Abstract
Although mouse models of Alzheimer’s disease (AD) have provided tremendous breakthroughs, the etiology of later onset AD remains unknown. In particular, tau pathology in the association cortex is poorly replicated in mouse models. Aging rhesus monkeys naturally develop cognitive deficits, amyloid plaques, and the same qualitative pattern and sequence of tau pathology as humans, with tangles in the oldest animals. Thus, aging rhesus monkeys can play a key role in AD research. For example, aging monkeys can help reveal how synapses in the prefrontal association cortex are uniquely regulated compared to the primary sensory cortex in ways that render them vulnerable to calcium dysregulation and tau phosphorylation, resulting in the selective localization of tau pathology observed in AD. The ability to assay early tau phosphorylation states and perform high-quality immunoelectron microscopy in monkeys is a great advantage, as one can capture early-stage degeneration as it naturally occurs in situ. Our immunoelectron microscopy studies show that phosphorylated tau can induce an “endosomal traffic jam” that drives amyloid precursor protein cleavage to amyloid-β in endosomes. As amyloid-β increases tau phosphorylation, this creates a vicious cycle where varied precipitating factors all lead to a similar phenotype. These data may help explain why circuits with aggressive tau pathology (e.g., entorhinal cortex) may degenerate prior to producing significant amyloid pathology. Aging monkeys therefore can play an important role in identifying and testing potential therapeutics to protect the association cortex, including preventive therapies that are challenging to test in humans.
Keywords: PKA, ryanodine, prefrontal cortex, entorhinal cortex
Research in rhesus monkeys can play a unique role in helping us discover why the primate association cortex is especially vulnerable to neurodegeneration in late-onset Alzheimer’s disease (LOAD), including the hidden molecular changes that initiate the degenerative process with advancing age. LOAD afflicts nearly a third of those over 85 y of age, causing massive degeneration of the association cortices and profound dementia. While the rare, early onset, autosomal dominant form of AD arises from genetic insults in amyloid precursor protein (APP) processing (1), the etiology of LOAD is complex and largely unknown. The greatest risk factor for LOAD is advanced age, but there are additional environmental (e.g., head injury, stress, insulin resistance) and genetic (e.g., apoe4, sorl1, trem2) factors that increase risk of disease (2). The elegant genetics of autosomal dominant AD have bolstered the amyloid hypothesis, which suggests amyloid triggers AD pathology, given that a variety of genetic insults lead to increased amyloid-β (Aβ) production or clearance (1). However, the etiology of LOAD is likely more complex; for example, Braak et al. (3) report that tau pathology precedes amyloid pathology in human brains, and strategies to reduce Aβ have limited benefit to benefit patients in early stages of LOAD (4).
A wide variety of approaches to reduce amyloid pathology have been tested based on their success in reducing pathology in transgenic mouse models of AD, yet to date most have failed in large trials of mild or prodromal LOAD patients (4). Mouse models may be inadequate for several fundamental reasons: 1) They are based on the genetic insults in amyloid processing that cause early onset autosomal dominant disease (1) but are not associated with LOAD; 2) mice have relatively few cortical–cortical connections and little association cortex, the circuits most afflicted by AD pathology; and 3) they are short-lived, which may limit the time available for the emergence of some types of pathology. Although mouse models have provided a powerful window into how genetic insults can cause amyloid pathology, mice generally require non-AD–related mutant human tau to develop tau pathology, a major hurdle for learning what causes tau pathology in LOAD (5) [although mice with humanized tau develop fibrillar tau with advanced age (6)].
The etiology of tau pathology is also challenging to study in human brains, as some sites on tau may rapidly dephosphorylate postmortem, and formalin fixation can destroy early-stage tau pathology (7). A long postmortem interval (PMI) also degrades cellular membranes, obscuring important etiological details. Thus, there is a great need for an appropriate animal model of LOAD with a well-developed association cortex, where phosphorylation state and ultrastructure can be preserved with little or no PMI. It has been appreciated for decades that aged rhesus macaques develop amyloid plaques (8) and cognitive deficits (9, 10). Our data have now shown that aging rhesus monkeys also develop the same qualitative pattern and sequence of tau pathology as humans, with neurofibrillary tangles in the oldest animals (7). Thus, they provide a unique opportunity to discover what causes LOAD and to test strategies for prevention.
Amyloid Plaques and Neurofibrillary Tangles: The Diagnostic Neuropathology of LOAD
The neuropathological hallmarks of LOAD are 1) extracellular amyloid plaques arising from the fibrillation of Aβ oligomers (11), and 2) intracellular tangles formed from fibrillated, hyperphosphorylated tau (12, 13). There has been tremendous progress in our understanding of amyloid pathology, where plaques are formed from the accumulation and fibrillation of the Aβ peptide. Aβ is cleaved from APP by β and γ secretases. This cleavage occurs with greater frequency when APP is sequestered in endosomes, which contain β secretase (see, for example, Fig. 7). Indeed, several genetic risk factors for LOAD (e.g., SORL1) are thought to impede endosomal trafficking and increase the time APP spends in endosomes exposed to Aβ, what is known as the “traffic jam” hypothesis (14). This hypothesis is strengthened by the finding that apoe4 genotype increases the numbers of early endosomes (15), and endosomal changes precede Aβ pathology (16). Aβ monomers cleaved from APP can combine to form oligomers, which are thought to be the toxic species: For example, interacting with a prion protein/mGluR5 receptor complex to drive phosphorylation of tau and synapse loss (17). Tau pathology progresses within neurons, ultimately disrupting cellular functions, and likely killing neurons (18). The number of tangles correlates with cognitive impairment (19), emphasizing the importance of understanding tau pathology. Tau’s normal function is to stabilize microtubules in axons and dendrites. However, it loses this function when phosphorylated, for example, by protein kinase A (PKA) at serine 214 (pS214Tau). Under healthy conditions, tau is in an equilibrium of phosphorylation by kinases vs. dephosphosphorylation by phosphatases (12). Pathology occurs when this balance is lost and tau becomes hyperphosphorylated, first aggregating and then fibrillating to form the paired helical filaments that constitute neurofibrillary tangles. Fibrillated tau was traditionally labeled by silver stains (20), but is now analyzed by antibodies like AT8 often used to diagnose AD (21).
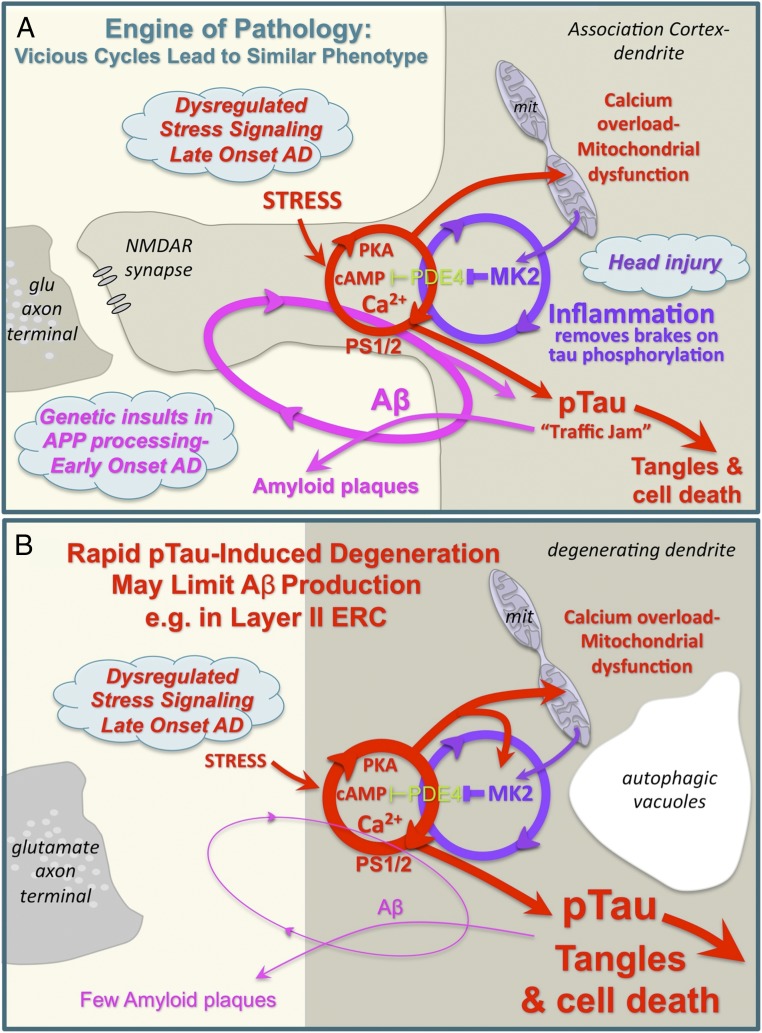
Fig. 7.
(A) Schematic diagram showing how interacting vicious cycles can drive amyloid and tau pathology in the aging association cortex. Thus, multiple starting points can lead to a similar phenotype. Inflammation removes the brakes on phosphorylation of tau, and can also drive synapse loss. (B) Schematic showing how conditions that drive rapid tau pathology, for example in layer II ERC, would destroy the “engine” for Aβ production and thus generate few amyloid plaques. As glutamatergic synapses in layer II ERC are primarily on dendrites, not spines, the pathways are shown in a degenerating dendrite with autophagic vacuoles, for example as in Fig. 2H.
Discovery of the Pattern and Sequence of Tau Pathology in Human LOAD Brains
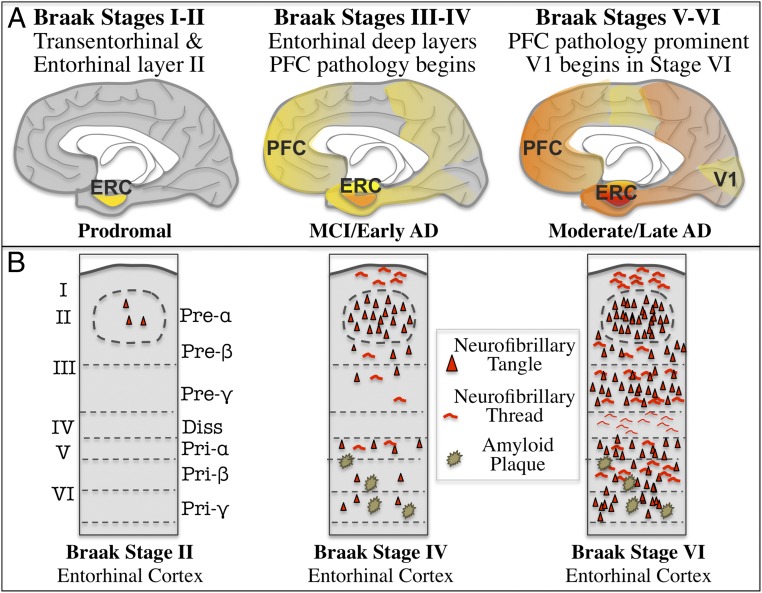
The specific pattern and sequence of tau pathology in LOAD, and its relationship to interconnected cortical networks, was discovered more than 30 y ago by monkey neuroanatomists whose knowledge of the macaque cortex allowed them to decipher their significance (22–26). They saw that very early in AD, tangles afflict the entorhinal cortex (ERC) (Fig. 1A), the gateway between the association cortices and the hippocampus needed for the formation of long-term memories (22, 26). In particular, tau pathology targets the cell islands of layer II ERC (Fig. 1B) (20), the cells that receive massive inputs from the association cortex (27). Some of these ERC cells are now known to include the layer II grid cells that dynamically map space and time (28, 29). Tau pathology next appears in the deep layers of the ERC, in the hippocampus, and the association cortices, targetting highly interconnected glutamatergic neurons with the most cortical–cortical connections (30). Importantly, tau pathology spares the primary visual (V1) and auditory cortex until very end-stage disease (24). This pattern and sequence was then codified by the Braaks (20, 21), whose extensive characterizations of human brains over the lifespan created the staging of tau pathology now used to diagnose LOAD, as summarized in Fig. 1. Their careful studies of younger brains revealed AT8 labeling of neuropil threads beginning in layer pre-α of the transentorhinal (i.e., perirhinal) cortex and then in layer II cell islands of the ERC (20), and in brainstem monoaminergic nuclei, such as the locus coeruleus, even in middle age, warning that tau pathology begins early in the aging process (21). In contrast to tau, amyloid pathology is more diffuse (31), consistent with Aβ release from the axon terminals of tau-afflicted neurons that project throughout the cortex. Amyloid pathology proceeds from cortex to subcortical structures (31). Intriguingly, the striking absence of plaques in layer II of the ERC may relate to the rapid degeneration of these cells (see Vicious Cycles in the Aging Association Cortex Drive Pathology over a Long Life Span). There are some plaques in the deep layers of the ERC, and many throughout association and sensory cortices, consistent with the widespread axon terminations of the association cortex. The regional and laminar specificity of these degenerative markers (e.g., Fig. 1B), especially that of hyperphosphorylated tau, allows for precise comparisons between animal models and human neuropathology.
Fig. 1.
The stages of Braak tau pathology, showing AT8-labeled, fibrillated tau distribution on a midsagital view of the human brain (A), and in a coronal section of entorhinal cortex (B). Adapted by permission from ref. 20, Springer Nature: Acta Neuropathologica, copyright 1991.
The Pattern and Sequence of Tau Pathology in Aging Rhesus Monkeys Is Qualitatively Similar to Human, Resembling Braak Stage III/IV
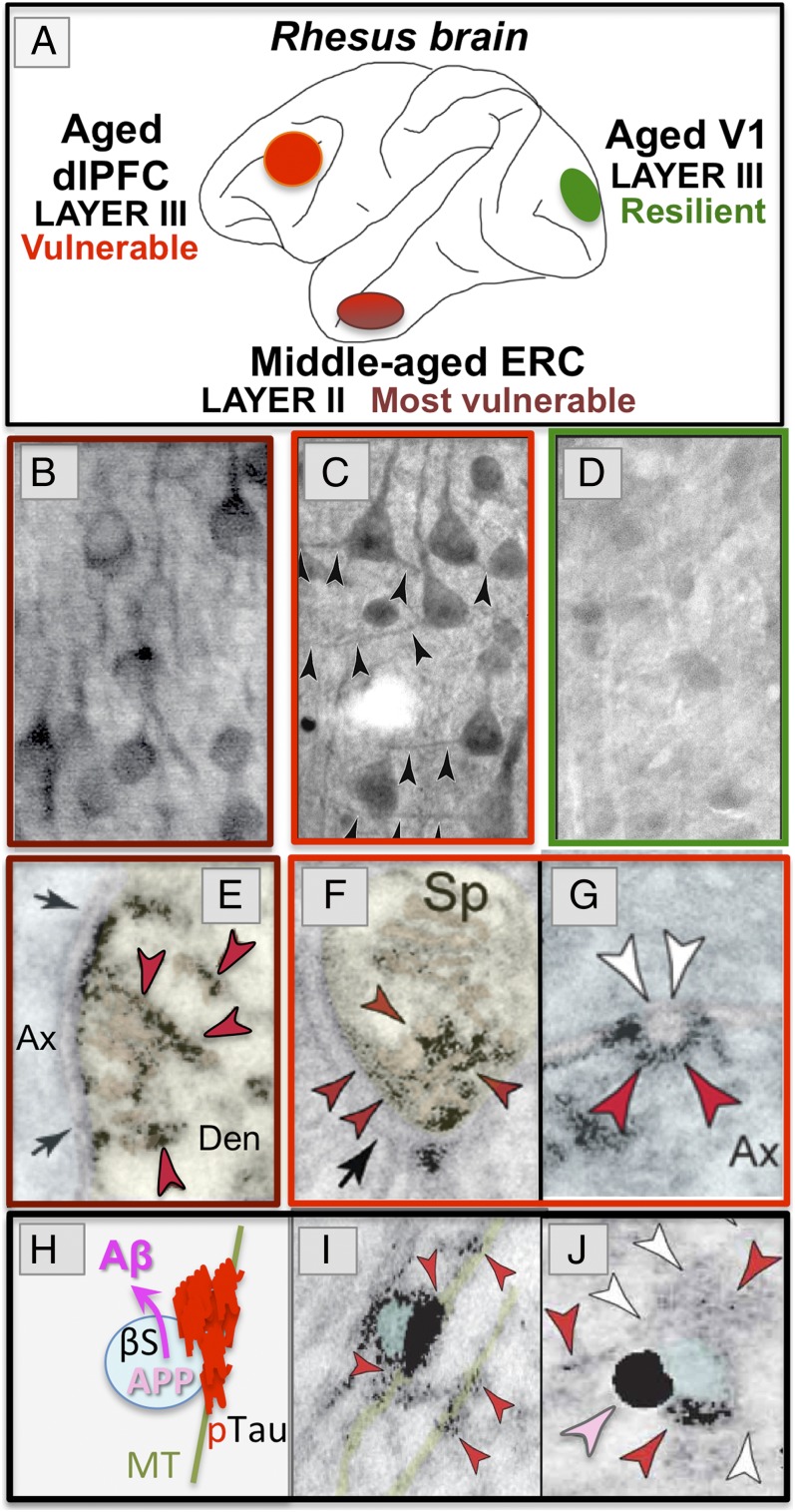
Aging rhesus monkeys have long been used to study the aging brain, as they have cognitive and neuropathological changes very similar to humans, including individual differences in the aging process (32). It has been appreciated for decades that aged rhesus macaques exhibit amyloid pathology (8). It was originally thought that aged rhesus monkeys do not express neurofibrillary tangles (33); however, these studies did not examine monkeys over 30 y of age, and did not appear to have examined the vulnerable ERC. More recent studies have examined rhesus monkeys of extreme age and have focused on the ERC and dorsolateral prefrontal cortex (dlPFC), and compared these vulnerable regions to the V1, which is resilient in LOAD. These data show that aging rhesus monkeys exhibit the same qualitative pattern and sequence of tau pathology as that seen in human brains (7). Although the quantity of the pathology is much less than in human, and thus monkeys do not have LOAD, the cortical regions, layers, and cell types where tau pathology emerges are the same as in humans. As can be seen in Fig. 2, the pattern of AT8 labeling is remarkably similar to human, starting in the layer II cell islands of the ERC (Fig. 2A). In the oldest rhesus monkeys (Fig. 2B), the pathology in layer II cell islands is extensive, while it begins to emerge in the deeper ERC layers and in the dlPFC association cortex (7), but not in V1 (see Aging Rhesus Monkeys: Unique Opportunity to Study the Etiology of LOAD).
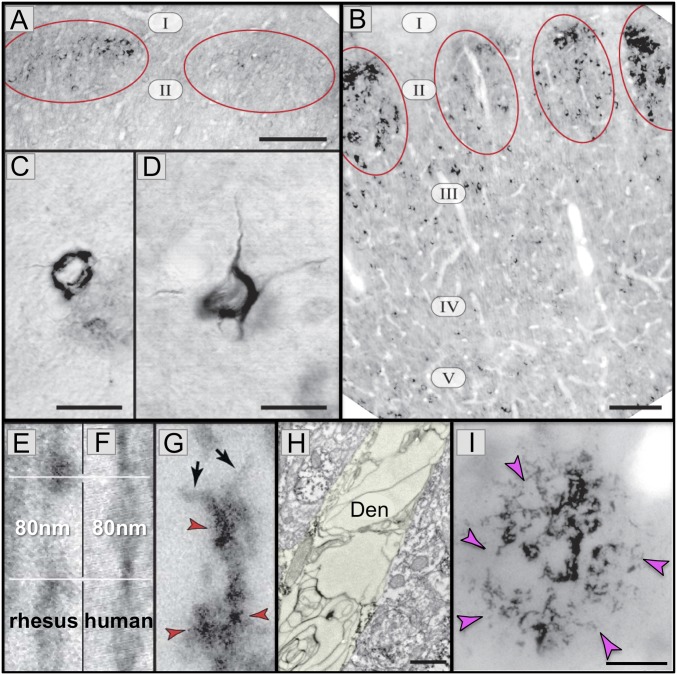
Fig. 2.
AT8 pTau and amyloid pathology in aged rhesus monkeys (26 to 38 y). (A) AT8 label is first seen in layer II cell islands in the ERC in a 26-y-old monkey. (B) In an older monkey (33 y), AT8 labeling is more extensive in layer II and now extends to deeper layers. (C and D) Higher-magnification view of neurofibrillary tangles in layer II (C) and layer V (D) from a 38-y-old monkey. (E–G) High-magnification view of AT8-labeled (red arrrowheads) paired helical filaments from an aged monkey (E and G) or human (F). Note the same width (10 nm) and helical frequency (80 nm). Black arrows in G show the abrupt ends of each filament. (H) A degenerating dendrite (Den) from an AT8-labeled neuron in aged monkey layer II ERC. The dendrite is devoid of normal organelles and is filled with autophagic vacuoles similar to those in LOAD. (I) An Aβ-labeled (magenta arrowheads) amyloid plaque from layer V ERC in an aged monkey. Reprinted from ref. 7, with permission from Elsevier. (Scale bars: A and B, 100 μm; C and D, 10 μm; E–G, 40 nm; H, 500 nm; I, 20 μm.)
Biochemical studies also show a rise in insoluble, fibrillated tau in the aging ERC (7). A closer view of the neurofibrillary tangles from the oldest monkeys illustrates how similar they are to those in human, using either light microscopy (Fig. 2 C and D) or high-magnification immunoelectron microscopy (immunoEM) (Fig. 2 E–G), where the size and helical frequency of the paired helical filaments are identical between species (Fig. 2 E and F). Similar tangles have recently been reported in the ERC of very old African green monkeys (34). Importantly, the accumulation of paired helical filaments within dendrites is associated with vacuolar degeneration, where the neuron becomes a “ghost’” tangle (7), similar to that in LOAD. For example, Fig. 2H shows a degenerating dendrite in layer II ERC, completely filled with autophagic vacuoles. Thus, even though the cell bodies remain intact, and would be counted as normal in most stereological studies (35), the function of these neurons likely would be completely abnormal. These qualitative similarities between monkey and human provide construct validity for using the aging rhesus monkey to study the etiology of tau pathology with advancing age, with focus on the early stages of pathology that are difficult to study in humans. Note that these same studies observed amyloid plaques in aged rhesus monkeys that are very similar to those in human in their size, shape, and distribution, localized in deep, but not superficial layers of the ERC (Fig. 2I) (7). Thus, the aging rhesus monkey can also be used to explore the emergence of Aβ production with advancing age, and potential interactions between tau and amyloid pathology.
Aging Rhesus Monkeys: Unique Opportunity to Study the Etiology of LOAD
The etiology of tau pathology is challenging to study in the human brain due to the variable dephosphorylation of tau and degradation of neuronal membranes postmortem. While AT8-labeled, fibrillated tau is preserved, earlier-stage tau phosphorylation may be lost. In contrast, the availability of perfused, fixed tissue, or fresh tissue with very short (minutes) PMI from monkeys allows the study of early tau phosphorylation states, and the preserved ultrastructure needed to see organelles and molecular interactions with great clarity. The opportunity to detect early tau phosphorylation states in perfusion-fixed monkey tissue also warrants a note of caution: Antibodies such as AT8 or AT100 generally label only insoluble, fibrillated phosphorylated tau in human brain, as the soluble, phosphorylated tau species are rapidly dephosphorylated postmortem and often not present in postmortem human tissue with long PMI. Thus, these antibodies have come to be known as agents that selectively label tangles. However, in the perfusion-fixed monkey brain, both soluble and insoluble forms of phosphorylated tau are preserved, and these antibodies can label both forms. This can lead to great confusion, for example, when researchers describe increases in diffuse, soluble AT8 labeling as tangles. Thus, we must be careful in describing the tau pathology detected in perfused, fixed monkey brains, distinguishing early stage pathology from later-stage fibrillated tangles. However, monkeys can be invaluable for studying these early stages that are usually destroyed by the longer PMI in human brains.
Monkey studies allow us to ask what it is about the aging process that renders the association cortices, but not the primary sensory cortex, so vulnerable to tau pathology, even in the absence of genetic alterations, a question that cannot be easily addressed in mouse models. The following is a brief review of the ideas emerging from the comparison of vulnerable vs. resilient circuits in the aging monkey cortex, and the dysregulated molecular events associated with the emergence of tau pathology in the aging association cortex.
Unique Molecular Modulation of Association Cortex vs. V1.
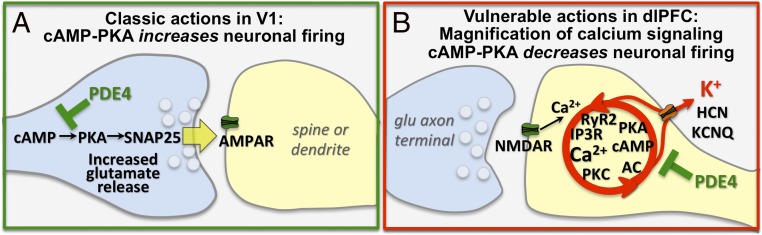
Why are highly interconnected glutamatergic neurons in the association cortex so vulnerable to tau pathology, while parallel neurons in V1 are so resilient? This question can be addressed in rhesus monkeys by comparing the molecular regulation of cells in the dlPFC and ERC with those in V1. Studies of V1 in rhesus monkeys show classic neurotransmission and neuromodulatory mechanisms (Fig. 3A) (36). V1 neurons are especially reliant on AMPAR rather than NMDAR neurotransmission (36), and show beneficial effects of cAMP signaling, where PKA increases sensory-evoked firing, consistent with cAMP-related proteins positioned to enhance glutamate release in axon terminals (36). These experiments also showed classic location and function of hyperpolarization-activated cyclic nucleotide (HCN) channels on the distal apical dendrite, where blockade reduced neuronal firing (36).
Fig. 3.
The primary visual cortex (V1) and dlPFC are regulated differently at the molecular level. (A) Schematic diagram of a layer III glutamate AMPAR synapse in V1, where PDE4A is presynaptic, consistent with PKA’s role in enhancing synaptic release. (B) In contrast to V1, layer III dlPFC NMDAR synapses on spines express feedforward, calcium-cAMP-K+ channel signaling, which reduces firing. Reprinted from ref. 36, by permission of Oxford University Press.
In contrast to V1, neurons in the dlPFC can maintain firing to represent a visual stimulus even in the absence of sensory stimulation, for example, during a working memory task. The persistant firing of these “Delay” cells is generated by a microcircuit of layer III pyramidal cells, exciting each other through glutamate synapses on spines to keep information “in mind.” Delay cell firing relies heavily on glutamate stimulation of NMDAR (Fig. 3B), including those with NR2B subunits that are found exclusively in the synapse (37). NMDAR2B close slowly and transfer large concentrations of calcium into the cell, a likely factor in their vulnerability to degeneration. Remarkably, dlPFC Delay cells are much less affected by AMPAR blockade (37), and rely on cholinergic depolarization of the membrane to permit NMDAR actions (38). dlPFC Delay cells also have nonclassic neuromodulation, where spines contain the molecular machinery to magnify calcium actions, and cAMP signaling reduces rather than increases neuronal firing by opening K+ channels (Fig. 3B). cAMP-related proteins concentrate near the calcium-storing smooth endoplasmic reticulum (SER, called the spine apparatus when it extends into the spine), positioned to regulate calcium release (39, 40), and create feedforward signaling (41). High levels of calcium-cAMP-PKA signaling open nearby HCN and KCNQ channels on spines to rapidly reduce firing, a process termed “dynamic network connectivity” (42). This may serve as important negative feedback in a recurrent excitatory circuit, but also allows for arousal state to rapidly alter cognitive state, for example, rapidly eroding representations and taking dlPFC “off-line” during uncontrollable stress (41).
It is currently unknown whether dynamic network connectivity mechanisms are a general feature of association cortex or specific to the PFC. However, it appears that layer II ERC grid cells share much in common with dlPFC Delay cells, as they rely on NMDAR (43), and cAMP-HCN channel signaling reduces the grid scale (44, 45), similar to actions in the dlPFC. There is greatly elaborated SER under glutamate synapses in the ERC, similar to the SER spine apparatus in dlPFC spines (7). Thus, both the dlPFC and ERC appear to use cAMP-HCN mechanisms to dynamically alter the representations of sensory experience (ERC) and memories (dlPFC), in ways that are opposite to cAMP mechanisms in V1. The dynamic reduction of synaptic strength in the association cortex may have survival value during stress, but must be tightly regulated, for example, by phosphodiesterases (PDE4s), to prevent detrimental signaling, including increased calcium-cAMP-PKA phosphorylation of tau. The finding that psychological stress drives feedforward calcium-cAMP-PKA signaling in dlPFC circuits may help to explain why stress is a risk factor for LOAD (46, 47), and is associated with worse cognition during mild cognitive impairment (48). As females have a lower threshold for stress-induced PFC dysfunction than males (49, 50), this underlying mechanism may also contribute to the preponderance of LOAD in women (51, 52). Intriguingly, the locus coeruleus also contains high levels of cAMP-PKA signaling that is activated by stress (53), suggesting that early tau pathology in this nucleus may also involve these signaling pathways.
Background on the Signaling Events Leading to the Hyperphosphorylation of Tau.
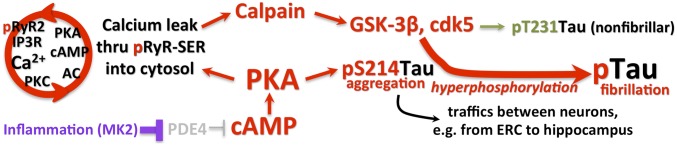
In vitro studies have detailed the mechanisms underlying the successive hyperphosphorylation of tau, leading to its fibrillation into paired helical filaments. Importantly, this work has shown that dysregulated calcium and cAMP-PKA signaling play major roles in tau hyperphosphorylation. PKA phosphorylation of tau contributes to early stages of paired helical filament formation (54). As summarized in Fig. 4, PKA phosphorylation primes tau for hyperphosphorylation by GSK3β (13, 55), including the truncated, activated form of GSK3β that is associated with tau pathology in LOAD (55). Calcium dysregulation exacerbates this process, in that high levels of cytosolic calcium can activate calpain, which in turn truncates GSK3β into its more active form (55). This signaling pathway is a focal point for multiple risk factors. In addition to psychological stress (described above), these signaling events are activated by traumatic brain injury (56), another risk factor for LOAD. Tau phosphorylation may also be increased by metabolic dysregulation and genetic alterations: For example, insulin receptor insensitivity disinhibits GSK3β (57). Importantly, advancing age also dysregulates this pathway, as described in the next section.
Fig. 4.
The key roles of dysregulated feedforward calcium-cAMP-PKA signaling in priming tau for hyperphosphorylation by GSK3β near NMDAR synapses in the association cortex.
Dysregulated Calcium-cAMP Signaling in the Aging Association Cortex Drives Tau Phosphorylation.
A variety of evidence finds that dysregulation of calcium-cAMP-PKA signaling in the aging association cortex leads to cognitive deficits and the phosphorylation of tau. Earlier studies showed that excessive cAMP-PKA-K+ signaling in the aging PFC reduces neuronal firing (58) and impairs cognition (59). More recent studies revealed a loss of PDE4 regulation with advancing age (40). PDE4s are usually anchored to the correct location (e.g., the SER) by DISC1, but can become “unanchored” by MK2 inflammatory signaling (60, 61), which may contribute to age-related loss of PDE4s from the dendritic compartment. Notably, inhibition of PDE4s can create an aged-like phenotype, reducing dlPFC neuronal firing (62) and increasing the production of pS214Tau in vitro (40). In addition, systemic administration of the PDE4 inhibitor, rolipram, worsens working memory in aged monkeys (59). These findings have immediate clinical significance, as PDE4 inhibitors are currently under development for the treatment of LOAD, based on studies of rodent hippocampus (63). However, hippocampal neurons from LOAD patients and from mouse AD models, like aged PFC, show evidence of excessive PKA actions on calcium release (64), as described in the following section. These data caution that prolonged treatment with PDE4 inhibitors may actually worsen rather than attenuate LOAD pathology.
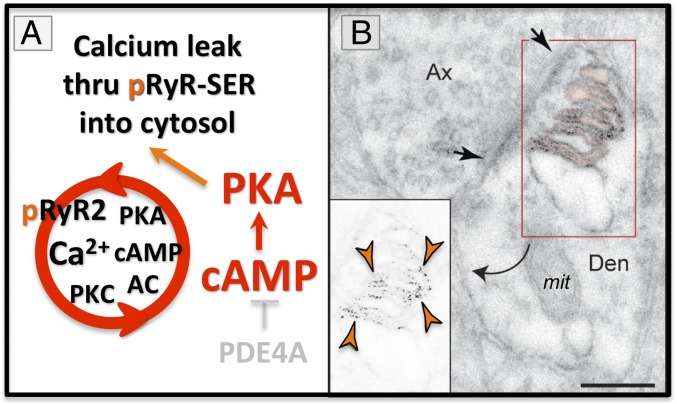
Calcium dysregulation has long been appreciated as an important etiological factor in the rise of LOAD pathology (65–67), and recent evidence from patients and mouse models have substantiated this early hypothesis (64). Excessive cAMP-PKA signaling can play a central role in calcium dysregulation, where PKA phosphorylates ryanodine receptors (RyR2) on the SER, rendering these channels “leaky,” increasing calcium concentrations in the cytosol (68). This mechanism has been studied extensively in cardiac muscle, where loss of PDE4D with age (69) increases PKA phosphorylation of pRyR2, inducing calcium leak and calcium overload of mitochondria, which in turn drives inflammation and heart failure (70). A similar process appears to occur in the aging association cortex, where we see PKA phosphorylated pRyR2 (7) and misshapen mitochondria associated with SER (71). PKA phosphorylated pRyR2 are concentrated on the SER under vulnerable glutamate synapses as part of the aging process. For example, the exquisite immunoEM images by Paspalas et al. (7) illustrate the expression of PKA phosphorylated pRyR2 on the highly elaborated SER, next to a mitochondrion, under a glutamate-like synapse on an ERC dendrite, already evident in middle age (Fig. 5A). This is the same age and subcellular location where tau pathology may begin. The unusually extensive SER under these synapses suggests that there would be massive calcium leak into the cytosol, which could drive pathology. As mentioned above, PKA phosphorylated pRyR2s have been documented in the hippocampus from human patients with LOAD and from AD transgenic mouse models (64), and also can be induced by stress exposure in mice (72). Aβ oligomers can also increase inctracellular calcium release (73). Thus, dyregulated calcium signaling can be seen across species in association with LOAD and its risk factors (age, stress). Intriguingly, presenillins (PS1/2) are also associated with the SER, and the PS1 mutations that cause early-onset, autosomal-dominant AD cause calcium leak (74–76), indicating that calcium dysregulation may be common to both autosomal-dominant and sporadic forms of disease. Calcium dysregulation may drive tau pathology in multiple ways, including activation of kinases that phosphorylate tau, and by inducing inflammatory processes that remove the brakes on tau phosphorylation. For example, high levels of calcium signaling may increase MK2 activity through activation of upstream kinases like p38 (77, 78), which can inhibit and unanchor PDE4s (60, 61) (Fig. 4). This would drive a vicious cycle, leading to still greater PKA and calcium signaling, and thus further inflammation (as illustrated, for example, in Fig. 7).
Fig. 5.
(A) High levels of PKA activity phosphorylate ryanodine receptors (pS2808RyR2) on the SER and cause calcium leak into the cytosol. (B) pS2808RyR2 can be seen on the elaborated SER under a glutamate synapse in layer II EHC from a middle-aged monkey (9 y). Orange arrowheads depict pS2808RyR2 labeling, black arrows delineate a synapse. mit = mitochondrion; Den = dendrite; Ax = axon. Reprinted from ref. 7, with permission from Elsevier. (Scale bar: 200 nm.)
Dysregulated PKA signaling also leads to increased pS214Tau (Fig. 6), a phosphorylated site that primes tau for hyperphosphorylation by other kinases and ultimately fibrillation (Fig. 4). Levels of soluble, pS214Tau markedly increase with age in the primate dlPFC (40). This early stage of tau phosphorylation is currently not captured with PET imaging, and can be challenging to measure in human brain, although recently has been seen to increase in the cerebrospinal fluid in LOAD (79). Thus, research in monkeys is helpful for understanding this early stage of tau phosphorylation that can initiate many toxic processes. The pattern and sequence of PKA phosphorylation of tau is consistent with the regional sequence of AT8 emergence in monkeys and humans, but as would be expected, occurs at earlier ages (Fig. 6A). Thus, pS214Tau is very prominent in the layer II ERC cell islands in middle age (Fig. 6 B and E), and at later ages in the dlPFC (Fig. 6 C and F), but not in aged V1 (Fig. 6D), consistent with the resilience of V1 in the human brain.
Fig. 6.
PKA phosphorylated tau (pS214Tau, red arrowheads) can be seen in layer II ERC in middle-aged monkeys, and in the dlPFC in aged monkeys, but not in V1. (A) Lateral view of the rhesus monkey brain, showing vulnerable vs. resilient cortical regions. (B) pS214tau-labeled stellate cells in layer II ERC of a middle-aged monkey (7 y) resembles early pathology in middle-aged humans. (C) pS214tau-labeled pyramidal cells in layer III dlPFC of an aged monkey (31 y). Black arrowheads indicate labeled dendrites. (D) Layer III in V1 from the same aged monkey (31 y) as shown in C shows little pS214Tau label. (E) pS214Tau aggregated on the elaborated SER and in the PSD of a glutamate-like synapse on a dendrite (Den) in layer II ERC of a middle-aged monkey. Black arrows delineate the synapse. (F) pS214Tau aggregating on the SER spine apparatus and in the PSD of a glutamate-like synapse on a dlPFC spine (Sp) from an aged monkey. (G) An example of pS214 in an ω-body, presumably trafficking between axons (Ax) in the aged dlPFC. (H) Schematic showing how aggregated tau on a microtubule (MT) can trap endosomes containing APP and β secretase (βS), causing a “traffic jam” that increases cleavage of APP to Aβ. (I) An example of aggregated pS214Tau (red arrowheads) on a microtubule (pseudocolored green) trapping an endosome (pseudocolored blue) in middle-aged ERC layer II. (J) Dual immunoEM showing AT8-labeled tau (red arrowheads) trapping an APP (pink arrowhead) containing endosomes in aged ERC. White arrowheads highlight the tau fibrils. B, E, I, and J reprinted from ref. 7, with permission from Elsevier; C, D, F, and G reprinted with permission from ref. 40. Pink pseudocoloring highlights the SER; presynaptic axons are pseudocolored blue; postsynaptic compartments in yellow. The magnification was altered for this paper; for details on magnification information, please refer to refs. 7 and 40.
PKA phosphorylation of tau contributes to its detachment from microtubules (80). Pre-embedding immunoEM, which preserves membranes and organelles, shows pS214Tau aggregation in distinct subcellular locations in the aging monkey association cortex. pS214Tau aggregates on microtubules in dendrites (7, 40), where it interferes with endosomal trafficking (Fig. 7B) (7). pS214Tau also accumulates on the SER in dendrites in both the ERC (Fig. 6E) and dlPFC (Fig. 6F), often near distorted mitochondria. Thus, pS214Tau accumulates near the SER at the same locations and ages as PKA phosphorylated pRyR2 (Fig. 5). pS214Tau also aggregates in and near the postsynaptic density (PSD) of glutamate-like synapses in middle-aged ERC (Fig. 6E) and aged dlPFC (Fig. 6F) (7, 40). In young monkeys, there is delicate pS214Tau labeling in axons (40), and in the PSD that appears to play a beneficial role (81). However, the large aggregations of pS214Tau near the synapse in aging (7, 40) may interfere with synaptic transmission. For example, a recent study of transgenic mice with humanized tau showed that it is the soluble rather than the fibrillar form, phosphorylated tau, that reduces neuronal activity (82). Accumulation of pS214Tau on the SER may add to ER stress, and its aggregation on microtubules interferes with intracellular trafficking, including trafficking of APP.
In summary, the accumulation of pS214Tau in the ERC is predominately seen on the SER and microtubules in dendrites under glutamate-like synapses near mitochondria, and is already evident in middle age. These toxic actions may be particularly deleterious in dendrites, where there is an extensive network of SER and mitochondria to magnify pathology. In contrast, pS214Tau accumulates at a later age in the dlPFC, associated with glutamate-like synapses predominately on spines, and this may make the process more gradual. As aggregated pS214Tau is seen within dlPFC neurons over a large extent of the aged lifespan (e.g., aged monkeys 20 to 34 y), it provides a long period for producing pathology.
pS214Tau Traffics Between Neurons.
Data from mouse models have demonstrated that phosphorylated tau can traffic within neural networks (83), for example from the ERC to “infect” hippocampal neurons (84). ImmunoEM has captured this process in the aging primate cortex, revealing pS214Tau trafficking between neurons in middle-aged ERC (7) and in aged dlPFC (40). For example, Fig. 6G shows pS214Tau in an ω-body—consistent with neuronal trafficking—between axons in aged dlPFC. These data indicate that tau pathology can arise from both internal generation within a neuron, and from transneuronal “infection” that captures a network of glutamatergic neurons starting in the association cortex and progressively afflicting sensory cortices as the process continues over many years. This progressive “infection” was intuited by primate neuroanatomists decades ago (22–26), and has now been confirmed through immunoEM.
Phosphorylated Tau Causes Endosomal “Traffic Jams” that May Drive Aβ Production
Research has established that Aβ oligomers can increase the phosphorylation of tau (17, 85), and recent data from monkeys suggests that the converse may also be true, and that phosphorylated tau might drive the production of Aβ by “trapping” APP in endosomes (7). A variety of evidence indicates that APP cleavage to Aβ is greatly increased when there are endosomal “traffic jams,” where APP spends more time sequestered in endosomes that contain β secretase. A variety of genetic alterations (e.g., apoe4, sorl1, or picalm) may increase the risk of LOAD through this mechanism: Increasing the numbers of endosomes, increasing APP sequestration in endosomes, and slowing endosomal trafficking (14, 15). As shown in Fig. 6 H–J, the immunoEM data from aging rhesus monkeys illustrate how phosphorylated tau could play this same important role, trapping APP in endosomes where it can be cleaved to Aβ (Fig. 6H). The ultrastructural data show that both pS214Tau (Fig. 6I), and AT8-labeled fibrillated tau (Fig. 6J), can “jam” endosomes (e.g., Fig. 6J captures an APP-containing endosome entangled by AT8 labeled fibrils). Given the extensive pS214Tau labeling in the aging association cortex, this may be a major factor driving Aβ production and amyloid pathology. This may be particularly true for areas like the dlPFC, where pS214Tau is expressed over a prolonged period in the aged brain, with extensive pS214Tau build-up on microtubules (e.g., as shown in Fig. 6I), with fibrillation and neuronal degeneration occurring more slowly and at a later age.
These data increase our understanding of the etiology of LOAD, as they suggest that tau phosphorylation in dendrites and spines could initiate the degenerative process, with downstream production of amyloid pathology. pS214Tau aggregations (e.g., in the aging PFC) may generate large amounts of Aβ at a time when fibrillated tau (labeled by AT8) is still not evident. As these early stages of tau pathology are difficult to assay in the human brain, early-stage, aggregated tau may be a major driver of Aβ production and yet be invisible to researchers using current technologies. This could give the impression that Aβ pathology precedes tau pathology, when in some individuals it could actually be the reverse. Studies of the aging monkey cortex can help address this important question.
Vicious Cycles in the Aging Association Cortex Drive Pathology over a Long Life Span
Our primate research indicates that aging unleashes multiple, interacting vicious cycles in the association cortices, whereby a tightly regulated, healthy physiology falls out of equilibrium and spirals into a similar pathological phenotype, irrespective of the initiating event (Fig. 7A). Thus, increases in 1) Aβ oligomers [e.g., from APP mutations (1)], 2) feedforward calcium-PKA signaling [e.g., from loss of PDE4 regulation and stress exposure (46, 47)], and 3) inflammation [e.g., from head injury (86)], can all lead to the same neuropathological phenotype of neurofibrillary tangles and amyloid plaques. The data indicate that the glutamatergic synapse in the association cortex serves as the “engine of pathology,” and that the larger the number of cortical–cortical glutamatergic connections across a longer lifespan, the greater the extent of AD-like pathology. This hypothesis is consistent with surveys across species, where tau pathology is minimal in wild-type rodents, is more extensive in rhesus monkeys (7, 40), still greater in chimpanzees (87), and markedly evident in aging humans (21).
Interacting vicious cycles may also explain why there is so little amyloid pathology in circuits, such as layer II ERC, where rapid tau pathology is associated with early neurodegeneration and “ghost” neurons. As schematized in Fig. 7B, the rapid degeneration in layer II ERC would destroy the “engine” before there would be time to generate high levels of Aβ. It is possible that aggressive tauopathies, like chronic traumatic encephalopathy, are devoid of amyloid pathology for the same reason: The source of Aβ production would be rapidly killed off. In contrast, the massive amyloid accumulation in LOAD in cortical regions such as the dlPFC may be related to the more gradual progression of tau pathology and slower degeneration, and thus the continued presence of the “engine” generating Aβ peptides.
Strategies for Treatment
Studies of the primate association cortex can also identify novel strategies for regulating feedforward cAMP-calcium signaling that may protect the high-order circuits from the detrimental effects of age. It is clear that these interventions must begin early, as tau phosphorylation in the ERC begins in middle age. Given this, a “baby aspirin” approach would be necessary, where a treatment must have minimal side effects to be taken by healthy individuals as a preventive therapy to reduce risk. As preventive treatments are challenging to test in humans, aging rhesus monkeys may also be helpful to assay treatment efficacy, as measures of early stage tau pathology are possible in aged monkeys. Assessments could also utilize novel primate models of LOAD pathology, where infusion of Aβ oligomers induces LOAD-like pathology in monkey cortex [ref. 88; see also Beckman et al. (89)]. This may be a more practical approach, given the relative paucity of aged monkeys. Given the similarities between the monkey and human cortex, testing in monkeys can be especially helpful, for example to guide dosing.
Future Directions: Transgenic Monkeys
Genetic tools in monkeys have lagged far behind those in mice, but are at last are having success [see El-Shamayleh and Horwitz (90)]. Thus, we are now positioned to learn how a genetic alteration that increases risk for LOAD alters molecular events in the aging association cortex to exacerbate pathology. Transgenic marmosets (91) and macaques (92–95) have already been created, and have begun to inform developmental and neurodegenerative disorders. Transgenic monkeys provide the unique opportunity to assess the effects of genetic mutations on higher cognitive functions, including physiological recordings and pharmacological manipulations especially important for disorders such as LOAD. As monkeys naturally develop tau pathology, transgenic monkey models may be especially important for determining how single genetic alterations impact the emergence of tau pathology with age, and their impact on higher cognitive functioning.
In summary, research in monkeys has played, and can continue to play, a vital role in revealing the etiology of LOAD. Although this review has focused on research from aging rhesus monkeys, studies of other primate species, such as African green monkeys (34, 96) and marmosets (97), can expand our knowledge of how the aging association cortex becomes vulnerable to LOAD-like pathology. This view can complement existing strategies using canine models (98) and mouse models to produce a more comprehensive understanding of LOAD etiology. In particular, the aging monkey can be unique in revealing the age-related changes that cause LOAD pathology in the absence of genetic mutations, a fundamental question for the field. Understanding the special neuromodulatory needs of the primate association cortex may also provide an innovative window on therapeutics to protect these newly evolved circuits from the ravages of disease.
Data Availability.
This paper contains no original data.
Acknowledgments
Much of the work reviewed in this paper was funded by NIH Grants Pioneer DP1 AG 047744, R01AG043430-05, and R01AG061190 (to A.F.T.A.). Support was also obtained from the NIH (AG047270, Training Grant T32 NS41228), the State of Connecticut Department of Mental Health and Addiction Services, Alzheimer’s Association Fellowship AARF-17-533294 (to D.D.), and by a Gruber fellowship (to S.L.).
Footnotes
Competing interest statement: A.F.T.A. and Yale University receive royalties from the United States sales of Intuniv by Shire/Takeda Pharmaceuticals. They do not receive royalties from generic or international sales.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Using Monkey Models to Understand and Develop Treatments for Human Brain Disorders,” held January 7–8, 2019, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. NAS colloquia began in 1991 and have been published in PNAS since 1995. From February 2001 through May 2019 colloquia were supported by a generous gift from The Dame Jillian and Dr. Arthur M. Sackler Foundation for the Arts, Sciences, & Humanities, in memory of Dame Sackler’s husband, Arthur M. Sackler. The complete program and video recordings of most presentations are available on the NAS website at http://www.nasonline.org/using-monkey-models.
This article is a PNAS Direct Submission. E.A.B. is a guest editor invited by the Editorial Board.
References
- 1.Selkoe D. J., Hardy J., The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hersi M., et al. , Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology 61, 143–187 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Braak H., Zetterberg H., Del Tredici K., Blennow K., Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol. 126, 631–641 (2013). [DOI] [PubMed] [Google Scholar]
- 4.van Dyck C. H., Anti-Amyloid-β monoclonal antibodies for Alzheimer’s disease: Pitfalls and promise. Biol. Psychiatry 83, 311–319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drummond E., Wisniewski T., Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 133, 155–175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andorfer C., et al. , Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 86, 582–590 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Paspalas C. D., et al. , The aged rhesus macaque manifests Braak stage III/IV Alzheimer’s-like pathology. Alzheimers Dement. 14, 680–691 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uno H., Walker L. C., The age of biosenescence and the incidence of cerebral beta-amyloidosis in aged captive rhesus monkeys. Ann. N. Y. Acad. Sci. 695, 232–235 (1993). [DOI] [PubMed] [Google Scholar]
- 9.Rapp P. R., Amaral D. G., Evidence for task-dependent memory dysfunction in the aged monkey. J. Neurosci. 9, 3568–3576 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herndon J. G., Moss M. B., Rosene D. L., Killiany R. J., Patterns of cognitive decline in aged rhesus monkeys. Behav. Brain Res. 87, 25–34 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Haass C., Selkoe D. J., Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Wang J. Z., Grundke-Iqbal I., Iqbal K., Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 25, 59–68 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal K., Liu F., Gong C. X., Alonso Adel. C., Grundke-Iqbal I., Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 118, 53–69 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Small S. A., Simoes-Spassov S., Mayeux R., Petsko G. A., Endosomal traffic jams represent a pathogenic hub and therapeutic target in Alzheimer’s disease. Trends Neurosci. 40, 592–602 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuriel T., et al. , The endosomal-lysosomal pathway is dysregulated by APOE4 expression in vivo. Front. Neurosci. 11, 702 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cataldo A. M., et al. , Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and down syndrome: Differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 157, 277–286 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Um J. W., et al. , Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron 79, 887–902 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Usenovic M., et al. , Internalized tau oligomers cause neurodegeneration by inducing accumulation of pathogenic tau in human neurons derived from induced pluripotent stem cells. J. Neurosci. 35, 14234–14250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannakopoulos P., et al. , Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 60, 1495–1500 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Braak H., Braak E., Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Braak H., Thal D. R., Ghebremedhin E., Del Tredici K., Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 70, 960–969 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Hyman B. T., Van Hoesen G. W., Damasio A. R., Barnes C. L., Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science 225, 1168–1170 (1984). [DOI] [PubMed] [Google Scholar]
- 23.Pearson R. C. A., Esiri M. M., Hiorns R. W., Wilcock G. K., Powell T. P. S., Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 82, 4531–4534 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis D. A., Campbell M. J., Terry R. D., Morrison J. H., Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: A quantitative study of visual and auditory cortices. J. Neurosci. 7, 1799–1808 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold S. E., Hyman B. T., Flory J., Damasio A. R., Van Hoesen G. W., The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex 1, 103–116 (1991). [DOI] [PubMed] [Google Scholar]
- 26.Van Hoesen G. W., Hyman B. T., Damasio A. R., Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus 1, 1–8 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Gómez-Isla T., et al. , Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J. Neurosci. 16, 4491–4500 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killian N. J., Jutras M. J., Buffalo E. A., A map of visual space in the primate entorhinal cortex. Nature 491, 761–764 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus B. J., et al. , During running in place, grid cells integrate elapsed time and distance run. Neuron 88, 578–589 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bussière T., et al. , Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: Stereologic analysis of prefrontal cortex area 9. J. Comp. Neurol. 463, 281–302 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Thal D. R., Rüb U., Orantes M., Braak H., Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Morrison J. H., Baxter M. G., The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 13, 240–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gearing M., Rebeck G. W., Hyman B. T., Tigges J., Mirra S. S., Neuropathology and apolipoprotein E profile of aged chimpanzees: Implications for Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 91, 9382–9386 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer P. E., et al. , Aging African green monkeys manifest transcriptional, pathological, and cognitive hallmarks of human Alzheimer’s disease. Neurobiol. Aging 64, 92–106 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Gazzaley A. H., Thakker M. M., Hof P. R., Morrison J. H., Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol. Aging 18, 549–553 (1997). [DOI] [PubMed] [Google Scholar]
- 36.Yang S. T., et al. , Core differences in synaptic signaling between primary visual and dorsolateral prefrontal cortex. Cereb. Cortex 28, 1458–1471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M., et al. , NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77, 736–749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y., et al. , Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 110, 12078–12083 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paspalas C. D., Wang M., Arnsten A. F. T., Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex: Potential substrate for working memory deficits in schizophrenia. Cereb. Cortex 23, 1643–1654 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlyle B. C., et al. , cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc. Natl. Acad. Sci. U.S.A. 111, 5036–5041 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnsten A. F., Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat. Neurosci. 18, 1376–1385 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnsten A. F. T., Wang M. J., Paspalas C. D., Neuromodulation of thought: Flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76, 223–239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gil M., et al. , Impaired path integration in mice with disrupted grid cell firing. Nat. Neurosci. 21, 81–91 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Giocomo L. M., et al. , Grid cells use HCN1 channels for spatial scaling. Cell 147, 1159–1170 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Heys J. G., Hasselmo M. E., Neuromodulation of I(h) in layer II medial entorhinal cortex stellate cells: A voltage-clamp study. J. Neurosci. 32, 9066–9072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansson L., et al. , Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer’s disease: A 38-year longitudinal population study. BMJ Open 3, e003142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flatt J. D., Gilsanz P., Quesenberry C. P. J. Jr, Albers K. B., Whitmer R. A., Post-traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimers Dement. 14, 28–34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katz M. J., et al. , Influence of perceived stress on incident amnestic mild cognitive impairment: Results from the Einstein Aging Study. Alzheimer Dis. Assoc. Disord. 30, 93–98 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shansky R. M., et al. , Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol. Psychiatry 9, 531–538 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Bale T. L., Epperson C. N., Sex differences and stress across the lifespan. Nat. Neurosci. 18, 1413–1420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viña J., Lloret A., Why women have more Alzheimer’s disease than men: Gender and mitochondrial toxicity of amyloid-beta peptide. J. Alzheimers Dis. 20 (suppl. 2), S527–S533 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Altmann A., Tian L., Henderson V. W., Greicius M. D.; Alzheimer’s Disease Neuroimaging Initiative Investigators , Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 75, 563–573 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nestler E. J., Alreja M., Aghajanian G. K., Molecular control of locus coeruleus neurotransmission. Biol. Psychiatry 46, 1131–1139 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Jicha G. A., et al. , cAMP-dependent protein kinase phosphorylations on tau in Alzheimer’s disease. J. Neurosci. 19, 7486–7494 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin N., et al. , Truncation and activation of GSK-3β by calpain I: A molecular mechanism links to tau hyperphosphorylation in Alzheimer’s disease. Sci. Rep. 5, 8187 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobori N., Moore A. N., Dash P. K., Altered regulation of protein kinase a activity in the medial prefrontal cortex of normal and brain-injured animals actively engaged in a working memory task. J. Neurotrauma 32, 139–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bello-Chavolla O. Y., Antonio-Villa N. E., Vargas-Vázquez A., Ávila-Funes J. A., Aguilar-Salinas C. A., Pathophysiological mechanisms linking type 2 diabetes and dementia: Review of evidence from clinical, translational and epidemiological research. Curr. Diabetes Rev., 10.2174/1573399815666190129155654 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Wang M., et al. , Neuronal basis of age-related working memory decline. Nature 476, 210–213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramos B. P., et al. , Dysregulation of protein kinase a signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron 40, 835–845 (2003). [DOI] [PubMed] [Google Scholar]
- 60.MacKenzie K. F., et al. , Phosphorylation of cAMP-specific PDE4A5 (phosphodiesterase-4A5) by MK2 (MAPKAPK2) attenuates its activation through protein kinase A phosphorylation. Biochem. J. 435, 755–769 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Houslay K. F., et al. , Identification of a multifunctional docking site on the catalytic unit of phosphodiesterase-4 (PDE4) that is utilised by multiple interaction partners. Biochem. J. 474, 597–609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang M., et al. , Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129, 397–410 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Prickaerts J., Heckman P. R. A., Blokland A., Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin. Investig. Drugs 26, 1033–1048 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Lacampagne A., et al. , Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol. 134, 749–767 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Khachaturian Z. S., Overview of basic research on Alzheimer disease: Implications for cognition. Alzheimer Dis. Assoc. Disord. 5 (suppl. 1), S1–S6 (1991). [DOI] [PubMed] [Google Scholar]
- 66.Mattson M. P., Calcium and neurodegeneration. Aging Cell 6, 337–350 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Stutzmann G. E., The pathogenesis of Alzheimers disease is it a lifelong “calciumopathy”? Neuroscientist 13, 546–559 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Marx S. O., et al. , PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell 101, 365–376 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Lehnart S. E., et al. , Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 123, 25–35 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santulli G., Xie W., Reiken S. R., Marks A. R., Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. U.S.A. 112, 11389–11394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morozov Y. M., Datta D., Paspalas C. D., Arnsten A. F. T., Ultrastructural evidence for impaired mitochondrial fission in the aged rhesus monkey dorsolateral prefrontal cortex. Neurobiol. Aging 51, 9–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X., et al. , Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell 150, 1055–1067 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alberdi E., et al. , Ca(2+) -dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid β-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell 12, 292–302 (2013). [DOI] [PubMed] [Google Scholar]
- 74.Guo Q., et al. , Alzheimer’s PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid beta-peptide. Neuroreport 8, 379–383 (1996). [DOI] [PubMed] [Google Scholar]
- 75.Begley J. G., Duan W., Chan S., Duff K., Mattson M. P., Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J. Neurochem. 72, 1030–1039 (1999). [DOI] [PubMed] [Google Scholar]
- 76.Zhang H., Sun S., Herreman A., De Strooper B., Bezprozvanny I., Role of presenilins in neuronal calcium homeostasis. J. Neurosci. 30, 8566–8580 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gardner O. S., Dewar B. J., Graves L. M., Activation of mitogen-activated protein kinases by peroxisome proliferator-activated receptor ligands: An example of nongenomic signaling. Mol. Pharmacol. 68, 933–941 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Javadov S., Jang S., Agostini B., Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives. Pharmacol. Ther. 144, 202–225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barthélemy N. R., Mallipeddi N., Moiseyev P., Sato C., Bateman R. J., Tau phosphorylation rates measured by mass spectrometry differ in the intracellular brain vs. extracellular cerebrospinal fluid compartments and are differentially affected by Alzheimer’s disease. Front. Aging Neurosci. 11, 121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu F., et al. , Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur. J. Neurosci. 26, 3429–3436 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crimins J. L., et al. , Synaptic distributions of pS214-tau in rhesus monkey prefrontal cortex are associated with spine density, but not with cognitive decline. J. Comp. Neurol. 527, 856–873 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Busche M. A., et al. , Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo. Nat. Neurosci. 22, 57–64 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gibbons G. S., Lee V. M. Y., Trojanowski J. Q., Mechanisms of cell-to-cell transmission of pathological tau: A review. JAMA Neurol. 76, 101–108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu L., et al. , Trans-synaptic spread of tau pathology in vivo. PLoS One 7, e31302 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Felice F. G., et al. , Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol. Aging 29, 1334–1347 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sivanandam T. M., Thakur M. K., Traumatic brain injury: A risk factor for Alzheimer’s disease. Neurosci. Biobehav. Rev. 36, 1376–1381 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Edler M. K., et al. , Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease. Neurobiol. Aging 59, 107–120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forny-Germano L., et al. , Alzheimer’s disease-like pathology induced by amyloid-β oligomers in nonhuman primates. J. Neurosci. 34, 13629–13643 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beckman D., et al. , Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc. Natl. Acad. Sci. U.S.A. 116, 26239–26246 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El-Shamayleh Y., Horwitz G. D., Primate optogenetics: Progress and prognosis. Proc. Natl. Acad. Sci. U.S.A. 116, 26195–26203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park J. E., et al. , Generation of transgenic marmosets expressing genetically encoded calcium indicators. Sci. Rep. 6, 34931 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chan A. W., et al. , A two years longitudinal study of a transgenic Huntington disease monkey. BMC Neurosci. 15, 36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Niu Y., et al. , Early Parkinson’s disease symptoms in α-synuclein transgenic monkeys. Hum. Mol. Genet. 24, 2308–2317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chan A. W., et al. , Progressive cognitive deficit, motor impairment and striatal pathology in a transgenic Huntington disease monkey model from infancy to adulthood. PLoS One 10, e0122335 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y., et al. , Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature 570, 326–331 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Latimer C. S., et al. , A nonhuman primate model of early Alzheimer’s disease pathologic change: Implications for disease pathogenesis. Alzheimers Dement. 15, 93–105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodriguez-Callejas J. D., Fuchs E., Perez-Cruz C., Evidence of tau hyperphosphorylation and dystrophic microglia in the common marmoset. Front. Aging Neurosci. 8, 315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Head E., McCleary R., Hahn F. F., Milgram N. W., Cotman C. W., Region-specific age at onset of beta-amyloid in dogs. Neurobiol. Aging 21, 89–96 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This paper contains no original data.