Abstract
Attention is a common but highly complex term associated with a large number of distinct behavioral and perceptual phenomena. In the brain, attention-related changes in neuronal activity are observed in widespread structures. The many distinct behavioral and neuronal phenomena related to attention suggest that it might be subdivided into components corresponding to distinct biological mechanisms. Recent neurophysiological studies in monkeys have isolated behavioral changes related to attention along the 2 indices of signal detection theory and found that these 2 behavioral changes are associated with distinct neuronal changes in different brain areas. These results support the view that attention is made up of distinct neurobiological mechanisms.
Keywords: attention, nonhuman primate, single unit, visual cortex, signal detection theory
Attention has captured great interest in psychological and neuroscience research for over 100 y (1–3). This fascination is related to the broad explanatory power that is commonly invested with the concept of attention. Attention is generally used to indicate any brain function that influences the behavioral performance of an animal. One aspect of attention involves sustaining vigilance over multiple hours or even days to maintain performance across a task (4). A different aspect of attention involves switching engagement with different stimuli within a fraction of a second (5). Comparing just these 2 aspects of attention, it is clear that phenomena embraced by the term span broad timescales and goals, ranging from glancing at a stimulus to completing a long-lasting task. As expected from its expansive scope, its definitions vary substantially across neurophysiological studies of attention—the focus of this review. Definitions of attention commonly involve the concepts of limited capacity for sensory encoding and the resulting need for selective processing (6, 7). A popular account defines attention as a biased competition that occurs in multiple brain structures and stages of processing, with the goal of appropriately assigning limited resources for sensory representation for the immediate interest of the subject (6). Other definitions do not depend on the concept of limited resources for stimulus representation and, instead, see attention’s primary role as dealing with too many resources for the limited number of effectors (8). Some views emphasize that a limited quantity being selected or competed for could be either sensory or motor (9). The loci of competition or selection are controversial. Some emphasize that selection is first resolved in the cortical areas associated with higher processing, such as the prefrontal and parietal cortices, and then broadcasted to areas associated with earlier processing (9, 10). Others emphasize that selection is resolved in the basal ganglia, and modulations of firing rates of neurons in neocortex result from this subcortical selection process (11).
The lack of a clear, consistent definition of attention suggests that we lack a clear understanding of what attention is. Given the breadth of the phenomena embraced by attention, it likely depends on multiple distinct constituent mechanisms. Many conceptual subdivisions of attention have been proposed, task procedures have been developed to separately operationally define the components of those subdivisions, and the behavioral effects associated with those subdivisions have been extensively measured. However, their biological correlates are rarely as discrete and segregated. Therefore, we currently do not have an understanding of attention in terms of distinguishable biological mechanisms. A promising approach for progress is to seek out biological mechanisms related to conceptual components of attention that are reliably distinguished.
The study of the neuronal mechanisms supporting memory provides a useful analogy. At one point, it was thought that memory was distributed across cortex, and different brain regions contributed to memory in ways that are largely equivalent (12). It is now recognized that different components of memory depend on separate brain structures (13, 14). The acquisition of facts and events depends preferentially on the medial temporal lobe and the diencephalon, while the gradual, feedback-guided learning that results in skills and habits depends preferentially on the neostriatum. Delayed eyeblink conditioning depends critically on the cerebellum, and is entirely preserved after hippocampal lesions. Emotional learning, such as fear conditioning, depends essentially on the amygdala. Therefore, while the mechanisms of memory are distributed in the brain, different features are contributed by separate structures.
Vision is similarly supported by distinct functional components that depend on different brain structures (15, 16). The visual cortex and superior colliculus (SC) mediate different visual functions, and within visual cortex, there are 2 diverging streams of processing. One projects ventrally and eventually reaches inferotemporal cortex, and the other projects dorsally and passes through posterior parietal cortex (17, 18). The ventral pathway processes visual attributes important for the discrimination and recognition of colors, patterns, and objects, whereas the dorsal stream extracts the features of visual stimuli relevant to actions on those objects, such as visually guided grasping, reaching, or foveating. The example of vision is particularly relevant. Given the range of behaviors associated with attention, it would be unsurprising if visual attention similarly manifests from distinct circuits and mechanisms that contribute to separate behavioral and perceptual functions related to vision. The diversity of behavioral changes currently related to visual attention might be a direct consequence of the specialized functions of separate visual structures.
Our focus is here to discuss how attention can be divided into separable components that work together to produce the observed behavioral effects. We discuss recent efforts to identify component mechanisms using signal detection theory to isolate the effects on behavioral performance associated with visual spatial attention. These studies demonstrate that neurobiological mechanisms of attention can be distinguished according to attention-related behavioral effects, and that signal detection theory is a useful starting point for this approach.
We primarily consider electrophysiological studies of visual attention in nonhuman primates, which include most of the single-neuron studies on attention. The visual system has been more extensively studied compared with other sensory modalities, thereby providing a strong foundation for the experiments on design and interpretation of attention-related modulations of neuronal activity. Primates have many visual cortical areas (19), which may mean that separate areas have more specialized representation and thereby facilitate interpretation of signals. However, it is possible that attention to different modalities or in different species consists of different mechanisms. While studies of visual attention in monkeys consist of a valuable domain of attention, exploration of attention in other systems might reveal mechanistic principles not shared in primate vision.
Many Conceptual but Fewer Biological Distinctions
The notion that attention includes distinct components and forms is well established. First, attention can be more or less selective. Selective attention refers to improvements in performance for a specific task, object, or stimulus feature that is significant or salient to an animal, and it is commonly studied in neurophysiological studies. Nonselective attention is related to effort or arousal for producing and maintaining a high level of performance in a broad range of tasks. Second, attention is also subdivided according to what caused it to be deployed. Attention can be drawn by the physical salience of a stimulus such as an abrupt flash (“bottom-up” attention), driven by internal factors under voluntary control such as a rule (“top-down”), or due to the lingering effects of what the subject previously attended (7, 8). Third, attention is further subdivided between overt attention, when it is associated with a detectable movement, typically of a sensory structure, such as the eye or the pinna, and covert attention, when attention shifts with no outward movement. Finally, attention can be directed to a spatial location or to a sensory feature, such as a color, tone, or odor.
Despite these clear conceptual separations, their biological correlates observed so far are less distinct. A biological distinction involves differences in the physiological activity of the neurons in anatomically separate structures or differences in behavioral deficit after a perturbation of those structures. Therefore, these conceptual dimensions remain opportunities for dissecting mechanisms of attention.
The changes in neuronal activity in visual cortex related to either nonselective or selective visual attention are qualitatively similar, involving increases in firing rates (20) and decreases in spike count correlation (21). Multiple neuromodulators are involved in attention (22). One such neuromodulator, acetylcholine, is involved in not only global state changes but also the more selective visual spatial attention (23), but acetylcholine transmission is also involved in nonselective attention.
Goal-directed and salience-directed attention are associated with partially segregated groups of brain areas, as assessed in human imaging and clinical studies (24, 25). In psychophysical studies, these 2 aspects of attention have been described as having shared but distinct behavioral effects and underlying mechanisms (11–13). Changes in neuronal firing rates related to either bottom-up or top-down attention have been observed in widespread brain regions, spanning the earlier and later stages of cortical processing, from V1 (26) to dorsolateral prefrontal cortex (27), and it remains unknown whether separate brain areas preferentially contribute to either of these forms of attention.
When shifting attention to a visual location, one can do so with or without an eye movement. Selective changes in the firing rates related to either aspect of attention have been observed across widespread brain areas, including not only association areas with a mix of visual and motor responses (9, 28, 29) but also visual cortical areas with predominantly sensory-driven responses (30, 31). While it is clear that these 2 aspects of attention share the common neural correlate of involving the increases in firing rates of visual and visuomotor neurons across brain structures (32), the mechanism that controls whether attention is accompanied by a saccade is unclear.
Comparison between attention to a location and attention to a stimulus feature, such as color or direction of motion, provides an example of how distinct components of attention might involve a common mechanism at the level of a single neuron’s firing rates, but nonetheless depend on different contributions from separate brain structures. When attention is directed to either a location or a stimulus feature, a neuron’s activity undergoes a gain change, typically increasing in responsiveness when the attended location or feature is preferred by the neuron and decreasing when it is not preferred (33, 34). This similarity has led to the proposal that these 2 types of neuronal modulations involve the same mechanism (35). In this view, a location in space is simply one of many features to which attention might be directed. The gain of a neuron’s attention-related modulation for any feature, including spatial location, is proportional to the extent to which the features being attended correspond to those that the neuron prefers (36). This view is supported by the finding that the relationship between different aspects of attention-related changes in neuronal firing rates is similar for spatial and feature-based attention (37). Furthermore, it is hypothesized that the perceptual effects of either spatial or feature-based attention depend on the shared mechanism of differentially modulating the gain of neuronal responses according to the similarity between a neuron’s sensory preference and the attended features, amplifying the responses of neurons that prefer the attended feature and reducing the gain for those with the opposite preference (38). However, the relative level of neuronal selectivity for space or a particular stimulus feature, such as color, differs across different brain structures. It is therefore expected that separate brain areas provide different relative levels of contribution. Consistent with this expectation, a recent study found that separate prefrontal cortical regions show different neuronal modulations related to spatial and feature-based attention, and inactivation of these regions has distinct effects on feature-based attention (39). However, other views propose very different modes of routing visual information for spatial and feature-based attention (40), and it remains unresolved at what mechanistic level these 2 aspects of attention diverge.
Visual Spatial Cueing Paradigm
Neurophysiological studies of attention generally seek to vary the subject’s attentional state while maintaining identical sensory stimulation. One popular paradigm that satisfies this goal involves orienting the subjects’ attention to a restricted position of visual space while the subjects keep their gaze fixed on a central location. This procedure is often referred to as the Posner cueing paradigm for its elegant implementation by Posner et al. (26) to demonstrate attention-related changes in reaction times. However, many of the studies we discuss here deviate from this particular implementation in the form of the cue, the contingency between the stimulus and the cue, and the readout of the effects of attention. We therefore refer to this family of tasks as the visual spatial cueing paradigm (Fig. 1).
Fig. 1.
In a typical variant of the visual spatial cueing paradigm, an observer must detect a signal that could appear at either of 2 locations in visual space. The subject begins a trial by fixating on a marker in the center of a display. A cue appears briefly to indicate the position where a signal is more likely to appear. In a majority of trials (e.g., 80%), the cue is valid in indicating the position of the signal (in this example, a low-contrast grating pattern). In valid trials compared with invalid trials, the observer’s reaction time is typically shorter and the probability of detecting the signal is higher, even when the stimulus configurations are identical between the 2 types of trials. These behavioral differences are attributed to greater attention directed to a given location during a valid trial compared with an invalid trial.
The simple design of visual spatial cueing tasks avoids confounds related to different stimulus conditions and can yield large, highly reproducible behavioral effects. Moreover, it is easily adapted for monkey subjects, which can readily attend to different parts of visual space without moving their eyes (3). By directing an animal’s attention to different visual positions while keeping the stimulation on the retina identical, this paradigm makes it possible to measure how responses of a single neuron to a given stimulus vary depending on whether the subject’s attention is directed toward or away from that stimulus, as well as to relate neuronal changes to changes in the observer’s performance.
This approach has long been used to study the behavioral and perceptual effects related to visual spatial attention. When attending to a visual location, compared with when attending elsewhere, stimuli are typically detected more quickly (26), discriminated with greater behavioral sensitivity (27), and reported with a more liberal criterion (41). Moreover, at the attended location, contrast sensitivity is increased (42); spatial resolution is enhanced, even in tasks where further increases in spatial resolution impair accuracy (43); and apparent contrast is increased (33).
Widespread Involvement of Brain Areas in Visual Spatial Attention
Attention-related changes in visual responses have been reported in many brain areas, including most of visual cortex, prefrontal cortex, and nuclei in the thalamus and midbrain (8, 28, 29). With the exception of the retina, every brain structure investigated that has spatially selective visual responses has been found to have modulations of neuronal activity related to visual spatial attention.
When attention is directed to a neuron’s receptive field, neuronal responses to visual stimuli are typically stronger than when attention is directed elsewhere (8, 28, 29). Because the variance of spike counts is typically proportional to the mean number of spikes elicited by a stimulus, the increase in the mean response related to attention increases the ratio of the mean to SD (hence, the signal to noise) of visual cortical responses (34). Attention is also related to a reduction in the variability in spike count (in windows on the order of 100 ms) across trials (36), and this reduction has been observed across visual cortex from V1 (37) to lateral prefrontal cortex (44). The across-trial variability in spike counts is weakly correlated between pairs of nearby neurons (often referred to as “noise correlations”), and this correlation is typically reduced when attention is directed to the receptive fields of those neurons (45, 46). These attention-related changes in neuronal activity can improve the readout of the visual stimulus based on the neuronal responses, depending on the decoding scheme (45); therefore, attention-related improvements in neuronal discriminability are often thought to contribute to the changes in behavioral performance that define attention.
While qualitatively similar attention-related neuronal changes can be observed across many brain areas, quantitative distinctions have been observed. Attention-related increases in firing rates are stronger in later levels of the visual processing hierarchy (47), even when the same manipulation is used to vary attention. When the latency of the modulation of firing rates in different visual cortical areas is compared, changes occur earlier in later stages of visual cortex (e.g., the lateral intraparietal area, LIP) than in earlier stages of visual cortex (e.g., the middle temporal visual area, MT) (48). Despite these quantitative distinctions, it is unknown to what extent attention-related modulations in different brain areas contribute to distinct behavioral effects related to visual spatial attention.
Experimental perturbations of brain areas in which attention-related neuronal changes have been observed are associated with changes that mimic attentional-related changes in behavioral performance. Electrical microstimulation of the frontal eye fields, lateral parietal cortex, or SC can produce behavioral improvements in visual detection or discrimination tasks similar to those associated with attention (49–52). Similarly, inactivating or lesioning some of these brain areas can impair behavioral performance in a spatially selective way (53–56). However, the contributions of these brain areas are not equivalent. A recent study compared the attention-related behavioral impairment using a γ-aminobutyric acid type A (GABAA) receptor agonist (muscimol) to inactivate the SC and the frontal eye fields (57). When the strength of the inactivation was balanced based on changes in saccade metrics, inactivation of the SC resulted in greater reduction in the attention-related improvements in target detection rates than inactivation of the frontal eye fields. Varying contributions from different brain areas suggest that they might contribute to different components of visual spatial attention.
Visual Spatial Attention Consists of Distinct Mechanisms
The uncertainty of the consequences of attention-related enhancement of neuronal firing rates for behavior was noted in one of the earliest single-neuron studies of attention: “It would be more likely to lead to a motor response, or it could present more information that could be stored as a memory trace” (3). However, whether neuronal modulations in any given brain area are associated with an increased tendency to respond or, alternatively, improved encoding of the stimulus has only been recently addressed. In most studies using a visual spatial cueing paradigm, observed changes in neuronal activity are attributed to the general process of attention, using terms such as “filtering” or “selection.” Neuronal changes in visual cortex are generally thought to improve the behavioral sensitivity of the animal. This interpretation is reasonable because the encoding or decoding of the stimulus can be improved through attention-related increases in firing rates (34), reductions in variability (36), and decreases in the correlations in spike count between pairs of nearby neurons (45, 46).
One approach to characterizing the changes in behavioral performance produced by visual spatial cueing is to decompose them into different underlying causes using the framework of signal detection theory (58) (Fig. 2). The 2 core parameters of signal detection theory, criterion (c) and sensitivity (d′), are defined to be orthogonal, meaning each can change independently without affecting the other. Any cueing-related increase in the probability of detecting the target (“hit rate”) might arise from either a more lax criterion (i.e., an increased tendency to respond) or an increase in d′ (i.e., better discrimination between signal and noise). Different human behavioral studies using variants of this paradigm have reported behavioral changes involving criterion, sensitivity, or both (27, 41, 59–61). Using separate, appropriately designed task manipulations for human observers, however, these 2 parameters can be independently controlled and selectively varied at different visual locations (62). Because behavioral criterion and sensitivity are orthogonally defined and can be independently controlled, they provide an opportunity to test whether these separate behavioral components of visual spatial attention depend on distinct networks of brain structures. One prediction of this hypothesis is that attention-related neuronal modulations in separate regions are differentially related in these 2 behavioral changes.
Fig. 2.
Orthogonal components of visual spatial attention can be defined using signal detection theory. (A) Signal detection theory supposes that when an observer is engaged in a detection task, each stimulus is transformed into an internal representation of evidence for whether a signal has occurred. However, the value of this evidence variable will vary from trial to trial due to noise. The distribution associated with the stimulus that contains a signal (“Signal + noise”) has a larger mean (i.e., stronger evidence) than the distribution associated with the stimulus containing only noise “Noise only”). In its simplest form (i.e., assuming the distributions are well- approximated by Gaussians and have equal SD), the signal detection model is fully specified by 2 parameters. Sensitivity, or d′, is the difference between the means of the 2 distributions normalized by their SD. Intuitively, one can see that as the 2 distributions (“Noise only” vs. “Signal + noise”) overlap less (larger d′), behavioral performance will improve. Criterion, or c, is the fixed value used by the observer to categorize a stimulus as either signal or noise. A stimulus that triggers an evidence value greater than the criterion is reported as signal and otherwise is reported as noise. (B) Parameters c and d′ of a signal detection model can be inferred based on the relative frequencies of different responses to stimuli: hits, misses, false alarms (FA), and correct rejections (CR). The relative frequency of each of these 4 responses corresponds to a separate area of the 2 distributions and fully specifies the model, and therefore c and d′. Since there are only 2 degrees of freedom (i.e., when expressed as probabilities, each column must sum to 1), the model is completely specified with a single number from each column (traditionally, “hit rate” and “false alarm rate”). (C) If one considers only the hit rate, any change between different cueing conditions might be due to a change in only criterion, only sensitivity, or both. It is thus crucial to also consider changes in the false alarm rate. (Left) If only the criterion has changed, an improvement in the hit rate can only come at the expense of an increased false alarm rate. (Right) If only sensitivity has improved, an increase in hit rate can be achieved with a decrease in false alarms.
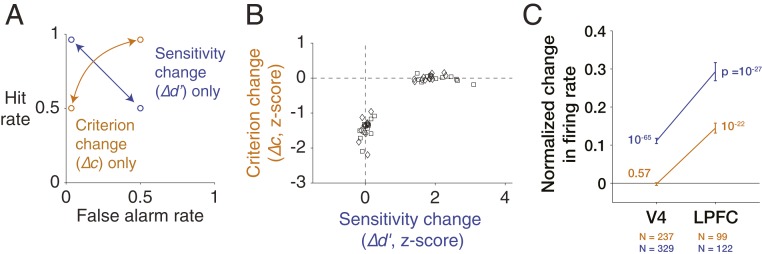
To examine this possibility, Luo and Maunsell (44, 63) trained rhesus monkeys to perform a standard variant of the visual spatial cueing paradigm in which a signal (orientation change) had to be detected at either of 2 stimulus locations. In each daily session, the animal switched in blocks of trials from attending to one stimulus versus another stimulus. Between these blocks, the reward contingencies were changed to encourage the animal to vary either its criterion or its sensitivity, but not both. A decrease in criterion consists of an increase in hit rate with an increase in false alarm rate, whereas an increase in sensitivity consists of an increase in hit rate and a decrease in false alarm rate (Fig. 3A). The animal’s criterion and sensitivity at the 2 stimulus locations were controlled by titrating reward values to different trial outcomes. Trained animals reliably changed only their criterion or only their sensitivity at a specific visual location, thereby allowing for neurophysiological examination of the correlates of isolated components (Fig. 3B).
Fig. 3.
Attention-related behavioral changes in either criterion or sensitivity are associated with distinct neural correlates. (A) Hypothetical example of a change in only behavioral criterion (orange) in terms of hit rate and false alarm rate and a hypothetical example of a change in only behavioral sensitivity (blue). (B) Isolated attention-related behavioral change in either criterion or sensitivity. Each marker indicates behavioral performance of one of 2 monkeys (different markers) in a single daily session. (C) Attention-related neuronal modulations in 2 cortical areas have distinct relationships with the 2 kinds of behavioral changes. Changes in neuronal firing rates in area V4 are associated with behavioral changes in sensitivity and not criterion, whereas changes in firing rates in lateral prefrontal cortex (LPFC) are associated with either behavioral change. Adapted with permission from ref. 44.
Single neurons were recorded from area V4 in visual cortex as well as the lateral prefrontal cortex (areas 45 and 46), where neurons exhibit robust visually evoked responses that are modulated by visual spatial attention (34, 64), motor preparation (31, 65), or visual working memory (66, 67). Lesions of either V4 or lateral prefrontal cortex can impair attention-related behavioral performance (54, 68). In V4, the neuronal modulations characteristically associated with attention, including stronger and less variable firing rates, were observed only in sessions when the animal changed its sensitivity, and not when the animal changed its criterion. This result implies that V4 does not contribute to attention-related behavioral changes in criterion.
In contrast to V4, neuronal responses in lateral prefrontal cortex were modulated when the animal changed either its criterion or sensitivity (Fig. 3C). In prefrontal cortex, the effects associated with these 2 shifts in behavior were not found in separate populations of neurons but, instead, were highly correlated on a cell-by-cell basis, typically with stronger modulations related to sensitivity changes rather than criterion changes. Taken together with the finding from V4, the result from prefrontal cortex indicates that attention-related neuronal modulations in different brain areas are not associated with a single component of attention but, instead, can be meaningfully distinguished according to their relationship to distinct behavioral changes. Prefrontal cortex participates in a set of brain areas separate from V4 that are involved in the control of behavioral criterion during changes in visual spatial attention.
Recent studies from other laboratories support the idea that different brain structures have distinct relationships to behavioral changes in criterion and sensitivity (69, 70). These reports focused on the SC, and, together, they indicate that neurons in the SC contribute to behavioral changes in either criterion or sensitivity, but with criterion being the more prominent component.
Lovejoy and Krauzlis (70) trained monkeys to discriminate the direction of a pulse of motion that could appear randomly at one of 4 locations (uncued condition) or at a cued location (cued condition). The effect of attention on performance was defined as the increase in d′ between the uncued and cued conditions. When the SC was pharmacologically inactivated, the cue-induced increase in d′ could not be detected in the affected region of visual space, indicating that SC is necessary for attention-related enhancement in behavioral sensitivity. SC inactivation also affected the subject’s criterion (termed “response bias” in this study), in that the subject exhibited a strong bias against making a saccade into the affected region, indicating that at least the motor component of the subject’s criterion was strongly affected by inactivation. However, a direct comparison between the impact of SC inactivation on cue-induced changes in d′ and cue-induced changes in criterion was not made in this study.
In another study, Sridharan et al. (69) analyzed 4 previous studies in which the SC was perturbed, through either microstimulation or pharmacological inactivation, while subjects performed tasks that required spatial attention (50, 51, 56, 71). In all cases, the changes in behavioral performance resulting from collicular perturbation could be accounted for by a change in behavioral criterion with no change in sensitivity. Consistent with this, for the 2 studies whose data were directly fit to models (56, 71), the effect of SC inactivation could be well explained by a combination of mostly changes in criterion and smaller changes in sensitivity.
These results from inactivation of the SC indicate that it contributes to attention-related changes in either criterion or sensitivity, with a greater contribution to criterion changes, and that its contribution to visual spatial attention is therefore distinct from that of area V4. The distinction in attention-related functions between SC and visual cortex is consistent with the little impact that SC inactivation has on the attention-related modulations of spike counts of visual cortical areas MT and the medial superior temporal area (MST) (71). Future studies that directly compare the attention-related neuronal changes or inactivation-induced behavioral deficits in terms of criterion and sensitivity can better distinguish the contributions of these structures to visual spatial attention.
Criterion and Sensitivity
The concepts of criterion and sensitivity provide a basis for demonstrating that visual spatial attention can be subdivided into distinct mechanisms based on behavioral effects. However, it is almost certain that they fail to provide a precise and complete description of the components’ mechanisms of attention. While the data described above suggest that criterion and sensitivity are at least correlated with neurobiologically meaningful components of attention, it remains to be seen how closely they align to such mechanisms. Additionally, there is little reason to believe that criterion and sensitivity are not themselves divisible into more basic components. Moreover, like the umbrella term attention, criterion and sensitivity often overlap with other constructs of cognitive behavior, such as reward and movement preparation. These issues are by no means unique to this scheme to subdivide attention, and they provide opportunities for achieving a more precise understanding of the organization of attention in brain.
Different Contributions to Behavioral Sensitivity.
In the psychophysical literature, a number of computational mechanisms have been proposed to explain how attention-related improvements in behavioral sensitivity might arise (72). The contributions of a subset of these mechanisms to sensitivity improvement have been compared directly within the framework of the perceptual template model (42). In this model, a signal is analyzed using a perceptual template in a way that is limited both by noise internal to the subject and by external noise that can experimentally manipulated. Cueing a visual location can improve behavioral sensitivity at that location in distinct ways, and these mechanisms are disambiguated by their different effects at varying levels of external noise. Studies using this approach have revealed that depending on the cueing procedure, a reduction of external noise, an enhancement of stimulus strength, or a combination of both mechanisms at the cued visual position can explain attention-related enhancement in behavioral sensitivity (42, 73). Given that distinct mechanisms of sensitivity improvement can be inferred based on behavioral performance, it is probable that multiple mechanisms distinguishable at the neuronal level contribute to attention-related changes in behavioral sensitivity.
Different Contributions to Behavioral Criteria.
Criterion can be an even more complex concept. In signal detection theory, criterion is an index of the observer’s tendency to categorize a stimulus as either only noise versus noise plus signal. The simplest view is that criterion depends on a mechanism that is essentially independent of sensory and motor influences. In this view, a possible implementation of behavioral changes in criterion is that 2 distinct groups of neurons represent the abstract categories of a noise-only stimulus and a signal-added stimulus, and the relative gain of these 2 groups is modulated to implement a criterion change. This is plausible because individual neurons have been shown to be able to represent an abstract category learned by a subject (74). However, in most tasks, the measured behavioral criterion is unlikely to depend on a mechanism that excludes sensory or motor influences.
In some tasks, criterion can depend strongly on sensory mechanisms. In the task used by Ferrera et al. (75), monkeys categorized the speed of a moving stimulus as either fast or slow. Across trials, the boundary across which the subject categorized was cued as either a slower boundary or a faster boundary, and the subject’s behavioral criterion changed accordingly. Conceptually, these behavioral changes in criterion could depend on a decision-related mechanism that changes the neural representation of the categorization boundary. Alternatively, independent of the decision boundary, the changes in the behavioral criterion could depend on biasing the sensory representation of the stimulus. The latter possibility is supported by recordings from neurons in the frontal eye fields. Neurons that preferred faster speeds increased the gain of their responses to the stimulus when the animal adopted the slower criterion and decreased their gain when the animal adopted the faster criterion. Neurons that prefer slower speeds exhibited the opposite modulation pattern. These neuronal changes are conceptually similar to those related to feature attention observed in earlier stages of visual cortical processing, such as MT (76). Therefore, changes in an animal’s behavioral criterion could depend on modulations of sensory representations.
Even in a detection task, behavioral criterion can be sensitive to the spike counts of sensory neurons. In a recent study (77), mice were trained to detect an orientation change from a static visual grating, and the strategy of the mice could be explained by a downstream circuit that sums the spike counts of V1 neurons, assigning higher weights to those more strongly preferring the target orientations. Therefore, the decision variable of the mice in this task could be approximated in units of V1 spike counts. When V1 excitability was either increased or decreased optogenetically, the mice showed no detectable change in sensitivity but showed changes in their criterion in the direction predicted by optogenetic changes in V1 firing rates.
A task might engage several criteria simultaneously. The task used by Luo and Maunsell (44, 63) concerned a criterion for reporting the presence or absence of a signal at a specific location. However, because the animal reported a signal at a visual location with a saccade to that location, the behavioral criterion measured in this task could depend on premotor mechanisms that underlie the tendency to make saccades of a particular direction. A different recent study shows that the SC contributes to multiple distinct behavioral criteria (78). In this study, monkeys decided whether there is structure in a noisy visual stimulus that fell on the center of gaze, and they reported their decision by making a saccade to one of 2 spots of different colors. The choice spots changed locations across trials; therefore, a given choice was not fixed to a particular motor response. The animal’s criterion was primed using a block of trials in which the proportion of structured stimuli was increased (or decreased) to induce a liberal (or conservative) criterion, while keeping sensitivity fixed. When single neurons were recorded in the intermediate layer of the SC in this task, their premovement firing rates increased when the animal was biased toward making a saccade into the neurons’ receptive field. Because the location of the choice target was randomized, the sign of the modulation did not directly reflect the animal’s criterion for categorizing whether a stimulus contains structure. However, microstimulation of the SC during this task shifted the criterion in a way that is independent of saccades, consistent with the previous finding of representations of an abstract perceptual decision in the neuronal spike counts in SC (79). This study indicates that the premovement firing rates of SC neurons correlate with the motor component of the subject’s criterion, and that the SC is also involved controlling a nonmotor component of the subject’s criterion, although perhaps not directly through the gain of premovement activity of intermediate-layer SC neurons. Moreover, this study suggests that inactivations of SC (69, 70) might simultaneously disrupt multiple distinct behavioral criteria.
Overlap with Other Cognitive Concepts.
In most tasks that manipulate any component of a subject’s attention, other cognitive processes, such as reward expectation, motor preparation, or working memory, are also affected. These cognitive variables are associated with many of the same neuronal changes, such as changes in the gain of responses to stimuli. A typical neurophysiological study of cognitive behavior provides separable operational definitions of only a subset of these variables. Therefore, a neurobiological mechanism described as related to a cognitive construct, such as criterion or sensitivity, might be more closely aligned with another. In some cases, constructs used to describe different cognitive processes can be indistinguishable, devolving to semantic arguments. For example, when the broadest definition of “reward” is used, there is little basis for distinguishing the location where a subject directs its attention from the location a subject expects to be most rewarding (80). While overlap might be inevitable when concepts are understood at a high level of abstraction, it is possible that more precisely specified concepts can be dissociated.
A recent study distinguished between the relative reward associated with a stimulus that is normalized to the reward for other stimuli and the absolute reward associated with a stimulus that is independent of other stimuli. This study found that whereas the behavioral sensitivity of monkeys in visual orientation discrimination depended more closely on the relative reward of a stimulus than on its absolute reward, neuronal activity in area V4 more closely correlated with absolute than relative reward (81). This finding indicates that changes in behavioral sensitivity cannot be accounted for solely by characteristic attention-related changes in V4 responses but also depend on selection mechanisms in downstream brain areas. It is expected that modulation of V4 neuronal responses cannot improve a subject’s sensitivity in all task conditions, and it is important to determine under what conditions attention-related modulations of visual cortical responses can contribute to behavioral sensitivity.
The dissociation between criterion and sensitivity in recent neurophysiological studies of attention parallels a line of work in attention studies largely conducted in humans that distinguishes between expectation and attention (82). In this work, expectation is defined by behavioral changes due to knowledge of the probability that a signal will occur and attention is defined by behavioral changes due to knowledge of stimulus relevance. When signal probability is manipulated, the subject’s criterion typically changes with little or no change in behavioral sensitivity. When stimulus relevance is varied, the subject’s sensitivity typically changes with minimal changes in criterion (62). Therefore, even though the cognitive terms are defined primarily by task manipulation, the resulting behavioral dissociation is between criterion and sensitivity. When electroencephalogram markers of early sensory processing were measured while strictly varying the signal probability (and not stimulus relevance) of visual features, little or no effect was found accompanying large changes in criterion, suggesting that behavioral changes in criterion even for visual features do not depend on early visual cortex (83).
The difference in terminology regarding whether manipulations of signal probability should be considered to vary attention reflects the continuing uncertainty of how attention should be defined. We suggest that because manipulations of signal probability have been part of operational definitions of attention throughout the research on attention, it would help in relating to this previous literature to see them as having manipulated distinct mechanisms of attention. Because attention has been used so broadly and its definition is still controversial, discussions about terminology could be minimized by treating distinct phenomena related to attention as its components and avoiding labels of what is, and what is not, attention. As long as terms related to (or excluded from) attention are distinguished by operational definitions, the understanding of attention will eventually depend less on terminology and more on biological structure and mechanisms.
Conclusion
Attention often has the appearance of a unitary system, perhaps because many of the behavioral consequences that define attention often covary. However, some attention-related effects are reliably dissociable in animal subjects, which can be used to dissect its neurobiological mechanisms. Other brain functions that appear unitary depend, in fact, on anatomically distinct mechanisms. In vision, multiple distinct processes often cooccur. When we reach to grasp a coffee mug, we typically also recognize that it is a coffee mug. However, the action on and the recognition of an object rely on largely separate visual cortical pathways. Similarly, different forms of learning often cooccur. When we learn to ride a bicycle, we acquire not only the motor skills but also the episodic experiences; however, again, these 2 forms of learning depend on largely separate brain structures. It is therefore likely that attention can be subdivided into neurobiological mechanisms, and such efforts can deepen our understanding and refine the terms we use to describe attention and related brain functions.
Subdividing attention according to its associated effects on performance is a promising approach for understanding attention in biological terms. Many important questions remain to be addressed. For example, how does the neuronal activity in the SC relate to behavioral changes in criterion or sensitivity? How do the behavioral deficits of inactivating either area V4 or lateral prefrontal cortex compare with those of inactivating the SC? Attention-related improvements in a viewer’s sensitivity can depend on multiple distinct mechanisms that can be inferred from performance using noisy stimuli (42). Do these behaviorally inferred mechanisms correspond to distinct neurobiological mechanisms? Similarly, attention-related changes in an observer’s criterion can depend on modulations of neuronal representations of stimulus features, abstract categories, or motor intentions. Is this a useful way to dissect the mechanisms of attention-related change in criterion? Future experiments aimed at questions such as these will bring the neuronal correlates of attention into sharper focus.
Acknowledgments
We thank Richard T. Born, Jackson J. Cone, and Supriya Ghosh for comments. This work was supported by NIH Grants R01EY005911 and F32MH115416.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Using Monkey Models to Understand and Develop Treatments for Human Brain Disorders,” held January 7–8, 2019, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. NAS colloquia began in 1991 and have been published in PNAS since 1995. From February 2001 through May 2019 colloquia were supported by a generous gift from The Dame Jillian and Dr. Arthur M. Sackler Foundation for the Arts, Sciences, & Humanities, in memory of Dame Sackler's husband, Arthur M. Sackler. The complete program and video recordings of most presentations are available on the NAS website at http://www.nasonline.org/using-monkey-models.
This article is a PNAS Direct Submission.
References
- 1.Nakayama K., Mackeben M., Sustained and transient components of focal visual attention. Vision Res. 29, 1631–1647 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Hubel D. H., Henson C. O., Rupert A., Galambos R., Attention units in the auditory cortex. Science 129, 1279–1280 (1959). [DOI] [PubMed] [Google Scholar]
- 3.Goldberg M. E., Wurtz R. H., Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J. Neurophysiol. 35, 560–574 (1972). [DOI] [PubMed] [Google Scholar]
- 4.Fortenbaugh F. C., DeGutis J., Esterman M., Recent theoretical, neural, and clinical advances in sustained attention research. Ann. N. Y. Acad. Sci. 1396, 70–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kröse B. J., Julesz B., The control and speed of shifts of attention. Vision Res. 29, 1607–1619 (1989). [DOI] [PubMed] [Google Scholar]
- 6.Desimone R., Duncan J., Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Knudsen E. I., Fundamental components of attention. Annu. Rev. Neurosci. 30, 57–78 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Maunsell J. H. R., Neuronal mechanisms of visual attention. Annu. Rev. Vis. Sci. 1, 373–391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisley J. W., Goldberg M. E., Attention, intention, and priority in the parietal lobe. Annu. Rev. Neurosci. 33, 1–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore T., Armstrong K. M., Fallah M., Visuomotor origins of covert spatial attention. Neuron 40, 671–683 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Krauzlis R. J., Bollimunta A., Arcizet F., Wang L., Attention as an effect not a cause. Trends Cogn. Sci. (Regul. Ed.) 18, 457–464 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lashley K. S., Brain Mechanisms and Intelligence: A Quantitative Study of Injuries to the Brain (University of Chicago Press, Chicago, IL, 1929). [Google Scholar]
- 13.Squire L. R., Mechanisms of memory. Science 232, 1612–1619 (1986). [DOI] [PubMed] [Google Scholar]
- 14.Squire L. R., Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 82, 171–177 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Schneider G. E., Two visual systems. Science 163, 895–902 (1969). [DOI] [PubMed] [Google Scholar]
- 16.Mishkin M., Ungerleider L. G., Macko K. A., Object vision and spatial vision: Two cortical pathways. Trends Neurosci. 6, 414–417 (1983). [Google Scholar]
- 17.Goodale M. A., Milner A. D., Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25 (1992). [DOI] [PubMed] [Google Scholar]
- 18.Ungerleider L. G., Mishkin M., “Two cortical visual systems” in Analysis of Visual Behavior, Ingle D. J., Goodale M. A., Mansfield R. J. W., Eds. (The MIT Press, Cambridge, MA, 1982), pp. 549–586. [Google Scholar]
- 19.Felleman D. J., Van Essen D. C., Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991). [DOI] [PubMed] [Google Scholar]
- 20.Spitzer H., Desimone R., Moran J., Increased attention enhances both behavioral and neuronal performance. Science 240, 338–340 (1988). [DOI] [PubMed] [Google Scholar]
- 21.Ruff D. A., Cohen M. R., Global cognitive factors modulate correlated response variability between V4 neurons. J. Neurosci. 34, 16408–16416 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiele A., Bellgrove M. A., Neuromodulation of attention. Neuron 97, 769–785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrero J. L., et al. , Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 454, 1110–1114 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbetta M., Shulman G. L., Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Shomstein S., Lee J., Behrmann M., Top-down and bottom-up attentional guidance: Investigating the role of the dorsal and ventral parietal cortices. Exp. Brain Res. 206, 197–208 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posner M. I., Snyder C. R., Davidson B. J., Attention and the detection of signals. J. Exp. Psychol. 109, 160–174 (1980). [PubMed] [Google Scholar]
- 27.Bashinski H. S., Bacharach V. R., Enhancement of perceptual sensitivity as the result of selectively attending to spatial locations. Percept. Psychophys. 28, 241–248 (1980). [DOI] [PubMed] [Google Scholar]
- 28.Krauzlis R. J., Lovejoy L. P., Zénon A., Superior colliculus and visual spatial attention. Annu. Rev. Neurosci. 36, 165–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore T., Zirnsak M., Neural mechanisms of selective visual attention. Annu. Rev. Psychol. 68, 47–72 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Fischer B., Boch R., Enhanced activation of neurons in prelunate cortex before visually guided saccades of trained rhesus monkeys. Exp. Brain Res. 44, 129–137 (1981). [DOI] [PubMed] [Google Scholar]
- 31.Steinmetz N. A., Moore T., Eye movement preparation modulates neuronal responses in area V4 when dissociated from attentional demands. Neuron 83, 496–506 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson K. G., Bichot N. P., A visual salience map in the primate frontal eye field. Prog. Brain. Res. 147, 251–262 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Carrasco M., Ling S., Read S., Attention alters appearance. Nat. Neurosci. 7, 308–313 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAdams C. J., Maunsell J. H. R., Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron 23, 765–773 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Treue S., Martínez Trujillo J. C., Feature-based attention influences motion processing gain in macaque visual cortex. Nature 399, 575–579 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Mitchell J. F., Sundberg K. A., Reynolds J. H., Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55, 131–141 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Herrero J. L., Gieselmann M. A., Sanayei M., Thiele A., Attention-induced variance and noise correlation reduction in macaque V1 is mediated by NMDA receptors. Neuron 78, 729–739 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maunsell J. H. R., Treue S., Feature-based attention in visual cortex. Trends Neurosci. 29, 317–322 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Bichot N. P., Heard M. T., DeGennaro E. M., Desimone R., A source for feature-based attention in the prefrontal cortex. Neuron 88, 832–844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olshausen B. A., Anderson C. H., Van Essen D. C., A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. J. Neurosci. 13, 4700–4719 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Downing C. J., Expectancy and visual-spatial attention: Effects on perceptual quality. J. Exp. Psychol. Hum. Percept. Perform. 14, 188–202 (1988). [DOI] [PubMed] [Google Scholar]
- 42.Lu Z. L., Dosher B. A., External noise distinguishes attention mechanisms. Vision Res. 38, 1183–1198 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Yeshurun Y., Carrasco M., Attention improves or impairs visual performance by enhancing spatial resolution. Nature 396, 72–75 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo T. Z., Maunsell J. H. R., Attentional changes in either criterion or sensitivity are associated with robust modulations in lateral prefrontal cortex. Neuron 97, 1382–1393 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen M. R., Maunsell J. H. R., Attention improves performance primarily by reducing interneuronal correlations. Nat. Neurosci. 12, 1594–1600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell J. F., Sundberg K. A., Reynolds J. H., Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63, 879–888 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maunsell J. H. R., Cook E. P., The role of attention in visual processing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 1063–1072 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrington T. M., Assad J. A., Temporal sequence of attentional modulation in the lateral intraparietal area and middle temporal area during rapid covert shifts of attention. J. Neurosci. 30, 3287–3296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore T., Fallah M., Microstimulation of the frontal eye field and its effects on covert spatial attention. J. Neurophysiol. 91, 152–162 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Cavanaugh J., Wurtz R. H., Subcortical modulation of attention counters change blindness. J. Neurosci. 24, 11236–11243 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Müller J. R., Philiastides M. G., Newsome W. T., Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc. Natl. Acad. Sci. U.S.A. 102, 524–529 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cutrell E. B., Marrocco R. T., Electrical microstimulation of primate posterior parietal cortex initiates orienting and alerting components of covert attention. Exp. Brain Res. 144, 103–113 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Wardak C., Ibos G., Duhamel J.-R., Olivier E., Contribution of the monkey frontal eye field to covert visual attention. J. Neurosci. 26, 4228–4235 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossi A. F., Bichot N. P., Desimone R., Ungerleider L. G., Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J. Neurosci. 27, 11306–11314 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monosov I. E., Thompson K. G., Frontal eye field activity enhances object identification during covert visual search. J. Neurophysiol. 102, 3656–3672 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovejoy L. P., Krauzlis R. J., Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat. Neurosci. 13, 261–266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bollimunta A., Bogadhi A. R., Krauzlis R. J., Comparing frontal eye field and superior colliculus contributions to covert spatial attention. Nat. Commun. 9, 3553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green D. M., Swets J. A., Signal Detection Theory and Psychophysics (John Wiley, Oxford, England, 1966). [Google Scholar]
- 59.Müller H. J., Findlay J. M., Sensitivity and criterion effects in the spatial cuing of visual attention. Percept. Psychophys. 42, 383–399 (1987). [DOI] [PubMed] [Google Scholar]
- 60.Hawkins H. L., et al. , Visual attention modulates signal detectability. J. Exp. Psychol. Hum. Percept. Perform. 16, 802–811 (1990). [DOI] [PubMed] [Google Scholar]
- 61.Müller H. J., Humphreys G. W., Luminance-increment detection: Capacity-limited or not? J. Exp. Psychol. Hum. Percept. Perform. 17, 107–124 (1991). [DOI] [PubMed] [Google Scholar]
- 62.Wyart V., Nobre A. C., Summerfield C., Dissociable prior influences of signal probability and relevance on visual contrast sensitivity. Proc. Natl. Acad. Sci. U.S.A. 109, 3593–3598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo T. Z., Maunsell J. H. R., Neuronal modulations in visual cortex are associated with only one of multiple components of attention. Neuron 86, 1182–1188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lennert T., Martinez-Trujillo J., Strength of response suppression to distracter stimuli determines attentional-filtering performance in primate prefrontal neurons. Neuron 70, 141–152 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Funahashi S., Bruce C. J., Goldman-Rakic P. S., Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J. Neurophysiol. 61, 331–349 (1989). [DOI] [PubMed] [Google Scholar]
- 66.Miller E. K., Li L., Desimone R., Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J. Neurosci. 13, 1460–1478 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrera V. P., Rudolph K. K., Maunsell J. H. R., Responses of neurons in the parietal and temporal visual pathways during a motion task. J. Neurosci. 14, 6171–6186 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Weerd P., Peralta M. R. 3rd, Desimone R., Ungerleider L. G., Loss of attentional stimulus selection after extrastriate cortical lesions in macaques. Nat. Neurosci. 2, 753–758 (1999). [DOI] [PubMed] [Google Scholar]
- 69.Sridharan D., Steinmetz N. A., Moore T., Knudsen E. I., Does the superior colliculus control perceptual sensitivity or choice bias during attention? Evidence from a multialternative decision framework. J. Neurosci. 37, 480–511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lovejoy L. P., Krauzlis R. J., Changes in perceptual sensitivity related to spatial cues depends on subcortical activity. Proc. Natl. Acad. Sci. U.S.A. 114, 6122–6126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zénon A., Krauzlis R. J., Attention deficits without cortical neuronal deficits. Nature 489, 434–437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carrasco M., Visual attention: The past 25 years. Vision Res. 51, 1484–1525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dosher B. A., Lu Z. L., Noise exclusion in spatial attention. Psychol. Sci. 11, 139–146 (2000). [DOI] [PubMed] [Google Scholar]
- 74.Freedman D. J., Assad J. A., Experience-dependent representation of visual categories in parietal cortex. Nature 443, 85–88 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Ferrera V. P., Yanike M., Cassanello C., Frontal eye field neurons signal changes in decision criteria. Nat. Neurosci. 12, 1458–1462 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Treue S., Maunsell J. H. R., Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J. Neurosci. 19, 7591–7602 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin M., Glickfeld L. L., Contribution of sensory encoding to measured bias. J. Neurosci. 39, 5115–5127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crapse T. B., Lau H., Basso M. A., A role for the superior colliculus in decision criteria. Neuron 97, 181–194.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horwitz G. D., Batista A. P., Newsome W. T., Representation of an abstract perceptual decision in macaque superior colliculus. J. Neurophysiol. 91, 2281–2296 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Maunsell J. H. R., Neuronal representations of cognitive state: Reward or attention? Trends Cogn. Sci. (Regul. Ed.) 8, 261–265 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Baruni J. K., Lau B., Salzman C. D., Reward expectation differentially modulates attentional behavior and activity in visual area V4. Nat. Neurosci. 18, 1656–1663 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Summerfield C., de Lange F. P., Expectation in perceptual decision making: Neural and computational mechanisms. Nat. Rev. Neurosci. 15, 745–756 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Rungratsameetaweemana N., Itthipuripat S., Salazar A., Serences J. T., Expectations do not alter early sensory processing during perceptual decision-making. J. Neurosci. 38, 5632–5648 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]