Abstract
Purpose
To describe the pharmacokinetics of orally administered ABT-751 and its conjugated metabolites in children with neuroblastoma and other solid tumors and to relate pharmacokinetic parameters to toxicity and therapeutic outcomes.
Methods
Patients (median age, 11 years) with neuroblastoma (n = 37) or other solid tumors (n = 25) had pharmacokinetic sampling after the first dose of ABT-751 (75–250 mg/m2/day) on a 7-day or 21-day schedule. ABT-751 and its glucuronide and sulfate metabolites were quantified with an HPLC/MS/MS assay. Pharmacokinetic parameters were derived with non-compartmental methods. The relative bioavailability of more water soluble capsule and suspension formulations was assessed.
Results
ABT-751 peaked in plasma at 2 h and declined monoexponentially with a t1/2 of 5.1 h. The apparent clearance was 33 ml/min/m2 and was age-independent. The AUC0–∞ increased in proportion to the dose, and at 200 mg/m2 the median AUC0–∞ was 91 mcg h/ml and the Cave was 3.9 mcg/ml. Inter-and intra-patient variability was low. The metabolites were detected in plasma 30 min post-dose and peaked 3–5 h after the dose. The glucuronide:sulfate molar AUC0–∞ ratio was 0.57. Less than 1% of the dose was excreted in urine as parent drug; 13% of the dose was excreted as sulfate metabolite and 10% as glucuronide metabolite. The relative bioavailability of the water soluble capsule and suspension formulations was 105 and 93%, respectively. AUC0–∞ was higher in patients experiencing dose-limiting toxicity.
Conclusions
Oral ABT-751 pharmacokinetics was dose-proportional and age-independent with minimal intra- and inter-patient variability in children.
Keywords: Pharmacokinetics, Tubulin binding agent, Neuroblastoma
Introduction
ABT-751 (Abbott Laboratories, Abbott Park, IL) is an orally bioavailable sulfonamide that inhibits microtubule polymerization by binding to the colchicine binding site on β-tubulin and preferentially to β3-tubulin [1, 2]. Phase 1 clinical trials evaluated 7- and 21-day schedules with once or twice daily oral administration [3–6]. The recommended dose in adults on the 7-day schedule was 250 mg/day (equivalent to ~140 mg/m2). ABT-751 was rapidly absorbed (Tmax ~2 h) after oral administration and in adults receiving 250 mg the AUC0–last was 61 mcg h/ml and the trough concentration at 24 h was 0.5 mcg/ml. Inter-patient variability in pharmacokinetic parameters in adults was low. ABT-751 is eliminated by glucuronidation and sulfation, and these conjugated metabolites are measurable in plasma and urine. In adults, ~50% of an orally administered dose is eliminated in urine as these conjugated metabolites [5].
The phase 1 trial of ABT-751 in children with refractory solid tumors evaluated two schedules—daily for seven consecutive days every 21 days (7-day schedule) and daily for 21 consecutive days every 28 days (21-day schedule) [3, 4]. The maximum tolerated dose (MTD) was 200 mg/m2/day (equivalent to ~360 mg/day in adults, based on BSA = 1.8 m2) on the 7-day schedule and 100 mg/m2/day on the 21-day schedule. The 21-day schedule was poorly tolerated in children. Clinical development (phase 2) of ABT-751 in children is currently focused on neuroblastoma because pre-clinical in vitro and in vivo studies and the clinical experience on a phase 1 trial suggested selective activity in this tumor [3, 4, 7, 8]. The aim of this work was to perform pharmacokinetic studies in the children enrolled on this phase 1 trial and in children with relapsed neuroblastoma enrolled on a subsequent pilot study of 200 mg/m2/day on the 7-day schedule, compare the pharmacokinetics in children and adults, and correlate the pharmacokinetic parameters with toxicity and outcome in children.
Materials and methods
Patient population
Seventy-six patients were enrolled on our phase 1 trial of ABT-751 between May 2002 and April 2007 at the three participating sites (Pediatric Oncology Branch, NCI; Children’s Hospital of Philadelphia; Children’s Memorial Hospital, Chicago). The trial included dose-finding studies for the 7- and 21-day dosing schedules and a pilot study in children with neuroblastoma. The eligibility, design, and results of the dose-finding and pilot studies were previously reported [3, 4, 9]. The protocol was approved by the Institutional Review Boards of the participating institutions, and all patients or their parents consented to participation in the optional pharmacokinetic studies. Assent was obtained according to institutional guidelines.
Drug supply and administration
ABT-751 was supplied by Abbott Laboratories as 25 and 100 mg capsules in two tautomeric forms (Form 1 and 2) and as a 25 mg/ml suspension (Form 2) for oral administration. The suspension formulation, which is taken up in purified or sterile water, contains Form 2 ABT-751 and sucrose, xanthan gum, colloidal silicon dioxide, and an orange flavor. Pharmacokinetic studies were performed with the first dose of ABT-751 administered orally after breakfast. Dose was normalized to body surface area and rounded to the nearest 25 mg. Dose levels for the 7- and 21-day schedules are listed in Table 1. The 33 patients enrolled on the neuroblastoma pilot portion of the trial were treated on the 7-day schedule at a dose of 200 mg/m2/day.
Table 1.
Dose levels studied on the 7- and 21-day schedules
| Dose level | 7-Day schedule | 21-Day schedule | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/m2/day) | Maximum dose (mg/day) | na | No. (%) with DLTb | Dose (mg/m2/day) | Maximum dose (mg/day) | na | No. (%) with DLTb | |
| 1 | 100 | 150 | 4 | 0 (0) | 75 | 125 | 3 | 0 (0) |
| 2 | 130 | 200 | 5 | 1 (20) | 100 | 150 | 6c | 1 (20) |
| 3 | 165 | 250 | 6 | 1 (17) | 130 | 200 | 4 | 2 (50) |
| 4 | 200 | 300 | 29 | 6 (21) | 165 | 250 | 3 | 3 (100) |
| 5 | 250 | 375 | 2 | 2 (100) | ||||
Toxicity data from the dose-finding portion of the trial on the 7- and 21-day schedule have been previously reported [3, 4]
Number of patients who participated in pharmacokinetic studies at each dose level
DLT, dose-limiting toxicity occurring on the first treatment cycle
One patient was not evaluable for toxicity
Plasma and urine pharmacokinetic sampling and drug assay
Pharmacokinetic samples were collected after the first dose on cycle 1. On the dose escalation portion of the trial on the 7- and 21-day schedules, 3 ml blood samples were collected in EDTA tubes immediately prior to the dose of ABT-751 and 0.5, 1, 2, 3, 5, 8, 10–12, and 24 h after the dose. Trough blood samples were also drawn prior to the 5th and 7th doses. Blood samples were placed immediately into ice, and plasma was separated by centrifugation in a pre-chilled centrifuge within 1 h and frozen at −20°C. A 24-h urine sample was collected after the first dose when feasible. A less extensive sampling schedule was used in patients enrolled on the neuroblastoma pilot arm. Blood samples were drawn prior to the dose and 1, 3, 6, 10–12, and 24 post-dose with a single trough sample on day 5. This limited sampling schedule was also used to study the relative bioavailability of a more water soluble tautomer of ABT-751 (Form 2) in capsules and a suspension formulation of ABT-751. On day 1 and day 2 of cycle 1, two subsets of patients were studied after equal doses of either the two capsule forms (Form 1 vs. Form 2, n = 8) or capsules versus suspension (n = 6) with the order of administration randomly assigned. Patients on the Form 1 versus Form 2 study also had pharmacokinetic sampling performed on day 1 of cycle 2 with the alternate Form of the drug that was studied on day 1 of cycle 1 when feasible (n = 3).
ABT-751 and its two conjugated metabolites were quantified in plasma and urine using a previously described HPLC tandem mass spectrometry assay [10].
Pharmacokinetic and pharmacodynamic analyses
The ABT-751 and metabolite plasma concentration–time data were analyzed using non-compartmental methods. The peak concentration (Cmax) and time to peak concentration (Tmax) were determined from a concentration–time curve for each patient. Area under the concentration curve to the last measured time point (AUC0–last) was calculated with the linear trapezoidal method and extrapolated to infinity (AUC0–∞) by adding the final measured plasma concentration divided by the terminal rate constant, which was derived from the slope of the natural log-transformed concentrations and times on the terminal elimination phase of the decay curve. The day 2 AUC0–∞ on the Form 1 versus Form 2 and capsule versus suspension relative bioavailability studies were corrected for residual drug exposure from the day 1 dose by subtracting the concentration in the day 2 pre-dose sample divided by the day 1 terminal rate constant. The half-life (t1/2) was calculated by dividing 0.693 by the terminal rate constant. Apparent clearance (CL/F) was calculated by dividing the dose by the AUC0–∞, and the relationship between patient age and CL/F was assessed using Spearman correlation. Accumulation of ABT-751 over the 7- or 21-day dosing interval was estimated from the ratio of the AUC0–∞, which equals the AUC0–24 h at steady state, to the AUC0–24 h after the first dose. The average plasma concentration (Cave) is the AUC0–∞ divided by the dosing interval (24 h). The ratio of the plasma AUC0–∞ for the glucuronide and sulfate metabolites and the fraction of the ABT-751 dose excreted in the urine over 24 h as the parent drug and metabolites were calculated after conversion to molar units. The relationship between patient age and glucuronide:sulfate molar ratio was assessed using Spearman correlation.
The AUC0–∞ in 16 patients who experienced a dose-limiting toxicity (DLT) on the first treatment cycle was compared to the AUC0–∞ in 45 patients who had tolerable toxicity during cycle 1 using the non-parametric Mann–Whitney test separately for the 7- and 21-day schedules.
Results
Patient characteristics
Sixty-two (82%) of the 76 patients enrolled on our pediatric phase 1 trial of ABT-751 participated in pharmacokinetic studies, including 39 (91%) of the 43 patients enrolled on the dose-finding portion of the trial for the 7- and 21-day schedules and 23 (70%) of the 33 enrolled on the neuroblastoma pilot portion. The median (range) age of the 62 patients was 11 years (2–18 years), and 40 were male and 22 were female. Thirty-seven patients had neuroblastoma (median age, 9 years), 18 had a sarcoma, and seven had other cancer types.
Pharmacokinetics of ABT-751
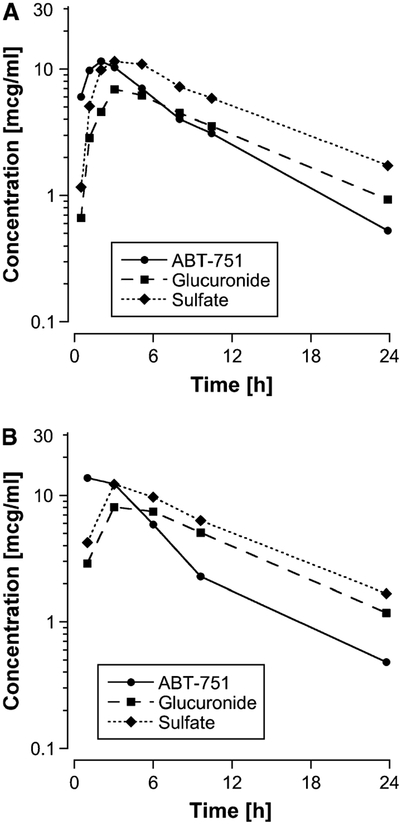
The plasma concentration–time profiles of ABT-751 and its metabolites are shown in Fig. 1. ABT-751 was rapidly absorbed after an oral dose (median time to peak concentration, 2.1 h) and plasma concentration declined monoexponentially with a median half-life of 5.1 h. Pharmacokinetic parameters in the 62 patients studied at 6 dose levels are listed in Table 2. Similar results were obtained using the two sampling schedules (Fig. 1); therefore, data generated from the detailed and limited sampling schedules are combined for most analyses.
Fig. 1.
Mean plasma concentration–time curve for ABT-751 and its glucuronide and sulfate metabolites at 200 mg/m2 in (a) 6 patients studied on the detailed sampling schedule and (b) 24 patients studied on the limited sampling schedule
Table 2.
Median (range) pharmacokinetic parameters for ABT-751 by dose level
| Dose level (mg/m2/day) | n | Cmax (mcg/ml) | Tmax (h) | AUC0–24 h (mcg h/ml) | AUC0−∞ (mcg h/ml) | T1/2 (h) | CL/F (ml/min/m2) |
|---|---|---|---|---|---|---|---|
| 75 | 3 | 8.1 (6.3–9.7) | 3.0 (2.0–3.0) | 40 (34–72) | 44 (36–76) | 6.0 (5.8–7.0) | 29 (18–33) |
| 100 | 8 | 11 (5.6–15) | 2.0 (0.9–4.0) | 55 (35–71) | 57 (41–92) | 6.0 (2.4–25) | 29 (16–39) |
| 100a | 2b | 7.2, 11 | 1.0, 3.0 | 48, 53 | 49, 58 | 5.0, 6.2 | 28, 30 |
| 130 | 6 | 9.6 (8.6–16) | 2.0 (1.1–3.1) | 58 (42–93) | 64 (43–96) | 4.8 (3.5–19) | 33 (22–39) |
| 130a | 3 | 9.6 (7.8–12) | 3.0 (1.0–6.0) | 68 (44–74) | 70 (45–78) | 4.8 (3.4–5.1) | 30 (26–55) |
| 165 | 9 | 11 (8.1–21) | 3.0 (1.0–5.0) | 94 (50–119) | 96 (53–122) | 5.0 (3.8–11) | 29 (23–47) |
| 200 | 6 | 17 (8.5–29) | 2.5 (1.2–5.7) | 83 (56–118) | 87 (64–123) | 5.4 (2.3–8.4) | 37 (25–43) |
| 200a | 23 | 17 (4.6–34) | 3.0 (1.0–6.0) | 90 (28–156) | 95 (29–183) | 4.8 (2.2–9.0) | 34 (17–122) |
| 250 | 2b | 19, 28 | 1.9, 2.1 | 106,134 | 114, 144 | 2.5, 6.2 | 30, 37 |
Monitored with the limited sampling schedule
Data presented in this row represents the values for the 2 patients studied at this dose level
Cmax peak plasma concentration; Tmax time that the peak concentration in plasma was achieved; AUC area under the plasma concentration–time curve; T1/2 half-life in plasma; CL/F apparent clearance
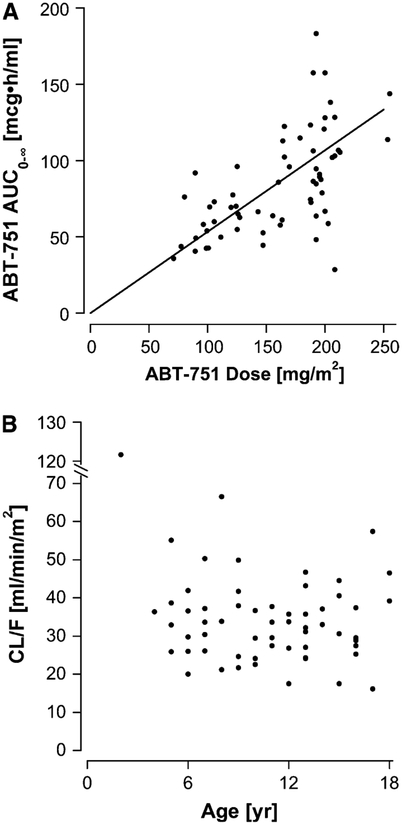
The AUC0–∞ increased in proportion to the dose over the dosage range from 75 to 250 mg/m2 (Fig. 2a). At the recommended dose for children of 200 mg/m2/day on the 7-day schedule, the median AUC0–∞ was 91 mcg h/ml, which is equivalent to a Cave in plasma of 3.9 mcg/ml (10 mcM). For an orally administered agent, the inter-patient variability of the parent drug pharmacokinetic parameters was low, as evidenced by the observation that the median deviation of the AUC0–∞ from the line of proportionality in Fig. 2a was 19%.
Fig. 2.
Relationship between (a) the administered dose normalized to body surface area and the AUC0–∞ (line represents the line of proportionality) and (b) patient age and apparent clearance (CL/F)
The median (range) apparent clearance (CL/F) was 33 (16–120) ml/min/m2, and CL/F was similar in males (33 ml/min/m2) and females (30 ml/min/m2). CL/F did not appear to be age-dependent (Spearman correlation coefficient, −0.09; Fig. 2b), but the youngest patient, who was 2 years old, had a substantially higher CL/F of 120 m/min/m2, which was not a result of poor absorption because the glucuronide and the sulfate metabolite AUCs were similar to those of other patients treated at the same dose level. In addition, this patient was studied on two consecutive days as part of the Form 1 versus Form 2 study with identical results (AUC0–∞ of 29 mcg h/ml on both days). The glucuronide to sulfate plasma AUC0–∞ molar ratio in this patient was 0.78.
The mean ratio of AUC0–∞:AUC0–24 h after the first dose was 1.06 ± 0.08, indicating that the AUC0–24 h at steady state would only be 6% higher than the AUC0–24 h after the first dose. Median trough (24 h post-dose) plasma concentrations on days 2, 5 and 7 were < 1 mcg/ml at all dose levels, and there was no evidence of significant drug accumulation. At the 200 mg/m2/day dose level, median (range) trough concentrations were 0.47 (0.14–2.42) mcg/ml on day 2, 0.53 (0.03–3.27) mcg/ml on day 5 and 0.68 (0.24–0.69) mcg/ml on day 7. Therefore, accumulation of ABT-751 with once daily dosing is minimal.
The glucuronide and sulfate metabolites of ABT-751 were measurable in plasma 30 min after the dose and plasma concentration of the sulfate peaked at a median of 3 h post-dose and the glucuronide peaked at 5 h. The median molar AUC0–∞ ratio of glucuronide to sulfate metabolites in plasma was 0.57 (range, 0.24–1.6), and the sulfate metabolite AUC0–∞ exceeded the glucuronide metabolite AUC0–∞ in 56 of 62 patients. The median plasma AUC0–∞s of the glucuronide and the sulfate metabolites were 98 and 160 mcg h/ml, respectively at the 200 mg/m2 dose level. The glucuronide:sulfate AUC0–∞ molar ratio in plasma was not age-dependent (Spearman r = 0.10). The median (range) percentage of the dose excreted in the urine over 24 h after the first dose in 28 patients was 0.05 (0–0.40%) for ABT-751, 10 (1.0–39%) for ABT-751 glucuronide, and 13 (2.3–51%) for ABT-751 sulfate.
Relative bioavailability of form 2 and suspension
The bioavailability of the more water soluble tautomer of ABT-751 (Form 2) relative to Form 1 was studied in eight patients, and the results are listed in Table 3. The mean relative bioavailability of Form 2 was 105%. The molar glucuronide:sulfate ratio was lower with Form 2 compared to Form 1 in all eight patients (p < 0.01) but only by a median of 12 (range, 1–21%), and the CL/Fs of Form 1 (median, 33 ml/min/m2) and Form 2 (36 ml/min/m2) were similar (p = 0.64).
Table 3.
Relative bioavailability of two tautomeric capsule formulations of ABT-751
| Patient No. | Dose (mg/m2) | Cycle | ABT-751 AUC0−∞ (mcg h/ml) | Form 2: Form 1 (%) | |
|---|---|---|---|---|---|
| Form 1 | Form 2 | ||||
| 38 | 130 | 1 | 44.5 | 55.0 | 124 |
| 2 | 53.6 | ||||
| 39 | 200 | 1 | 107 | 88.4 | 83 |
| 40 | 130 | 1 | 69.6 | 77.6 | 111 |
| 100 | 2 | 110a | |||
| 41 | 200 | 1 | 29.0 | 28.5 | 98 |
| 42 | 130 | 1 | 70.0 | 78.2 | 111 |
| 43 | 100 | 1 | 58.2 | 50.1 | 86 |
| 44 | 100 | 1 | 44.4 | 49.3 | 111 |
| 46 | 200 | 1 | 93.9 | 106 | 113 |
| 2 | 108 | ||||
Form 1 was used in the first 37 patients enrolled on the trial
Patient had a dose reduction from 130 to 100 mg/m2 on cycle 2. AUC0–∞ on cycle 2 is normalized to a dose of 130 mg/m2 for comparison
The bioavailability of the suspension formulation was compared to capsules (Form 2) in 6 patients who received the two formulations on consecutive days in random order. The mean ± SD relative bioavailability of the suspension was 93 ± 17%.
The intra-patient variability in the 14 patients who were studied on two consecutive day in these relative bioavailability studies was minimal. The mean (±SD) difference in the AUC0–∞ on days 1 and 2 was 14 ± 6%.
Pharmacodynamic pharmacokinetic relationships
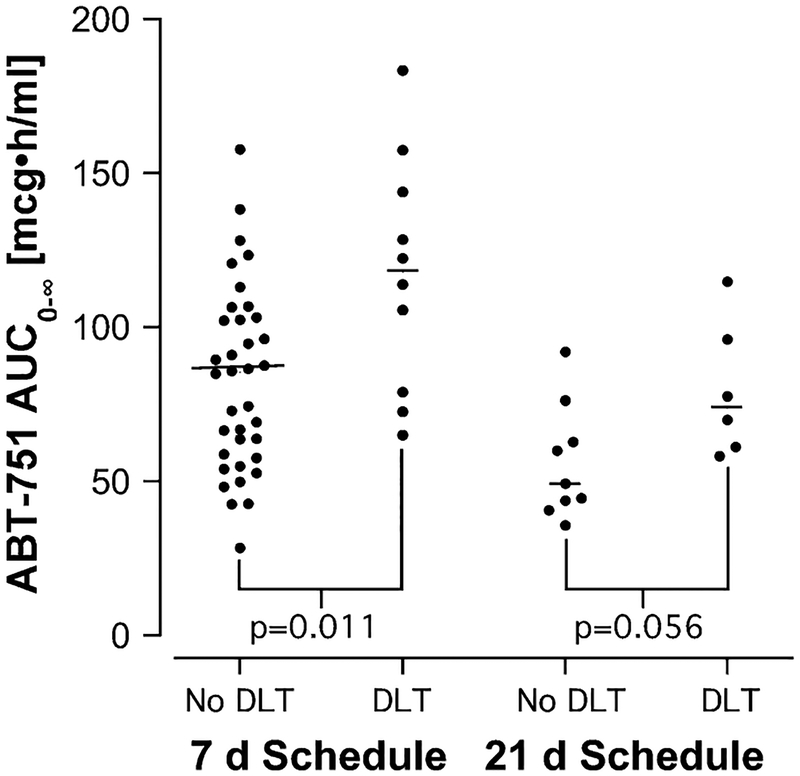
Dose-limiting toxicity (DLT) on cycle 1 occurred in 16 of 61 patients who underwent pharmacokinetic sampling (one patient on the 21-day schedule was not evaluable for toxicity). Dose-limiting toxicities included sensory and motor neuropathy, fatigue, hypertension, nausea, vomiting, dehydration, abdominal pain, and constipation [3, 4, 9]. The distribution of patients with a DLT by dose level is shown in Table 1, and the relationship between ABT-751 AUC0–∞ and DLT is shown in Fig. 3. On the 7-day schedule, patients who experienced DLT on cycle 1 had a significantly higher AUC0–∞ (median, 120 mcg h/ml) than those with tolerable toxicity (median, 85 mcg h/ml). The incidence of cycle 1 DLT overall on the 7-day schedule was 10/46 (22%), but in patients with an AUC0–∞ > 100 mcg h/ml the incidence was 7/18 (39%) and with an AUC0–∞ >120 mcg h/ml 5/10 patients (50%) experienced a DLT.
Fig. 3.
Relationship between AUC0–∞ and dose-limiting toxicity (DLT) on the first cycle of therapy on the two treatment schedules. The horizontal bars represent the median AUC0–∞ for each group. Two-tailed P values were generated using the Mann–Whitney test
Five of 37 patients with neuroblastoma received ≥50 cycles of ABT-751 and had no evidence of disease progression. The median (range) AUC0–∞ in these 5 patients was 75 (43–180) mcg h/ml compared to 93 (29–160) mcg h/ml in the remaining 32 patients with shorter times to progression. Two patients with no evidence of disease progression for greater than 50 cycles experienced a DLT during cycle 1 (AUC0–∞ were 78 and 180 mcg h/ml). Both had an initial dose reduction of 30% and continued on protocol therapy. The patient with the highest AUC0–∞ (180 mcg h/ml) required a second dose reduction in subsequent cycles.
Discussion
We studied the pharmacokinetics of ABT-751 and its conjugated metabolites over a dosage range of 75–250 mg/m2 in a large group of children ranging in age from 2 to 18 years (6 children < 6 years, 27 children 6–11 years, 29 children 12–18 years). Except for the youngest patient (2 years), who had a CL/F that was 3.5-fold higher than the population mean, CL/F was not age-dependent. Our results were also comparable to the results from the trial of ABT-751 in adults. The mean AUC0–24 h of ABT-751 in adults treated at a dose of 250 mg (~140 mg/m2) was 61 mcg h/ml, [5] and the AUC0–24 h in the 9 children (median age, 11 years) treated at the 130 mg/m2 dose level was 62 mcg h/ml.
As in adult subjects, the sulfate conjugate was the predominant metabolite of ABT-751 in the plasma and urine. Renal excretion was not a route of elimination for the parent drug and on average <25% of the dose of ABT-751 was excreted in the urine as conjugated metabolites. Incomplete 24-h urine collections in these children could account for the variability seen in the fraction of the dose excreted in urine as metabolites (range, 2.3–51% for the sulfate metabolite) and for underestimation of the urinary excretion of the conjugated metabolites. In plasma, the combined molar AUC0–∞s of the glucuronide (median, 180 mcM h at 200 mg/m2) and the sulfate (350 mcM h) metabolites exceeded the molar AUC0–∞ of the parent drug (240 mcM h). These high plasma concentrations of the conjugated metabolites and their detection in plasma 30 min after the ABT-751 dose are suggestive that some pre-systemic (first-pass) metabolism of ABT-751 is occurring after oral administration.
The relative bioavailability of the three dosing formulations used on this trial was equivalent. Although the number of patients who were studied on two consecutive days to compare Form 1 to Form 2 capsules (n = 8) and Form 2 capsules to Form 2 suspension (n = 6) was small, the intra-patient variability was low, and in most cases the differences between AUC0–∞s of the two dosing forms being compared was <15%. Therefore, the formulations can be used interchangeably without adjusting the dose.
Seven of the 10 patients who experienced a dose-limiting toxicity from ABT-751 on the 7-day schedule had an ABT-751 AUC0–∞ > 100 mcg h/ml, and the incidence of DLT in the 18 patients with an AUC0–∞ > 100 mcg h/ml on this schedule was 39% compared to 11% in the 28 patients with an AUC0–∞ < 100 mc h/ml. Although efficacy is difficult to quantify on a phase 1 trial, five of the 37 patients with neuroblastoma survived progression-free for more than 3 years and the median AUC0–∞ in these 5 patients was 75 mcg h/ml, suggesting that a therapeutic effect can be achieved with this drug at drug exposures (AUC0–∞) that are tolerable. At the recommended dose of ABT-751 for children (200 mg/m2/day for 7 days repeated every 21 days), the median AUC0–∞ was 91 mcg h/ml and 16 of the 29 patients had an AUC0–∞ < 100 mcg h/ml. This analysis may provide a basis for dose reductions and individualized dosing of ABT-751 in children.
ABT-751 has a number of favorable pharmacological characteristics. It is orally bioavailable, it has minimal inter- and intra-patient pharmacokinetic variability, the AUC is proportional to the administered dose so that dose modifications result in a predictable changes in drug exposure, and it is eliminated by conjugation to both glucuronide and sulfate conjugates, which minimizes the risk of drug interactions. An ongoing Phase 2 study of ABT-751 through the Children’s Oncology Group is designed to determine whether this agent is active against refractory neuroblastoma.
Acknowledgments
This research was supported, in part, by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. Additional funding to Children’s Hospital of Philadelphia and Children’s Memorial Hospital was provided by Abbott Laboratories.
Footnotes
Preliminary results of this study were reported at the Annual ASCO meeting in 2007: ASCO Annual Proceedings 25 (18S):540s (Abstract 9557).
Contributor Information
Elizabeth Fox, Pediatric Oncology Branch, National Cancer Institute, 10 Center Drive, Bldg. 10/Rm. 1-5750, Bethesda, MD 20892-1101, USA.
John M. Maris, Center for Childhood Cancer Research at The Children’s Hospital of Philadelphia and University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Susan L. Cohn, University of Chicago, Chicago, IL, USA
Wendy Goodspeed, Pediatric Oncology Branch, National Cancer Institute, 10 Center Drive, Bldg. 10/Rm. 1-5750, Bethesda, MD 20892-1101, USA.
Anne Goodwin, Pediatric Oncology Branch, National Cancer Institute, 10 Center Drive, Bldg. 10/Rm. 1-5750, Bethesda, MD 20892-1101, USA.
Marie Kromplewski, Center for Childhood Cancer Research at The Children’s Hospital of Philadelphia and University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Diane Medina, Abbott Laboratories, Abbott Park, IL, USA.
Hao Xiong, Abbott Laboratories, Abbott Park, IL, USA.
Andrew Krivoshik, Abbott Laboratories, Abbott Park, IL, USA.
Brigitte Widemann, Pediatric Oncology Branch, National Cancer Institute, 10 Center Drive, Bldg. 10/Rm. 1-5750, Bethesda, MD 20892-1101, USA.
Peter C. Adamson, Center for Childhood Cancer Research at The Children’s Hospital of Philadelphia and University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Frank M. Balis, Pediatric Oncology Branch, National Cancer Institute, 10 Center Drive, Bldg. 10/Rm. 1-5750, Bethesda, MD 20892-1101, USA Center for Childhood Cancer Research at The Children’s Hospital of Philadelphia and University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
References
- 1.Iwamoto Y, Nishio K, Fukumoto H, Yoshimatsu K, Yamakido M, Saijo N (1998) Preferential binding of E7010 to murine beta 3-tubulin and decreased beta 3-tubulin in E7010-resistant cell lines. Jpn J Cancer Res 89(9):954–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimatsu K, Yamaguchi A, Yoshino H, Koyanagi N, Kitoh K (1997) Mechanism of action of E7010, an orally active sulfonamide antitumor agent: inhibition of mitosis by binding to the colchicine site of tubulin. Cancer Res 57(15):3208–3213 [PubMed] [Google Scholar]
- 3.Fox E, Maris JM, Widemann BC et al. (2008) A phase I study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 21 days every 28 days in pediatric patients with solid tumors. Clin Cancer Res 14(4):1111–1115 [DOI] [PubMed] [Google Scholar]
- 4.Fox E, Maris JM, Widemann BC et al. (2006) A phase 1 study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 7 days every 21 days in pediatric patients with solid tumors. Clin Cancer Res 12(16):4882–4887 [DOI] [PubMed] [Google Scholar]
- 5.Hande KR, Hagey A, Berlin J et al. (2006) The pharmacokinetics and safety of ABT-751, a novel, orally bioavailable sulfonamide antimitotic agent: results of a phase 1 study. Clin Cancer Res 12(9):2834–2840 [DOI] [PubMed] [Google Scholar]
- 6.Yee KW, Hagey A, Verstovsek S et al. (2005) Phase 1 study of ABT-751, a novel microtubule inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res 11(18):6615–6624 [DOI] [PubMed] [Google Scholar]
- 7.Meany H, Fox E, Hagey AE, Balis FM (2006) In vitro cytotoxicity of ABT-751, a novel tubulin inhibitor, in pediatric solid tumor cell lines. Proc Am Assoc Cancer Res 47:1021 (Abstr #4351) [Google Scholar]
- 8.Morton CL, Favours EG, Mercer KS et al. (2007) Evaluation of ABT-751 against childhood cancer models in vivo. Invest New Drugs 25(4):285–295 [DOI] [PubMed] [Google Scholar]
- 9.Fox E, Adamson PC, Hagey A, et al. (2005) Phase I trial of oral ABT-751 in pediatric patients: preliminary evidence of activity in neuroblastoma (NBL). J Clin Oncol. ASCO Ann Meeting Proc 2005; 23(June 1 Suppl) (16S, Part 1 of 2: Abstr # 8527) [Google Scholar]
- 10.Rudek MA, Zhao M, He P, Messersmith WA, Baker SD (2006) Validation and implementation of a liquid chromatography/tandem mass spectrometry assay to quantitate ABT-751, ABT-751 glucuronide, and ABT-751 sulfate in human plasma for clinical pharmacology studies. J Pharm Biomed Anal 42(2):253–260 [DOI] [PubMed] [Google Scholar]