Significance
In the present study, we provide evidence of a cellular mechanism of breach of tolerance in an in vivo model of spontaneous autoimmune thyroiditis (AIT). We observe that self-antigen (Ag) specific Treg circulate in all lymphatic tissues except for the lymph nodes (LNs) draining the thyroid, which represents the site of inflammation. By contrast, cognate effector T cells accumulate in the draining LNs and thyroid. The absence of Treg unleashes cognate Teff, which promote tissue destruction. We demonstrate that the organ-restricted disappearance of Treg is driven by the self-Ag, aberrantly expressed in a highly inflammatory environment, by a mechanism of activation-induced cell death.
Keywords: regulatory T cells, autoimmunity, tolerance, AICD
Abstract
Activation of self-reactive T cells is a major driver to autoimmunity and is suppressed by mechanisms of regulation. In a humanized model of autoimmune thyroiditis, we investigated the mechanism underlying break of tolerance. Here, we found that a human TCR specific for the self-antigen thyroid peroxidase (TPO) is positively selected in the thymus of RAG KO mice on both T effector (Teff) and T regulatory (Treg) CD4+Foxp3+ cells. In vivo Teff are present in all immune organs, whereas the TPO-specific Treg are present in all lymphoid organs with the exception of the thyroid-draining lymph nodes. We suggest that the presence of TPO in the thyroid draining lymph nodes induces the activation of Teff and the depletion of Treg via activation-induced cell death (AICD). Our findings provide insights on the failure of the mechanisms of immune tolerance, with potential implications in designing immunotherapeutic strategies.
Regulatory CD4+CD25+Foxp3+ T cells (Treg) play a key role in the maintenance of immune tolerance by exerting cell-to-cell and cytokine-mediated suppression (1, 2), while impaired regulation by Treg triggers multiorgan autoimmunity in both mice and human (3, 4). The mechanisms leading to the loss of immune homeostasis and breakdown of peripheral tolerance, and the role that T cell recognition of immunodominant self-antigens, might play in these mechanisms remain still elusive due to the limitations of autoimmune animal models (5).
The TAZ10 mouse model is transgenic for a human T cell receptor (TCR) isolated from the thyroid infiltrate of a patient with autoimmune thyroiditis and specific for the immunodominant cryptic peptide of thyroid peroxidase (TPO536–547) (6, 7). T cell recognition of cryptic epitopes has been shown to play a pivotal role in triggering break of tolerance, as cryptic epitopes are poorly presented in the thymus but aberrantly displayed in the periphery under inflammatory conditions (8). We reported that the TAZ10 mice, both on Rag+ and Rag−/− backgrounds, spontaneously develop autoimmune hypothyroidism, which resembles Hashimoto’s disease with its clinical, hormonal, and histological signs (7, 9). In TAZ10 Rag−/− mice, clinical manifestations of the disease occur as early as 12 wk of age and are more severe than in Rag+ littermates (7, 9).
Here, we describe a unique and unexpected population of thymus-derived CD4+CD25+Foxp3+ Treg in the TAZ10 Rag−/− mice. Interestingly, despite displaying strong suppressive functions on effector T cells in vitro, these TPO-specific Treg fail to protect TAZ10 mice from autoimmunity in vivo. In this study, we underpin the mechanism responsible for the initiation and exacerbation of autoimmunity.
Results
Presence of CD4+CD25+Foxp3+ T Cells in Lymphoid Tissues of TAZ10 Mice.
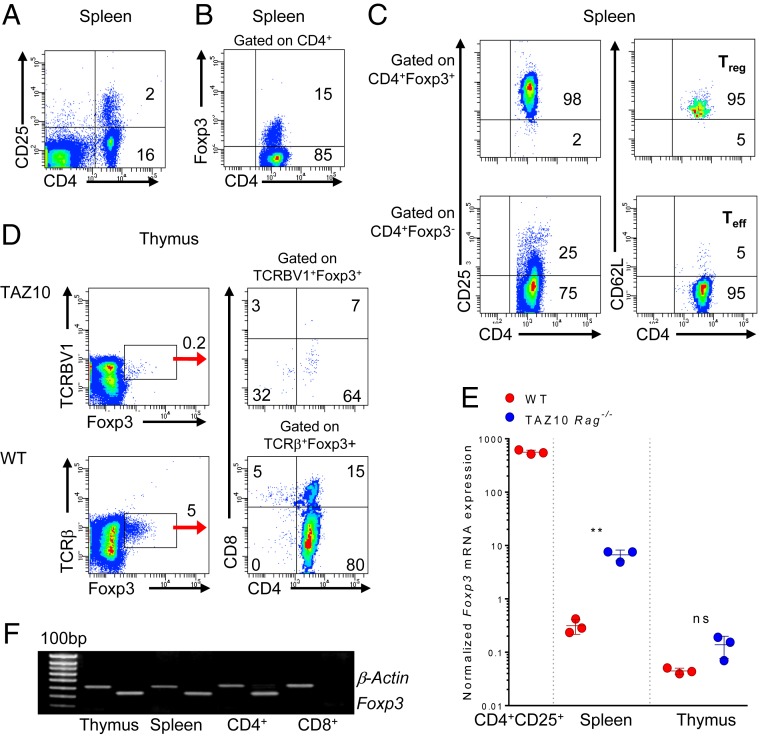
To underpin the mechanisms leading to the spontaneous activation of TAZ10 Rag−/− (from now on referred to as TAZ10) TPO-specific T cells, we initially characterized the phenotype of peripheral T cells. As expected, in the spleen we observed the presence of a discrete proportion of CD4+ T cells expressing CD25 (Fig. 1A), a surface marker up-regulated by activated Teff but also constitutively expressed by Treg (10). We expected that the detected CD4+CD25+ T cells were spontaneously activated self-reactive Teff since the transgenic T cells were on the Rag−/− background (11). Surprisingly, about 15% of CD4+ T cells expressed high levels of Foxp3, the specific marker for Treg in mice (Fig. 1B) (12). The majority of CD4+Foxp3+ T cells expressed the phenotype of Treg being CD25highCD62L+, whereas CD4+Foxp3− cells had the classic phenotype of activated effector memory CD25-/lowCD62L− T cells (Fig. 1C and SI Appendix, Fig. S1). Morphologically, these CD4+CD25+ T cells were also smaller compared to the CD4+CD25– T cell population, in keeping with a previously published report (SI Appendix, Fig. S2) (13).
Fig. 1.
Phenotypic and genotypic characterization of CD4+CD25+ T cells from TAZ10 mice. (A) Flow cytometry of TAZ10 spleen stained for CD4 and CD25. Density plot is gated on FSC/SSC. (B) Staining for surface CD4 and intracellular Foxp3 of splenocytes gated on the CD4+ population. (C) Staining of TAZ10 splenocytes for CD4, CD25, CD62L, and Foxp3. Cells were gated on the CD4+Foxp3+ and CD4+Foxp3− populations, further gated on CD25highCD62L+ Treg and CD25-/lowCD62L− T effector (Teff) cells. (D) Staining of TAZ10 and WT thymus for human TCRBV1 or mouse TCRβ, respectively, CD4, CD8, and Foxp3. TAZ10 and WT thymocytes are gated on human TCRBV1+ and mouse TCRβ+, respectively. (E) Real-time PCR for Foxp3 in the spleen and thymus of TAZ10 (blue, n = 3) and WT mice (red, n = 3). CD4+CD25+ splenocytes from WT mice were used as positive controls (n = 3). Foxp3 mRNA expression was normalized against GAPDH and CD8+CD25− sorted cells were used as calibrator. Dots represent individual mice. (F) RT-PCR for the expression of mouse Foxp3 and β-Actin on cellular extract of whole thymus, spleen, or purified CD4+ and CD8+ T cells from TAZ10 mice. Data are from 1 experiment representative of more than 10 mice analyzed (A–C) or 4 independent experiments (D and F). (E) Statistical analysis comparing the trends of expression of Foxp3 between WT and TAZ10 was performed using 2-tailed unpaired Student’s t test (nonsignificant [n.s.], P > 0.05; **P < 0.01). Cell percentages are indicated in each quadrant (A–D).
We then questioned whether TAZ10 Treg were naturally occurring Treg (nTreg) selected in the thymus (12, 14) or induced Treg (iTreg), which develop extrathymically in the periphery (15, 16). TAZ10 T cells, which express the human TCR VB1/VA15, can be stained by the human TCRBV1 monoclonal antibody (mAb). A small proportion of TCRBV1+ TAZ10 thymocytes expressed Foxp3 mainly within the CD4 single-positive population. TCRβ+ thymocytes from wild type (WT) syngenic mice were used as control (Fig. 1D). We also confirmed that splenocytes and thymocytes from TAZ10 mice express Foxp3 at the RNA level (Fig. 1E), and this expression was confined to CD4+ T cells (Fig. 1F). Our data strongly indicated that TAZ10 mice presented a population with the phenotype of thymus-derived regulatory CD4+CD25+Foxp3+ T cells.
TAZ10 CD4+CD25+ T Cells Suppress Effector T Cell Responses In Vitro.
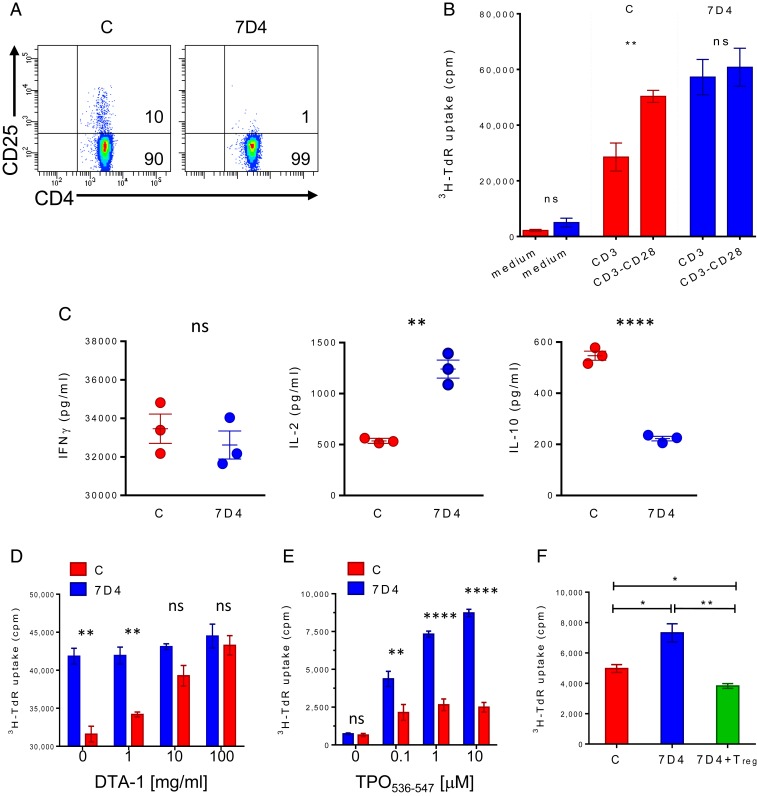
To prove that TAZ10 CD4+CD25+Foxp3+ Treg were bona fide regulatory cells, we first addressed whether their depletion would have any impact on immune responses. TAZ10 splenocytes were therefore in vitro-depleted of Treg using the α-CD25 mAb antibody clone 7D4 (Fig. 2A). These cells showed a significantly increased proliferation to α-CD3, thus indicating that the TAZ10 Foxp3+ have a suppressive function (Fig. 2B and SI Appendix, Fig. S3A). As expected, Treg-mediated suppression was abrogated upon addition of an agonistic α-CD28 (17) (Fig. 2B and SI Appendix, Fig. S3A). The depletion of Treg also resulted in a change in the cytokine profile in response to α-CD3, with levels of secreted IL-10 drastically reduced and much higher amounts of IL-2 (Fig. 2C and SI Appendix, Fig. S3B), further demonstrating that Treg promoted an antiinflammatory microenvironment (18). Interestingly, the levels of IFNγ did not significantly change after depletion (Fig. 2C and SI Appendix, Fig. S3B) with Treg unable to abrogate IFNγ release by Teff, possibly due to the large amount of IFNγ produced by the Teff.
Fig. 2.
TAZ10 CD4+CD25+Foxp3+ Treg suppress Teff proliferation in vitro. (A) Depletion of CD25+ T cells was done by incubating TAZ10 LNs and splenocytes (from pooled mice, n = 6) with the anti-CD25 monoclonal antibody clone 7D4 in conjunction with rabbit complement. Complement only was used as control. Depletion’s efficiency was verified by costaining with CD4 and CD25 (clone PC61). Numbers in quadrants indicate cell percentages. Untreated (C, red) or CD25 depleted (7D4, blue) TAZ10 splenocytes were challenged with: plate-bound CD3 antibody alone or in conjunction with CD28 for 18 h (B), and the levels of IFNγ, IL-2, and IL-10 were assessed by ELISA (C); CD3 in the presence of increasing concentrations of blocking anti-mouse DTA-1 mAb [0–100 mg/mL] (D); increasing concentrations of TPO peptide (TPO536–547) [0.1–10 μM] (E); TPO536–547 [10 μM] in the presence or absence of sorted TAZ10 CD4+CD25+ Treg (green) (F). (B–F) Data show 1 representative of 3 (B and D–F) or 4 (C) independent experiments, each done in triplicate with 3 independent wells (mean + SEM). Proliferation was assessed by 72-h thymidine incorporation assay adding Tritiated Thymidine in the last 18 h of the assay. (B–F) Statistical analysis was performed by using 2-tailed unpaired Student’s t test (nonsignificant [n.s.], P > 0.05; *P < 0.05; **P < 0.01; ****P < 0.0001).
Blockade of the glucocorticoid-induced TNF receptor (GITR), another functional marker of Treg (19, 20), also abolishes the suppressive activity of CD4+CD25+ Treg leading to exacerbation of autoimmunity in several animal models (20, 21). The selective blockade of GITR, expressed by TAZ10 Treg (SI Appendix, Fig. S4), has the ability to block the proliferation of TAZ10 T cells to α-CD3, except when they have been previously Treg-depleted (Fig. 2D and SI Appendix, Fig. S3C) (21). All these data confirm the hypothesis that the isolated CD4+CD25highFoxp3+ TAZ10 T cells are indeed regulatory T cells, not only as per phenotype but also function.
TAZ10 CD25+ Treg Suppress Antigen-Specific T Cell Responses In Vitro.
We then assessed whether TAZ10 Treg were able to impair TAZ10 Teff response to its peptide antigen, the human cryptic TPO536–547 peptide. Indeed, in vitro depletion of Treg (7D4) led to a strong increased T cell proliferation compared to unmanipulated splenocytes (C), in an antigen-dependant manner (Fig. 2E and SI Appendix, Fig. S3D). Similarly, we were able to block proliferation by the reintroduction of sorted TAZ10 CD4+CD25+ Treg cells (Fig. 2F and SI Appendix, Fig. S3E).
Treg Suppress Autoimmune Thyroiditis In Vivo.
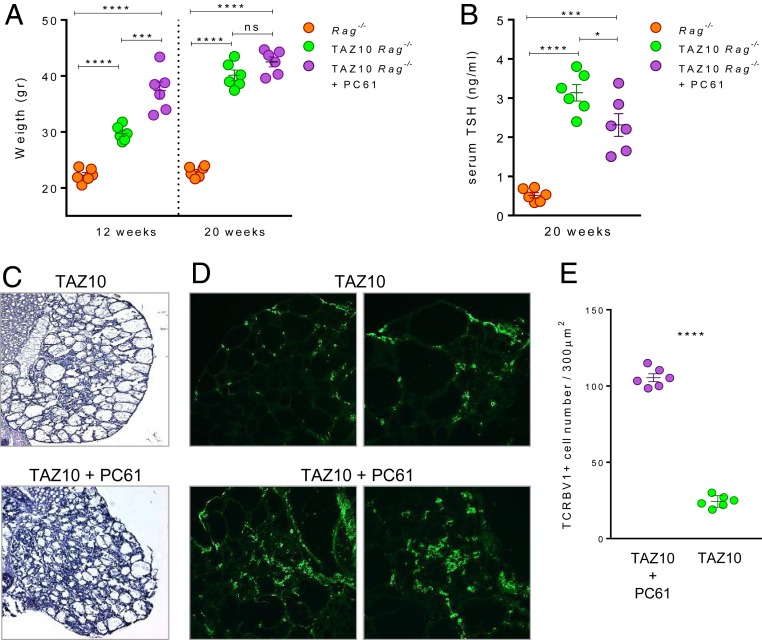
The data so far highlighted that the transgenic TAZ10 TCR (on the Rag−/− background) can select a single TCR on both Treg and classic Teff able to trigger autoimmunity (22). The spontaneous development of severe autoimmune thyroiditis in TAZ10 transgenic mice suggested, however, that the TAZ10 Treg could not provide complete protection in vivo (7). To understand their function in vivo, 3-wk-old TAZ10 mice were depleted of CD25+ Treg twice a week from 3 to 20 wk of age using the α-CD25 mAb clone PC61 injected intraperitoneally (IP) (SI Appendix, Fig. S5). Weight gain was used as readout of thyroiditis progression since it correlates with disease severity (7). At 12 wk of age, the TAZ10 transgenic mice were already overweight compared to the WT Rag−/− littermates. Noteworthy, at 12 wk of age the TAZ10 mice injected with α-CD25 mAb significantly gained more weight than the untreated TAZ10 mice (37.4 g ± 3.7 and 29.73 g ± 1.3, respectively) (Fig. 3A). At 20 wk of age, however, the α-CD25–treated and untreated TAZ10 littermates presented similar average weights (42.5 g ± 2.1 and 40.1 g ± 2.2, respectively) (Fig. 3A). The levels of thyroid-stimulating hormone (TSH) in the sera at 20 wk of age were also in line with the changes in weight with both groups of mice presenting a significant increase in thyroid stimulating hormone (TSH) compared to WT Rag−/− littermates (Fig. 3B).
Fig. 3.
CD4+CD25+Foxp3+ Treg cells suppress autoimmune thyroiditis in vivo. (A) Three-week-old TAZ10 mice (n = 6; purple) were injected periodically with anti-mouse CD25 mAb (clone PC61) and weighted at 12 and 20 wk of age. PC61-treated TAZ10 mice showed a significantly higher weight at 12 wk of age (mean 37.4 ± 3.7 g), than age-matched nontransgenic (n = 6; orange; mean 22.2 ± 1.2 g) and untreated TAZ10 littermates (n = 6, green, mean 29.7 ± 1.3 g). No significant difference was observed between untreated and PC61-treated TAZ10 mice at 20 wk, scoring close average weights (mean 40.1 ± 2.2 g and 42.5 ± 2.1 g, respectively), and both significantly higher than WT Rag−/− littermates. Age-matched WT Rag−/− mice showed no significant weight increase at 12 or 20 wk (mean 22.2 ± 1.2 g and 22.8 ± 0.9 g, respectively). (B) Serum TSH levels of 20-wk-old Rag−/− (orange), untreated (green), and PC61-treated TAZ10 mice (purple) (n = 6). PC61-treated and PC61-untreated transgenic mice showed significantly higher TSH levels than Rag−/− littermates. (C and D) At 20 wk, mice were killed and thyroids observed by H/E staining for sign of thyroiditis (D) and immunofluorescence with anti-human TCRBV1-FITC antibody to mark infiltrating pathogenic T cells. (Magnification: C and D, Left, 20×; D, Right, 40×.) The thyroid of 1 representative mouse of 6 is shown. (E) Mean infiltration score of TCRBV1+ cells of thyroid sections of TAZ10 mice untreated or injected with PC61 mAb (n = 6). (A and B) Data are done on the same mice at 12 and 20 wk of age (mean + SEM). Statistical analysis was calculated using ordinary 1-way ANOVA with Tukey’s multiple comparisons test (A and B) or 2-tailed unpaired Student’s t test (E) (nonsignificant [n.s.], P > 0.05; *P < 0.05; ***P < 0.001; ****P < 0.0001).
These observed clinical and serological signs of hypothyroidism were confirmed by histological analysis of thyroids from untreated and α-CD25–treated TAZ10 mice at 20 wk of age. Indeed, the architecture of thyroid lobes in Treg-depleted mice was even more severely compromised with fibrotic follicles and extensive cellular infiltrates (Fig. 3C and SI Appendix, Fig. S6). To demonstrate that cellular infiltrate was represented by pathogenic TAZ10 T cells, thyroid sections were stained with the α-human TCRBV1 antibody (Fig. 3D). We found a significantly higher degree of TCRBV1+ cell staining in the thyroid of PC61-treated mice (Fig. 3E). This in vivo data clearly demonstrated that TPO-specific TAZ10 Treg were bona fide functional regulatory cells capable of in vivo delaying, albeit not entirely preventing, the progression of the autoimmune process.
The Sites of Autoimmune Response Are Devoid of Treg Cells.
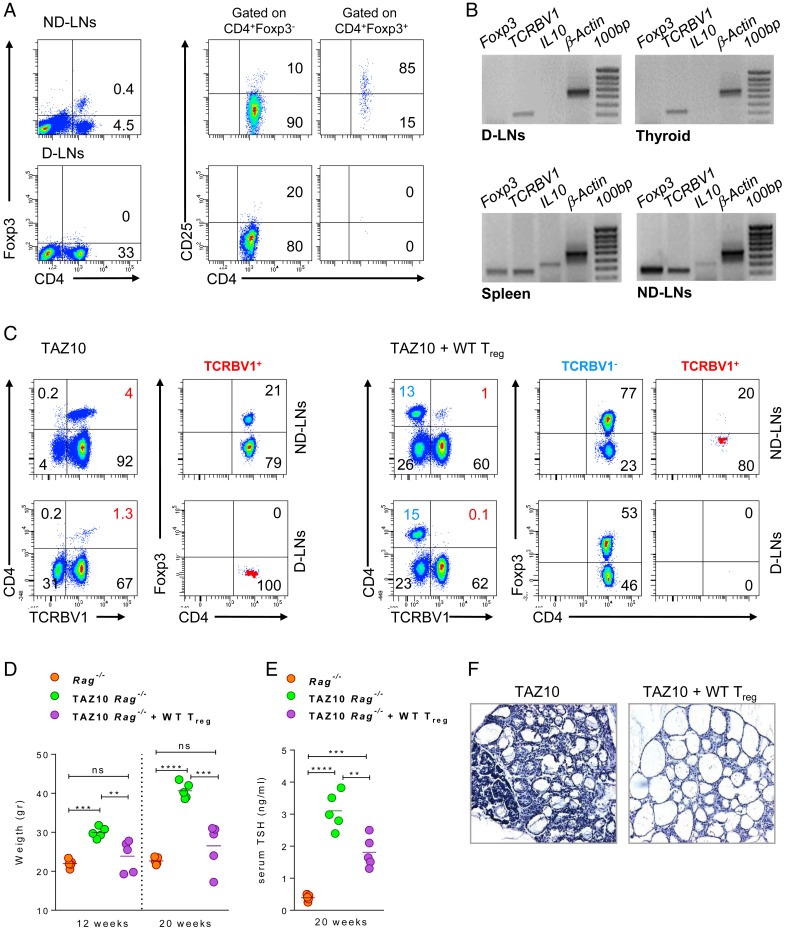
Considering the observed lack of protection from autoimmunity in vivo, we questioned if Treg were present at the sites of inflammation where they would be most needed. Strikingly, contrary to a distinct population of CD4+CD25+Foxp3+ Treg present in the spleen (Fig. 1), all nondraining lymph nodes (ND-LNs) but not the thyroid draining lymph nodes (D-LNs) presented a discrete population of Treg (Fig. 4A). This absence of Treg in the D-LNs was confirmed at the RNA level also in the thyroid and correlated with the inability to detect IL10 and Foxp3. This was in sharp contrast to their detection in the spleen and ND-LNs (Fig. 4B). As positive control, the human TCRBV1 was detected in all immune tissues tested, thus confirming the presence of infiltrating pathogenic Teff at the sites of inflammation (7).
Fig. 4.
WT Treg home to the sites of inflammation and protect TAZ10 mice from autoimmunity. (A) CD4, CD25, and Foxp3 expression in ND-LNs and D-LNs of TAZ10 mice. Numbers indicate cell percentages. (B) RT-PCR for Foxp3, IL10, TCRBV1, and β-Actin in the spleen, ND-LNs, D-LNs, and thyroid of TAZ10 mice. (C) Adoptive transfer of CD4+CD25+ cells from WT syngeneic mice into 3-wk-old TAZ10 Rag−/− mice (n = 5). Density plots of 1 representative mouse show the distribution of WT TCRBV1− or TAZ10 TCRBV1+ CD4+Foxp3+ Treg and CD4+Foxp3– Teff in ND-LNs and D-LNs of Treg-injected TAZ10 mice. (D) Weight record at 12 and 20 wk of age of Rag−/− (orange), TAZ10 (green), and Treg-injected TAZ10 mice (purple). At 12 wk and 20 wk, Treg-injected TAZ10 mice showed no significant weight gain compared to age-matched nontransgenic Rag−/− (mean 23.88 ± 4.1 g; P = 0.3339, ns; and 26.5 ± 5.9 g; P = 0.1901, ns, respectively). Weight of untreated transgenic littermates increased as to 29.96 ± 1.4 g at 12 wk and 40.66 ± 5.9 g at 20 wk being significantly higher of Rag−/− and Treg-injected littermates. (E) Serum TSH levels of Rag−/− (orange), untreated (green), and Treg-injected TAZ10 mice (purple) at 20 wk of age. Untreated TAZ10 mice had the highest TSH levels, but injection of Treg was accompanied by a drastic reduction of TSH levels compared to the untreated mouse control. TSH levels in Treg-injected TAZ10 compared to control Rag−/− littermates was also increased. (F) H/E staining of thyroids from untreated and Treg-injected TAZ10 mice. (Magnification 20×.) (A and B) One representative mouse of 10 is shown. (D) Data are done on the same mice at 12 and 20 wk of age (mean + SEM). (D and E) Statistical analysis was performed with ordinary 1-way ANOVA with Tukey’s multiple comparisons test (nonsignificant [n.s.], P > 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
WT Treg Harbor in D-LNs of TAZ10 Mice and Protect from Autoimmunity.
The absence of Treg in D-LNs prompted us to assess whether the D-LNs of TAZ10 mice represented a hostile microenvironment for their homing, expansion, and survival. We adoptively transferred 3-wk-old TAZ10 mice with purified CD4+CD25+ Treg from syngeneic WT mice. Mice were killed 17 wk later and the distribution of Treg in D-LNs and ND-LNs assessed. Interestingly, while CD4+Foxp3+ WT Treg were present in both ND-LNs and D-LNs, the transgenic TCRBV1+ TAZ10 Treg were still not detectable in D-LNs (Fig. 4C and SI Appendix, Figs. S7 and S8). The impact of the adoptive transfer of WT Treg in TAZ10 mice could be further seen as mice did not gain weight over time compared to age-matched untreated Rag−/− littermates both at early (12 wk of age, 27.1 g ± 1.2) and late stages of disease (20 wk, 30.4 g ± 0.6) (Fig. 4D). Changes in the serum TSH also confirmed the protection conferred by WT Treg to TAZ10 mice (Fig. 4E). Histological analysis of the thyroid of Treg-treated TAZ10 mice also revealed that the architecture was preserved, with follicles filled with colloid and no cellular infiltrate. On the contrary, the follicular structure of untreated TAZ10 thyroids appeared compromised with abundant cellular infiltrates (Fig. 4F). Altogether, these data indicated that polyclonal WT Treg could traffic to the D-LNs and confer protection to TAZ10 mice against the autoimmune process. This also importantly highlighted that TAZ10 Teff were sensible to immune regulation by Treg and, therefore, raised questions on the failure of TPO-specific TAZ10 Treg to naturally prevent the disease.
Altered Expression of Foxp3 by CD4+ T Cells in the Presence of TPO Self-Ag.
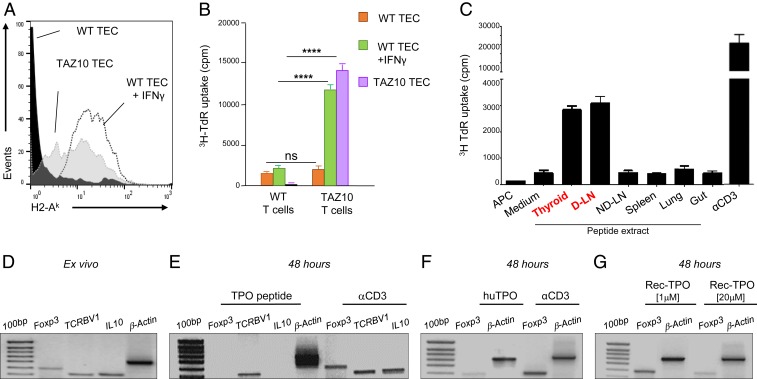
The selective absence of TAZ10 Treg in the D-LNs drove us to investigate whether the cognate antigen had any role in driving their disappearance at the sites of inflammation (23, 24). We first investigated if TAZ10 T cells could recognize the immunogenic TPO antigen presented in the thyroid and LNs. Interestingly, follicular thyroid epithelial cells (TEC) of TAZ10 mice constitutively express high levels of MHC class II (Fig. 5A) and can act as antigen-presenting cells (APC) inducing the proliferation of TAZ10 T cells (Fig. 5B). By contrast, WT TEC needs the addition of IFNγ to up-regulate MHC class II molecules and stimulate T lymphocytes (Fig. 5 A and B) (25). These results confirmed that the TAZ10 Teff cells can recognize autologous thyroid epithelial cells, as shown in human autoimmune thyroiditis (26).
Fig. 5.
Stimulation with the TPO cognate antigen induces a reduction of Foxp3 expression. (A) Medullary thyroid epithelial cells (mTEC) of WT mice express H2-Ak molecules under stimulation with IFNγ (dotted line), while TAZ10 mice express H2-Ak constitutively (shaded line). (B) Seventy-two-hour proliferation assay of TAZ10 T cells stimulated with TAZ10 mTEC (purple) and WT mTEC activated with IFNγ (green). No proliferation is observed with resting WT mTEC (orange). WT T cells were used as negative control. (C) Protein extract obtained from tissue homogenate of thyroid, cervical D-LNs, ND-LNs, spleen, lung, and gut from 80 mice was loaded on professional mDCs and used as APC on a 72-h thymidine incorporation assay to stimulate TAZ10 T cells. Coated aCD3 antibody was used as positive control. The error bars represent variation of 3 technical replicates. (D–G) Purified CD4+ T cells isolated from mesenteric LN and spleen of pooled TAZ10 mice (n = 6) were added to autologous mature DC challenged with αCD3, TPO536–547 peptide [0.1 μM], thyroid purified human TPO [0.1 μM], or different concentrations of recombinant human TPO [1 and 20 μM]. RT-PCR for the expression or Foxp3, IL10, human TCRBV1, and β-actin ex vivo (D) and after stimulation with α-CD3 or human TPO (E–G). (B) Statistical analysis using 2-tailed unpaired Student’s t test (nonsignificant [n.s.], P > 0.05; ****P < 0.0001).
It remained to be assessed whether the TPO antigen was present in the thyroid D-LNs as its expression would not naturally occur in any other LNs. We observed that TAZ10 T lymphocytes proliferated when challenged with autologous DC loaded with peptide extracts derived from thyroid and thyroid D-LNs. However, proliferation was not observed in response to peptide extracts from any other lymphoid tissues such as ND-LNs, spleen, and lung or gut (Fig. 5C).
These results show that the immunogenic peptide recognized by TAZ10 T cells was present in the thyroid and thyroid D-LNs. We assessed whether this peptide had any impact on the survival of TAZ10 Treg and Teff. mRNA expression of Foxp3, IL10, and human TCRBV1 was determined after TAZ10 CD4+ T cells were stimulated for 48 h with either the TPO cognate peptide or α-CD3. While these genes were expressed ex vivo (Fig. 5D), exposure to the cryptic TPO536–547 peptide, but not to α-CD3, prompted a simultaneous loss of Foxp3 and IL10. The continuous presence of TCRBV1 independently of the stimulation suggested that Treg cells but not Teff were sensitive to TPO536–547 stimulation (Fig. 5E). Similarly, a strong reduction of expression of Foxp3 was observed when TAZ10 CD4+ T cells were stimulated with purified human TPO (Fig. 5F). We could exclude that any impurity generated during the purification of the TPO protein from human thyroid was responsible for immunologic response as the use of recombinant TPO protein (Rec TPO) led to a similar reduction of Foxp3 expression. This observation correlated to the concentration of Rec TPO (Fig. 5G).
TAZ10 Treg Are Prone to Antigen-Induced Cell Death When Exposed to the Cognate Antigen.
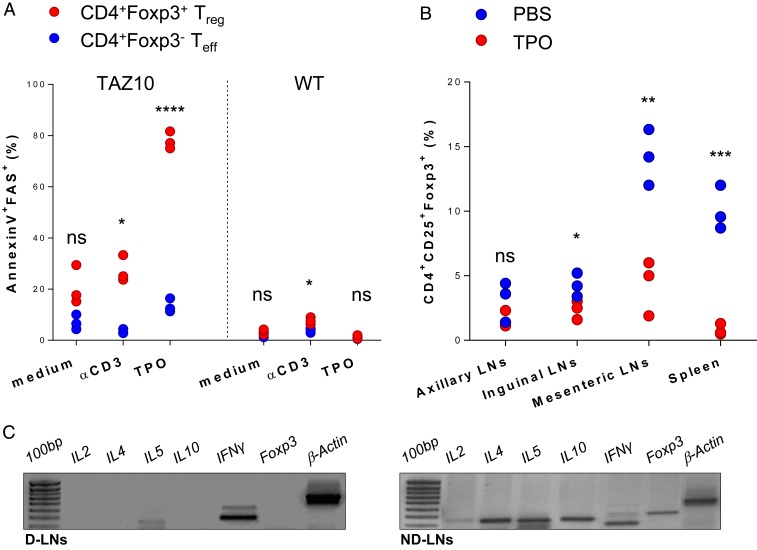
The data shown so far suggested that Treg are highly sensitive to the cognate antigen. This was a challenging hypothesis as TAZ10 Teff and Treg express the same TCR, thus leading us to investigate their ultimate fate following TPO antigen stimulation. After in vitro exposure to TPO536–547 or Rec TPO, up to 80% of TAZ10 Treg up-regulated phosphatidylserine and FAS, initiating a process of activation-induced cell death (AICD) (Fig. 6A and SI Appendix, Fig. S9). This was in stark contrast to TAZ10 Teff, with only a minority (less than 15%) of these cells expressing these apoptotic markers (Fig. 6A and SI Appendix, Fig. S10). Polyclonal Treg from WT mice were used as control and showed no major fluctuations in expression of early death markers when stimulated with TPO antigen (Fig. 6A).
Fig. 6.
Treg display higher sensitivity to the cognate TPO antigen than effector T cells. (A) Proportion (%) of CD4+Foxp3+ Treg (red) and CD4+Foxp3− Teff cells (blue) positive for Annexin V and FAS after 48-h stimulation with aCD3 or mDCs loaded with TPO536–547 peptide [0.1 μM]. The experiment was repeated 3 times in similar conditions. Each dot represents the experimental replicate. (B) Proportion (%) of CD25+CD4+Foxp3+ Treg in different lymphoid districts of TAZ10 mice immunized with thyroid purified human TPO protein (n = 3, red) or PBS (n = 3, blue). (C) RT-PCR for IL2, IL4, IL5, IL10, IFNγ, and Foxp3 in D-LNs and ND-LNs of 1 TAZ10 mouse representative of 3. (A and B) Statistical analysis was performed using 2-tailed unpaired Student’s t test (nonsignificant [n.s.], P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
To confirm that the Treg are sensitive to AICD, we immunized the mice at the base of the tail with TPO purified from human thyroid. Five days after immunization with TPO, a significant reduction of TAZ10 Treg was observed in all LNs and, in particular, the mesenteric LNs and spleen of TPO-immunized mice compared to PBS-treated controls (Fig. 6B). To have an insight of the immunological microenvironment at the sites of chronic inflammation, we compared the cytokine milieu of D-LNs and ND-LNs from TAZ10 mice, looking for common proinflammatory and antiinflammatory markers. Interestingly, we observed that IFNγ was produced in both D-LNs and ND-LNs, but cytokines fundamental for the survival, function, recruitment, and expansion of Treg such as IL-2, IL-4, IL-5, and IL-10 were not detected ex vivo in the D-LNs, although present in ND-LNs (Fig. 6C). Altogether, these data suggest that the selective reduction in TAZ10 Treg observed in the D-LNs, and their absence in the thyroid of TAZ10 mice, was driven by the presence/recognition of the cognate TPO antigen.
Discussion
Mouse models have been used extensively to provide insight into the mechanisms underlying many diseases, to explore the efficacy of candidate drugs, and to predict patient responses. The TAZ10 transgenic mice, with a human TCR isolated from a thyroiditis patient and specific for the self-antigen TPO, is a mouse model that spontaneously develops autoimmunity (6, 7). Due to the high degree of homology between the human DQB1*0602 and mouse A2-K, the human TAZ10 TCR can recognize the human TPO peptide and the highly homologous mouse TPO in the context of mouse MHC (7).
Here we show that the TAZ10 TCR can be naturally selected in the thymus on both Treg and Teff subsets. It has been previously shown that thymocytes could be directed to differentiate into Treg in response to thymic self-antigen expression (27).
Studies on Treg with known antigen specificity have however been hampered by their low frequencies. Furthermore, the development of Treg in TCR transgenic mice on a RAG-deficient background does not usually occur as endogenous α-chain recombination is required to shape the Treg repertoire (11). With the TAZ10 model, we had the unique opportunity to unravel the initiation of autoimmunity, in a model in which Teff coexist with Treg. These are also naturally occurring Treg (nTreg) as we detected Foxp3 in the thymus of TAZ10 Rag−/− (28). We therefore speculated that the shaping of this Treg repertoire in the thymus is likely orchestrated by the homologous murine TPO, which it is known to be also expressed in the thymus (29). Whether TAZ10 T cells sharing the same transgenic human TCR are selected in the thymus into Treg and Teff on the same or different peptides remains still unknown, but this is likely to ultimately influence their respective activation status and function in the periphery (22, 30).
Self-antigen–specific regulatory T cells are difficult to study since suitable specific tools to isolate and characterize these cells are lacking; they comprise only a small fraction of the total T cell population and are difficult to culture and maintain in vitro (31, 32). The compelling demonstration that the CD25highFoxp3+ T cell detected in TAZ10 mice were in fact Treg was that they elicited robust suppression in standard coculture assays.
The in vivo disappearance of the Treg only from the thyroid draining lymph nodes begs the question if the cognate antigen has any role in this process and what are the cells that present the antigen to T cells. In this regard, most attention to date has focused on DCs as main APCs in the LNs. We have shown, however, that in TAZ10 mice, the thyroid epithelial cells aberrantly up-regulate MHC class II molecules, as also previously demonstrated in patients with autoimmune thyroiditis (33). The aberrant expression of MHC class II molecules makes TEC able to present the endogenously processed TPO to T cells (6). It is possible that cells with scavenger function may engulf dead thyrocytes and present TEC-TPO in the draining LNs (34). Upon recognition of the cognate TPO peptide in the thyroid-draining LNs, Treg activate a process of AICD. That recognition of the cognate peptide is the triggering factor for AICD of Treg is also confirmed by the fact that immunization with whole TPO leads to Treg cell death in peripheral lymphoid tissues. Immunization with whole TPO has no effect, however, on the Teff cells.
These data indicate that Treg homeostasis is positively regulated by encounter with self-antigen expressed on peripheral tissues, a process likely to be relevant for reshaping the Treg repertoire across the different anatomical locations. This is confirmed by the fact that while TAZ10 Treg have little effect on the progression of autoimmunity, adoptive transfer of WT Treg strongly inhibits the onset and progression of disease, as shown by the lack of weight gain and only weak increase in serum TSH. Furthermore, these data demonstrate that TAZ10 Teff are sensitive to suppression by WT Treg, strongly suggesting that breach of tolerance is more likely due to a dysfunction of the endogenous Treg pool rather than to a refractoriness of Teff to suppression (35).
These data confirm but also add complexity to the “buddy hypothesis” that postulates that autoreactive conventional T cells would have a Treg cell “buddy,” with the same antigen specificity, to prevent unwanted T cell activation and autoimmunity (27, 36). In this model, we demonstrated that the in vivo depletion of Treg in very young TAZ10 mice led to a faster onset of autoimmune thyroiditis, confirming the active role of Treg in preventing autoimmunity. In this transgenic model, and possibly in bona fide autoimmunity, however, the massive imbalance between self-reactive T cell and Treg lead to disease. When this occurs, the tissue destruction produces even more debris that are engulfed by scavenger cells, and the self-antigen(s) are going to be processed and presented in the regional/draining LNs. This triggers a program of cell death in the Treg, until they finally disappear from these draining LNs. It has been reported that, compared to conventional T cells, Treg cells are also highly sensitive to apoptosis, correlating with lower expression levels of c-FLIP (37). Unleashed by the controls mediated by TAZ10 Treg, TAZ10 Teff would then overcome regulatory and protective mechanisms in the thyroid and leading to an amplification of the autoimmune process.
Our results support the notion that the establishment and maintenance of tolerance to self-antigen would rely on the generation of Treg with high TCR diversity (38), but the use of antigen-specific Treg as therapy might raise some issues for the treatment of autoimmune diseases. Current therapies do not only try to dampen proinflammatory cytokines but also restore homeostasis by promoting Treg stability and function (39). In the era of personalised medicine, some strategies are also under investigations, such as adoptive transfer of ex vivo expanded self-reactive Treg or in vivo expansion of self-reactive Treg, specific antigen-driven conversion of Teff into Treg in vivo or ex vivo (40) or chimeric antigen receptors (CAR)-engineered Treg (41). The identification of the self-antigen landscape in each patient affected by autoimmunity could help to select those Treg expanded in vitro, which undergo depletion by AICD or conversion to Th17. This could prove difficult as this landscape is complex, and the nature of the antigens generated by epitope spreading is difficult to predict. The strong beneficial effect of the adoptive transfer of Treg from WT mice into TAZ10 mice shows that choosing a polyclonal TCR population of Treg from healthy individual with the same haplotype could be an ideal strategy, especially in conjunction with current immunotherapeutic approaches.
Materials and Methods
Mice.
WT (CBA), Rag−/−, and TAZ10 TCR+Rag−/− mice were housed at the Tenovus animal facility at the University of Southampton (United Kingdom). All mice were used between 3 and 20 wk of age and immunocompromised transgenic mice maintained under specific pathogen-free conditions. All experiments were carried out according to local ethical committee guidelines under United Kingdom Home Office license number PIL70/13192. Additional details are described in SI Appendix, Supplemental Materials and Methods. Additional data, associated protocols, code, and materials in the paper can be available upon request through the corresponding author or following the link DOI: https://doi.org/10.5281/zenodo.3552497.
Flow Cytometry.
Cell suspensions were obtained as previously described (7). Briefly, cells obtained from thymuses, spleens, lymph nodes, and bone-marrows of 3- to 20-wk-old mice were passed through a 70-μm cell-filter before red blood cells were lysed in ammonium–chloride–potassium (ACK) buffer. Cells were collected in ice-cold 1% fetal calf serum, 0.05% sodium azide in phosphate-buffered saline (PBS) and stained at 4 °C. Flow cytometry analysis was performed using a FACSCalibur flow cytometer, and data were analyzed using Cell Quest or fluorescence-activated cell sorting (FACS) Diva software (Becton Dickinson). Cells were stained using antibodies to CD4, CD8α, CD25 (clone 7D4), CD62L, TCRβ, H-2k, CD11c, Annexin V (BD Pharmingen). Mouse CD25 (clone PC61) and GITR (clone DTA-1) were made in-house. Anti-human TCRBV1 was obtained from lmmunotech. CD95/Fas, CD86, and Foxp3 were from eBioscience.
Cell Isolations and Purifications.
Thymus, spleen, and lymph nodes of TAZ10 or WT mice were passed through a 70-μm cell strainer and red blood cells lysed in ACK buffer. CD4+, CD8+, and CD4+CD25+ T cell populations were purified with by magnetic cell separation (Miltenyi Biotec). In vitro depletion of CD25+ T cells was achieved with anti-mouse CD25 clone 7D4 and rabbit complement (Harlan Seralab). Efficiency of depletion was assessed by flow cytometry using the anti-mouse CD25 clone PC61.
Generation of Bone Marrow-Derived Dendritic Cells.
Bone marrow-derived dendritic cells (DC) from 4- to 12-wk-old CBA mice were generated as previously described (42). Briefly, bone marrow cells depleted of red blood cells were cultured with 200–400 ng/mL GM-CSF for 10 d. Then immature DC were harvested and seeded at 0.5 × 106 cells per well and pulsed with 100 ng/mL (0.1 μM) of purified human TPO (kind gift from B. Rapoport, Department of Medicine, University of California, Los Angeles [UCLA], CA) and then matured overnight with 10 ng/mL LPS (Sigma) before being cocultured with TAZ10 T cells. The phenotype of the DC generated was assessed by FACS before and after LPS maturation (SI Appendix, Fig. S11).
T Cell Activation and Proliferation Assays.
Proliferation assays were done as previously described (9). Untreated or CD25-depleted TAZ10 lymphocytes were pulsed with increasing doses of the human TPO536–547 N-DPLIRGLLARPA-C peptide (0.1–10 μM), anti-mouse CD3 alone (10 μg/mL) or in with anti-mouse CD28 (2 μg/mL). Where specified, titrating amounts of the blocking anti-mouse GITR antibody (0.1–100 mg/mL) were used. Cells seeded at 105 cells per well in triplicate were pulsed with [3H] thymidine for the last 16 h of culture. [3H] thymidine incorporation was measured with a microplate scintillation counter (Packard).
ELISA.
Primary coating antibodies used were purified anti-mouse IFNγ (rat lgG1, clone R4-6A2); IL-2 (rat lgG2a, clone JES6-1A12); and IL-10 (rat lgG2b, clone JES5-16E3). Secondary detecting antibodies used were biotin anti-mouse IFNγ (rat lgG1, clone XMG1.2); IL-2 (rat lgG2b, clone JES6-5H4), or IL-10 (rat lgG1, clone JES5-2A5) (eBioscience). Each well was incubated with streptavidin–horseradish peroxidase (HRP) and then with 30% H202/TMB peroxidase substrate. The reaction was stopped with 2 M sulfuric acid. Reading at 450 nm was then measured.
Mouse Serum TSH Assay.
Serum TSH was quantified as previously described (7). Briefly, TAZ10 serum TSH was quantified using a mouse TSH kit (Mesoscale Discovery-MSD) on 96-well plates coated with anti-mouse TSH Ab. Ten microliters of MSD Sulfo-tag Mouse TSH Ab were plated into each well, and 25 μL of either TSH standard or mouse serum were plated in duplicate. Signal was revealed with high sensitivity Reading Buffer T, and plate was read at the MSD reading machine MS 6000.
In Vivo Depletion and Adoptive Transfer of CD25+ Treg.
Three-week-old TAZ10 mice (n = 6 per group) were injected twice per week IP with 400 μg of the anti-mouse mAb PC61 (43) or PBS. Readouts of weight was taken at 12 and 20 wk of age. Serum TSH levels were determined at 20 wk of age. The efficiency of depletion was assessed by flow cytometry and RT-PCR.
Macs purified CD4+CD25+ cells (5 × 106 cells, Miltenyi Biotec) from WT CBA mice were injected i.v. into the lateral tail vein of 3-wk-old TAZ10 mice, followed a month later by a second injection of 5 × 106 CD4+CD25+ T cells. Organs and blood were collected 17 wk later for further FACS and RT-PCR analysis. The thyroids of treated mice were cryopreserved in OCT for further histological or RT-PCR analysis. Readouts of weight was taken at 12 and 20 wk of age. Serum TSH levels were determined at 20 wk of age.
Immunization of TAZ10 Mice with TPO.
Three-week-old TAZ10 mice were immunized at the base of the tail with thyroid-purified TPO protein or PBS in CFA (n = 3 per group). Mice were culled a week later, and their spleen, axillary, inguinal, and mesenteric lymph nodes isolated separately for further flow cytometry and RT-PCR analysis.
Thyroid Digestion and Culture.
Thyroid primary cultures were obtained as previously described (44, 45). Briefly, thyroid lobes separated with a scalpel from the trachea were digested in 112 U/mL of collagenase-A and 1.2 U/mL dispase-I (Roche). Digestion was carried out at 37 °C, the single-cell suspension was gently passed through a 70-μm cell strainer. Thyroid lobes were aseptically removed from the trachea of WT (CBA), Rag−/−, or TAZ-10 transgenic mice, placed in Eagle’s Minimum Essential Medium (EMEM) (AutogenBioclea) and cut into pieces. Disrupted thyroid lobes were digested in 1 mL of EMEM containing type I collagenase (5 mg/mL; Sigma) and Dispase I (2.5 mg/mL; Roche) for 45 min at 37 °C. The digested mix was spun down before the pellet containing partially digested single thyroid follicles was resuspended in complete thyroid medium. The culture medium was Nu-Serum IV (BD Biosciences) diluted 2.5 times in Hams F-12 medium (AutogenBioclear) and supplemented with 10 ng/mL somatostatin (Sigma) and 2 ng/mL glycyl-l-histidyl-l-lysine acetate (Sigma). Medium was changed 1 d after the start of the culture and replaced after 2 d. IFNγ (Peprotech) was added at 200 U/mL.
Peptide Extraction.
Peptides were isolated from thyroids, LNs draining and not draining the thyroid, spleens, and other tissues such as the trachea or the gut as previously described (45, 46). Briefly, tissues from 80 WT (CBA) mice were passed through a cell strainer, cells were lysed by 3 cycles of gentle freeze-thaw cycle. Lysed mixes were centrifuged to pellet the membranes, and supernatant was passed through a 30-kDa cutoff filter by centrifugation (Millipore).
RNA Extraction, cDNA Synthesis, RT-PCR, and Real-Time PCR.
Total RNA isolation, cDNA synthesis, and PCR were performed from up to 5 × 106 total lymphocytes from cervical, axillary, inguinal, mesenteric lymph nodes, splenocytes, thymus, or thyroid from either WT or TAZ10 transgenic mice.
Real-time PCR was done as previously described (12). All reported mRNA levels are normalized to the GAPDH mRNA level (GAPDH = 1). Primer pairs are described in SI Appendix, Table S1. In other experiments, the relative expression of Foxp3 was normalized by semiquantitative RT-PCR for mouse β-actin. PCRs were performed on a PTC-200TM cycler (MJ Research Inc.). The primers used are reported in SI Appendix, Table S2. All primers were designed to span an intron. PCR products were visualized by ethidium bromide 2% agarose gel.
Hystological Analysis.
Histology on mouse thyroids was done as previously described (7, 9). Briefly, thyroids were embedded in OCT. Four- to five-micrometer frozen sections were adhered to glass slides. For hematoxylin/eosin (H/E) staining, sections were fixed for 10 min 4% PFA, stained in Harris’ Haematoxylin for 10 min and in eosin (BDH) for 10 min. For immunofluorescence, air-dry frozen sections were fixed in acetone (BDH) for 10 min, blocked for Streptavidin/Biotin and for another 30 min with Normal Goat Serum (Vector Lab). Sections were incubated O/N at 4C with anti-human TCRBV1-FITC mAb (rat IgG1, BL37.2) Pictures of sections were taken with an Axioskop 2 Plus microscope (Zeiss). The mean infiltration score was calculated using LCmicro Imaging software (Olympus) and expressed as number of TCRBV1+ cells/300 μm2 area.
Statistical Analysis.
Statistical analysis considered the sample size for each data point as well as technical and experimental replicates, if any. Specific tests are described in this section as well as in the legends and were performed using the GraphPad Prism (GraphPad v6 and v8 software). Statistical analysis was performed using 2-tailed unpaired Student’s t test. The in vivo data were analyzed using ordinary 1-way ANOVA with Tukey’s multiple comparisons test. Not significant (n.s.), P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Supplementary Material
Acknowledgments
We thank Dr. Claudia Coronnello (Fondazione RiMED, Palermo, Italy) and Dr. Paolo Vicini (Kymab, Cambridge, UK) for the statistical analysis of the data, Prof. Basil Rapoport (Cedars-Sinai Medical Center, UCLA) for providing the human TPO and Richard Reid, and staff at the Tenovus Animal Research Facility for technical support. This work was supported by Cancer Research UK Grants C7056/A3110 and C8624/A4912.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910281116/-/DCSupplemental.
References
- 1.Kim J. M., Rasmussen J. P., Rudensky A. Y., Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S., Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6, 345–352 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Khattri R., Cox T., Yasayko S. A., Ramsdell F., An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Dominguez-Villar M., Hafler D. A., Regulatory T cells in autoimmune disease. Nat. Immunol. 19, 665–673 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner J. H., Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 10, 849–859 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quaratino S., Feldmann M., Dayan C. M., Acuto O., Londei M., Human self-reactive T cell clones expressing identical T cell receptor beta chains differ in their ability to recognize a cryptic self-epitope. J. Exp. Med. 183, 349–358 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quaratino S., et al. , Degenerate self-reactive human T-cell receptor causes spontaneous autoimmune disease in mice. Nat. Med. 10, 920–926 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Collado J. A., Guitart C., Ciudad M. T., Alvarez I., Jaraquemada D., The repertoires of peptides presented by MHC-II in the thymus and in peripheral tissue: A clue for autoimmunity? Front. Immunol. 4, 442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badami E., Maiuri L., Quaratino S., High incidence of spontaneous autoimmune thyroiditis in immunocompetent self-reactive human T cell receptor transgenic mice. J. Autoimmun. 24, 85–91 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M., Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995). [PubMed] [Google Scholar]
- 11.Itoh M., et al. , Thymus and autoimmunity: Production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162, 5317–5326 (1999). [PubMed] [Google Scholar]
- 12.Hori S., Nomura T., Sakaguchi S., Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Peng Y., Laouar Y., Li M. O., Green E. A., Flavell R. A., TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl. Acad. Sci. U.S.A. 101, 4572–4577 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontenot J. D., Gavin M. A., Rudensky A. Y., Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Curotto de Lafaille M. A., Lafaille J. J., Natural and adaptive foxp3+ regulatory T cells: More of the same or a division of labor? Immunity 30, 626–635 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Badami E., et al. , Defective differentiation of regulatory FoxP3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes 60, 2120–2124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt A., Oberle N., Krammer P. H., Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 3, 51 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhry A., et al. , Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34, 566–578 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHugh R. S., et al. , CD4(+)CD25(+) immunoregulatory T cells: Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16, 311–323 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Shimizu J., Yamazaki S., Takahashi T., Ishida Y., Sakaguchi S., Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3, 135–142 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Gavin M., Rudensky A., Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr. Opin. Immunol. 15, 690–696 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Wojciech L., et al. , The same self-peptide selects conventional and regulatory CD4+ T cells with identical antigen receptors. Nat. Commun. 5, 5061 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey-Bucktrout S. L., et al. , Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39, 949–962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su L. F., Del Alcazar D., Stelekati E., Wherry E. J., Davis M. M., Antigen exposure shapes the ratio between antigen-specific Tregs and conventional T cells in human peripheral blood. Proc. Natl. Acad. Sci. U.S.A. 113, E6192–E6198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Londei M., Bottazzo G. F., Feldmann M., Human T-cell clones from autoimmune thyroid glands: Specific recognition of autologous thyroid cells. Science 228, 85–89 (1985). [DOI] [PubMed] [Google Scholar]
- 26.Dayan C. M., et al. , Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc. Natl. Acad. Sci. U.S.A. 88, 7415–7419 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh C. S., Lee H. M., Lio C. W., Selection of regulatory T cells in the thymus. Nat. Rev. Immunol. 12, 157–167 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Kraj P., Ignatowicz L., The mechanisms shaping the repertoire of CD4+ Foxp3+ regulatory T cells. Immunology 153, 290–296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misharin A. V., Nagayama Y., Aliesky H. A., Rapoport B., McLachlan S. M., Studies in mice deficient for the autoimmune regulator (Aire) and transgenic for the thyrotropin receptor reveal a role for Aire in tolerance for thyroid autoantigens. Endocrinology 150, 2948–2956 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pohar J., Simon Q., Fillatreau S., Antigen-specificity in the thymic development and peripheral activity of CD4+FOXP3+ T regulatory cells. Front. Immunol. 9, 1701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevach E. M., Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Smigiel K. S., Srivastava S., Stolley J. M., Campbell D. J., Regulatory T-cell homeostasis: Steady-state maintenance and modulation during inflammation. Immunol. Rev. 259, 40–59 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Londei M., Lamb J. R., Bottazzo G. F., Feldmann M., Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature 312, 639–641 (1984). [DOI] [PubMed] [Google Scholar]
- 34.Elliott M. R., Ravichandran K. S., The dynamics of apoptotic cell clearance. Dev. Cell 38, 147–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basdeo S. A., et al. , Polyfunctional, pathogenic CD161+ Th17 lineage cells are resistant to regulatory T cell-mediated suppression in the context of autoimmunity. J. Immunol. 195, 528–540 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Kurd N., Robey E. A., T-cell selection in the thymus: A spatial and temporal perspective. Immunol. Rev. 271, 114–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plaza-Sirvent C., et al. , c-FLIP expression in Foxp3-expressing cells is essential for survival of regulatory T cells and prevention of autoimmunity. Cell Rep. 18, 12–22 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Adeegbe D., Matsutani T., Yang J., Altman N. H., Malek T. R., CD4(+) CD25(+) Foxp3(+) T regulatory cells with limited TCR diversity in control of autoimmunity. J. Immunol. 184, 56–66 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fasching P., Stradner M., Graninger W., Dejaco C., Fessler J., Therapeutic potential of targeting the Th17/Treg Axis in autoimmune disorders. Molecules 22, E134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyara M., Ito Y., Sakaguchi S., TREG-cell therapies for autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 10, 543–551 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Boardman D. A., et al. , Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am. J. Transplant. 17, 931–943 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Lutz M. B., et al. , An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999). [DOI] [PubMed] [Google Scholar]
- 43.McGeachy M. J., Stephens L. A., Anderton S. M., Natural recovery and protection from autoimmune encephalomyelitis: Contribution of CD4+CD25+ regulatory cells within the central nervous system. J. Immunol. 175, 3025–3032 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Jeker L. T., Hejazi M., Burek C. L., Rose N. R., Caturegli P., Mouse thyroid primary culture. Biochem. Biophys. Res. Commun. 257, 511–515 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Cexus O., “Immunological mechanisms controlling chronic inflammatory diseases,” PhD thesis, University of Southampton, Southampton, UK (2009). [Google Scholar]
- 46.Schwab S. R., Li K. C., Kang C., Shastri N., Constitutive display of cryptic translation products by MHC class I molecules. Science 301, 1367–1371 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.