 30 y, the substantia nigra pars reticulata has been recognized as a potential target, but the efferent pathways mediating the suppression of seizures have remained obscure. Here, we show that silencing the projection from the substantia nigra to the superior colliculus fully recapitulates the antiseizure effects evoked from cell bodies within the substantia nigra. By contrast, inhibition of the projection to the pedunculopontine nucleus exacerbates some seizures, reduces others, and is without effect on still others. The functional divergence of these pathways highlights a key role for projections to the superior colliculus in the control of seizures.
Keywords: seizure, deep brain stimulation, basal ganglia, optogenetics, substantia nigra
Abstract
Three decades of studies have shown that inhibition of the substantia nigra pars reticulata (SNpr) attenuates seizures, yet the circuits mediating this effect remain obscure. SNpr projects to the deep and intermediate layers of the superior colliculus (DLSC) and the pedunculopontine nucleus (PPN), but the contributions of these projections are unknown. To address this gap, we optogenetically silenced cell bodies within SNpr, nigrotectal terminals within DLSC, and nigrotegmental terminals within PPN. Inhibition of cell bodies in SNpr suppressed generalized seizures evoked by pentylenetetrazole (PTZ), partial seizures evoked from the forebrain, absence seizures evoked by gamma-butyrolactone (GBL), and audiogenic seizures in genetically epilepsy-prone rats. Strikingly, these effects were fully recapitulated by silencing nigrotectal projections. By contrast, silencing nigrotegmental terminals reduced only absence seizures and exacerbated seizures evoked by PTZ. These data underscore the broad-spectrum anticonvulsant efficacy of this circuit, and demonstrate that specific efferent projection pathways differentially control different seizure types.
Brain circuits that exert endogenous seizure-suppressive effects have been a topic of investigation for over 30 y. One group of circuits has received particular attention in this regard: the basal ganglia. While most commonly considered in the context of posture, movement control, and movement disorders, discrete nodes of the basal ganglia can also be harnessed to control seizures in a broad-spectrum, multipotent manner. For example, inhibition of the substantia nigra pars reticulata (SNpr) exerts profound antiseizure effects in models of generalized (both convulsive and absence) and partial seizures (1–10), and a failure of GABA release in SNpr is associated with seizure susceptibility in genetically epilepsy-prone rats (GEPRs) (11).
Despite decades of study, the circuit mechanisms by which SNpr suppresses seizure activity have remained unclear. The SNpr is a GABAergic projection nucleus with several major output pathways; thus, inhibition of SNpr leads to disinhibition of multiple target structures. These output targets include the thalamus, the deep and intermediate layers of the superior colliculus (DLSC), and the pedunculopontine nucleus (PPN) (12–15). Prior studies using conventional lesion, microinjection, and electrical recording methods have suggested a role for each of these target regions in nigra-evoked seizure control (16–18). However, prenigral hemitransections which sever rostral-going projections from SNpr to the forebrain do not impair the anticonvulsant effect of SNpr inhibition. Therefore, we focused on 2 caudal projections: the nigrotectal pathway, terminating in DLSC, and the nigrotegmental pathway, terminating in PPN.
With conventional microinjection or lesion approaches, it was impossible to selectively manipulate these highly collateralized projection pathways; pharmacological methods would inhibit or disinhibit all of the output pathways of SNpr simultaneously. Optogenetic approaches to silencing have enabled this dissection. Here, we sought to address 2 unresolved questions. First, to what extent does selective silencing of the nigrotectal as compared to the nigrotegmental pathway recapitulate the effect of silencing SNpr? Second, are the seizure-suppressive effects of SNpr silencing mediated by divergent pathways depending on the seizure type?
We optogenetically silenced either SNpr, nigrotectal, or nigrotegmental projections in 4 models of experimental epilepsy in rats: the gamma-butyrolactone model of absence (thalamocortical) seizures, pentylenetetrazole (PTZ)-evoked forebrain tonic-clonic seizures, piriform cortex-evoked limbic seizures, and brainstem tonic-clonic seizures in the GEPRs. The human epilepsies are diverse and present with a variety of seizure types; these models were selected to each reflect a different type of seizure seen in the epilepsies, providing a measure of whether our manipulations are seizure type-specific or more generally effective against seizures writ large. Optogenetic inhibition of SNpr suppressed seizures in all models, and, strikingly, these effects were completely recapitulated by selective silencing of the nigrotectal projection. By contrast, inhibition of nigrotegmental projections was effective only against absence seizures and, conversely, exacerbated seizures evoked by PTZ.
Results
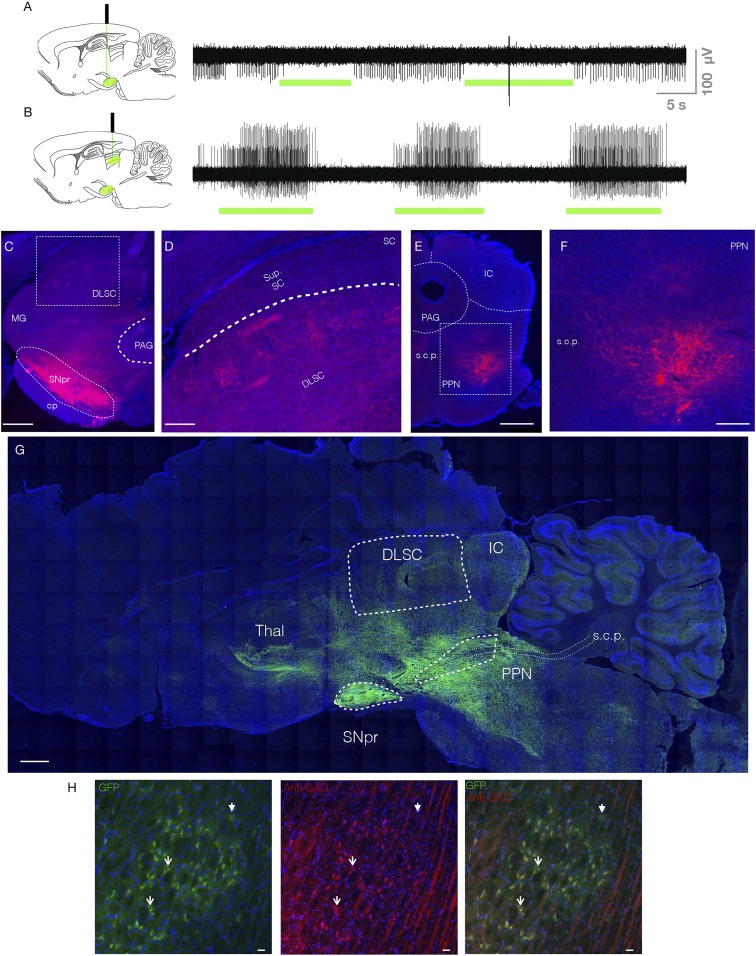
We microinjected rAAV8-CAG-ArchT-GFP coding for the inhibitory opsin, ArchT, into SNpr of wild-type Sprague–Dawley (SD) rats. To verify optogenetic silencing, we performed multiunit recordings in anesthetized rats. Neurons in SNpr displayed tonic baseline firing, which was suppressed by light delivery (Fig. 1A). Optogenetic silencing of nigrotectal terminals disinhibited neurons in DLSC, resulting in burst firing during light delivery (Fig. 1B). In both cases, moving the optrode 1.5 mm dorsal from the site of virus injection eliminated optogenetic modulation of neuronal activity. This is in keeping with the modeled decay in power with increasing distance from the tip of the fiber (19, 20), which suggests that we were inhibiting neurons and terminals within a cone of tissue extending ∼1 mm radially from the tip and ∼1 to 1.5 mm below the fiber tip. With our fiber placed 0.2 mm dorsal to SNpr at its approximate midpoint in the rostrocaudal and mediolateral planes, we were thus positioned to deliver light to approximately half of the structure. The light delivery parameters we employed for DLSC mirror those we had previously used for optogenetic activation experiments (21). While we did not record from DLSC while inhibiting cell bodies in SNpr, our data inhibiting nigrotectal terminals within DLSC are in keeping with the well-described functional architecture of nigrotectal pathways, best described in the context of saccadic eye movements (22–24), i.e., suppression of activity in SNpr disinhibits DLSC.
Fig. 1.
Electrophysiological and histological verification of opsins in the SNpr and its tectal and tegmental terminals. (A) Unit activity within SNpr. Green bars indicate optogenetic inhibition, which decreased in unit activity. (B) Unit activity within DLSC. Green bars indicate optogenetic inhibition of the nigrotectal terminals, which increased activity within DLSC. (C) Coronal section through SNpr and DLSC. Red indicates tdTomato fluorescence from the virus. Box indicates expanded view showing terminals in DLSC in D. (E) Coronal section through PPN. Box indicates expanded view showing terminals in F. (G) Parasagittal montage showing expression of GFP reporter in a GAD-Cre rat. Dense fluorescence is evident in SNpr, with fibers extending to known projections sites (DLSC, PPN, thalamus). Dotted lines outline structures of interest. (H) GFP+ cells (Left), anti-GAD immunofluorescence (Middle), and overlay (Right) showing that the majority of GFP+ cells colocalize with GAD. Solid arrowhead indicates a GAD−/GFP+ cell, open arrowheads indicate representative GAD+/GFP+ cells. PAG, periaqueductal gray; MG, medial geniculate nucleus; cp, cerebral peduncle; IC, inferior colliculus; Sup. SC, superficial layers of SC; s.c.p., superior cerebellar peduncle; PPN, pedunculopontine nucleus; Thal, thalamus. (Scale bars: C, E, and G, 1 mm; F and D, 330 μm; H, 25 μm.)
For these and all other experiments, we verified viral expression by immunofluorescence. Fiber optic placement was verified by damage associated with the fiber optic (Fig. 1 C–G). For experiments using transgenic (GAD-Cre) rats, we verified the selectivity of the Cre-driver line by immunofluorescence (Fig. 1H). We counted 58 fields through SNpr (range, 4 to 10 per rat) of 7 GAD-Cre rats injected with AAV-Cre-GFP and found an 83.5% colocalization between anti-GAD67 immunofluorescence and GFP positivity. In GAD-Cre rats, we observed minimal retrograde uptake of the virus (i.e., cell bodies were not seen in the striatum). In wild-type rats, we observed both terminals in the striatum (consistent with viral infection of adjacent substantia nigra pars compacta dopamine neurons) and some labeled somata, indicating retrograde uptake of the virus after injection into SNpr.
Inhibition of SNpr or Nigrotectal Projections Suppresses PTZ-Evoked Seizures.
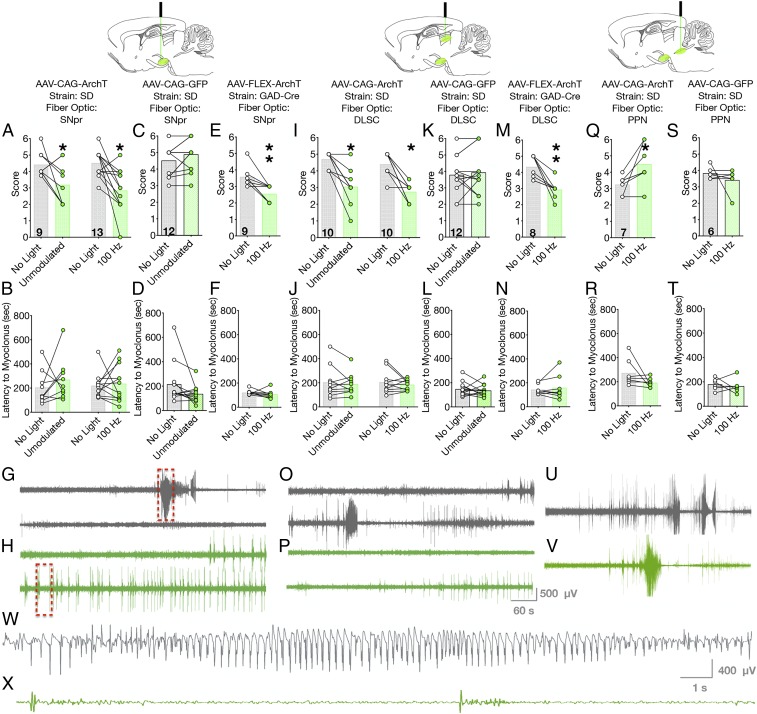
We next examined the ability of optogenetic silencing of SNpr cell bodies to attenuate seizures. We first turned to seizures evoked by systemic injection of PTZ. PTZ is a model of generalized seizures with multifocal onset and evokes both forebrain (clonic) and hindbrain (tonic) activity similar to that seen in human secondarily generalized seizures. PTZ administration, in the absence of light delivery, triggered behavioral and electrographic seizures in rats characterized by bilateral forelimb clonus followed by rearing. When the same rats were tested with unmodulated 532-nm light delivery, the median seizure score was significantly decreased, and was primarily characterized by bilateral forelimb clonus (Wilcoxon sign-rank test, P = 0.047; Fig. 2A). We next examined a reduced light delivery paradigm at 100 Hz (50% duty cycle); as with unmodulated light delivery, 100 Hz light significantly attenuated the severity of PTZ-evoked seizures (Wilcoxon sign-rank test, P = 0.0024; Fig. 2A).
Fig. 2.
Optogenetic silencing of SNpr or nigrotectal terminals reduces PTZ seizures, while optogenetic silencing of nigrotegmental terminals worsens PTZ seizures. Optogenetic silencing of cell bodies within SNpr, with either unmodulated or 100-Hz light delivery, reduces the severity of PTZ-evoked seizures (A) without impacting latency (B). In opsin-negative rats, light delivery was without effect (C and D). Optogenetic inhibition of GABAergic neurons in SNpr suppressed seizure severity (E) without affecting latency (F). Representative electrographic responses from a rat without (G) and with (H) optogenetic inhibition of SNpr. Red boxes indicate expanded view in W and X. (I) Optogenetic silencing of nigrotectal terminals reduced seizure severity, but not (J) latency to seizure. Light delivery to nigrotectal terminals was without effect in opsin-negative rats (K and L). Inhibition of GABAergic nigrotectal terminals reduced seizure severity (M) without affecting latency (N). Antiseizure effects obtained by silencing nigrotectal projections were evident both behaviorally and electrographically (O, without optogenetic silencing; P, with optogenetic silencing). Optogenetic silencing of nigrotegmental terminals increased seizure severity (Q) without effect on latency (R). In opsin-negative rats, light delivery was without effect on seizure severity (S), latency (T), or electrographic activity (U and V). (W and X) Expanded view of the segments indicated by the red boxes in G and H, respectively. Bars indicate means, with individual animal data indicated by circles and lines (*P < 0.05, **P < 0.01).
The latency to first myoclonic jerk did not vary as a function of treatment for either unmodulated light (paired t test, t = 0.8347, P = 0.4281; Fig. 2B) or 100-Hz light delivery (paired t test, t = 0.3282, P = 0.7489; Fig. 2B). In available, artifact-free electrographic recordings, the durations of fast ictal activity were 111.7 s without light delivery and 64.04 s with light delivery. Duration decreased in 4 of 4 animals, but did not reach the level of statistical significance (paired t test, t = 1.979, df = 4, P = 0.1189; SI Appendix, Fig. S1A).
To control for off-target effects of light delivery (e.g., heating artifacts), we tested rats injected with a control vector (rAAV-CAG-GFP). Light delivery in these rats had no effect on seizure severity (P = 0.1094, Wilcoxon test; Fig. 2C), latency to first myoclonic jerk (paired t test, t = 1.829, P = 0.0947; Fig. 2D), or duration of electrographic activity (paired t test, t = 0.06432, df = 4, P = 0.9512; SI Appendix, Fig. S1B), thus confirming the specificity of our manipulation (Fig. 2 A and B). The suppression in seizure activity observed in active-vector SD rats was evident both behaviorally (Fig. 2A) and electrographically (Fig. 2G vs. Fig. 2H). In the absence of light delivery, PTZ administration resulted in high-amplitude, fast ictal discharges on the cortical EEG (Fig. 2G); by contrast, in the presence of light delivery, this activity was reduced to short bursts (Fig. 2H).
From a clinical neurostimulation standpoint, reduced duty cycle offers advantages such as prolonged battery life. Moreover, reduced total integrated light delivered minimizes concerns regarding heating and tissue damage with optogenetics. To determine if a strategy that used minimal light delivery would also be effective, we injected wild-type SD rats with a step-waveform inhibitory channelrhodopsin (rAAVDJ-hSyn-SwiChR-eYFP) into SNpr. This opsin is an anion-conducting variant of channelrhodopsin with slow off-rate kinetics, allowing brief pulses of blue light to trigger sustained neuronal hyperpolarization (25). We applied 0.2 Hz light stimulation, and, as with our other experiments, detected a significant decrease in seizure severity without alterations in latency to first myoclonic jerk compared within subject to no-light-delivery sessions (SI Appendix, Fig. S2).
The substantia nigra contains both GABAergic neurons in SNpr and dopaminergic neurons in the SNpc. However, the nigrotectal and nigrotegmental pathways are comprised exclusively of GABAergic neurons; thus, terminal-restricted illumination would impact only GABAergic projections. To confirm that our effects were due to selective silencing of SNpr GABAergic neurons, and because our CAG promoter did not confer selectivity to one population or the other, we next turned to a transgenic rat strategy.
We injected GAD-Cre transgenic Long–Evans (LE) rats with rAAV8-FLEX-ArchT-tdTomato to selectively express ArchT in GABA neurons of SNpr. When challenged with PTZ in the absence of light delivery, rats displayed a median seizure score of 3.5, which was significantly reduced by the 100-Hz light delivery (P = 0.0078, Wilcoxon paired sign-rank test; Fig. 2E). The duration of electrographic fast ictal activity was significantly decreased following light delivery as compared to the no-light session (paired t test, t = 4.418, df = 6, P = 0.0045; SI Appendix, Fig. S1C). As with the other conditions, light delivery did not affect latency to first myoclonic jerk (paired t test, t = 0.9545, P = 0.3678; Fig. 2F). These results mirror our findings in the wild-type rats using the CAG promoter and confirm that selective silencing of GABAergic neurons within SNpr is sufficient to attenuate seizure activity. This is consistent with prior suggestions that SNpr, and not the adjacent SNpc dopamine neurons, are required for anticonvulsant effects (26).
We have previously shown that optogenetic activation of DLSC is potently anticonvulsant (21). Because inhibition of SNpr disinhibits DLSC, we hypothesized that DLSC would be a prime candidate for mediating the anticonvulsant effects of nigral inhibition. To test this hypothesis, we injected wild-type SD and GAD-Cre LE rats with AAV encoding ArchT in SNpr, and placed fiber optics in the nigrotectal terminal fields within DLSC. Following PTZ administration and in the absence of light delivery, wild-type rats displayed a median seizure score of 5, corresponding to bilateral forelimb clonus, rearing, and loss of balance; the severity of seizures was significantly attenuated by unmodulated light delivery (Wilcoxon test, P = 0.0039; Fig. 2I). Similarly, 100-Hz light delivery to DLSC significantly suppressed seizure activity (Wilcoxon test, P = 0.0039; Fig. 2I). Neither unmodulated nor 100-Hz light delivery affected the latency to first myoclonic jerk (paired t test, t = 0.6661, P = 0.5221; t = 0.8549, P = 0.4148, respectively; Fig. 2J). In 4 rats, we quantified the duration of fast ictal electrographic discharge. In all 4, the discharge duration was numerically decreased, and, in 3 of the 4, it was nearly abolished. However, due to the limited sample size, this analysis did not reach the level of statistical significance (paired t test, t = 1.84, df = 3, P = 0.1630; SI Appendix, Fig. S1D). Seizure-suppressive effects were absent in rats injected with a control (rAAV8-CAG-GFP) virus and subjected to light delivery into DLSC (Wilcoxon sign-rank test, P = 0.6445; Fig. 2K). There were also no effects on latency to myoclonic jerk (paired t test, t = 0.6042, df = 11, P = 0.5580; Fig. 2L) or electrographic seizure duration (paired t test, t = 0.6334, df = 5, P = 0.5543; SI Appendix, Fig. S1E). We again confirmed the selectivity of this manipulation in GAD-Cre rats. In the absence of light delivery, PTZ triggered seizures with a median score of 4.5; the severity of the seizures was significantly reduced by 100-Hz light delivery (Wilcoxon sign-rank test, P = 0.0078; Fig. 2M). The duration of fast ictal electrographic discharge was significantly decreased following light delivery in the GAD-Cre rats (paired t test, t = 7.314, df = 3, P = 0.0053; SI Appendix, Fig. S1F). Latency to first myoclonic jerk did not differ as a function of treatment (paired t test, t = 0.6481, P = 0.5376; Fig. 2N). As with silencing of cell bodies in SNpr, silencing of nigrotectal terminals was associated with a suppression of both behavioral seizures (Fig. 2 I and M) and electrographic seizures (Fig. 2 O and P). A representative electrographic recording from a wild-type rat expressing ArchT shows the baseline response to PTZ, with multiple high-amplitude, fast ictal discharges (Fig. 2O). In the same subject, light delivery to nigrotectal terminals, present throughout the observation period, reduced electrographic discharges evoked by PTZ (Fig. 2P).
The other major caudal GABAergic projection from SNpr terminates in the pedunculopontine nucleus (PPN). Prior studies have reported that pharmacological activation of PPN can attenuate seizures (16, 18, 27). As with DLSC, silencing SNpr is expected to disinhibit PPN. Thus, to determine if the projection from SNpr to PPN was sufficient to prevent PTZ-evoked seizures, we injected wild-type (SD) rats with rAAV8-CAG-ArchT-GFP into SNpr and placed a fiber optic in the nigrotegmental terminal fields in PPN. Based on prior studies using unmodulated and 100-Hz light delivery, we focused on the use of 100-Hz light delivery for this experiment. PTZ, in the absence of light delivery, produced clonus equivalent to that which we observed in the other PTZ-treated groups, with a median score of 3.5. When the same rats were tested with 100-Hz delivery of 532-nm light, seizure severity score was significantly increased (Wilcoxon paired sign-rank test, P = 0.0312; Fig. 2Q), without a change in latency to the first myoclonic jerk (paired t test, t = 1.704, P = 0.1392; Fig. 2R). The duration of electrographic ictal activity did not differ between light and no-light delivery conditions. Of the 5 animals, 2 showed an increase in discharge duration, 2 were unchanged, and 1 decreased (paired t test, t = 0.2850, P = 0.7898; SI Appendix, Fig. S1C). Light delivery was without effect in animals injected with control virus (rAAV8-CAG-GFP) and tested with 100 Hz light (Wilcoxon paired sign-rank, P = 0.75; Fig. 2S). Light delivery was without effect on latency to seizure onset (paired t test, t = 0.4758, P = 0.6543; Fig. 2T) or electrographic seizure duration (paired t test, t = 0.02516, df = 6, P = 0.9807; SI Appendix, Fig. S1H) in control animals. Thus, inhibition of the nigrotegmental projections from SNpr to PPN exacerbated seizures evoked by PTZ, a profile markedly different from that observed with silencing of either cell bodies within SNpr or nigrotectal terminals in DLSC.
We interpret, with caution, the duration of electrographic activity after PTZ administration for the following reasons: 1) PTZ produces multiple forms of electrographic discharge, ranging from spike-and-wave to fast ictal responses; 2) a shorter duration of electrographic activity, when coupled with a severe behavioral seizure (tonic seizure) may be explained by postictal depression; and 3) conversely, brief electrographic seizures associated with less severe behavioral seizure (myoclonic jerk, clonic seizure) may sum over a session to produce larger electrographic burden as compared to more severe behavioral seizures. However, in sum, the behavioral and electrographic data produce a consistent profile in the PTZ model: inhibition of SNpr or its projections to DLSC, but not to PPN, suppresses seizure activity.
Inhibition of SNpr or Nigrotectal Projections Suppresses Area Tempestas-Evoked Seizures.
We next sought to determine if the same profile of anticonvulsant effects would be evident in a model of temporal lobe seizures evoked from area tempestas (AT), an ictogenic trigger zone located in the anterior piriform cortex (28, 29). Picomole administration of GABA-A receptor antagonist (bicuculline methiodide) into AT triggers recurrent clonic seizures over ∼1 h. Therefore, we examined both the severity and the number of seizures. As with PTZ, rats were tested within-subject in a counterbalanced manner in the absence of light delivery or with either unmodulated or 100-Hz delivery of 532-nm light.
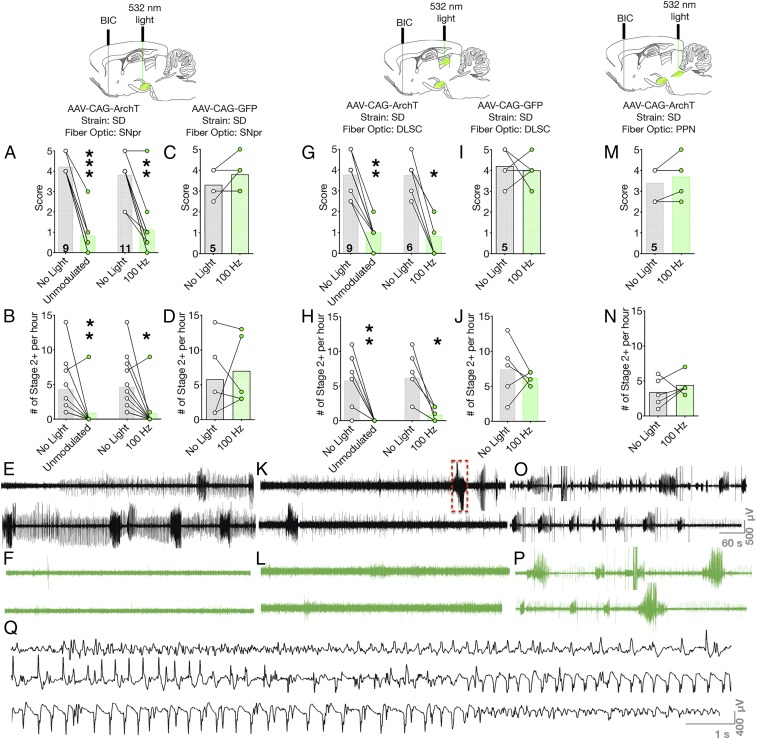
We placed a cannula in AT, injected rAAV8-CAG-ArchT-GFP in SNpr, and implanted fiber optics above SNpr in wild-type rats. In the absence of light delivery, infusion of bicuculline into AT triggered multiple seizures over the course of a 1-h observation period. The median severity of these seizures was a score of 4, corresponding to bilateral forelimb clonus and rearing. Unmodulated light delivery significantly reduced behavioral seizure severity to a median seizure score of 0.5, which correlates to facial/jaw clonus (Wilcoxon sign-rank test, P = 0.0010; Fig. 3A). Similarly, 100-Hz light delivery significantly reduced the behavioral seizure severity from a median score of 4 to a median score of 1 (corresponding to myoclonic jerks; Wilcoxon sign-rank test, P = 0.002; Fig. 3A). Concurrent with a reduction in the severity of seizures, we found that optogenetic silencing with either unmodulated light (Wilcoxon sign-rank, P = 0.0039) or 100-Hz light delivery (Wilcoxon sign-rank, P = 0.0211) significantly reduced the number of seizure episodes observed during the observation period (Fig. 3B). The anticonvulsant effect of this manipulation was evident both behaviorally and electrographically. Electrographic seizure activity was abolished in all animals analyzed (SI Appendix, Fig. S3A). Under baseline conditions, multiple high-amplitude, fast ictal events were observed on the cortical EEG (Fig. 3E); in the presence of light delivery, this was completely abolished, with only isolated interictal spikes observed (Fig. 3F).
Fig. 3.
Optogenetic silencing of SNpr or nigrotectal terminals, but not nigrotegmental terminals, suppresses area tempestas-evoked seizures. (A) Optogenetic inhibition of cell bodies within SNpr reduced the severity of seizures evoked by bicuculline injection. (B) The number of seizures were also reduced by optogenetic inhibition of SNpr. (C and D) These effects were not seen in opsin-negative rats. (E and F) Electrographic trace without and with light delivery to SNpr. (G) Optogenetic inhibition of nigrotectal terminals reduced behavioral seizure severity and (H) numbers of seizures. In opsin-negative rats, light delivery was without effect on (I) seizure severity or (J) latency. (K and L) Optogenetic inhibition of SNpr attenuates electrographic seizures (K, no light delivery vs. L, optogenetic inhibition); red box indicates expanded view in Q. (M and N) Optogenetic inhibition of nigrotegmental projections does not protect against AT-evoked behavioral or (O and P) electrographic (O, no light vs. P, optogenetic inhibition) seizures. (Q) Expanded view of the discharge indicated in K showing high-amplitude repetitive spike and poly-spike and wave discharge. Bars indicate means, with individual animal data indicated by circles and lines (*P < 0.05, **P < 0.01, and ***P < 0.001). BIC = microinfusion of bicuculline methiodide.
These seizure-suppressive effects of light delivery were not seen in animals injected with control virus (rAAV8-CAG-GFP) and tested with 100-Hz light delivery (Wilcoxon paired sign-rank test, P = 0.50; Fig. 3 C and D). Light delivery also had no effect on the number of episodes observed during the observation period (Wilcoxon paired sign-rank test, P > 0.99; Fig. 3D). In control animals, the total electrographic ictal duration did not differ with light as compared to no light delivery (SI Appendix, Fig. S3B).
To determine if nigrotectal projections also restrained seizures evoked from the piriform cortex, we injected rAAV8-CAG-ArchT-GFP in SNpr and implanted fiber optics in DLSC of wild-type SD rats. In the absence of light delivery, bicuculline injection into AT evoked seizures characterized by bilateral forelimb clonus and rearing (median score, 4). Delivery of either unmodulated or 100-Hz modulated 532-nm light significantly reduced seizure severity (Wilcoxon sign-rank test, P = 0.0039 and P = 0.0312, respectively; Fig. 3G) and electrographic seizure activity (Fig. 3 K and L). Consistent with the anticonvulsant effect, the number of behavioral seizures observed during the 1-h observation window was reduced by both light delivery paradigms (P = 0.0078 and P = 0.0312 for unmodulated and 100-Hz light delivery, respectively; Fig. 3H). In the absence of light delivery, there was an average of 120 s of fast ictal electrographic activity. Light delivery abolished fast ictal activity in all animals analyzed, but did not reach the level of statistical significance (paired t test, t = 2.429, P = 0.0934; SI Appendix, Fig. S3C). This effect mirrored that observed with silencing cell bodies within SNpr, suggesting that silencing the projections from SNpr to DLSC is sufficient to account for the antiseizure effects of SNpr inhibition in this model. In animals injected with control virus (rAAV8-CAG-GFP), neither seizure severity (Wilcoxon paired sign-rank test, P > 0.99; Fig. 3I), number of seizures (Wilcoxon paired sign-rank test, P = 0.50; Fig. 3J), nor electrographic seizure duration differed (paired t test, t = 1.673, df = 4, P = 0.1696; SI Appendix, Fig. S3D) as a function of light delivery (100 Hz).
We next injected rAAV8-CAG-ArchT-GFP in SNpr, and placed fiber optics in PPN of wild-type SD rats. In the absence of light delivery, rats displayed seizures characterized by forelimb clonus and rearing. Unlike the anticonvulsant effect we observed following selective silencing of cell bodies in SNpr or nigrotectal projections, inhibition of nigrotegmental projections did not affect behavioral seizure severity (Wilcoxon paired sign-rank test, P = 0.50; Fig. 3M), number of seizure episodes (Wilcoxon paired sign-rank test, P = 0.6875; Fig. 3N), or the pattern of electrographic activity (Fig. 3 O and P). Similarly, the duration of ictal activity also did not differ between light and no-light delivery conditions (paired t test, t = 0.8053, P = 0.4658; SI Appendix, Fig. S3E).
Inhibition of SNpr or Nigrotectal Projections Suppresses Seizures in Genetically Epilepsy-Prone Rats.
The GEPR-3s are a well-established model of genetic reflex epilepsy (30, 31). Seizures in these rats are triggered by acoustic stimulation and consist of wild running developing into bouncing generalized tonic-clonic seizures (clonus) and tonic extension of both forelimbs and hindlimbs (tonus). The networks mediating these audiogenic seizures (AGSs) are localized to the brainstem and reflect the networks mediating tonic components of generalized tonic-clonic seizures.
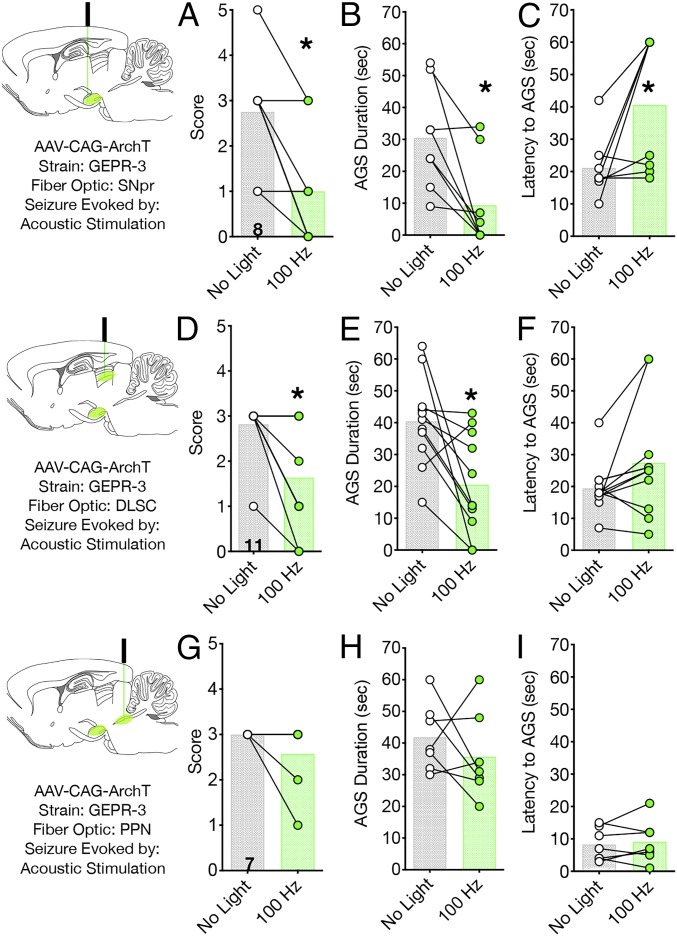
We injected GEPR-3s with rAAV8-CAG-ArchT-GFP in SNpr and implanted fiber optics in either SNpr, DLSC, or PPN. In the absence of light delivery, GEPR-3s displayed wild running seizures and clonus. Inhibition of cell bodies within SNpr with 100-Hz light delivery significantly reduced the severity of AGSs (Wilcoxon test, P = 0.0312; Fig. 4A), as well as the duration of AGSs (Wilcoxon sign-rank test, P = 0.0156; Fig. 4B). Moreover, the latency to seizure onset was significantly increased by this treatment (Wilcoxon sign-rank test, P = 0.0469; Fig. 4C). We detected a similar profile of anticonvulsant action when we stimulated the nigrotectal terminals in DLSC: seizure score (Wilcoxon sign-rank test, P = 0.01256; Fig. 4D) and seizure duration (Wilcoxon test, P = 0.0068; Fig. 4E) were significantly decreased. Latency to seizure onset, while numerically higher with light delivery, did not reach the level of statistical significance (Wilcoxon sign-rank, P = 0.0859; Fig. 4F). Thus, as with the other models, silencing nigrotectal projections protected against seizures in a manner similar to that observed with silencing cell bodies within SNpr. In contrast to the seizure-suppressive effect of nigrotectal inhibition, optogenetic silencing of the nigrotegmental pathway was without effect on AGSs in the GEPR-3s. Light delivery did not modulate AGS severity (Wilcoxon paired sign-rank test, P = 0.50; Fig. 4G), duration (Wilcoxon test, P = 0.50; Fig. 4H), or latency (Wilcoxon sign-rank, P = 0.7344; Fig. 4I). Due to the limited availability of this strain, we were unable to evaluate the effects of light delivery in opsin-negative animals, but, given the consistent lack of effect in opsin-negative animals in the other models (as described here earlier and later), we are confident in the specificity of our manipulation.
Fig. 4.
Optogenetic silencing of SNpr or nigrotectal terminals, but not nigrotegmental terminals, suppresses seizures in GEPR-3 rats. Optogenetic inhibition of cell bodies within SNpr reduces AGS (A) severity, (B) duration, and (C) latency to AGS onset. Similarly, optogenetic inhibition of nigrotectal terminals reduces AGS (D) severity, (E) duration, and (F) latency to AGS onset. Optogenetic inhibition of nigrotegmental projections was without effect on AGS (G) severity, (H) duration, or (I) latency. Bars indicate means, with individual animal data indicated by circles and lines (*P < 0.05).
Inhibition of SNpr, Nigrotectal, or Nigrotegmental Projections Suppresses Absence-Like Seizures.
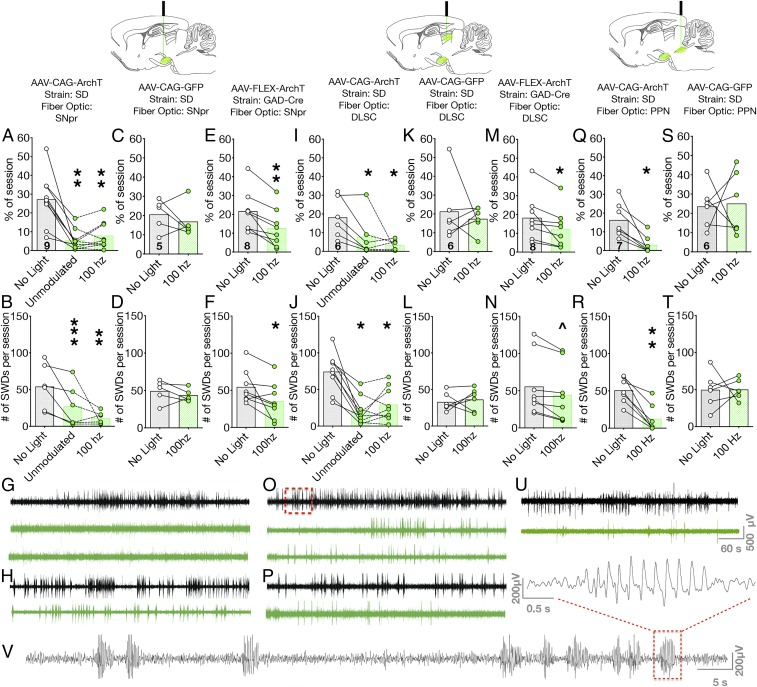
Having found that inhibition of SNpr was effective against seizures evoked in both limbic forebrain networks and hindbrain networks, we next sought to determine the effect of SNpr and its targets against seizures evoked in a thalamocortical network. For this purpose, we selected the gamma-butyrolactone (GBL) model of absence seizures. GBL is a prodrug for gamma-hydroxybutyrate and produces reliable discharges in mice, rats, monkeys, and humans (32–34). Seizures in this model rely on the canonical corticothalamocortical circuit that underlies absence seizures in humans. As in human absence epilepsy, the primary feature of GBL-induced seizures is repetitive spike-and-wave discharges (SWDs).
We injected rAAV8-CAG-ArchT-GFP into SNpr of wild-type SD rats or AAV8-FLEX-ArchT-tdTomato or AAV8-FLEX-ArchT-GFP into SNpr of GAD-Cre transgenic rats. We placed fibers in SNpr, the nigrotectal terminal fields within DLSC, or the nigrotegmental terminal fields within PPN.
After injection of GBL, SWDs emerge within minutes; these discharges display a high-amplitude, crescendo-decrescendo pattern (Fig. 5V). In the absence of light delivery, SD rats expressing ArchT in SNpr displayed SWDs for an average of 27.2% of the 20-min observation period. Both unmodulated and 100-Hz light delivery to cell bodies in SNpr significantly reduced the seizure burden (ANOVA, F2.205,9.639 = 18.84, P = 0.0012; Fig. 5A). With unmodulated light delivery, rats displayed SWDs for an average of 5.6% of the session (P = 0.0027; Holm–Sidak corrected). When tested with 100-Hz light delivery, rats displayed SWDs for an average of 8.0% of the session (P = 0.0032, Holm–Sidak corrected).
Fig. 5.
Optogenetic silencing of SNpr, nigrotectal, or nigrotegmental terminals attenuates spike-and-wave discharge evoked by GBL. Optogenetic silencing of cell bodies in SNpr significantly reduces (A) the percentage of the session showing SWD and (B) the number of SWDs. In opsin-negative rats, light delivery to SNpr was without effect on (C) percentage of session displaying SWD or (D) number of SWDs. (E) Selective optogenetic silencing of GABAergic neurons in SNpr reduces the percentage of session showing SWD and (F) number of SWDs. (G) Representative EEG from a rat injected with AAV-CAG-ArchT-GFP in SNpr. Top (black) trace shows GBL-evoked seizures with no light delivery, middle trace shows unmodulated light delivery, and bottom trace shows 100-Hz light delivery with GBL. Optogenetic manipulations suppressed high-amplitude discharges. (H) Representative EEG from a GAD-Cre rat injected with AAV-FLEX-ArchT-tdTomato. Top (black) trace shows GBL-evoked seizures with no light delivery; bottom trace shows a reduction in high-amplitude discharges when the rat received 100-Hz light delivery. Optogenetic silencing of nigrotectal terminals suppresses (I) percentage of session displaying SWD and (J) number of SWDs. In opsin-negative rats, light delivery to DLSC was without effect on (K) percentage of session displaying SWD or (L) number of SWDs per session. Selective optogenetic silencing of GABAergic terminals reduced (M) percentage of session displaying SWD and (N) number of SWDs. (O) Representative EEG from a rat injected with AAV-CAG-ArchT-GFP in SNpr with light delivered to DLSC. Top (black) trace, GBL-evoked seizures with no light delivery; middle trace, unmodulated light delivery with GBL; and bottom trace, 100-Hz light delivery. Red box indicates the section expanded in V. (P) Representative EEG from a GAD-Cre rat injected with AAV-FLEX-ArchT-tdTomato. Top (black) trace, GBL-evoked seizures with no light delivery; bottom trace, 100-Hz light delivery. Optogenetic silencing of nigrotegmental terminals reduced (Q) percentage of session displaying SWD and (R) number of SWDs. In opsin-negative rats, light delivery to PPN was without effect on (S) percentage of session displaying SWD or (T) number of SWDs. (U) Electrographic suppression of SWDs by inhibition of nigrotegmental terminals. (V) Expanded view from O showing repeated spike-and-wave discharge with the typical crescendo-decrescendo pattern observed for absence-like seizures in rodents. Red box indicates a single discharge shown with an expanded timescale above V. For A–T, bars indicate means, with individual animal data indicated by circles and lines (^P = 0.0625, *P < 0.05, **P < 0.01, and ***P < 0.001).
Paralleling the reduction in total duration of SWDs, we also observed a reduction in the number of SWDs (Friedman test, X2 = 14, P = 0.0002; Fig. 5B). In the absence of light stimulation, rats displayed an average of 74.3 SWDs per session; the number of SWDs per session was reduced by either unmodulated (20.4 SWDs; P = 0.0008, Dunn’s test) or 100-Hz light delivery (29.4 SWDs; Holm–Sidak corrected P = 0.0094, Dunn’s test). In animals injected with control virus (rAAV8-CAG-GFP), there was no difference in total duration of SWDs (paired t test, t = 0.8032, P = 0.4669; Fig. 5C) or the number of SWDs per session (Wilcoxon paired sign-rank test, P = 0.3125; Fig. 5D) as a function of light delivery.
As with the PTZ model, we sought to determine if a step-function opsin approach would be effective against GBL-evoked seizures (SI Appendix, Fig. S4). We found a significant decrease in the percentage of the session displaying SWDs (paired t test, t = 2.8, df = 8, P = 0.0231) and the total number of SWDs (Wilcoxon sign-rank test, P = 0.0039).
To determine if the same effects would be seen when selectively targeting GABA neurons, we injected AAV8-FLEX-ArchT-tdTomato into SNpr of GAD-Cre rats. In the absence of light delivery, rats displayed SWDs for 21.7% of the session (Fig. 5E). This was significantly reduced by 100-Hz light delivery (11.1% of the session; paired t test, t = 4.692, P = 0.0022; Fig. 5E). Under baseline conditions, rats had an average of 54.6 SWDs, which was reduced to 31.0 SWDs following 100-Hz light delivery (Wilcoxon paired sign-rank test, P = 0.0234; Fig. 5F). Representative EEG traces from a Sprague–Dawley rat are shown in Fig. 5G, and representative traces from a GAD-Cre rat are shown in Fig. 5H. In both cases, the number of SWDs was reduced. Interestingly, the effect of stimulation, while significant and present in every animal tested, was less robust in the GAD-Cre rats. This may be due to the genetic background of this strain; the GAD-Cre transgenic line was developed on a Long–Evans background, and Long–Evans rats display a heightened propensity for GBL-evoked SWDs as well as spontaneous SWDs (32, 35).
We next examined the ability of selective silencing of nigrotectal projections to reduce SWDs. In the absence of light delivery, GBL treatment resulted in an average of 18.1% of the session spent displaying SWDs. Light delivery significantly reduced the percentage of the session displaying SWDs (ANOVA, F1.66,8.3 = 6.185, P = 0.0261). This effect was present for both unmodulated light delivery to DLSC (7.8% of the session; P = 0.0399, Holm–Sidak; Fig. 5I) as well as 100-Hz light delivery to DLSC (3.2%; P = 0.0399, Holm–Sidak corrected; Fig. 5I). Consistent with the reduction in the percentage of the session displaying SWDs, we also detected a significant difference in the number of SWDs following inhibition of the nigrotectal projections (Friedman test, X2 = 9.478, P = 0.0054; Fig. 5J). This reached the level of statistical significance for both unmodulated (P = 0.0283, Dunn’s test) and 100-Hz light delivery (P = 0.0122, Dunn’s test). In animals injected with control virus (rAAV8-CAG-GFP) and tested with 100-Hz light delivery, there was no difference in percent duration of SWDs (paired t test, t = 0.5092, P = 0.6323; Fig. 5K) or the number of SWDs per session (Wilcoxon paired sign-rank test, P = 0.6875; Fig. 5L).
We again verified our findings in GAD-Cre transgenic rats. In the absence of light delivery, rats displayed an average of 18.3% of the session with SWD, which was significantly reduced to 12.4% during 100-Hz light stimulation trials (paired t test, t = 3.112, P = 0.0170; Fig. 5M). This was associated with a nonsignificant trend toward a decrease in the number of SWDs (55.6 vs. 40.8; Wilcoxon test, P = 0.0625, Fig. 5N); the number of discharges was numerically decreased in 7 of 8 of the rats tested. Representative electrographic traces from a wild-type (SD rat) and a GAD-Cre transgenic animal are shown in Fig. 5 O and P, respectively.
Finally, we evaluated the impact of selective silencing of nigrotegmental projections on GBL-evoked SWDs. Whereas silencing of nigrotegmental projections exacerbated seizures in the PTZ model, it had no effect on seizures in the AT and GEPR models, and significantly attenuated GBL-evoked SWDs. This was evident in both the percentage of time within the session the rats displayed SWDs between conditions (paired t test, t = 4.1, df = 6, P = 0.0063; 16.3% vs. 3.1% for no light and 100-Hz light delivery, respectively; Fig. 5Q) and the number of SWDs (Wilcoxon test, P = 0.0156; Fig. 5R). These effects were not seen in animals injected with control virus (rAAV8-CAG-GFP) tested with 100-Hz light delivery. In these animals, neither the percent duration of SWDs (paired t test, t = 0.1510, P = 0.8859; Fig. 5S) nor the number of SWDs per session (Wilcoxon paired sign-rank test, P = 0.6875; Fig. 5T) differed as a function of light delivery. Representative electrographic traces from a Sprague–Dawley rat with nigrotegmental targeting are shown in Fig. 5U.
Discussion
Here we report that optogenetic inhibition of SNpr exerts potent control over seizure activity in 4 animal models of seizures, including 1) the PTZ model of secondarily generalized convulsive seizures, 2) the AT model of complex partial seizures evoked in the forebrain, 3) brainstem generalized tonic-clonic seizures in the GEPR-3 (a model of inherited epilepsy), and 4) the GBL model of absence (thalamocortical) seizures. The efficacy of this manipulation across diverse seizure types underscores the power of this approach; nigral inhibition exerts broad-spectrum antiseizure action. This finding may have a significant translational implication for individuals with unknown or multifocal sites of seizure onset or multiple seizure types; inhibiting SNpr may be a particularly attractive approach for seizure suppression.
While the efficacy of inhibition of SNpr against seizures has been known since the 1980s, the circuit-level mechanisms have remained poorly defined. Here we show, strikingly, that the full spectrum of antiseizure effects achieved by silencing cell bodies in SNpr can be recapitulated by selective inhibition of projections from SNpr to the deep and intermediate layers of the superior colliculus (DLSC). By contrast, selective inhibition of projections from SNpr to the pedunculopontine nucleus (PPN) produced mixed results: absence seizures were reduced by this treatment, PTZ-evoked seizures were exacerbated by this treatment, and seizures evoked from AT or AGSs in GEPR-3 rats were unaffected by this treatment.
Our present findings on the effects of inhibition of SNpr using optogenetic manipulations reinforce the findings of many prior studies that used focal pharmacological manipulations across a wide number of seizure models (1, 5, 7, 8, 10, 17, 18, 36, 37). Our findings differ from a prior report in the Wistar Audiogenic Rat (WAR) model, in which pharmacological inhibition of SNpr failed to suppress AGS (38), but are consistent with the effects of pharmacological inhibition of SNpr in GEPR-9 rats (39). Moreover, by selectively targeting GABA neurons within SNpr, we avoided issues associated with drug diffusion and potential impacts on the adjacent SNpc that limited the interpretation of some of the prior microinjection experiments. The major gap that we sought to address, however, was the role of nigral output pathways. The SNpr has several major targets, including thalamus, PPN, and DLSC. Of these targets, DLSC has been examined most thoroughly for its effects on seizure control.
Transient disinhibition of DLSC induced by physiological pausing of nigral firing is vital for the control of immediate reactions to unexpected stimuli, including control of gaze and eye movements; this disinhibition can trigger cortical and autonomic arousal (40) in concert with orienting to appetitive stimuli and escape from aversive stimuli (41, 42). Neurons of SNpr are GABAergic; therefore, SNpr, which displays high basal firing rates, tonically inhibits DLSC (23). Inhibition of SNpr thus has the net effect of activating DLSC.
Because SNpr inhibition disinhibits DLSC, one might expect that activation of DLSC would exert anticonvulsant effects. This is precisely the pattern that has been previously been found by several groups (43–46). Pharmacological stimulation of DLSC (either by infusion of GABA-A receptor antagonists such as bicuculline methiodide or picrotoxin) or by direct infusion of glutamate has been shown to yield anticonvulsant effects against maximal electroshock seizures (MES), seizures evoked from AT, and thalamocortical (absence-like) seizures (17, 18, 43, 45, 47, 48). Moreover, we have shown that optogenetic activation of DLSC reduces seizures in the PTZ, AT, GBL, and GEPR-3 models (21). In addition to these studies, which suggest that activation of DLSC is sufficient to suppress seizures, it has been shown that lesions to DLSC abolish the antiseizure effect of nigral inhibition in the MES seizure model (17) and in an absence seizure model (2), suggesting that DLSC is necessary for nigral-mediated seizure control. Thus, our findings are consistent with prior findings but, notably, show that activation of this pathway was sufficient to fully recapitulate the effects evoked from SNpr.
The other major output target of SNpr is PPN, a heterogenous structure located in the ventrolateral pons, comprised of glutamatergic, GABAergic, and a high density of cholinergic neurons (49). The PPN receives input both from SNpr and from DLSC, and has extensive ascending projections back to the basal ganglia, to the thalamus, and to forebrain cholinergic cell groups in nucleus basalis (50–53). The PPN also sends projections to other brainstem targets within the pontine reticular formation including the nucleus reticularis pontis oralis (54), which has been associated with the control of tonic seizures (55). Stimulation of PPN triggers cortical desynchronization (56–59). Moreover, activity in PPN is suppressed during seizures, and this suppression has been hypothesized to contribute to the loss of consciousness seen in temporal lobe seizures (60, 61).
Pharmacological activation of PPN yields anticonvulsant effects against MES-induced seizures (62), against thalamocortical absence-like seizures (63), and against PTZ-evoked seizures (64). Inhibition of PPN, by contrast, produces mixed results, with one study reporting a worsening of PTZ-evoked seizures (64) and another reporting a suppression of seizures evoked by systemic administration of pilocarpine (27). Moreover, Garant and Gale found that pharmacological inhibition of PPN failed to disrupt the anticonvulsant effects of SNpr inhibition against MES-induced seizures (17). One possible explanation for the divergent findings across these prior studies was methodological variability (i.e., precise coordinates, volumes, and concentrations of drugs). Another explanation is that PPN is differentially involved in the regulation of different seizure types. Consistent with the latter hypothesis, we found that, while inhibition of nigrotegmental terminals suppressed absence seizures, it also exacerbated PTZ-evoked seizures, and was without effect against seizures triggered from AT and AGSs in GEPR-3s. Given that we used identical methodology to target inputs to PPN across these models, we favor the hypothesis that PPN may be a viable target for some but not all seizure types. It is thus clear that this structure cannot and does not account for the seizure-suppressive effects of nigral inhibition. These data also suggest that, given the heterogeneity of cell types within PPN and the diversity of synaptic inputs to PPN, a careful dissection of this region in the context of seizure control would be merited.
While there have been several reports regarding the topography of nigral-mediated anticonvulsant effects in rodents, we targeted a region that has proved consistently effective across studies (i.e., the anterior SNpr, at a level in which the region sometimes described as the “pars lateralis” is present) (18, 65). Moreover, this region robustly projects to the deep and intermediate layers of the SC (18). Within the rat SNpr, there is a high degree of collateralization of nigral output pathways (i.e., single neurons project to DLSC and to PPN); by contrast, there is a much greater degree of segregation of outputs in the primate SNpr (12, 66). The degree to which regional topography might hold true in the primate brain is uncertain, as, in primates, cells that project to each of the major nigral target regions are dispersed throughout SNpr. Deep brain stimulation in SNpr and the adjacent STN has shown clinical promise for the treatment of epilepsy (67); however, the feasibility of silencing the entire SNpr in a human (either using electrical or optogenetic DBS) is questionable. For these reasons, projection-specific targeting of terminal fields may represent a more refined and translatable approach for neurostimulation.
The SNpr, DLSC, and PPN are not part of canonical seizure initiation pathways (but see refs. 68 and 69). Rather, SNpr and its targets potential remote “choke points” for seizure activity. While we have demonstrated a clear seizure type-specific architecture, the mechanisms by which focal manipulations in these regions translate to antiseizure effects remain partially obscure. Activation of DLSC or PPN both potently trigger cortical desynchronization (70, 71), a state that is not conducive to the synchronous activity seen during seizures. Collicular efferent targets (72, 73), project rostrally (e.g., to hypothalamus and thalamus), caudally (to brainstem motor nuclei), and ventrally (to the reticular formation, including PPN). Similarly, PPN has both ascending and descending projections (74). A final layer of complexity is that DLSC and PPN are reciprocally connected. The target regions of both collicular and PPN projections are well-suited, through both monosynaptic and polysynaptic relays, to alter activity in key ictogenic areas. Further dissection of these pathways is expected to elucidate further the mechanisms mediating seizure control via the SN-DLSC-PPN network.
In sum, our data provide clear evidence for the suggestion made by Depaulis et al. (75) that “different outputs are involved according to the type of seizures under consideration.” Put another way, not all output pathways from SNpr are equal with respect to seizure control. We aimed to clarify a long-standing question in the field: what role do these individual output pathways play in the control of seizures? The divergent effects of optogenetic silencing of nigrotectal, as compared to nigrotegmental, terminals highlight a dominant role for the projection from SNpr to DLSC in the control of a wide range of seizure types.
Methods
Experimental Model and Subject Details.
Animals and surgery.
Adult male rats were used for the present experiments. Details regarding the strains, husbandry, and surgery are provided in SI Appendix, SI Methods.
Electroencephalography.
EEG recordings were performed in awake, unrestrained rats as we have previously described (32). Additional details are provided in SI Appendix, SI Methods. Animal procedures were conducted under a protocol approved by the Georgetown University Animal Care and Use Committee.
Multiunit recording.
Information about multiunit recording is provided in SI Appendix, SI Methods.
Optogenetic stimulation.
Optogenetic stimulation was performed in a within-subject manner; details of stimulation are provided in SI Appendix, SI Methods.
Pentylenetetrazole (PTZ) seizures.
For PTZ seizure experiments, 59 SD rats and 15 GAD-Cre LE rats were used, of which 3 rats were excluded for poor virus expression and 2 were excluded for not reaching behavioral criteria. All rats received baseline seizure testing as well as at least one experimental test session unmodulated or with 100-Hz stimulation. The seizure rating scale for PTZ is described in SI Appendix, SI Methods.
Electrographic fast ictal discharge was scored by a treatment-blind, experienced observer (P.A.F.). Activity was considered fast ictal when it was of large amplitude (>5× the baseline signal) with an abrupt onset of repetitive spike, polyspike, or spike-and-wave discharge. Only bursts lasting at least 5 s were included in this analysis.
Piriform cortex (area tempestas) seizures.
Thirty-four SD rats were used for piriform cortex seizure experiments. Bicuculline methiodide was infused into AT to elicit a seizure as described in SI Appendix, SI Methods. Seven SD rats were used for EEG confirmation of seizure activity. Electrographic seizure burden was scored as described for the PTZ model.
Audiogenic seizure (AGS) testing.
Twenty-one GEPR-3s were used for AGS testing. Four weeks after surgery, GEPR-3s were tested for AGSs (SI Appendix, SI Methods includes testing details and seizure rating scale).
Gamma-butyrolactone (GBL) seizures.
Details of GBL seizures are provided in SI Appendix, SI Methods. Forty-four SD rats were used for these experiments, of which 5 rats were excluded for poor GBL response and 3 rats were excluded for poor EEG quality. Twelve GAD-Cre LE rats were also used in this experiment, of which 2 rats were excluded for poor viral expression.
Histology.
Information about histology is provided in SI Appendix, SI Methods.
Statistics and data analysis.
Information about statistics and data analysis is provided in SI Appendix, SI Methods.
Data Availability.
Data are available upon request to the corresponding author.
Supplementary Material
Acknowledgments
Thank you to Drs. Megan Huizenga and Dr. Victor Santos for technical assistance; and Drs. Norberto Garcia-Cairasco and Ludise Malkova for feedback on a draft of this manuscript. Research was supported by grants to P.A.F.: R01NS097762 and pilot grants from the Georgetown–Howard University Clinical and Translational Science Award (UL1TR000101), Georgetown University Dean for Research, and the American Epilepsy Society/Epilepsy Foundation of America (no. 367405). S.K.H. was supported by grant F30NS110318. P.N. was supported by grants R21AA027171 and R01AA027660. P.A.F. was supported in part by grant KL2TR001432.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1908176117/-/DCSupplemental.
References
- 1.Iadarola M. J., Gale K., Substantia nigra: Site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science 218, 1237–1240 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Depaulis A., Vergnes M., Liu Z., Kempf E., Marescaux C., Involvement of the nigral output pathways in the inhibitory control of the substantia nigra over generalized non-convulsive seizures in the rat. Neuroscience 39, 339–349 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Deransart C., Lê-Pham B. T., Hirsch E., Marescaux C., Depaulis A., Inhibition of the substantia nigra suppresses absences and clonic seizures in audiogenic rats, but not tonic seizures: Evidence for seizure specificity of the nigral control. Neuroscience 105, 203–211 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Doretto M. C., Garcia-Cairasco N., Differential audiogenic seizure sensitization by selective unilateral substantia nigra lesions in resistant Wistar rats. Physiol. Behav. 58, 273–282 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Garant D. S., Gale K., Lesions of substantia nigra protect against experimentally induced seizures. Brain Res. 273, 156–161 (1983). [DOI] [PubMed] [Google Scholar]
- 6.Löscher W., Ebert U., Lehmann H., Rosenthal C., Nikkhah G., Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J. Neurosci. Res. 51, 196–209 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Maggio R., Gale K., Seizures evoked from area tempestas are subject to control by GABA and glutamate receptors in substantia nigra. Exp. Neurol. 105, 184–188 (1989). [DOI] [PubMed] [Google Scholar]
- 8.McNamara J. O., Galloway M. T., Rigsbee L. C., Shin C., Evidence implicating substantia nigra in regulation of kindled seizure threshold. J. Neurosci. 4, 2410–2417 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morimoto K., Goddard G. V., The substantia nigra is an important site for the containment of seizure generalization in the kindling model of epilepsy. Epilepsia 28, 1–10 (1987). [DOI] [PubMed] [Google Scholar]
- 10.Gale K., Iadarola M. J., Seizure protection and increased nerve-terminal GABA: Delayed effects of GABA transaminase inhibition. Science 208, 288–291 (1980). [DOI] [PubMed] [Google Scholar]
- 11.Doretto M. C., et al. , A microdialysis study of amino acid concentrations in the extracellular fluid of the substantia nigra of freely behaving GEPR-9s: Relationship to seizure predisposition. Epilepsy Res. 17, 157–165 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Beckstead R. M., Edwards S. B., Frankfurter A., A comparison of the intranigral distribution of nigrotectal neurons labeled with horseradish peroxidase in the monkey, cat, and rat. J. Neurosci. 1, 121–125 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Childs J. A., Gale K., Neurochemical evidence for a nigrotegmental GAbAergic projection. Brain Res. 258, 109–114 (1983). [DOI] [PubMed] [Google Scholar]
- 14.Deniau J. M., Hammond C., Riszk A., Feger J., Electrophysiological properties of identified output neurons of the rat substantia nigra (pars compacta and pars reticulata): Evidences for the existence of branched neurons. Exp. Brain Res. 32, 409–422 (1978). [DOI] [PubMed] [Google Scholar]
- 15.Yasui Y., Tsumori T., Ando A., Domoto T., Demonstration of axon collateral projections from the substantia nigra pars reticulata to the superior colliculus and the parvicellular reticular formation in the rat. Brain Res. 674, 122–126 (1995). [DOI] [PubMed] [Google Scholar]
- 16.De Sarro G., Meldrum B. S., De Sarro A., Patel S., Excitatory neurotransmitters in the lateral habenula and pedunculopontine nucleus of rat modulate limbic seizures induced by pilocarpine. Brain Res. 591, 209–222 (1992). [DOI] [PubMed] [Google Scholar]
- 17.Garant D. S., Gale K., Substantia nigra-mediated anticonvulsant actions: Role of nigral output pathways. Exp. Neurol. 97, 143–159 (1987). [DOI] [PubMed] [Google Scholar]
- 18.Redgrave P., Marrow L. P., Dean P., Anticonvulsant role of nigrotectal projection in the maximal electroshock model of epilepsy–II. Pathways from substantia nigra pars lateralis and adjacent peripeduncular area to the dorsal midbrain. Neuroscience 46, 391–406 (1992). [DOI] [PubMed] [Google Scholar]
- 19.Deisseroth Lab, Stanford University. Predicted irradiance values: Model based on direct measurements in mammalian brain tissue. web.stanford.edu/group/dlab/cgi-bin/graph/chart.php. Accessed 1 October 2019.
- 20.Stujenske J. M., Spellman T., Gordon J. A., Modeling the spatiotemporal dynamics of light and heat propagation for in vivo optogenetics. Cell Rep. 12, 525–534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soper C., Wicker E., Kulick C. V., N’Gouemo P., Forcelli P. A., Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol. Dis. 87, 102–115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hikosaka O., Wurtz R. H., Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J. Neurophysiol. 53, 292–308 (1985). [DOI] [PubMed] [Google Scholar]
- 23.Hikosaka O., Wurtz R. H., Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J. Neurophysiol. 49, 1285–1301 (1983). [DOI] [PubMed] [Google Scholar]
- 24.Hikosaka O., Wurtz R. H., Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J. Neurophysiol. 53, 266–291 (1985). [DOI] [PubMed] [Google Scholar]
- 25.Berndt A., Lee S. Y., Ramakrishnan C., Deisseroth K., Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science 344, 420–424 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Cairasco N., Trivińo-Santos H. F., Effects of both 6-hydroxydopamine-induced and electrolytic lesion of the substantia nigra on the rotational behavior and audiogenic seizures in the rat. Braz. J. Med. Biol. Res. 22, 617–629 (1989). [PubMed] [Google Scholar]
- 27.Patel S., Millan M. H., Meldrum B. S., Neurotransmission in the pedunculopontine nucleus and pilocarpine-induced motor limbic seizures in rats. Neurosci. Lett. 74, 243–249 (1987). [DOI] [PubMed] [Google Scholar]
- 28.Gale K., Zhong P., Miller L. P., Murray T. F., Amino acid neurotransmitter interactions in ‘area tempestas’: An epileptogenic trigger zone in the deep prepiriform cortex. Epilepsy Res. Suppl. 8, 229–234 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Piredda S., Gale K., A crucial epileptogenic site in the deep prepiriform cortex. Nature 317, 623–625 (1985). [DOI] [PubMed] [Google Scholar]
- 30.Jobe P. C., Dailey J. W., Genetically epilepsy-prone rats (GEPRs) in drug research. CNS Drug Rev. 6, 241–260 (2006). [Google Scholar]
- 31.Lee Y., et al. , Divergent brain changes in two audiogenic rat strains: A voxel-based morphometry and diffusion tensor imaging comparison of the genetically epilepsy prone rat (GEPR-3) and the Wistar audiogenic rat (WAR). Neurobiol. Dis. 111, 80–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos V. R., Kobayashi I., Hammack R., Danko G., Forcelli P. A., Impact of strain, sex, and estrous cycle on gamma butyrolactone-evoked absence seizures in rats. Epilepsy Res. 147, 62–70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snead O. C., 3rd, An investigation of the relationship between the dopaminergic and electroencephalographic effects of gamma-butyrolactone. Neuropharmacology 21, 539–543 (1982). [DOI] [PubMed] [Google Scholar]
- 34.Venzi M., Di Giovanni G., Crunelli V., A critical evaluation of the gamma-hydroxybutyrate (GHB) model of absence seizures. CNS Neurosci. Ther. 21, 123–140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor J. A., et al. , Voluntary control of epileptiform spike-wave discharges in awake rats. J. Neurosci. 37, 5861–5869 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Depaulis A., Snead O. C. 3rd, Marescaux C., Vergnes M., Suppressive effects of intranigral injection of muscimol in three models of generalized non-convulsive epilepsy induced by chemical agents. Brain Res. 498, 64–72 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Turski L., Cavalheiro E. A., Turski W. A., Meldrum B. S., Excitatory neurotransmission within substantia nigra pars reticulata regulates threshold for seizures produced by pilocarpine in rats: Effects of intranigral 2-amino-7-phosphonoheptanoate and N-methyl-D-aspartate. Neuroscience 18, 61–77 (1986). [DOI] [PubMed] [Google Scholar]
- 38.Rossetti F., Rodrigues M. C. A., de Oliveira J. A. C., Garcia-Cairasco N., EEG wavelet analyses of the striatum-substantia nigra pars reticulata-superior colliculus circuitry: Audiogenic seizures and anticonvulsant drug administration in Wistar audiogenic rats (War strain). Epilepsy Res. 72, 192–208 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Millan M. H., Meldrum B. S., Boersma C. A., Faingold C. L., Excitant amino acids and audiogenic seizures in the genetically epilepsy-prone rat. II. Efferent seizure propagating pathway. Exp. Neurol. 99, 687–698 (1988). [DOI] [PubMed] [Google Scholar]
- 40.Dean P., Simkins M., Hetherington L., Mitchell I. J., Redgrave P., Tectal induction of cortical arousal: Evidence implicating multiple output pathways. Brain Res. Bull. 26, 1–10 (1991). [DOI] [PubMed] [Google Scholar]
- 41.Dean P., Redgrave P., Westby G. W., Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 12, 137–147 (1989). [DOI] [PubMed] [Google Scholar]
- 42.DesJardin J. T., et al. , Defense-like behaviors evoked by pharmacological disinhibition of the superior colliculus in the primate. J. Neurosci. 33, 150–155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gale K., Pazos A., Maggio R., Japikse K., Pritchard P., Blockade of GABA receptors in superior colliculus protects against focally evoked limbic motor seizures. Brain Res. 603, 279–283 (1993). [DOI] [PubMed] [Google Scholar]
- 44.Redgrave P., Simkins M., overton P., Dean P., Anticonvulsant role of nigrotectal projection in the maximal electroshock model of epilepsy–I. Mapping of dorsal midbrain with bicuculline. Neuroscience 46, 379–390 (1992). [DOI] [PubMed] [Google Scholar]
- 45.Nail-Boucherie K., Lê-Pham B. T., Marescaux C., Depaulis A., Suppression of absence seizures by electrical and pharmacological activation of the caudal superior colliculus in a genetic model of absence epilepsy in the rat. Exp. Neurol. 177, 503–514 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Depaulis A., et al. , Suppression of spontaneous generalized non-convulsive seizures in the rat by microinjection of GABA antagonists into the superior colliculus. Epilepsy Res. 5, 192–198 (1990). [DOI] [PubMed] [Google Scholar]
- 47.Dean P., Gale K., Anticonvulsant action of GABA receptor blockade in the nigrotectal target region. Brain Res. 477, 391–395 (1989). [DOI] [PubMed] [Google Scholar]
- 48.Shehab S., Dean P., Redgrave P., The dorsal midbrain anticonvulsant zone–II. Efferent connections revealed by the anterograde transport of wheatgerm agglutinin-horseradish peroxidase from injections centred on the intercollicular area in the rat. Neuroscience 65, 681–695 (1995). [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Gonzalez C., Bolam J. P., Mena-Segovia J., Topographical organization of the pedunculopontine nucleus. Front. Neuroanat. 5, 22 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Redgrave P., Mitchell I. J., Dean P., Descending projections from the superior colliculus in rat: A study using orthograde transport of wheatgerm-agglutinin conjugated horseradish peroxidase. Exp. Brain Res. 68, 147–167 (1987). [DOI] [PubMed] [Google Scholar]
- 51.Semba K., Fibiger H. C., Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: A retro- and antero-grade transport and immunohistochemical study. J. Comp. Neurol. 323, 387–410 (1992). [DOI] [PubMed] [Google Scholar]
- 52.Woolf N. J., Butcher L. L., Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res. Bull. 16, 603–637 (1986). [DOI] [PubMed] [Google Scholar]
- 53.Saper C. B., Loewy A. D., Projections of the pedunculopontine tegmental nucleus in the rat: Evidence for additional extrapyramidal circuitry. Brain Res. 252, 367–372 (1982). [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Rill E., Skinner R. D., Miyazato H., Homma Y., Pedunculopontine stimulation induces prolonged activation of pontine reticular neurons. Neuroscience 104, 455–465 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Peterson S. L., Infusion of NMDA antagonists into the nucleus reticularis pontis oralis inhibits the maximal electroshock seizure response. Brain Res. 702, 101–109 (1995). [DOI] [PubMed] [Google Scholar]
- 56.Dringenberg H. C., Olmstead M. C., Integrated contributions of basal forebrain and thalamus to neocortical activation elicited by pedunculopontine tegmental stimulation in urethane-anesthetized rats. Neuroscience 119, 839–853 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Kleiner S., Bringmann A., Nucleus basalis magnocellularis and pedunculopontine tegmental nucleus: Control of the slow EEG waves in rats. Arch. Ital. Biol. 134, 153–167 (1996). [PubMed] [Google Scholar]
- 58.Vertes R. P., Colom L. V., Fortin W. J., Bland B. H., Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp. Brain Res. 96, 419–429 (1993). [DOI] [PubMed] [Google Scholar]
- 59.Rasmusson D. D., Clow K., Szerb J. C., Modification of neocortical acetylcholine release and electroencephalogram desynchronization due to brainstem stimulation by drugs applied to the basal forebrain. Neuroscience 60, 665–677 (1994). [DOI] [PubMed] [Google Scholar]
- 60.Furman M., et al. , Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: Effects on cortical physiology. Epilepsia 56, e198–e202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Motelow J. E., et al. , Decreased subcortical cholinergic arousal in focal seizures. Neuron 85, 561–572 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shehab S., Guadagno J., Ferguson K., Redgrave P., Regional distribution of the anticonvulsant and behavioural effects of bicuculline injected into the pontine reticular formation of rats. Eur. J. Neurosci. 9, 1875–1884 (1997). [DOI] [PubMed] [Google Scholar]
- 63.Danober L., Depaulis A., Vergnes M., Marescaux C., Mesopontine cholinergic control over generalized non-convulsive seizures in a genetic model of absence epilepsy in the rat. Neuroscience 69, 1183–1193 (1995). [DOI] [PubMed] [Google Scholar]
- 64.Okada R., Negishi N., Nagaya H., The role of the nigrotegmental GABAergic pathway in the propagation of pentylenetetrazol-induced seizures. Brain Res. 480, 383–387 (1989). [DOI] [PubMed] [Google Scholar]
- 65.Moshé S. L., et al. , Ontogeny and topography of seizure regulation by the substantia nigra. Brain Dev. 17 (suppl.), 61–72 (1995). [DOI] [PubMed] [Google Scholar]
- 66.Beckstead R. M., Frankfurter A., The distribution and some morphological features of substantia nigra neurons that project to the thalamus, superior colliculus and pedunculopontine nucleus in the monkey. Neuroscience 7, 2377–2388 (1982). [DOI] [PubMed] [Google Scholar]
- 67.Wille C., et al. , Chronic high-frequency deep-brain stimulation in progressive myoclonic epilepsy in adulthood–Report of five cases. Epilepsia 52, 489–496 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Faingold C. L., Randall M. E., Neurons in the deep layers of superior colliculus play a critical role in the neuronal network for audiogenic seizures: Mechanisms for production of wild running behavior. Brain Res. 815, 250–258 (1999). [DOI] [PubMed] [Google Scholar]
- 69.Maggio R., Liminga U., Gale K., Selective stimulation of kainate but not quisqualate or NMDA receptors in substantia nigra evokes limbic motor seizures. Brain Res. 528, 223–230 (1990). [DOI] [PubMed] [Google Scholar]
- 70.Redgrave P., Dean P., Tonic desynchronisation of cortical electroencephalogram by electrical and chemical stimulation of superior colliculus and surrounding structures in urethane-anaesthetised rats. Neuroscience 16, 659–671 (1985). [DOI] [PubMed] [Google Scholar]
- 71.Van Dort C. J., et al. , Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc. Natl. Acad. Sci. U.S.A. 112, 584–589 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benevento L. A., Fallon J. H., The ascending projections of the superior colliculus in the rhesus monkey (Macaca mulatta). J. Comp. Neurol. 160, 339–361 (1975). [DOI] [PubMed] [Google Scholar]
- 73.Harting J. K., Huerta M. F., Frankfurter A. J., Strominger N. L., Royce G. J., Ascending pathways from the monkey superior colliculus: An autoradiographic analysis. J. Comp. Neurol. 192, 853–882 (1980). [DOI] [PubMed] [Google Scholar]
- 74.Mena-Segovia J., Bolam J. P., Rethinking the pedunculopontine nucleus: From cellular organization to function. Neuron 94, 7–18 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Depaulis A., Vergnes M., Marescaux C., Endogenous control of epilepsy: The nigral inhibitory system. Prog. Neurobiol. 42, 33–52 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to the corresponding author.