Abstract
The use of animal models in brain aging research has led to numerous fundamental insights into the neurobiological processes that underlie changes in brain function associated with normative aging. Macaque monkeys have become the predominant nonhuman primate model system in brain aging research due to their striking similarities to humans in their behavioral capacities, sensory processing abilities, and brain architecture. Recent public concern about nonhuman primate research has made it imperative to attempt to clearly articulate the potential benefits to human health that this model enables. The present review will highlight how nonhuman primates provide a critical bridge between experiments conducted in rodents and development of therapeutics for humans. Several studies discussed here exemplify how nonhuman primate research has enriched our understanding of cognitive and sensory decline in the aging brain, as well as how this work has been important for translating mechanistic implications derived from experiments conducted in rodents to human brain aging research.
Keywords: cognitive aging, nonhuman primates, presbycusis
Advances in medicine, education, and nutrition over the past century have decreased mortality rates in populations across the globe, resulting in longer average life expectancies (1). Lifespan extensions reflect one of humanity’s most remarkable and underappreciated cultural accomplishments as the growing group of older individuals is one of few natural resources not diminishing. One often misunderstood aspect of the normative brain aging process is that while an enormous absolute number of individuals present with a dementing neurodegenerative disease at some time in their life (14% of people over the age of 70 y), the vast majority of people (86%) take healthier cognitive trajectories (2). Within this 86%, there exist significant interindividual differences in the ability to maintain healthy brain function, or to resist brain disease, across the lifespan (2–4).
A major goal within the field of the neuroscience of aging is to determine what factors allow certain individuals to evade neurodegenerative disease, while others succumb to them. Among those who do evade the pathological hallmarks of neurodegenerative disease, it is also of interest to determine why there remains significant individual variation in the quality of cognitive or sensory function across age. Insights into these basic questions are critical for devising strategies to maintain cognitive health in older individuals who fall along all points of the sensory and cognitive aging spectrum.
The ability to uncover the neurobiological changes responsible for dementing neurodegenerative disease requires knowledge of the “normal” trajectory that brain function takes across the lifespan. Animal models of cognitive and sensory aging have enriched our understanding of the mechanisms that contribute to different aspects of healthy brain aging. This is largely due to the availability of technologies in animals that provide a means of dissecting neuronal circuits with superior temporal, spatial, and genetic precision than is possible in humans. Nonhuman animals also do not spontaneously develop dementing neurodegenerative diseases, which eliminates a major confound often encountered in human brain aging research. Because cognitive problems arise relatively late in the progression of these diseases, some participants considered to be aging normally may actually have undetected pathophysiological brain markers during the period when behavioral testing is conducted (5). Finally, animal models help control for generational effects that sometimes influence human aging research (6) since young and aged animals can be exposed to similar testing and living conditions over the course of their lifespans. For example, a generational effect sometimes encountered in human cognitive neuroscience is differential exposure to technology, such as expertise with computers, between generations that can cause older individuals to perform more poorly than younger people on tasks, regardless of their actual cognitive status (6).
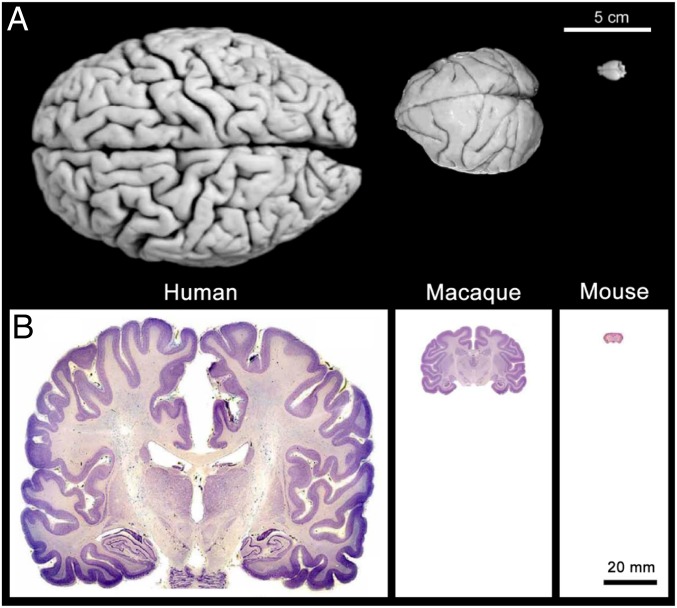
Nonhuman primates, in particular, have been central and arguably irreplaceable to cognitive aging research due to several critical features unique to these animals. First, monkeys possess cognitive and sensory repertoires that more closely resemble those of humans compared with other animal models (7). These characteristics allow different aspects of brain function to be tested with similar or even identical tests as those used in humans. Furthermore, cognitive and sensory brain structures in nonhuman primates share many more organizational principles with human brains than do the brains of phylogenetically more distant laboratory animals such as rodents (7–11) (Fig. 1). Both of these points emphasize how nonhuman primates can provide a fundamental link between nonprimate laboratory animals and humans.
Fig. 1.
Comparison of human, macaque, and mouse brains. (A) Images of the dorsal surface of a human brain, macaque brain, and mouse brain. Notice the striking differences in the size and convolution complexity of the cerebral cortex across the 3 species. (B) Coronal Nissl-stained sections of hippocampus-containing tissue in the 3 species. Reproduced with permission from ref. 104.
Unfortunately, public awareness of the importance of nonhuman primates in neuroscience research as a whole, and their importance in aging research in particular, is limited. The goal of this review is to begin to articulate the reasons that these research animals are a critical component of the experimental enterprise aimed at understanding the process of brain aging and to our quest to discover treatments that optimize brain and cognitive health. While such interventions would increase the quality of life for those individuals on normative cognitive trajectories, they may also result in postponing manifestations of neurodegenerative disease. In particular, this review will highlight studies that have utilized aged macaques to gain network-level and regional insights into age-related episodic memory decline and hearing loss, which are 2 of the most commonly experienced alterations in brain function experienced by older individuals. There will be a particular emphasis on conceptualizing the critical bridge that nonhuman primates provide between behavioral, neuroanatomical, and electrophysiological data obtained in aged rodents and cognitive, psychophysical, and functional imaging data from older humans.
Age-Associated Hyperexcitability in the Hippocampus and Episodic Memory Decline
Episodic memory refers to an individual’s recollection of a particular event in place and time and is considered among the most advanced forms of human memory. Compared with other types of memory, episodic memory is particularly vulnerable to the effects of normative brain aging, and a hallmark of Alzheimer’s disease is a drastic reduction in the capacity to form these representations (12). Lesion and functional imaging studies in humans indicate that the integrity of medial temporal lobe brain structures, including the hippocampus, is critical for episodic memory formation (13, 14). Consequently, age-related deficits in human episodic memory are thought to largely result from structural and functional alterations in these structures, although the precise neurobiological mechanisms involved have been difficult to pinpoint. An important clue to a critical brain change that occurs in aging came from a study that used functional magnetic resonance imaging (fMRI) to demonstrate that the CA3/dentate gyrus region of the hippocampus is more active in older individuals than in younger adults as they attempt to discriminate increasingly overlapping visual stimuli (15). Importantly, this study also demonstrated that the extent of CA3/dentate gyrus hyperactivation was significantly associated with discrimination abilities in these participants, regardless of age (15). Distinguishing similar, but nonidentical, experiences is a critical aspect of episodic memory that commonly becomes impaired in older individuals (16). These findings indicate that aging can lead to a disruption in the activity of circuits within the hippocampus that negatively impacts mnemonic processing.
While fMRI technologies can detect broad changes in activity, they do not provide detailed circuit-level insights into such changes. Studies using electrophysiological and neuroanatomical techniques in aging rats have provided a critical, yet incomplete, window into the neurobiological processes that might result in CA3/dentate gyrus hyperactivity in older people. Extracellular recordings across various hippocampal subfields have shown that neurons specifically in the CA3 region become hyperexcitable (higher firing rates) in older animals, whereas CA1 pyramidal cell firing rates are not different between adult and aged rats (17). This finding indicates a cross-species similarity in this age-associated hyperexcitability between rats and humans (15, 16). Other studies conducted in the rat hippocampus suggest that a neuronal change that may contribute to this is altered calcium homeostasis in older neurons (18). This physiological change would be expected to combine with known age-associated increases in L-type calcium channel conductance and calcium release from intracellular stores to result in higher calcium levels in hippocampal neurons of aged rats (19–22). The calcium hypothesis of brain aging posits that excess intracellular calcium levels in aging hippocampal cells result in altered cellular metabolism, gene expression, and neurotransmitter release that may ultimately result in mnemonic impairment (18, 23, 24).
Another observation in hippocampal circuits of aged rats is that there are significantly fewer inhibitory cells both within CA3 and the hilar region of the dentate gyrus compared with younger animals (25). Importantly, the number of a specific type of interneuron expressing the neuropeptide somatostatin was positively associated with memory function in aged rats (25). These findings indicate that regional hyperexcitability at the single-neuron level could result from a decreased level of inhibitory neurotransmission in older hippocampal networks. The calcium hypothesis suggests that rebalancing intracellular calcium levels, and perhaps hyperexcitability, by increasing inhibition to control excess neural activity may rebalance circuit activity and help preserve memory function in aging. Consistent with this prediction, normalizing excess neural activity in human participants using an anticonvulsant medication does partially rescue cognitive performance in patients with mild cognitive impairment (26). These findings exemplify how basic research in animal models of brain aging can reveal neurobiological mechanisms contributing to age-related cognitive impairments that are clinically targetable to maintain cognitive health later in life.
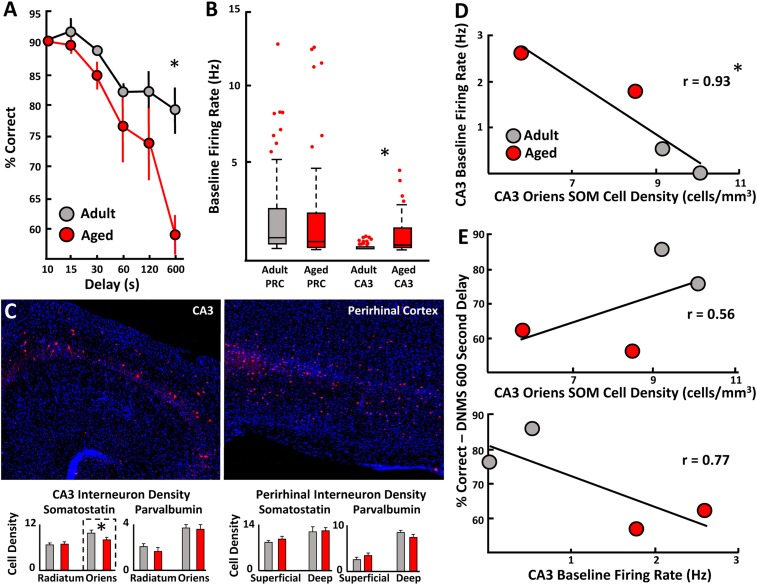
One study was able to bridge the gap between these cellular data in aged rats and imaging data from older humans by combining ensemble electrophysiological recordings with cell type-specific imaging in the medial temporal lobe of cognitively assessed aging rhesus macaques (27). Adult and aged monkeys underwent a test of object recognition memory. The older animals were shown to be significantly impaired relative to the younger animals (Fig. 2A). Tetrode recording probes were advanced individually with a hyperdrive device that allowed the acquisition of single action potentials from hippocampal CA3 pyramidal cells at more superficial depths. When the tetrodes were advanced further into the brain, perirhinal cortical cells were also recorded. Baseline firing rates were significantly higher in the CA3 region, but not in the perirhinal cortex, of the older monkeys compared with the adults, consistent with the regional specificity of the age-associated hyperactivity observed in aged humans (Fig. 2B). The brains from these animals were then harvested and serially sectioned for immunohistochemical labeling of 2 chemically distinct classes of inhibitory cells: somatostatin-positive and parvalbumin-positive interneurons. Critically, there was a selective decrease in somatostatin-positive interneuron density in the stratum oriens layer of CA3 (Fig. 2C). Whether the reduction observed reflects cellular degeneration or a biochemical down-regulation of the neuropeptide somatostatin remains an open question. Regardless, there was a strong negative correlation between the density of somatostatin-positive CA3 interneurons and principal neuron firing rates (Fig. 2D). Additionally, fewer somatostatin-positive interneurons and higher CA3 firing rates were associated with poorer cognition (Fig. 2E). Conversely, parvalbumin-positive interneuron numbers did not change with age, and they were not associated with firing rates or object recognition memory abilities. This study provides a within-animal link between age-associated declines in a molecularly defined population of inhibitory cells and principal cell excitability in older hippocampal networks. Together, these findings suggest that age-associated changes in hippocampal inhibitory circuits may lead to CA3 hyperexcitability and circuit dysfunction that impacts memory. These data also provide a possible mechanistic explanation for the excitability observed at the single-cell level in aged rats and in fMRI studies in older humans. One important translational implication of this work is the suggestion that age-related episodic memory decline may be alleviated by manipulating the function of specific interneuron subtypes to rebalance local network activity.
Fig. 2.
Macaques with object recognition memory deficits display hyperactivity in the CA3 region of the hippocampus that is associated with fewer somatostatin(SOM)-positive inhibitory interneurons. (A) Proportion of correct responses made by adult and aged rhesus macaques performing a delayed nonmatching-to-sample task at different delays. Aged animals were significantly impaired relative to adult animals at the 600-s delay condition. (B) Boxplots of pyramidal neuron baseline firing rates recorded from the perirhinal cortex (PRC) and CA3 in adult and aged monkeys. Baseline firing rates were significantly greater in the CA3 of aged animals relative to the adults. PRC firing rates were not different between age groups. (C) Magnified images of CA3 and PRC SOM inhibitory neurons. SOM interneuron densities were reduced in the stratum oriens layer of CA3 in aged animals, but not in the stratum radiatum or in the PRC. Parvalbumin-positive neuron density was not different between age groups in either region. (D) CA3 SOM neuron densities were significantly negatively correlated with CA3 baseline firing rates. (E) CA3 SOM neuron density and CA3 firing rates showed weak associations with object recognition performance. DNMS, delayed nonmatching-to-sample. *P < 0.05. Reproduced with permission from ref. 27.
The Prefrontal Cortex and Medial Temporal Lobe Are Impacted Uniquely by the Aging Process
Anatomical studies in aging macaques have provided substantial evidence that the total number of neurons in the prefrontal cortex (28–30) and medial temporal lobe (31–33) does not change across the lifespan in the absence of neurodegenerative disease. These observations indicate that the neurobiological processes driving functional changes in aged cognitive circuits (e.g., hyperexcitability) likely occur at subcellular levels. While cortical neurons are not lost with age, unbiased stereological assessments indicate that there is a 30 to 60% decrease in prefrontal cortical synapse density with age (34), and that both excitatory and inhibitory synapses are vulnerable (35). Additionally, axospinous, axosomatic, and axodendritic spine sizes appear to increase with age in the prefrontal cortex, and the extent of these structural changes in superficial cortical layers was significantly associated with performance measures on an object recognition task (36, 37). Hippocampal synapse density has also been carefully examined across the macaque lifespan, with the bulk of this work focusing on synapses formed by perforant path input from the superficial layers of the entorhinal cortex to the inner and outer molecular layers of the dentate gyrus (38). Unlike in the prefrontal cortex, the density of axospinous synapses, which are the predominant synapse type in the dentate gyrus, remains stable across the macaque lifespan (39, 40). Despite the apparent preservation of synapses in the dentate gyrus of aged monkeys, the density of perforated synapses in the outer molecular layer of this region was significantly associated with recognition memory accuracy (40).
Electrophysiological evidence from studies utilizing in vitro slice preparations further indicate that frontal cortical and medial temporal lobe networks are differentially impacted by normative aging. For example, intracellular recordings indicate that pyramidal neurons in the lateral prefrontal cortex become more excitable with age, as reflected by increases in membrane input resistance and action potential discharge to a given stimulation intensity (41). Furthermore, these electrophysiological changes were significantly associated with performance on an object recognition task (41). Recordings obtained from awake-behaving dorsolateral prefrontal cortex are inconsistent with these findings in that the firing rates in older monkeys performing a spatial working memory task have been observed to be reduced (42). Such differences obtained from in vitro versus in vivo electrophysiological recording experiments highlight the impact that modulation from external neuronal networks can have on firing patterns in awake-behaving animals. Additionally, both within and between brain regions, aged neurons can exhibit striking differences in excitability. In awake macaques, prefrontal cortical neurons show reduced firing rates (42), whereas temporal lobe neurons recorded in the CA3 region show increased excitability (27), but hippocampal granule cells recorded in vitro show no change in firing rate (43). Because the impact that age will have on a specific neural network cannot necessarily be predicted a priori, it is essential to examine regions independently as age-related changes in one circuit may not generalize to those observed in another.
Calcium Binding Proteins in the Aged Central Auditory System
Age-related hearing loss, or presbycusis, is among the more commonly encountered alterations in nervous system function in older individuals. Audiometric deficits can result from various insults to the cochlea that diminish this sensory organ’s capacity to transduce acoustic information (44–47). The data discussed here do not focus on age-associated changes in the cochlea, but rather on the central auditory system’s response to a weakened drive from the periphery.
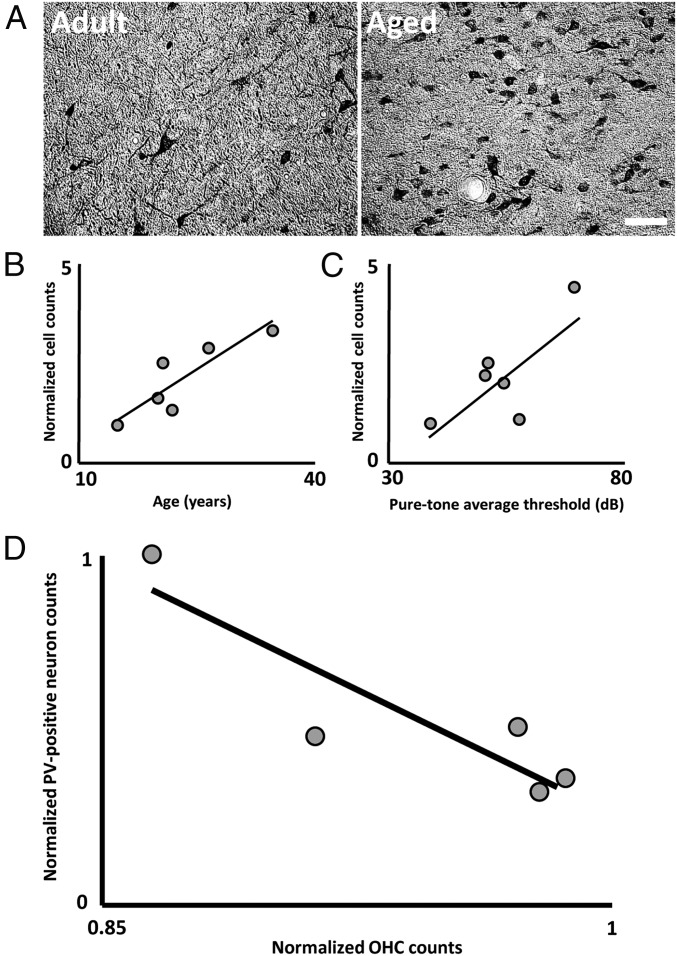
A consistent finding from this work is that decreases in inhibitory neurotransmission at the level of the auditory brainstem (48–50), midbrain (50–53), thalamus (50, 54, 55), and cortex (50, 56, 57) result in both hyperexcitability and changes in the tuning properties of central auditory neurons. Observations of central auditory system hyperexcitability led to the hypothesis that some aspects of presbycusis may result from altered calcium dynamics in aged auditory neurons that could lead to cell dysfunction and excitotoxicity that disrupts network function. As a result, histological experiments aimed at quantifying the number of neurons expressing calcium binding proteins (CaBPs) were conducted throughout the central auditory system. Numerous studies, first in rodents (both rats and mice) and then in macaques, have demonstrated that the number of neurons expressing CaBPs changes across the lifespan at multiple levels of the ascending auditory pathway (58–66). In rodents, there are contradicting reports as to whether CaBP density increases or decreases with age; however, every study in the macaque has reported age-associated increases in the number of auditory neurons expressing these proteins (Fig. 3A). Since most CaBPs operate as intracellular calcium buffers (67), the greater expression of these proteins in the auditory system of older animals has been proposed to reflect cellular response mechanisms to the potentially pathophysiological environment associated with greater calcium levels. In fact, the density of CaBP-expressing neurons has been found to be significantly associated with higher acoustic thresholds and fewer inner and outer hair cells at the level of the cochlea in macaques (58, 59, 65) (Fig. 3 B–D). These observations indicate that neurochemical changes occur within the central auditory system in response to a reduced acoustic drive from the periphery. Thus, a potentially promising therapeutic avenue to attenuate the effects of central auditory system aging may be to focus on reducing calcium levels in this system. This might be achieved either by finding a means to abolish hyperexcitability or by enhancing built-in cellular mechanisms that aged auditory neurons appear to employ to help maintain a healthier intracellular state in the face of pathophysiology elsewhere in the system.
Fig. 3.
Greater numbers of neurons expressing CaBPs in the central auditory system of aging macaques is associated with poorer peripheral auditory function. (A) Photomicrographs of parvalbumin-expressing neurons in an adult and aged macaque auditory brainstem. Note the qualitatively greater density of parvalbumin-positive cells in the older animal. Reprinted with permission from ref. 58. (B) Age is significantly associated with greater numbers of auditory neurons expressing parvalbumin in the core of the inferior colliculus (IC). Reprinted with permission from ref. 59, which is published under CC BY 3.0. (C) Animals with more parvalbumin-positive cells in the IC had higher auditory brainstem response pure-tone average thresholds. (D) Remarkably, animals with fewer outer hair cells (OHCs) in the cochlea had more neurons expressing parvalbumin (PV) in the dorsal cochlear nucleus of the auditory brainstem. Note that both auditory brainstem response thresholds and hair cell numbers reflect cochlear function. Together, these data indicate that chemical expression patterns of CaBPs in the central auditory system are associated with peripheral auditory dysfunction. (Scale bar, 100 μm.) Reprinted with permission from refs. 58; and 59, which is published under CC BY 3.0.
Research aimed at uncovering therapeutic approaches to alleviate the impact of presbycusis necessitates an animal model with an auditory system that anatomically and functionally approximates that of humans as closely as is possible. Both nonhuman primates and rodents have been used for these purposes, although critical species differences between the 2 species make monkeys a far superior animal model of the human auditory system. Among others, 2 differences will be discussed here: 1) distinct CaBP expression patterns of auditory neurons and 2) distinct hearing ranges between rodents and macaques.
With respect specifically to a calcium homeostasis approach, there is evidence that CaBP expression differs considerably between the auditory systems of primates and rodents. In macaques, 2 parallel ascending pathways that can be distinguished by immunohistochemical labeling of the CaBPs parvalbumin and calbindin traverse through the auditory system. Functional anatomical work has shown that neurons belonging to each pathway participate in vastly different aspects of acoustic processing, with the parvalbumin-rich direct pathway primarily acting as an information-relay system and the calbindin-rich indirect pathway acting more as a modulator (68). Critically, rodents do not appear to have these chemically defined parallel auditory pathways since the auditory thalamus of rats is almost completely devoid of parvalbumin and lacks calbindin in many areas where it is clearly expressed in primates (69, 70). Interspecies differences in the chemical composition of different brain regions highlight that not all aspects of brain physiology are the same across species. It becomes especially critical to use animal models that possess brains with chemical compositions similar to those of humans when potential therapies depend on targeting genetically defined cell types.
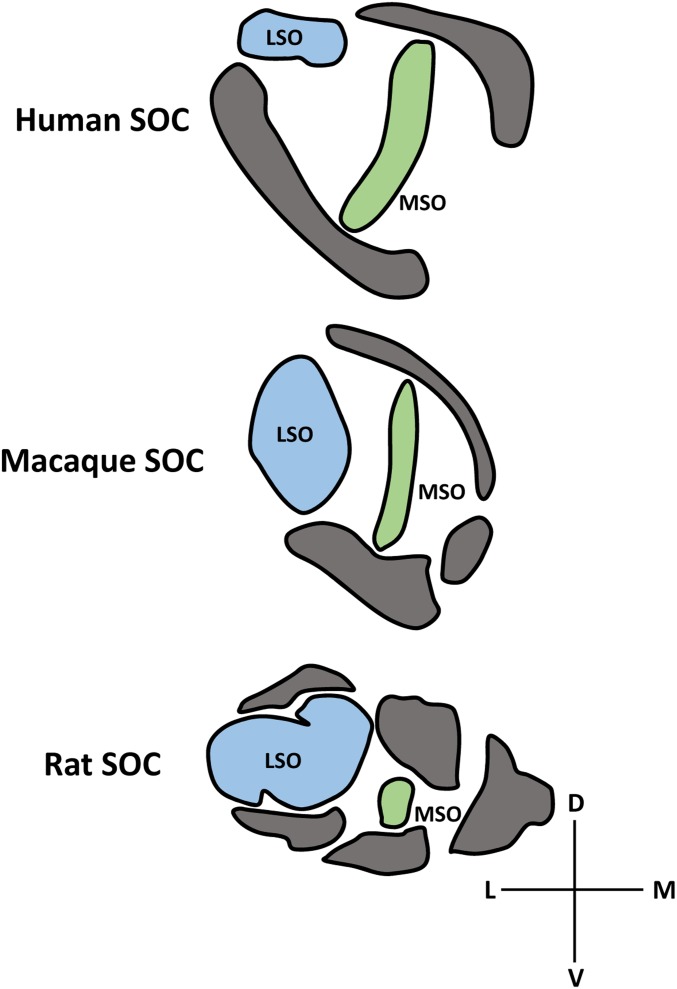
Perhaps the most important interspecies difference between rodents and primates with respect to presbycusis research is their vastly different hearing ranges. In particular, the human audiogram to 60-dB tone stimuli ranges roughly from 31 Hz to 17.6 kHz, and macaques show an acoustic range between 28 Hz and 34.5 kHz when given tones of the same intensity (71). Conversely, the domestic mouse cannot hear 60-dB tones below 2.3 kHz and can hear up to 85.5 kHz (71). These profound discrepancies in acoustic sensitivity between mice and primates are reflected by interspecies differences in auditory system anatomy. One location along the ascending auditory pathway where these interspecies differences are clear is within the superior olivary complex (SOC) in the ventral brainstem. The SOC is the first site of binaural integration in the auditory system, and is critical in computing the interaural time, intensity, and phase differences necessary for sound source localization in azimuth (72, 73). Interaural time differences are the primary cue used to localize lower frequency sounds, and these computations primarily involve the medial superior olivary (MSO) nucleus (74, 75). Localization of higher frequency sounds occurs primarily through interaural intensity difference calculations in the lateral superior olivary (LSO) nucleus (74–78). Based on acoustic sensitivity, the auditory system of mice should utilize calculations of interaural intensity differences in the LSO more so than the primate auditory system, which should utilize interaural time difference calculations in the MSO to a greater extent. In fact, mice and rats have a reduced MSO and more pronounced LSO compared with both macaques and humans, indicating that the anatomical representation of different auditory regions is unique to a species’ acoustic range (Fig. 4). Anatomical differences like these highlight the fact that sensory systems develop to process species-relevant information, and that differences in sensory transduction ranges will be reflected in the basic structure of an animal’s nervous system.
Fig. 4.
Schematic depiction of the relative size of auditory nuclei within the SOC of humans, macaques, and rats. (Top) Human SOC is characterized by a relatively small LSO nucleus (light blue) and an elongated MSO nucleus that is surrounded by other olivary nuclei (gray). (Middle) Organization of the macaque SOC follows very similar organizational principles, with a slightly larger LSO and slightly smaller MSO than humans. (Bottom) Rats, on the other hand, have a drastically expanded LSO relative to humans and macaques, as well as a significantly reduced MSO. Additionally, the shape and organization of the other olivary nuclei are very different in the rat compared with monkeys and humans. These anatomical differences between species are thought to reflect the drastically different acoustic sensitivities of primates and rodents. D, dorsal; L, lateral; M, medial; V, ventral. Adapted by permission from ref. 105 (Springer Nature: The Mammalian Auditory Pathway: Neuroanatomy, Copyright 1992).
Auditory Processing Deficits Are Selectively Associated with Medial Temporal Lobe Mnemonic Function and White Matter Connectivity in Aging Macaques
A relatively understudied aspect of brain aging is the impact that sensory decline has on cognitive function in older people. Despite evidence dating back several decades to suggest that individuals with poorer sensory function are more likely to have cognitive problems later in life, the neurobiological processes responsible for these associations have not been a subject of intense research (79–82). More recently, the impact that hearing loss, in particular, has on cognition has reemerged as a topic of research interest. This is largely due to evidence from large longitudinal studies that individuals with poorer acoustic function are at a higher risk of developing age-related cognitive decline and dementia (83, 84). A few functional imaging studies in older humans have shown compensatory recruitment of certain auditory, frontal, and temporoparietal brain regions when older people listen to human speech, even in subjects with clinically normal hearing thresholds (85–89). These observations indicate that the brains of older people functionally reorganize themselves to preserve auditory perception.
Recent work utilizing aging macaques provides a perspective on the association between auditory and cognitive function across the lifespan (90). Adult and aged macaques completed a battery of behavioral tests designed to probe the function of distinct aspects of frontal and temporal lobe-dependent cognition. Older monkeys were impaired relative to adults on some, but not all, of the cognitive functions tested in the battery, indicating that distinct aspects of cognition operate partially independent of one another in aged macaques, as is the case in older humans (91–95). This observation highlights a major challenge in cognitive aging research of understanding each individual’s unique behavioral and neurobiological profile. The same monkeys underwent electrophysiological assessments of auditory thresholds, auditory system temporal information processing, and visual system temporal information processing. Older monkeys showed drastic auditory processing deficits relative to adults despite auditory thresholds and visual system function not being different between age groups. These observations indicate that, like cognitive function, not all aspects of sensory function are equivalently impacted by the aging process in macaques.
The acquisition of multiple estimates of cognitive and sensory function within the same group of monkeys allowed an assessment of whether specific cognitive and sensory domains changed independent of one another or in tandem (90). Visual information processing and auditory thresholds were not associated with any aspect of cognition tested. Superior auditory processing, however, was related to better overall cognitive function. Critically, only specific cognitive functions tested drove the association between better auditory processing and higher overall cognition. In particular, superior auditory processing was associated with better performance on tests of concurrent reversal learning, object recognition memory, and discrimination of objects with high feature overlap, but not with performance on tests of reward devaluation, spatial short-term memory, or object discrimination. Based on previous lesion studies in macaques, the 3 tasks associated with auditory processing all require the integrity of medial temporal lobe brain structures, whereas the tasks in this battery that require frontal and occipital cortex integrity were not associated with auditory processing. Together, these observations indicate that auditory processing abilities functionally covary specifically with aspects of cognition driven by medial temporal lobe networks, regardless of auditory acuity.
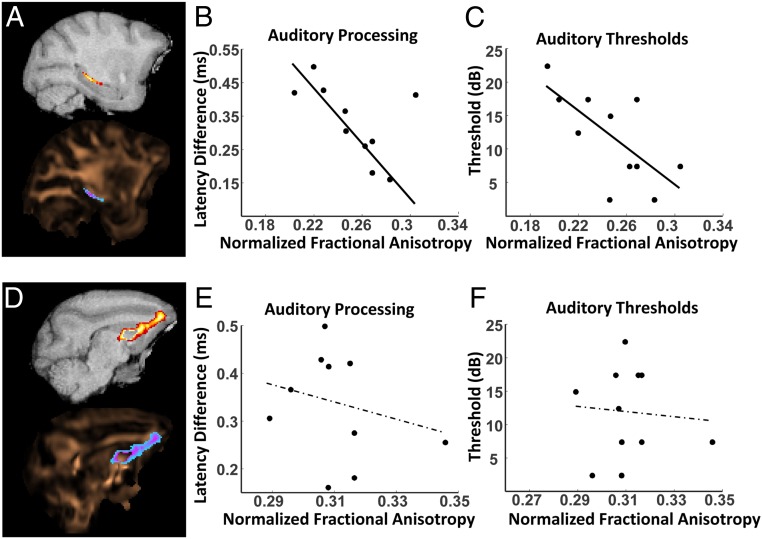
Diffusion tensor imaging analyses of frontal cortex- and medial temporal lobe-associated white matter microstructural condition were performed in the same monkeys to determine whether sensory and cognitive processing abilities are associated with the structural composition of these cognitive circuits. Monkeys with greater fractional anisotropy in the fimbria-fornix (Fig. 5A) and hippocampal commissure, both fiber tracts associated with hippocampus connectivity, were better able to discriminate stimuli with overlapping features, a cognitive process known to require medial temporal lobe circuits. The animals with better discrimination abilities and measures of medial temporal lobe tract fractional anisotropy also had higher auditory processing capacities (Fig. 5B) and lower acoustic thresholds (Fig. 5C). Importantly, auditory processing abilities were not associated with the microstructural condition of white matter in the frontal thalamic radiations (Fig. 5 D–F) or anterior commissure. This suggests that the relationships between the fractional anisotropy of hippocampus-associated white matter tracts and hearing function were not due to general changes in white matter systems across the aging brain. Together, these diffusion tensor imaging data indicate that auditory processing abilities and medial temporal lobe-dependent cognition become associated across an animal’s lifespan due, in part, to differential structural alterations to white matter in the temporal versus frontal lobes.
Fig. 5.
Animals with greater hippocampus-associated white matter integrity have better auditory processing capacities and lower auditory thresholds. (A) Representative probability map of the right hemisphere fimbria-fornix overlaid upon T1-weighted MRI (Top) and a fractional anisotropy (FA) map pseudocolored in copper (Bottom). (B) Animals with higher right hemisphere fimbria-fornix FA had better auditory processing abilities. (C) Similarly, animals with higher right hemisphere fimbria-fornix FA had lower auditory brainstem response (ABR) thresholds. Note that lower values indicate better function for both auditory measures. (D) Representative probability map of the right hemisphere frontal thalamic radiation overlaid upon T1-weighted MRI (Top) and an FA map pseudocolored in copper (Bottom). (E) Frontal radiation FA was not associated with auditory processing. (F) Frontal radiation FA was also not associated with ABR thresholds. Together, these findings indicate that auditory function is specifically associated with the structural composition of medial temporal lobe-associated, but not frontal lobe-associated, white matter.
An important consideration in interpreting these data is that auditory information processing in the primate forebrain primarily occurs along the dorsal and lateral banks of the superior temporal gyrus (9, 96). Thus, the striking specificity by which anatomical connectivity, auditory physiology, and cognitive outcome measures covaried across the lifespan in this study indicates that normative aging impacts neural networks contained within the temporal lobe more similarly than circuits residing in other brain regions (i.e., between the frontal and temporal lobes in this study).
A number of factors may drive the observed associations between white matter composition of specific brain regions and sensory and cognitive function during aging (90). First, it might be the case that embryological and developmental differences between the frontal and temporal lobes predispose each region to succumb to distinct risk factors associated with brain aging. For example, inhibitory interneurons destined for the frontal lobes are enriched with different combinations of transcription factors during development than those destined for the temporal lobes (97). Second, differential patterns of age-related neurovascular dysfunction between different brain regions also could bias circuits contained within a lobe to experience comparable physiological consequences associated with poorer vascular health. Under this hypothesis, the efficacy of neural circuits in relatively close proximity might become similarly impacted by vascular aging. In support of this idea, there is evidence that occlusions of distinct cerebral arteries result in different patterns of cognitive dysfunction, indicating that there is some level of regional specificity in the impact of a given vascular insult (98). Finally, another possibility is that the extent of neuronal connectivity between regions may determine the degree to which brain functions covary across the lifespan through mechanisms associated with hyperexcitability or changes in synaptic function. Critically, these hypotheses are not necessarily mutually exclusive, and each remains to be verified or ruled out.
Currently, most frameworks for understanding function in aging brains consider different cognitive and sensory operations in isolation from one another rather than taking into consideration the fact that different brains systems interact and influence each other’s functioning. The results discussed here call for a shift in focus in cognitive aging research toward understanding how different brain functions impact one another across a lifespan. Comparative approaches to understanding brain aging in humans, using nonhuman primates, will be critical for gaining a more complete understanding of the fundamental principles by which neuronal networks compensate for and adapt to patterns of functional decline that arise uniquely in older individuals.
Conclusion
Nonhuman primates provide the animal model that is closest to humans and remain an important bridge for testing the veracity of discoveries effective in rodents when clinical applications of these are being considered for human testing. Macaque monkeys, in particular, have been the predominant nonhuman primate model in neuroscience, although relatively recent advances in transgenic technologies for use in marmosets (99–101) have rapidly established this anthropoid as a promising additional nonhuman primate tool. With respect specifically to brain aging research, it will be critical to objectively determine whether the advantages associated with the novel experimental tools that marmoset research can provide outweigh the potential disadvantages of reduced gyral complexity and increased phylogenetic distance from humans (102). Furthermore, given the rich literature base on cognitive and sensory aging in the macaque that does not yet exist in the marmoset, combined with major advances in fast gene-editing technologies such as the CRISPR/Cas9 system (103), a parallel approach may be to expand the suite of tools available in the marmoset to macaques in order to take advantage of these past advances.
As societies worldwide continue to achieve longer life expectancies, it is becoming more critical to devise strategies to maintain sensory and cognitive health to reduce the burden of declines in function for older individuals themselves, as well as the impact that this has on their families. Nonhuman primates will be a critical component to the future of brain aging research since many aspects of cognitive aging simply cannot be properly modeled in other laboratory animals. As ethical debates continue to surround this work, the time has come for scientists, physicians, caretakers, and policy makers to engage in a dialogue, both among themselves and with the general public, directed toward devising the best and most humane nonhuman primate research models to achieve the common goal of optimizing cognitive health throughout life.
Acknowledgments
We thank Michelle Albert for assistance with the graphics. This work was supported by NIH Grants R01 AG050548 (to C.A.B.) and F31 AG055263 (to D.T.G.) and by the McKnight Brain Research Foundation.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Using Monkey Models to Understand and Develop Treatments for Human Brain Disorders,” held January 7–8, 2019, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. NAS colloquia began in 1991 and have been published in PNAS since 1995. From February 2001 through May 2019 colloquia were supported by a generous gift from The Dame Jillian and Dr. Arthur M. Sackler Foundation for the Arts, Sciences, & Humanities, in memory of Dame Sackler's husband, Arthur M. Sackler. The complete program and video recordings of most presentations are available on the NAS website at http://www.nasonline.org/using-monkey-models.
Author contributions: D.T.G. and C.A.B. wrote the paper.
This article is a PNAS Direct Submission. E.A.B. is a guest editor invited by the Editorial Board.
References
- 1.Hoyert D. L., Xu J., Deaths: Preliminary data for 2011. Natl. Vital Stat. Rep. 61, 1–51 (2012). [PubMed] [Google Scholar]
- 2.Plassman B. L., et al. , Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 29, 125–132 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyberg L., Lövdén M., Riklund K., Lindenberger U., Bäckman L., Memory aging and brain maintenance. Trends Cogn. Sci. 16, 292–305 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Luo L., Craik F. I. M., Aging and memory: A cognitive approach. Can. J. Psychiatry 53, 346–353 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Sperling R. A., Karlawish J., Johnson K. A., Preclinical Alzheimer disease-the challenges ahead. Nat. Rev. Neurol. 9, 54–58 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charness N., Boot W. R., Aging and information technology use potential and barriers. Curr. Dir. Psychol. Sci. 18, 253–258 (2009). [Google Scholar]
- 7.Hara Y., Rapp P. R., Morrison J. H., Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age (Dordr.) 34, 1051–1073 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackett T. A., Preuss T. M., Kaas J. H., Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J. Comp. Neurol. 441, 197–222 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Kaas J. H., Hackett T. A., Subdivisions of auditory cortex and processing streams in primates. Proc. Natl. Acad. Sci. U.S.A. 97, 11793–11799 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrides M., Pandya D. N., Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci. 11, 1011–1036 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Petrides M., Tomaiuolo F., Yeterian E. H., Pandya D. N., The prefrontal cortex: Comparative architectonic organization in the human and the macaque monkey brains. Cortex 48, 46–57 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Glisky E. L., “Changes in cognitive function in human aging” in Brain Aging: Models, Methods, and Mechanisms, Frontiers in Neuroscience, Riddle D. R., Ed. (CRC Press/Taylor & Francis, Boca Raton, FL, 2007). [PubMed] [Google Scholar]
- 13.Scoville W. B., Milner B., Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Squire L. R., The legacy of patient H.M. for neuroscience. Neuron 61, 6–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yassa M. A., Mattfeld A. T., Stark S. M., Stark C. E. L., Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 108, 8873–8878 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yassa M. A., et al. , Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus 21, 968–979 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson I. A., Ikonen S., Gallagher M., Eichenbaum H., Tanila H., Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci. 25, 6877–6886 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toescu E. C., Vreugdenhil M., Calcium and normal brain ageing. Cell Calcium 47, 158–164 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Thibault O., Gant J. C., Landfield P. W., Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: Minding the store. Aging Cell 6, 307–317 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thibault O., Hadley R., Landfield P. W., Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: Relationship to impaired synaptic plasticity. J. Neurosci. 21, 9744–9756 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell L. W., Hao S.-Y., Thibault O., Blalock E. M., Landfield P. W., Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons. J. Neurosci. 16, 6286–6295 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thibault O., Landfield P. W., Increase in single L-type calcium channels in hippocampal neurons during aging. Science 272, 1017–1020 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Disterhoft J. F., Moyer J. R. Jr, Thompson L. T., The calcium rationale in aging and Alzheimer’s disease. Evidence from an animal model of normal aging. Ann. N. Y. Acad. Sci. 747, 382–406 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Verkhratsky A., Toescu E. C., Calcium and neuronal ageing. Trends Neurosci. 21, 2–7 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Spiegel A. M., Koh M. T., Vogt N. M., Rapp P. R., Gallagher M., Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J. Comp. Neurol. 521, 3508–3523 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker A., et al. , Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomé A., Gray D. T., Erickson C. A., Lipa P., Barnes C. A., Memory impairment in aged primates is associated with region-specific network dysfunction. Mol. Psychiatry 21, 1257–1262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters A., et al. , Neurobiological bases of age-related cognitive decline in the rhesus monkey. J. Neuropathol. Exp. Neurol. 55, 861–874 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Peters A., Leahu D., Moss M. B., McNally K. J., The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb. Cortex 4, 621–635 (1994). [DOI] [PubMed] [Google Scholar]
- 30.Peters A., Sethares C., The effects of age on the cells in layer 1 of primate cerebral cortex. Cereb. Cortex 12, 27–36 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Amaral D. G., Morphological analyses of the brains of behaviorally characterized aged nonhuman primates. Neurobiol. Aging 14, 671–672 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Gazzaley A. H., Thakker M. M., Hof P. R., Morrison J. H., Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol. Aging 18, 549–553 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Keuker J. I. H., Luiten P. G. M., Fuchs E., Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol. Aging 24, 157–165 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Uemura E., Age-related changes in prefrontal cortex of Macaca mulatta: Synaptic density. Exp. Neurol. 69, 164–172 (1980). [DOI] [PubMed] [Google Scholar]
- 35.Peters A., Sethares C., Luebke J. I., Synapses are lost during aging in the primate prefrontal cortex. Neuroscience 152, 970–981 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumitriu D., et al. , Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J. Neurosci. 30, 7507–7515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soghomonian J. J., Sethares C., Peters A., Effects of age on axon terminals forming axosomatic and axodendritic inhibitory synapses in prefrontal cortex. Neuroscience 168, 74–81 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witter M. P., Van Hoesen G. W., Amaral D. G., Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J. Neurosci. 9, 216–228 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tigges J., Herndon J. G., Rosene D. L., Preservation into old age of synaptic number and size in the supragranular layer of the dentate gyrus in rhesus monkeys. Acta Anat. (Basel) 157, 63–72 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Hara Y., et al. , Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol. Aging 33, 421.e17–421.e28 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang Y.-M., Rosene D. L., Killiany R. J., Mangiamele L. A., Luebke J. I., Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb. Cortex 15, 409–418 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Wang M., et al. , Neuronal basis of age-related working memory decline. Nature 476, 210–213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luebke J. I., Rosene D. L., Aging alters dendritic morphology, input resistance, and inhibitory signaling in dentate granule cells of the rhesus monkey. J. Comp. Neurol. 460, 573–584 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Ryan A. F., Protection of auditory receptors and neurons: Evidence for interactive damage. Proc. Natl. Acad. Sci. U.S.A. 97, 6939–6940 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmiedt R. A., “The physiology of cochlear presbycusis” in The Aging Auditory System, Springer Handbook of Auditory Research, Gordon-Salant S., Frisina R. D., Popper A. N., Fay R. R., Eds. (Springer, New York, NY, 2010), pp. 9–38. [Google Scholar]
- 46.Schuknecht H. F., Presbycusis. Laryngoscope 65, 402–419 (1955).14392966 [Google Scholar]
- 47.Schuknecht H. F., Further observations on the pathology of presbycusis. Arch. Otolaryngol. 80, 369–382 (1964). [DOI] [PubMed] [Google Scholar]
- 48.Caspary D. M., Hughes L. F., Schatteman T. A., Turner J. G., Age-related changes in the response properties of cartwheel cells in rat dorsal cochlear nucleus. Hear. Res. 216-217, 207–215 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Caspary D. M., Schatteman T. A., Hughes L. F., Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: Role of inhibitory inputs. J. Neurosci. 25, 10952–10959 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caspary D. M., Ling L., Turner J. G., Hughes L. F., Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J. Exp. Biol. 211, 1781–1791 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milbrandt J. C., Hunter C., Caspary D. M., Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J. Comp. Neurol. 379, 455–465 (1997). [DOI] [PubMed] [Google Scholar]
- 52.Milbrandt J. C., Albin R. L., Turgeon S. M., Caspary D. M., GABAA receptor binding in the aging rat inferior colliculus. Neuroscience 73, 449–458 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Shaddock Palombi P., Backoff P. M., Caspary D. M., Responses of young and aged rat inferior colliculus neurons to sinusoidally amplitude modulated stimuli. Hear. Res. 153, 174–180 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Richardson B. D., Ling L. L., Uteshev V. V., Caspary D. M., Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J. Neurosci. 33, 1218–1227a (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richardson B. D., Hancock K. E., Caspary D. M., Stimulus-specific adaptation in auditory thalamus of young and aged awake rats. J. Neurophysiol. 110, 1892–1902 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Llano D. A., Turner J., Caspary D. M., Diminished cortical inhibition in an aging mouse model of chronic tinnitus. J. Neurosci. 32, 16141–16148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Juarez-Salinas D. L., Engle J. R., Navarro X. O., Recanzone G. H., Hierarchical and serial processing in the spatial auditory cortical pathway is degraded by natural aging. J. Neurosci. 30, 14795–14804 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engle J. R., Gray D. T., Turner H., Udell J. B., Recanzone G. H., Age-related neurochemical changes in the rhesus macaque inferior colliculus. Front. Aging Neurosci. 6, 73 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gray D. T., Engle J. R., Recanzone G. H., Age-related neurochemical changes in the rhesus macaque superior olivary complex. J. Comp. Neurol. 522, 573–591 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Idrizbegovic E., Bogdanovic N., Viberg A., Canlon B., Auditory peripheral influences on calcium binding protein immunoreactivity in the cochlear nucleus during aging in the C57BL/6J mouse. Hear. Res. 179, 33–42 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Idrizbegovic E., Bogdanovic N., Willott J. F., Canlon B., Age-related increases in calcium-binding protein immunoreactivity in the cochlear nucleus of hearing impaired C57BL/6J mice. Neurobiol. Aging 25, 1085–1093 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Idrizbegovic E., Salman H., Niu X., Canlon B., Presbyacusis and calcium-binding protein immunoreactivity in the cochlear nucleus of BALB/c mice. Hear. Res. 216-217, 198–206 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Ouda L., Burianova J., Syka J., Age-related changes in calbindin and calretinin immunoreactivity in the central auditory system of the rat. Exp. Gerontol. 47, 497–506 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Ouda L., Druga R., Syka J., Changes in parvalbumin immunoreactivity with aging in the central auditory system of the rat. Exp. Gerontol. 43, 782–789 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Gray D. T., Engle J. R., Recanzone G. H., Age-related neurochemical changes in the rhesus macaque cochlear nucleus. J. Comp. Neurol. 522, 1527–1541 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gray D. T., Rudolph M. L., Engle J. R., Recanzone G. H., Parvalbumin increases in the medial and lateral geniculate nuclei of aged rhesus macaques. Front. Aging Neurosci. 5, 69 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baimbridge K. G., Celio M. R., Rogers J. H., Calcium-binding proteins in the nervous system. Trends Neurosci. 15, 303–308 (1992). [DOI] [PubMed] [Google Scholar]
- 68.Jones E. G., Viewpoint: The core and matrix of thalamic organization. Neuroscience 85, 331–345 (1998). [DOI] [PubMed] [Google Scholar]
- 69.Celio M. R., Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 35, 375–475 (1990). [DOI] [PubMed] [Google Scholar]
- 70.Steriade M., Jones E. G., McCormick D. A. [Francis Crick], Thalamus (Elsevier, Oxford, ed. 1, 1997). D. G. Wills Books. https://www.abebooks.com/first-edition/Thalamus-Steriade-E.G.Jones-D.A-McCormick-Francis/3809194510/bd. Accessed 6 February 2019.
- 71.Heffner H. E., Heffner R. S., Hearing ranges of laboratory animals. J. Am. Assoc. Lab. Anim. Sci. 46, 20–22 (2007). [PubMed] [Google Scholar]
- 72.Reuss S., Introduction to the superior olivary complex. Microsc. Res. Tech. 51, 303–306 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Tollin D. J., The lateral superior olive: A functional role in sound source localization. Neuroscientist 9, 127–143 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Yin T. C. T., “Neural mechanisms of encoding binaural localization cues in the auditory brainstem” in Integrative Functions in the Mammalian Auditory Pathway, Springer Handbook of Auditory Research, Oertel D., Fay R. R., Popper A. N., Eds. (Springer, New York, NY, 2002), pp. 99–159. [Google Scholar]
- 75.Yin T. C., Chan J. C., Interaural time sensitivity in medial superior olive of cat. J. Neurophysiol. 64, 465–488 (1990). [DOI] [PubMed] [Google Scholar]
- 76.Jeffress L. A., A place theory of sound localization. J. Comp. Physiol. Psychol. 41, 35–39 (1948). [DOI] [PubMed] [Google Scholar]
- 77.Galambos R., Schwartzkopff J., Rupert A., Microelectrode study of superior olivary nuclei. Am. J. Physiol. 197, 527–536 (1959). [DOI] [PubMed] [Google Scholar]
- 78.Guinan J. J. Jr, Warr W. B., Norris B. E., Topographic organization of the olivocochlear projections from the lateral and medial zones of the superior olivary complex. J. Comp. Neurol. 226, 21–27 (1984). [DOI] [PubMed] [Google Scholar]
- 79.Baltes P. B., Lindenberger U., Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol. Aging 12, 12–21 (1997). [DOI] [PubMed] [Google Scholar]
- 80.Lindenberger U., Baltes P. B., Sensory functioning and intelligence in old age: A strong connection. Psychol. Aging 9, 339–355 (1994). [DOI] [PubMed] [Google Scholar]
- 81.Lindenberger U., Scherer H., Baltes P. B., The strong connection between sensory and cognitive performance in old age: Not due to sensory acuity reductions operating during cognitive assessment. Psychol. Aging 16, 196–205 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Humes L. E., Busey T. A., Craig J., Kewley-Port D., Are age-related changes in cognitive function driven by age-related changes in sensory processing? Atten. Percept. Psychophys. 75, 508–524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deal J. A., et al. ; Health ABC Study Group , Hearing impairment and incident dementia and cognitive decline in older adults: The health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 703–709 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin F. R., Hearing loss and cognition among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 66, 1131–1136 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peelle J. E., Troiani V., Grossman M., Wingfield A., Hearing loss in older adults affects neural systems supporting speech comprehension. J. Neurosci. 31, 12638–12643 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peelle J. E., Troiani V., Wingfield A., Grossman M., Neural processing during older adults’ comprehension of spoken sentences: age differences in resource allocation and connectivity. Cereb. Cortex 20, 773–782 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Profant O., et al. , Functional changes in the human auditory cortex in ageing. PLoS One 10, e0116692 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wingfield A., Grossman M., Language and the aging brain: Patterns of neural compensation revealed by functional brain imaging. J. Neurophysiol. 96, 2830–2839 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Wingfield A., Peelle J. E., How does hearing loss affect the brain? Aging Health 8, 107–109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gray D., Examining the relationship between auditory function and cognitive decline in aging macaque monkeys, PhD dissertation, The University of Arizona, Tucson, AZ (2019). https://repository.arizona.edu/handle/10150/631904. Accessed 31 May 2019.
- 91.Fernandes M. A., Davidson P. S. R., Glisky E. L., Moscovitch M., Contribution of frontal and temporal lobe function to memory interference from divided attention at retrieval. Neuropsychology 18, 514–525 (2004). [DOI] [PubMed] [Google Scholar]
- 92.Fisk J. E., Sharp C. A., Age-related impairment in executive functioning: Updating, inhibition, shifting, and access. J. Clin. Exp. Neuropsychol. 26, 874–890 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Glisky E. L., Rubin S. R., Davidson P. S., Source memory in older adults: An encoding or retrieval problem? J. Exp. Psychol. Learn. Mem. Cogn. 27, 1131–1146 (2001). [DOI] [PubMed] [Google Scholar]
- 94.Glisky E. L., Polster M. R., Routhieaux B. C., Double dissociation between item and source memory. Neuropsychology 9, 229–235 (1995). [Google Scholar]
- 95.Miyake A., et al. , The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognit. Psychol. 41, 49–100 (2000). [DOI] [PubMed] [Google Scholar]
- 96.Recanzone G. H., Sutter M. L., The biological basis of audition. Annu. Rev. Psychol. 59, 119–142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma T., et al. , Subcortical origins of human and monkey neocortical interneurons. Nat. Neurosci. 16, 1588–1597 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Fabiani M., et al. , Taking the pulse of aging: Mapping pulse pressure and elasticity in cerebral arteries with optical methods. Psychophysiology 51, 1072–1088 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burkart J. M., Finkenwirth C., Marmosets as model species in neuroscience and evolutionary anthropology. Neurosci. Res. 93, 8–19 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Tokuno H., Moriya-Ito K., Tanaka I., Experimental techniques for neuroscience research using common marmosets. Exp. Anim. 61, 389–397 (2012). [DOI] [PubMed] [Google Scholar]
- 101.Kishi N., Sato K., Sasaki E., Okano H., Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev. Growth Differ. 56, 53–62 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Tokuno H., Watson C., Roberts A., Sasaki E., Okano H., Marmoset neuroscience. Neurosci. Res. 93, 1–2 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Heidenreich M., Zhang F., Applications of CRISPR-Cas systems in neuroscience. Nat. Rev. Neurosci. 17, 36–44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DeFelipe J., The evolution of the brain, the human nature of cortical circuits, and intellectual creativity, Front. Neuroanat. 5, 29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz I. R., The Superior Olivary Complex and Lateral Lemniscal Nuclei, in The Mammalian Auditory Pathway: Neuroanatomy, Webster D. B., Popper A. N., Fay R. R., Eds. (Springer; 1992), pp 117–167. [Google Scholar]