Significance
Despite favorable therapeutic outcomes of 5-HT2A receptor agonists for stress- and alcohol-related disorders, the underlying neural mechanisms remain unclear. Stress is a commonly cited risk factor for initiation or resumption of excessive alcohol drinking. This behavioral interaction involves aberrant inhibitory plasticity in the midbrain ventral tegmental area (VTA). Therefore, we investigated the influence of the serotonin 2A receptor (5-HT2AR) agonist TCB-2 on this GABAergic circuitry. We found that stress-altered VTA inhibitory circuitry is corrected by 5-HT2AR agonist treatment via positive modulation of the chloride transporter KCC2. Our results implicate VTA KCC2 hypofunction as a critical target of 5-HT2AR agonists.
Keywords: alcohol, reward circuitry, KCC2, serotonin receptor, GABA
Abstract
Stress is known to alter GABAergic signaling in the ventral tegmental area (VTA), and this inhibitory plasticity is associated with increased alcohol self-administration. In humans, serotonin 2A receptor (5-HT2AR) agonists can treat stress- and alcohol-related disorders, but the neural substrates are ill-defined. Thus, we reasoned that 5-HT2AR pharmacotherapies may ameliorate the stress-induced dysregulated inhibitory VTA circuitry that contributes to subsequent alcohol abuse. We found that acute stress exposure in mice compromised GABA-mediated inhibition of VTA GABA neurons corresponding with increased ethanol-induced GABAergic transmission. This stress-induced inhibitory plasticity was reversible by applying the 5-HT2AR agonist TCB-2 ex vivo via functional enhancement of the potassium-chloride cotransporter KCC2. The signaling pathway linking 5-HT2AR activation and normalization of KCC2 function was dependent on protein kinase C signaling and phosphorylation of KCC2 at serine 940 (S940), as mutation of S940 to alanine prevented restoration of chloride transport function by TCB-2. Through positive modulation of KCC2, TCB-2 also reduced elevated ethanol-induced GABAergic signaling after stress exposure that has previously been linked to increased ethanol consumption. Collectively, these findings provide mechanistic insights into the therapeutic action of 5-HT2AR agonists at the neuronal and circuit levels of brain reward circuitry.
Stress is commonly cited as a reason for initiation or escalation of alcohol use (1, 2). However, there remains a lack of treatments targeting stress-subverted neural circuitry underlying this behavioral interaction. The midbrain ventral tegmental area (VTA) is an important substrate of acute stress and alcohol (ethanol) effects in the brain (3–5). Our prior work in rats demonstrated that GABAAR signaling in VTA GABA neurons undergoes stress-induced plasticity, resulting in aberrant ethanol-induced excitation of local GABA neurons (6). Diminished GABA-mediated inhibition or noncanonical excitation, reflected in a depolarized GABAAR reversal potential (EGABA), arises from impaired chloride homeostasis maintained by the potassium-chloride cotransporter KCC2 (7–11). Decreased functionality of KCC2 in the VTA contributes to increased ethanol consumption (6, 12), suggesting that targeting this form of stress-induced neural plasticity can mitigate problem drinking.
Interestingly, serotonin 2A receptor (5-HT2AR) agonists demonstrate potential in human patients as adjunctive pharmacotherapies for the treatment of neuropsychiatric disorders including alcohol abuse and depression (13–17). For this reason, the 5-HT2AR might be critically involved in the relationship between stress and alcohol drinking. However, the neural mechanisms linking 5-HT2AR activation to positive therapeutic outcomes remains unclear (18). Promotion of neuronal chloride homeostasis, dictated by KCC2 in VTA GABA neurons, may underlie the therapeutic success of 5-HT2AR agonists. Notably, TCB-2, a 5-HT2AR agonist (19), was shown to up-regulate KCC2 function in spinal cord neurons following injury (20–22), indicating that 5-HT2AR activation is one of a variety of mechanisms by which KCC2 can be modulated (23, 24). Furthermore, 5-HT2ARs and KCC2 are both expressed in VTA GABA neurons and have both been implicated in the regulation of inhibitory transmission in this brain area (25, 26). Therefore, we hypothesized that activation of 5-HT2ARs in VTA GABA neurons normalizes chloride homeostasis after stress exposure by boosting KCC2 function and thereby mitigates aberrant ethanol-induced GABAergic signaling that promotes increased ethanol drinking (6).
Indeed, we found that, after stress exposure, ex vivo administration of TCB-2 promoted recovery of chloride homeostasis and returned VTA GABAergic circuitry to the prestress condition. The 5-HT2AR activation boosted stress-impaired KCC2 function via protein kinase C signaling and phosphorylation of KCC2 at serine 940. At the circuit level, aberrant GABAergic transmission in response to ethanol was prevented by 5-HT2AR activation. These results indicate that VTA KCC2 up-regulation is an important component of 5-HT2AR agonist effects in the brain and suggest potential cellular and synaptic mechanisms that underlie the therapeutic success of 5-HT2AR activation in treating stress- and alcohol-related disorders.
Results
5-HT2AR Activation Restores Chloride Homeostasis in VTA GABA Neurons of Stress-Exposed Mice.
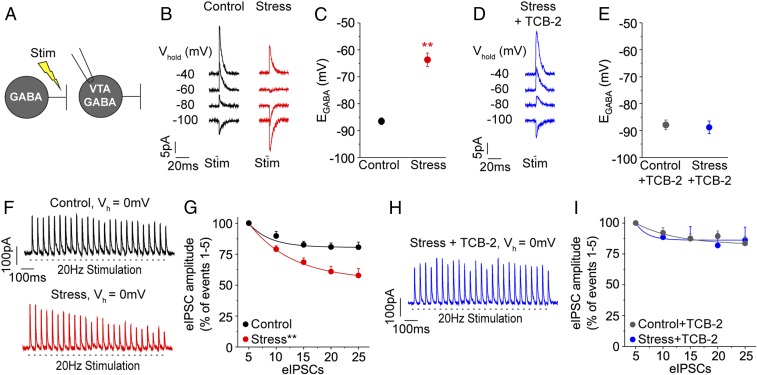
Adult mice were subjected to acute restraint stress 24 h before cutting midbrain slices to examine the effect of stress on VTA chloride homeostasis and GABAergic circuitry. Using gramicidin perforated patch recordings to preserve the intracellular anion concentration, EGABA was measured in VTA GABA neurons by evoking inhibitory postsynaptic currents (eIPSCs) with electrical stimulation (Fig. 1 A and B). Lateral VTA GABA neurons were identified as previously reported (SI Appendix, Fig. S1) (6, 12). EGABA was substantially depolarized in VTA GABA neurons of stressed mice relative to unstressed control mice (Fig. 1C): −63.7 ± 2.5 mV (Stress, red data) versus −86.5 ± 1.2 mV (Control, black data), n = 5, 6 cells/group, respectively, and P < 0.01 by unpaired, 2-tailed t test, which reinforces our prior findings in rats that stress alters the chloride gradient in these neurons (6). Total synaptic GABAAR conductances and resting membrane potentials were calculated for EGABA measurements and did not differ across experimental conditions (SI Appendix, Table S1).
Fig. 1.
Ex vivo 5-HT2AR agonist treatment restores chloride homeostasis in VTA GABA neurons following stress exposure. (A) GABAergic inputs were stimulated in VTA GABA neurons. Patched neurons were clamped at different holding potentials to record EGABA or chloride accumulation, which are both measures of chloride homeostasis. (B) Perforated patches were obtained to estimate EGABA in VTA GABA neurons from a control and a stressed mouse at the given holding potentials; eIPSCs reverse direction at EGABA. (C) Significantly depolarized EGABA was observed in VTA GABA neurons from stressed mice relative to controls: n = 5 to 6 cells/group; **P < 0.01. (D) Sample of EGABA recording from a stressed mouse in the presence of the 5-HT2AR agonist TCB-2 (1 μM). (E) TCB-2 hyperpolarized EGABA in VTA GABA neurons of stressed mice, which were indistinguishable from controls: n = 4 to 5 cells/group; P = 0.76. (F) Whole-cell patches were obtained to estimate chloride accumulation in VTA GABA neurons during repetitive GABAAR stimulation (20 Hz, Vh = 0 mV). Sample of a recording from a control mouse revealed minor depression in eIPSC amplitudes. Heightened eIPSC depression was observed in a VTA GABA neuron of a stressed mouse. (G) At 0 mV, VTA GABA neurons from stressed mice demonstrated a significantly higher rate of eIPSC depression compared to controls: n = 9 cells/group; **P < 0.01. (H) In the presence of TCB-2 (1 μM), repetitive eIPSCs in a VTA GABA neuron of a stressed mouse revealed minor depression. (I) Decreases in eIPSC amplitudes in VTA GABA neurons of stressed and control mice were not significantly different with TCB-2 treatment: n = 6 cells/group; P = 0.94.
In the spinal cord, agonist-mediated 5-HT2AR activation resulted in a hyperpolarizing shift in EGABA in motoneurons from animals exposed to injury (20). VTA GABA neurons also express 5-HT2ARs (25, 27, 28); however, it remains unknown whether 5-HT2ARs can similarly regulate EGABA in the VTA. To investigate, ex vivo slices containing the VTA were bathed in the 5-HT2AR agonist TCB-2 (1 μM) (19, 20) prior to and during measurement of EGABA in VTA GABA neurons of stressed and control mice. In the presence of TCB-2, EGABA was hyperpolarized back to the control potential in GABA neurons of stressed mice and had no effect on controls (Fig. 1 D and E): −88.8 ± 2.4 mV (Stress + TCB-2, blue data), −87.9 ± 1.8 mV (Control + TCB-2, gray data), n = 4, 5 cells/group, respectively, and P = 0.76 by unpaired, 2-tailed t test, suggesting that TCB-2 can restore normal chloride homeostasis after stress.
EGABA is determined by the anion gradient (7, 29, 30). Depolarization of EGABA is indicative of intracellular chloride accumulation following GABAAR activation. To observe chloride accumulation in slice, we applied repetitive GABAAR stimulation during whole-cell recordings. VTA GABA neurons were clamped at a holding potential (0 mV) that drives chloride influx (Fig. 1F). Under these conditions, chloride accumulation results in reduced amplitudes of eIPSCs (6, 12, 31). In VTA GABA neurons of stressed mice, we found that repetitive stimulation reduced eIPSC amplitudes more than in controls. An exponential fit of the eIPSC amplitudes revealed a significant difference in the rates of eIPSC depression between stressed and control groups (Fig. 1G: F = 65.5, n = 9 cells/group, P < 0.01). Next, we tested whether treatment with TCB-2 would prevent stress-induced chloride accumulation. In the presence of TCB-2 (1 μM), repetitive GABAAR stimulation did not produce substantial eIPSC depression in GABA neurons from stressed mice when compared to controls (Fig. 1 H and I: F = 0.12, n = 6 cells/group, P = 0.94), indicating that 5-HT2AR activation can reverse stress-induced chloride accumulation.
At a holding potential of −90 mV, chloride effluxes via GABAARs and presynaptic rundown can be observed independently of intracellular chloride accumulation. Presynaptic rundown was unchanged across all treatment groups (SI Appendix, Fig. S2), confirming that the effects of TCB-2 in stressed animals occurred through postsynaptic normalization of chloride extrusion.
5-HT2AR Activation Normalizes VTA Chloride Homeostasis Following Stress Exposure via Second Messenger Signaling and Site-Specific Phosphorylation of KCC2.
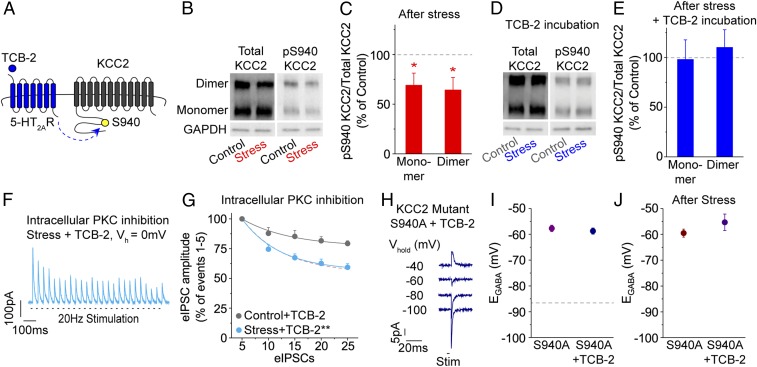
We previously revealed that stress-induced chloride accumulation in VTA GABA neurons resulted from functional down-regulation of KCC2, which is expressed in nondopaminergic VTA neurons (SI Appendix, Fig. S3A) (12, 32). Posttranslational modifications, such as reduced phosphorylation at serine 940 (S940), can diminish the chloride extrusion capacity of KCC2 (6, 23, 33) or dynamically regulate the functional state of KCC2 (23, 24). Therefore, we investigated whether TCB-2 normalizes chloride homeostasis in VTA GABA neurons of stressed mice by initiating an intracellular pathway that phosphorylates KCC2 at S940 (Fig. 2A). Using Western blot analysis of VTA tissue punches, we assessed changes in KCC2 protein levels in stressed and control mice (Fig. 2B). We found that stress diminished S940 phosphorylation (pS940) relative to controls (Fig. 2C): 69.1 ± 12.2% (monomer) and 64.3 ± 12.6% (dimer) of control, n = 8 mice/group, and P = 0.02 (monomer) and P = 0.03 (dimer) by paired, 2-tailed t test. In separate groups of stressed and control mice, VTA slices were incubated in TCB-2 (1 μM) for 20 to 30 min prior to sample preparation for immunoblotting. TCB-2 treatment increased pS940 in stressed mice to the control level (Fig. 2 D and E): 98.0 ± 20.3% (monomer) and 110.1 ± 17.5% (dimer) of control, n = 5 mice/group, and P = 0.79 (monomer) and P = 0.61 (dimer) by paired, 2-tailed t test. Total KCC2 protein was unchanged in stressed mice relative to controls, with or without TCB-2 treatment (SI Appendix, Fig. S3 B and C). These findings demonstrate that TCB-2 re-establishes normal KCC2 function by increasing phosphorylation at S940.
Fig. 2.
5-HT2AR activation normalizes stress-impaired chloride homeostasis via intracellular PKC signaling and phosphorylation of KCC2 S940. (A) In VTA GABA neurons of stressed mice, we hypothesized that restoration of chloride homeostasis by TCB-2 occurs via 5-HT2AR activation and subsequent phosphorylation of KCC2 S940 as illustrated. (B) Whole-cell lysates of VTA tissue were probed for total KCC2 and pS940, with GAPDH serving as the loading control. No difference in total KCC2 expression was detected between groups. However, a substantial reduction in pS940 was observed between stressed and control mice. (C) A significant decrease in the ratio of pS940 KCC2 to total KCC2 was found in stress samples relative to controls (100% dashed line): n = 8 mice/group; *P = 0.02 (monomer) and *P = 0.03 (dimer). (D) Sample of a Western blot of stressed and control VTA samples incubated in TCB-2 (1 μM) prior to tissue punching. (E) No significant difference in the ratio of pS940 KCC2 to total KCC2 was found in stress samples relative to controls (100% dashed line) when exposed to TCB-2: n = 5 mice/group; P = 0.79 (monomer), P = 0.61 (dimer). (F) Chloride accumulation was estimated in VTA GABA neurons from stressed and control mice in the presence of TCB-2 (1 μM) while intracellularly dialyzing the PKC inhibitor chelerythrine (20 μM). Under these conditions, a sample recording from a VTA GABA neuron in the stressed group demonstrated rapid eIPSC depression when held at 0 mV. (G) Chelerythrine prevented the effect of TCB-2 on eIPSC amplitude reduction in VTA GABA neurons from stressed mice when compared to the control group: n = 7 cells/group; **P < 0.01. Red dashed line represents the untreated stress group. (H) In KCC2 S940A heterozygous mice, haplodeficiency of the S940 phosphorylation site produced depolarized EGABA in a VTA GABA neuron, which was insensitive to bath application of TCB-2. (I) EGABA was comparably depolarized in VTA GABA neurons of unstressed S940A heterozygotes between untreated or TCB-2–treated slices: n = 5 to 6 cells/group; P = 0.56. Dashed line represents wild-type control EGABA. (J) Following stress exposure, EGABA remained depolarized in VTA GABA neurons of S940A heterozygotes, comparable to that observed in unstressed S940A mice. TCB-2 did not significantly alter EGABA relative to untreated slices after stress exposure: n = 4 to 6 cells/group; P = 0.23.
KCC2 function can be altered by kinase activity (33, 34), and kinases are a common mechanism by which proteins are phosphorylated. Upon activation by agonist, 5-HT2ARs engage the Gq-type G protein and modulate cellular function through a number of effectors, including protein kinase C (PKC). Previously, PKC signaling has been shown to phosphorylate KCC2 S940 (34), so we hypothesized that TCB-2–mediated rescue of chloride accumulation in the VTA GABA neurons of stressed mice might occur via 5-HT2AR–induced PKC signaling. Therefore, in whole-cell configuration, we recorded chloride accumulation in VTA GABA neurons of stressed and control mice in the presence of TCB-2 while also intracellularly dialyzing the PKC inhibitor chelerythrine (20 μM) (20, 35) (Fig. 2F, light blue trace). Blocking intracellular PKC function prevented TCB-2-mediated recovery of chloride extrusion in VTA GABA neurons of stressed mice (Fig. 2G, light blue data: F = 22.4, n = 7 cells/group, P < 0.01), indicating that 5-HT2AR agonism leads to phosphorylation of KCC2 S940 by activating PKC signaling.
Next, we examined whether reduced KCC2 S940 phosphorylation was sufficient to dysregulate chloride homeostasis. We also wanted to avoid any confounds that may have arisen from off-target pharmacological effects in our mechanistic experiments. Therefore, we used KCC2 transgenic mice in which S940 is mutated to alanine (S940A), rendering the 940 site insensitive to kinase activity and impairing KCC2 transport activity (36). We found that haplodeficiency of S940 phosphorylation in unstressed heterozygous S940A mice resulted in depolarized EGABA in VTA GABA neurons (Fig. 2H), similar to the effects of stress in wild-type mice. This demonstrates that KCC2 S940 phosphorylation is indeed sufficient to cause the aberrant chloride gradient observed after stress. Additionally, TCB-2 (1 μM) application during recordings did not correct this depolarized EGABA (Fig. 2I): −57.7 ± 1.2 mV (S940A, purple data), −58.8 ± 1.2 mV (S940A + TCB-2, dark blue data), n = 6, 5 cells/group, respectively, and P = 0.56 by unpaired, 2-tailed t test, further indicating that 5-HT2AR activation normalizes chloride homeostasis in VTA GABA neurons after stress via phosphorylation of KCC2 S940. Acute stress exposure in S940A heterozygotes resulted in no additional depolarization in EGABA and was unaffected by TCB-2 treatment (Fig. 2J): −59.5 ± 1.6 mV (S940A, dark red data), −55.3 ± 3.2 mV (S940A + TCB-2, indigo data), n = 6, 4 cells/group, respectively, and P = 0.23 by unpaired, 2-tailed t test. The occlusion of this stress-induced plasticity suggests that KCC2 S940 phosphorylation is indeed the mechanism by which stress dysregulates chloride homeostasis in VTA GABA neurons.
5-HT2AR Activation Reverses Aberrant GABA- and Ethanol-Induced Firing of VTA GABA Neurons in Stressed Mice.
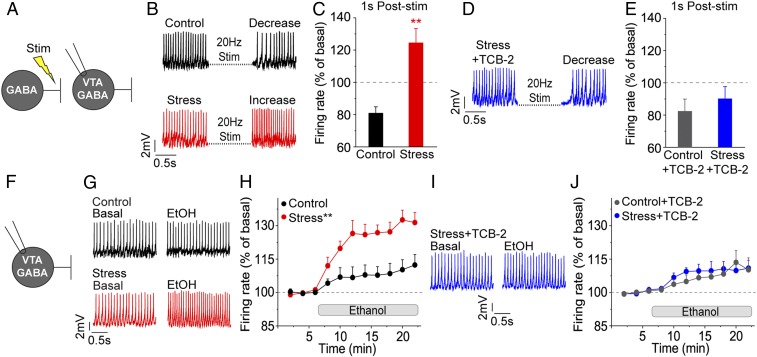
In our previous work in rats, we showed that stress resulted in compromised GABAAR-mediated inhibition of VTA GABA neurons and even caused paradoxical GABAAR-mediated excitation (6). We repeated those experiments in mice with the additional hypothesis that, because TCB-2 positively modulates KCC2 function, 5-HT2AR activation would recover GABA-mediated inhibition in the VTA GABA neurons of stressed mice. Thus, repetitive stimulation of GABAARs was performed during spontaneous action potential firing of VTA GABA neurons ex vivo (Fig. 3A). GABAergic inhibition of firing was observed in control mice (Fig. 3B), but as in our previous work, VTA GABA neurons from stressed mice were resistant to GABAergic inhibition and instead displayed paradoxical increased firing (Fig. 4 B and C): 124.5 ± 8.9% (Stress, red) vs. 80.9 ± 4.0% (Control, black), n = 9 cells/group, and P < 0.01 by unpaired, 2-tailed t test. Changes in firing rate were calculated for 1 s immediately following stimulation and normalized to the prestimulation period. TCB-2 application (1 μM) normalized GABAAR-mediated inhibition of VTA GABA neurons from stressed mice to control levels while having no impact on VTA GABA neurons from control mice (Fig. 3 D and E): 90.2 ± 7.4% (Stress + TCB-2, blue) vs. 82.5 ± 7.5% (Control + TCB-2, gray), n = 6 to 7 cells/group, and P = 0.48 by unpaired, 2-tailed t test, suggesting that 5-HT2AR activation corrects compromised GABAergic inhibition of VTA GABA neurons.
Fig. 3.
5-HT2AR activation normalizes GABA- and ethanol-induced VTA GABA neuron firing in stressed mice. (A) Cell-attached recordings of VTA GABA neurons were performed to observe changes in spontaneous action potential firing following electrical stimulation of GABAergic inputs. (B) Representative recordings from control and stressed mice revealed distinct poststimulation (20 Hz Stim) firing patterns. The control cell displayed canonical GABA-mediated inhibition, while the stress cell showed paradoxical excitation in response to stimulation. (C) When averaged over 1 s following the end of stimulation, normalized firing in VTA GABA neurons from stressed mice showed enhanced firing relative to the decrease in firing observed in controls: n = 9 cells/group; **P < 0.01. (D) In the presence of TCB-2 (1 μM), a VTA GABA neuron from a stressed mouse demonstrated GABA stimulation-induced decreased firing (i.e., restoration of GABAergic inhibition). (E) VTA GABA neurons exposed to TCB-2 from stressed and control mice revealed comparable GABA-mediated inhibition of firing: n = 6 to 7 cells/group; P = 0.48. (F) In a separate experiment, cell-attached recordings of VTA GABA neurons were made in the presence of ethanol (50 mM) to observe changes in ethanol-induced firing rate. (G) Ethanol produced a modest increase in firing of a control VTA GABA neuron relative to the baseline period, while a stress GABA neuron displayed a substantially greater increase in firing rate in response to ethanol. (H) We observed a significantly greater ethanol-induced increase in firing of GABA neurons from stressed mice when compared to controls: n = 10 to 12 cells/group; **P < 0.01. (I) TCB-2 application (1 μM) to a stressed slice revealed a marginal increase in GABA neuron firing in response to ethanol. (J) TCB-2 prevented the increased ethanol-induced firing in VTA GABA neurons from stressed mice relative to controls: n = 7 to 8 cells/group; P = 0.88.
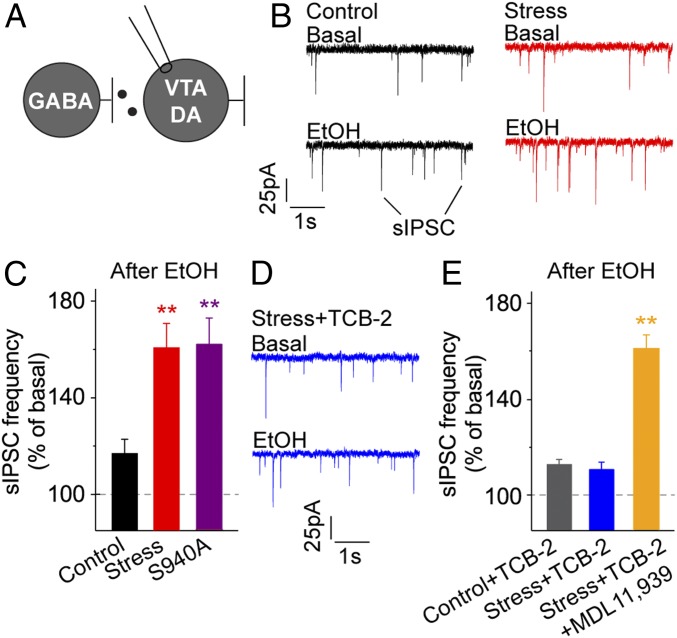
Fig. 4.
TCB-2 rescues normal ethanol-induced inhibitory drive onto VTA DA neurons following stress exposure. (A) In whole-cell configuration, sIPSCs in VTA DA neurons were recorded while holding at −60 mV. Changes in sIPSC frequency and amplitude were monitored in the presence of ethanol (50 mM). (B) Sample of sIPSC recordings in VTA DA neurons that demonstrated a robust enhancement of ethanol-induced sIPSC frequency in the stress group relative to controls. No differences in amplitude were observed between groups. (C) Ethanol-induced sIPSC frequency, relative to baseline, was significantly increased in the wild-type stressed and unstressed S940A groups when compared to wild-type controls: n = 5 to 7 cells/group; **P < 0.01. (D) TCB-2 (1 μM) decreased the sIPSC frequency in response to ethanol in VTA DA neurons from stressed mice. TCB-2 did not impact sIPSC amplitudes. (E) Ethanol-induced sIPSC frequency was reduced to the control level in VTA DA neurons from stressed mice in the presence of TCB-2. Blockade of 5-HT2ARs with the antagonist MDL11,393 (2 μM) prevented the effect of TCB-2 in the stressed group: n = 6 to 7 cells/group; **P < 0.01.
Our previous work has also shown that the acute stress procedure that we used resulted in an aberrant increase in VTA GABA neuron firing in response to ethanol, leading to a corresponding increase in ethanol-induced inhibitory drive to the VTA dopamine (DA) neurons (6). To first show that, in mice, stress results in the same irregular ethanol-induced inhibitory signaling in the VTA, we measured VTA GABA neuron action potential firing in the presence of ethanol (Fig. 3F). Bath application of ethanol (50 mM) (6, 12) onto control VTA slices resulted in a modest increase in firing rates (Fig. 3G, black traces). In contrast, a substantially greater increase in ethanol-induced firing was observed in VTA GABA neurons of stressed mice (Fig. 3G, red traces). Normalizing to the pre-ethanol baseline period revealed a significant difference between stressed (red data) and control (black data) firing in response to ethanol (Fig. 3H): group × time, F(10,438) = 5.20, P < 0.01, n = 12, and 10 cells/group, respectively.
Next, we hypothesized that, by correcting KCC2 function, 5-HT2AR activation would restore normal firing responses to ethanol. Slices were treated with TCB-2 (1 μM), which indeed normalized VTA GABA neuron firing in response to ethanol in stressed mice (Fig. 3I, blue traces). No significant difference was observed in normalized ethanol-induced firing rates in stressed (blue data) and control (gray data) groups in the presence of TCB-2 (Fig. 3J): group × time, F(10,19) = 0.48, P = 0.88, and n = 7, 8 cells/group, respectively, demonstrating that 5-HT2AR activation prevents stress-induced enhancement of VTA GABA neuron firing in response to ethanol.
5-HT2AR Activation in Stressed Mice Rescues Normal Ethanol-Induced GABAergic Inhibitory Drive to VTA DA Neurons.
VTA GABA neurons influence ethanol-related behavior, in part, by modifying DA neuron activity (6, 12, 37, 38). We hypothesized that stress-induced KCC2 impairment in VTA GABA neurons would correspond with enhanced inhibitory transmission onto DA neurons and that this circuit adaptation could be reversed by activation of 5-HT2ARs. We performed whole-cell voltage-clamp recordings in VTA DA neurons and monitored ethanol-induced changes in inhibitory transmission (Fig. 4A). Lateral VTA DA neurons were identified as previously reported (6, 12, 39) (SI Appendix, Fig. S1). In control mice, spontaneous IPSC (sIPSC) frequency onto control DA neurons moderately increased from baseline in response to ethanol (50 mM, Fig. 4B, black traces). In stressed mice, sIPSC frequency robustly increased relative to baseline in response to ethanol and significantly increased compared to controls (Fig. 4B, red traces). Similarly, in unstressed heterozygous S940A mice, sIPSC frequency was substantially increased in response to ethanol compared to wild-type controls, indicating that impaired KCC2 function is sufficient to cause aberrant inhibitory signaling to VTA DA neurons. Mean changes in the normalized sIPSC frequency during ethanol wash-in were significantly greater in the stressed and S940A groups relative to controls (Fig. 4C) by 1-way ANOVA: 116.5 ± 5.9% (Control, black data), 159.8 ± 10.0% (Stress, red data), and 160.63 ± 10.8% in the (S940A, purple data), F(2, 15) = 9.029, P < 0.01 (Dunnett’s post hoc test, **P < 0.01), n = 7, 6, 5 and cells/group, respectively. Bath application of TCB-2 (1 μM) prevented the ethanol-induced increase in sIPSC frequency in stressed mice (Fig. 4D, blue traces). Importantly, coapplication of the 5-HT2AR antagonist, MDL11,939 (2 μM) (20), blocked the effects of TCB-2 in stressed mice (Fig. 4E): 112.2 ± 2.7% (Control + TCB-2, gray data), 110.6 ± 3.2% in the (Stress + TCB-2, blue data), and 159.2 ± 5.5% (Stress + TCB-2 + MDL11,939, orange data), F(2, 17) = 49.96, P < 0.01 (Dunnett’s post hoc test, **P < 0.01), n = 7, 7, and 6 cells/group, respectively. Further demonstrating the specificity of TCB-2 action at the 5-HT2AR, bath application of a lower concentration of TCB-2 (0.1 μM) or a distinct 5-HT2AR agonist, 25CN-NBOH (1 μM), comparably suppressed the enhanced ethanol-induced sIPSC frequency in VTA DA neurons of stressed mice (SI Appendix, Fig. S4).
Discussion
Stress triggers neural plasticity that can promote addiction-related behaviors (40, 41). Here, we show that acute stress exposure in mice disrupts chloride homeostasis in the VTA by impairing KCC2 function and thereby altering midbrain GABAergic transmission in response to ethanol. Critically, these stress-induced alterations to VTA circuitry were pharmacologically reversible. Ex vivo administration of the 5-HT2AR agonist TCB-2 was sufficient to restore chloride homeostasis and normalize GABAergic responses to ethanol. At the molecular level, recovery of KCC2 function by TCB-2 was dependent on both intact second messenger signaling and phosphorylation of KCC2 S940. These data suggest that drugs, such as TCB-2, which can normalize the functional state of KCC2, may be particularly effective in treating conditions involving KCC2 impairment.
The 5-HT2AR agonist drugs are emerging as promising interventions for a number of neuropsychiatric conditions, including alcohol dependence and stress-related disorders (14, 15, 42, 43). While the mechanisms underlying the therapeutic outcomes associated with 5-HT2AR agonist treatment have remained unclear, evidence suggests that circuitry adaptations in the ventral midbrain are important. For example, the transition to escalating drug intake in rodents has been associated with inhibition-resistant VTA GABA neuron firing (38, 44), and intra-VTA infusion of a 5-HT2AR agonist can prevent such dependence-related transitions (45). We observed similar irregular VTA GABA neuron firing in response to GABAergic stimulation or ethanol application following acute stress exposure, which was ameliorated by TCB-2. Our findings are also consistent with a proposed regulatory role of 5-HT2ARs on VTA GABAergic transmission (26), as TCB-2 mitigated the stress-induced increase in inhibitory drive onto DA neurons in response to ethanol. Our previous work has also shown that correcting chloride homeostasis in the VTA decreases stress-induced ethanol self-administration (6), indicating that 5-HT2AR agonists may recruit similar mechanisms to achieve long-lasting therapeutic effects.
A growing preclinical literature has implicated KCC2 impairment in drug abuse (6, 12, 30, 32). KCC2 down-regulation renders VTA GABA neurons less sensitive to, or even excited by, GABA or GABA mimetics (6, 12). Under basal conditions, KCC2 deficiency does not overtly change GABAergic signaling, but repetitive GABAAR stimulation or exposure to ethanol overwhelms the limited chloride extrusion capacity of impaired KCC2 following stress (31, 41). This explains why we observed no differences in baseline GABA neuron firing rates or sIPSCs in DA neurons between stress and control groups. Dynamic in vivo regulation of KCC2 function occurs via posttranslational changes along the C-terminal, cytoplasmic domain (11, 33). Among these, phosphorylation of S940 positively modulates the transport function of KCC2 (34). While stress reduced the functionality of KCC2 in the VTA, ex vivo activation of 5-HT2ARs normalized the functional state of KCC2 by restoring phosphorylated S940 to the prestress level. To understand how 5-HT2AR activation modulates KCC2 phosphorylation, we pharmacologically inhibited PKC, which blocked the restorative effects of TCB-2. Additionally, we used a mouse with a genetic mutation of the KCC2 S940 site to alanine. This KCC2 deficiency in S940A heterozygous mice was also effective in preventing 5-HT2AR–mediated normalization of EGABA following acute stress exposure and recapitulated the effects of stress on ethanol-induced GABAergic transmission. These data suggest that stress-induced KCC2 impairment in VTA GABA neurons is mediated by PKC signaling and phosphorylation of S940 induced by 5-HT2AR activation and that disruption of S940 phosphorylation is sufficient to dysregulate GABA transmission in the VTA. Finally, while our results indicate KCC2 impairment as the critical mediator of aberrant GABAergic signaling in the VTA following stress, future work is needed to assess potential alterations in other transporters involved in chloride homeostasis, such as the chloride ion importer NKCC1 (11).
Negative motivational conditions, like stress, and reward states may converge in the VTA to modulate KCC2 function, thereby altering GABA signaling and promoting maladaptive behaviors (41). Our data implicate this VTA circuitry as an important locus of 5-HT2AR agonist effects. Taken together, we provide mechanistic insight into the therapeutic action of 5-HT2AR agonists through correction of stress-perturbed chloride homeostasis and inhibitory transmission in the ventral midbrain.
Materials and Methods
Detailed methods are provided in SI Appendix, SI Materials and Methods.
Animals.
Adult (8 to 16 wk of age) wild-type C57BL/6J and heterozygous S940A knock-in mice (36) were used in all studies in compliance with guidelines specified by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Slice Electrophysiology.
Electrophysiological recordings were performed as previously described (6, 12). In experiments using 5-HT2AR agonists, slices were perfused with agonist-containing artificial cerebrospinal fluid (aCSF) solution for at least 10 min prior to recordings.
Western Blot Analysis.
Whole-cell VTA lysates were probed with KCC2-specific antibodies as previously described (6, 12). In experiments with TCB-2 (1 μM), slices were incubated for 20 to 30 min in aCSF containing TCB-2 prior to punch collection.
Data Availability.
All manuscript and SI Appendix raw data have been uploaded to Open Science Framework, https://osf.io/abfu5/?view_only=ee1b7a7bd82b460e80942dfdcf709ba6 (DOI: 10.17605/OSF.IO/ABFU5).
Supplementary Material
Acknowledgments
The S940A knock-in mice were kindly provided by Dr. Stephen Moss and his colleagues (Sackler School of Graduate Biomedical Sciences, Tufts University, Boston, MA). This work was funded by NIH National Institute on Alcohol Abuse and Alcoholism Grants F31 AA026766 (to B.A.K.) and R01 AA026267 (to J.A.D.); National Institute of Neurological Disorders and Stroke Grant R01 NS021229 (to J.A.D.); and National Institute on Drug Abuse Grants R01 DA009411 (to J.A.D.) and K01 DA048134 (to A.O.). This work also was supported by a generous award from the Chernowitz Medical Research Foundation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All raw data have been deposited to Open Science Framework, https://osf.io/abfu5/?view_only=ee1b7a7bd82b460e80942dfdcf709ba6 (DOI: 10.17605/OSF.IO/ABFU5).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911446116/-/DCSupplemental.
References
- 1.Uhart M., Wand G. S., Stress, alcohol and drug interaction: An update of human research. Addict. Biol. 14, 43–64 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keyes K. M., Hatzenbuehler M. L., Grant B. F., Hasin D. S., Stress and alcohol: Epidemiologic evidence. Alcohol Res. 34, 391–400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodie M. S., Shefner S. A., Dunwiddie T. V., Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 508, 65–69 (1990). [DOI] [PubMed] [Google Scholar]
- 4.Saal D., Dong Y., Bonci A., Malenka R. C., Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37, 577–582 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Niehaus J. L., Murali M., Kauer J. A., Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur. J. Neurosci. 32, 108–117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostroumov A., et al. , Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron 92, 493–504 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaila K., Ionic basis of GABAA receptor channel function in the nervous system. Prog. Neurobiol. 42, 489–537 (1994). [DOI] [PubMed] [Google Scholar]
- 8.Rivera C., et al. , The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Coull J. A., et al. , Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Doyon N., et al. , Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis. PLoS Comput. Biol. 7, e1002149 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaila K., Price T. J., Payne J. A., Puskarjov M., Voipio J., Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 15, 637–654 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas A. M., et al. , Adolescent nicotine exposure alters GABAA receptor signaling in the ventral tegmental area and increases adult ethanol self-administration. Cell Rep. 23, 68–77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogenschutz M. P., Studying the effects of classic hallucinogens in the treatment of alcoholism: Rationale, methodology, and current research with psilocybin. Curr. Drug Abuse Rev. 6, 17–29 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Bogenschutz M. P., et al. , Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 29, 289–299 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Carhart-Harris R. L., et al. , Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 3, 619–627 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Nichols D. E., Johnson M. W., Nichols C. D., Psychedelics as medicines: An emerging new paradigm. Clin. Pharmacol. Ther. 101, 209–219 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Nielson E. M., May D. G., Forcehimes A. A., Bogenschutz M. P., The psychedelic debriefing in alcohol dependence treatment: Illustrating key change phenomena through qualitative content analysis of clinical sessions. Front. Pharmacol. 9, 132 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollenweider F. X., Kometer M., The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat. Rev. Neurosci. 11, 642–651 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Fox M. A., French H. T., LaPorte J. L., Blackler A. R., Murphy D. L., The serotonin 5-HT(2A) receptor agonist TCB-2: A behavioral and neurophysiological analysis. Psychopharmacology (Berl.) 212, 13–23 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Bos R., et al. , Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc. Natl. Acad. Sci. U.S.A. 110, 348–353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong R., Yu B., Chen L., Yu W., The 5-HT2A receptor potassium-chloride cotransporter 2 signaling pathway in a rat incision pain model. Exp. Ther. Med. 12, 3583–3588 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Brualla I., et al. , Activation of 5-HT2A receptors restores KCC2 function and reduces neuropathic pain after spinal cord injury. Neuroscience 387, 48–57 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Medina I., et al. , Current view on the functional regulation of the neuronal K(+)-Cl(−) cotransporter KCC2. Front. Cell. Neurosci. 8, 27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahadevan V., Woodin M. A., Regulation of neuronal chloride homeostasis by neuromodulators. J. Physiol. 594, 2593–2605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nocjar C., Roth B. L., Pehek E. A., Localization of 5-HT(2A) receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience 111, 163–176 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Bankson M. G., Yamamoto B. K., Serotonin-GABA interactions modulate MDMA-induced mesolimbic dopamine release. J. Neurochem. 91, 852–859 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Cornea-Hébert V., Riad M., Wu C., Singh S. K., Descarries L., Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J. Comp. Neurol. 409, 187–209 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Doherty M. D., Pickel V. M., Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Res. 864, 176–185 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Staley K. J., Soldo B. L., Proctor W. R., Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 269, 977–981 (1995). [DOI] [PubMed] [Google Scholar]
- 30.Ben-Ari Y., Khalilov I., Kahle K. T., Cherubini E., The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 18, 467–486 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Hewitt S. A., Wamsteeker J. I., Kurz E. U., Bains J. S., Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 12, 438–443 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Taylor A. M., et al. , Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology 41, 949–959 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahle K. T., et al. , Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci. 36, 726–737 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H. H., et al. , Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J. Biol. Chem. 282, 29777–29784 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Liu Y., Chen X., Cholinergic excitation of dopaminergic cells depends on sequential activation of protein kinase C and the L-type calcium channel in ventral tegmental area slices. Brain Res. 1245, 41–51 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Silayeva L., et al. , KCC2 activity is critical in limiting the onset and severity of status epilepticus. Proc. Natl. Acad. Sci. U.S.A. 112, 3523–3528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morikawa H., Morrisett R. A., Ethanol action on dopaminergic neurons in the ventral tegmental area: Interaction with intrinsic ion channels and neurotransmitter inputs. Int. Rev. Neurobiol. 91, 235–288 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson A. C., et al. , Ventral tegmental area GABA neurons are resistant to GABA(A) receptor-mediated inhibition during ethanol withdrawal. Front. Neurosci. 12, 131 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T. A., Placzek A. N., Dani J. A., In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology 59, 431–436 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koob G. F., Le Moal M., Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat. Neurosci. 8, 1442–1444 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Ostroumov A., Dani J. A., Convergent neuronal plasticity and metaplasticity mechanisms of stress, nicotine, and alcohol. Annu. Rev. Pharmacol. Toxicol. 58, 547–566 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogenschutz M. P., Johnson M. W., Classic hallucinogens in the treatment of addictions. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 250–258 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Carhart-Harris R. L., Goodwin G. M., The therapeutic potential of psychedelic drugs: Past, present, and future. Neuropsychopharmacology 42, 2105–2113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargas-Perez H., et al. , Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science 324, 1732–1734 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vargas-Perez H., et al. , A single administration of the hallucinogen, 4-acetoxy-dimethyltryptamine, prevents the shift to a drug-dependent state and the expression of withdrawal aversions in rodents. Eur. J. Neurosci. 45, 1410–1417 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All manuscript and SI Appendix raw data have been uploaded to Open Science Framework, https://osf.io/abfu5/?view_only=ee1b7a7bd82b460e80942dfdcf709ba6 (DOI: 10.17605/OSF.IO/ABFU5).