Abstract
Age-related hearing loss (AHL) or presbycusis is steadily increasing due to the overall aging of the Chinese population. Traditional Chinese medicine (TCM) has long been used to prevent and treat deafness, but its effectiveness and mechanism of action are still uncertain. The present study tested a TCM preparation called “Jian Er” in a mouse model of prebycusis.
Keywords: C57BL/6J mice, presbycusis, traditional Chinese medicine, auditory brainstem response
1. Introduction
The incidence of presbycusis, or age-related hearing loss (AHL) is steadily increasing with the overall aging of the general population in China. AHL takes the form of a bilateral sensorineural hearing loss (SHL), beginning with an inability to detect high frequencies. AHL is a serious human health problem that negatively affects the quality of life in many elderly individuals. AHL is the leading cause of total years lived with any disability in adults. According to the World Health Organization (WHO), by 2025 there will be approximately 1.2 billion people in the world over the age of 60, which marks a shift in the whole world population towards older people. An estimated 70–80% of adults between 65 and 75 years of age suffer from presbycusis [1]. According to a report in China [2], (due to the aging of the whole population) 51% of all people diagnosed with hearing loss suffered from AHL. Owing to the extremely complex etiology and pathogenesis of AHL, the prevention and treatment of AHL has proved extremely difficult. Up to the present day there is still no therapy that has definitively been proven to reverse damaged sensorineural cells, or rescue auditory neurons in human beings.
Therefore, it is important to search for effective treatments and methods of prevention for AHL. There have been many literature reports of the treatment of deafness by traditional Chinese medicine (TCM) approaches. These medicines have been used for thousands of years in China; however, up to now, their effectiveness has not been conclusively proven, and their mechanism(s) of action remain unclear. During decades of clinical practice, and taking into account modern pharmacological research in TCM, we introduced the new TCM compound, which was called “Jian Er preparation” (JEP). JEP showed good clinical ability to protect hearing in humans, and has been used for treating patients with SHL (including AHL). JEP received a patent [3] for the SHL application in China [4–7].
Jian Er TCM preparation is composed of the following herbs Radix astragali, Radix puerariae, Radix salviae miltiorrhizae, and Rhizoma drynariae. Previously, experimental injection of astragalus had shown protective effects against ototoxicity and renal toxicity in guinea pigs, caused by administration of the aminoglycoside antibiotic, gentamicin [8]. Subsequently, we also found that an astragalus compound, “Bu Shen Jian Pi” capsules, which is another TCM preparation used for treating AHL, had anti-apoptotic effects in cochlear hair cells in a mouse model of AHL [9, 10].
To further investigate the protective effects and mechanism of action of JEP on presbycusis, we chose C57BL/6J mice as an animal model of AHL. The expanding arsenal of transgenic mice has created powerful models for investigating the biological bases of presbycusis [11–15]. C57BL/6J is not a transgenic strain, but rather is a common inbred strain of laboratory mice. C57BL/6J mice exhibit a high frequency of hearing loss and cochlear degeneration in the middle of their life-span, beginning at about 5 months of age, and progressing to a profound hearing impairment by 15 months of age. The major gene responsible for AHL in C57BL/6J mice was mapped by analysis of a (C57BL/6J x CAST/Ei) x C57BL/6J backcross [16]. A locus contributing to the hearing loss in C57BL/6J mice was termed “age-related hearing loss” (ahl), and was mapped to chromosome 10 [17] on a splice variant of the cadherin 23 gene. Mutations in cadherin 23 have been shown to be correlated with congenital high frequency progressive hearing loss in humans, and some particular mutations caused late onset moderate hearing loss [18–20]
The goal of the present study was to study the effectiveness of “Jian Er” TCM in the C57BL/6J mouse model of AHL and to investigate its mechanism of action. We assessed the hearing ability of the mice by auditory brainstem response (ABR), and examined morphological changes in their cochlear hair cells, and spiral ganglion neurons by histological staining of a stretched preparation of the cochlear basilar membrane. Cyt-C and caspase-3 activity in cochlear tissues were measured by RT-PCR, and malondialdehyde (MDA) in cochlea, auditory cortex and hepatic tissue was measured by an assay kit, respectively.
2. Material and Methods
2.1. Animals
Fifty-four C57BL/6J mice (50% male and 50% female at 4 weeks of age) were used for this study. C57BL/6J mice were obtained from the Vital River Laboratory Animal Technology Company in Beijing, China [Batch number: SCXK (Beijing) 2012–0001]. All procedures described in the study were specifically approved by the Institutional Animal Care and Use Committee (IACUC) of Guangxi University of Chinese Medicine (license No. SYXK.GUI.2010–0001h).
2.2. Experimental groups
Fifty-four C57BL/6J mice were randomly divided into three groups. Twenty animals in the first group drank tap water from weaning (1 month of age) until 7 months of age as the control group, twenty animals in the second group drank tap water containing TCM daily from 1 month of age until 7 months of age as the treatment group. Six animals from each of these groups were used for evaluation of audiology, quantification of cochlear hair cells and spiral ganglion neurons. An additional eight animals from each group were used to measure Cyt-C, FasL and caspase-3 in the cochlear tissues. The remaining six animals from each group were used to measure MDA in cochlea, auditory cortex and hepatic tissue. In addition, fourteen untreated C57BL/6J mice drank tap water from weaning (1 month of age) until 2 months of age as a normal control group before disease development for the evaluation of audiology, quantification of cochlear hair cells (HCs) and spiral ganglion neurons (SGNs) from six animals and for the assay of Cyt-C, FasL and caspase-3 from eight animals.
2.3. Preparation of traditional Chinese medicine
Compound “Jian Er” preparation of TCM is composed mainly of the following herbs: Radix astragali, Radix puerariae, Radix salviae miltiorrhizae, Rhizoma drynariae. These botanical preparations were successively treated by cooking in water, filtering, concentrating, drying, pulverizing, and passing through a mesh to produce a powder according to the methods that had been previously used to prepare this herbal medicine in capsules for administration to patients. One gram of the final TCM powder was equal to 6.63 gram of the raw herbal materials. By applying the rule of dosage equivalence between the mouse and the adult human [21], the dosage for mice was calculated to be 1.83g /kg/day. The powder was added to tap water in drinking bottles for the animals to drink from 1 month of age until 3 months of age, so the animals could receive the calculated dosage each day without any other source of water. Subsequently the mice were daily administered the calculated dosage by gavage needle from 4 months of age until 7 months of age. The use of different methods of administration was because the esophagus and pharynx cavity of mice before 3 months of age is thin and delicate; therefore daily gavage may easily cause local damage and even lead to animal death.
2.4. Evaluation of auditory function in C57BL/6J mice
The auditory brain stem response (ABR) methodology has been described in our previous publications [12–15]. In brief, at the end of the experiment (7 months of age), the mice were anesthetized with ketamine (0.56 mg/kg) and acepromazine (36 mg/kg). Their body temperature was maintained at 37–38°C by placing the mice on a heating pad in a sound- attenuating chamber. The active needle electrode was inserted subcutaneously at the vertex, the reference electrode was inserted at the mastoid area of the test ear, and the ground electrode was inserted at the contralateral mastoid. The ABR was elicited with tone bursts (8, 12, 16, 32 kHz; 0.5 ms rise/fall time, no plateau, alternating phase) or clicks (10 ls) presented at 21/s. Stimuli were generated digitally (TDT system, Sig Gen, FL, USA), passed through a D/A converter (TDT, RP2.1, 100 kHz sampling rate) and presented through a high-frequency speaker (model: AS-TH400A), which was channeled through plastic tubes into the animals’ ear canals. The evoked brainstem responses to 8, 16 and 32 kHz tonebursts stimuli were amplified and averaged. ABR thresholds were obtained for each stimulus by reducing the sound pressure level (SPL) at 10 dB steps and finally at 5 dB steps up and down to identify the lowest level at which an ABR waveform could be detected. Stimulus presentation, data acquisition and analysis were performed using computerized equipment from Intelligent Hearing Systems (IHS; Miami, Florida). 100 dB was the maximum SPL presented for all stimuli. With our testing system, the average ABR thresholds (in dB SPL) for normal hearing in C57BL/6J mice at 2 months of age were about 50–55 dB for all tested frequencies (8, 12, 16, 32, and 48 kHz).
2.5. Cytocochleograms
Cochleae were prepared and evaluated as described in detail in our earlier publications [9, 15–17, 21]. After ABR testing, mice were decapitated immediately. The temporal bones were removed and fixed with 10% formalin in PBS for 24 hours. After decalcification with Decal solution (Baxter Scientific Products) for 3 days, one cochlea from each animal was infused with Harris’ hematoxylin staining solution for 5 min. The cochlear basilar membrane was micro-dissected out and mounted as a flat surface preparation in glycerin on a glass slide. Specimens were examined under a light microscope, and the numbers of missing inner hair cells (IHCs) and outer hair cells (OHCs) were counted over 0.24 mm intervals along the entire length of the cochlea. In comparison with experimental norms from young, healthy C57BL/6J mice, the cochleogram of the 7 month-old mice showing the percent hair cell loss as a function of percent distance from the apex, were constructed for each animal. The results from all the mice in each experimental group were averaged across animals in order to obtain a mean cochleogram for each group [12, 13, 15, 22–28]. The cochlear position was related to frequency using a cochlear frequency-place map [29].
2.6. Evaluation of cochlear spiral ganglion neurons
In order to assess the damage to spiral ganglion neurons (SGNs), the cochlea from the other ear was fixed with 10% formalin in 0.1 M phosphate buffered saline (pH 7.4), then immersed in fixative for approximately 24 h. The fixed cochleas were decalcified by immersion in Decal (Baxter Scientific Products) for 3 days, dehydrated, and embedded in Epon 812 resin. Serial sections were cut in a plane parallel to the modiolus at a thickness of 3 μm (Reichert Super Nova microtome), mounted on slides and stained with toluidine blue or hematoxylin - eosin (HE) staining [15, 28, 30–33].
2.7. Assays for Cyt-C mRNA, FasL mRNA and caspase-3 mRNA using Real-Time PCR
It was decided to measure Cyt-C mRNA, FasL mRNA and caspase-3 mRNA by RT-PCR rather than by measuring the expression of these proteins by immunohistochemistry. The coding gene of Cytochrome C (Cyt-C) from mouse represented somatic Cyt-C levels. RT-PCR has previously been used for this purpose [34, 35], but demonstration of increased protein expression in the tissues would also be necessary for final confirmation. Moreover Cyt-c is released from the mitochondria during apoptosis so its sub-cellular localization is also important as well as its expression levels. The FasL (fas ligand) is a Type II transmembrane glycoprotein from the cell membrane. It is known as an early key factor in the cell membrane pathway to induce apoptosis by activating members of the caspase family. The main experimental reagents and experimental apparatus were: mRNA synthesis of single strand amplified reverse transcription kit (Dalian Takara Biotechnology Co., Ltd.), fluorescence quantitative PCR primers (Beijing Liuhe Hua Gene Technology Co., Ltd.) and Goldview I type of nucleic acid staining agent (Shanghai Solarbio Biological Technology Co., Ltd.), total RNA Extraction Kit (Axygen company, USA), high speed freezing centrifuge (5810R, Eppendorf, Germany), conventional PCR (9700, ABI, USA), real-time quantitative PCR (Mastercycler ep realplex 4, Eppendorf, Germany), micro ultraviolet spectrophotometry (Nanodrop 2000, Thermo Fisher, USA).
ACTB (mouse, bp291) was selected as the PCR reference primer, using sequences of AGTGTGACGTTGACATCCGT (forward) and AGTAACAGTCCGCCTAGAAGC (reverse). Test PCR primers were respectively caspase-3 (mouse, bp224), which sequences were CCACGTGGGAAAGTGAACCA (forward) and CAGGGCTATTGCTGGATGCT (reverse), Cyt-C (mouse, bp190), which sequences were AATCTCCACGGTCTGTTCGG (forward) and GGTCTGCCCTTTCTCCCTTC (reverse), FasL (mouse, bp169), which sequences were GAGGTCTGTGACTGAGGGAC (forward) and AAACGGCCTCTGTGAGGTAG (reverse).
The cochleas from six animals in each group at 7 months of age in control and TCM groups were taken, and then placed in RNA protection solution and frozen in liquid nitrogen for preservation. Total RNA was extracted from cochlear cells according to the operating procedure of the total RNA Extraction kit. The ground cochlear samples were mixed and centrifuged successively in R-I Buffer solution and R-II Buffer solution, the supernatant was removed, and then centrifuged respectively in isopropyl alcohol, Buffer W1A and Buffer W2. Finally the RNA was obtained by centrifugation and eluted in RNase-free water. The total RNA was electrophoresed on an agarose gel to judge its integrity. The total RNA concentration was determined by micro ultraviolet spectrophotometry, and was in the range of 100–300 ng/μL. According to the instructions in the reverse transcription reaction kit, RNA was added into DNA Eraser Buffer, supplemented the total volume with RNase-free water, and then respectively added Prime Script Buffer, RNase-free water, RT Primer Mix, Prime Script RT Enzyme Mix I to conduct the reverse transcription reaction for synthesizing cDNA. Then added primers, and in the Real-Time PCR instrument, passed through respectively pre-denaturation, denaturation, annealing and extension reactions conducted by temperature cycling. Finally, Ct values of the amplified ACTB, caspase-3 and Cyt-C, FasL from the different samples were measured, and converted into 2-ΔΔCt value by calculating formula for expressing values of the mRNA.
2.8. Test for malondialdehyde concentration by UV spectrophotometry
A kit for malondialdehyde (MDA) determination was provided by the Nanjing Institute of Biological Engineering. In brief, the cochleas, temporal tissue of the brain (equivalent to the auditory cortex) and hepatic tissue of the animals were rapidly removed, and immersed in PBS (pH 7.4) with 1:9 volume ratio, stored in freezer at −20 °C. These tissues were respectively ground under liquid nitrogen and diluted tenfold in PBS, and then were respectively centrifuged at 4000 rpm for 10 min, and divided into two parts. One part of the supernatant, the MDA reagent in the kit (N-methyl-2-phenylindole) was added and the absorbance measured at 586 nm. To the other part of the supernatant, Coomassie brilliant blue was used to measure protein concentration. A calibration curve was prepared and the malondialdehyde content (nmol/mg protein) was calculated.
2.9. Statistical analysis
ABR data analysis was performed using the JMP 7.0 interactive statistics and graphics software program (www.JMP.com). Statistical significance of the differences among means was determined by one-way ANOVA with Tukey test for correction of multiple pairwise comparisons. The data of hair cell counts and spiral ganglion counts from the basal turn were analyzed using a 2-way ANOVA. A Tukey post-hoc analysis was used to identify significant differences (Sigma Stat 3.5 for Windows, version 3.5) [23–26, 28–32].
3. Results
3.1. Hearing loss
The results of the measured ABR thresholds are shown in Figure 1. The C57BL/6J mice at 2 months of age exhibited normal ABR thresholds for all tested frequencies (8, 12, 16, 32, and 48 kHz). However, the ABR thresholds in the untreated control group at 7 months of age (drinking tap water daily from 1–7 months of age) were significantly elevated at all tested frequencies in comparison with the normal control group (drinking tap water daily from 1–2 months of age) (p<0.05). In contrast, 7-month old C57BL/6J mice that had been treated with TCM (JEP) showed nearly normal ABR thresholds at all tested frequencies and there was no statistically significant difference compared to the normal control group (drinking tap water daily from 1–2 months of age) (p> 0.05); however there was a statistically significant difference compared to the untreated AHL control group (drinking tap water daily from 1–7 months of age) (p<0.05) (Figure 1). These results suggest that long-term treatment with traditional Chinese medicine can postpone the occurrence, or lessen the severity of presbycusis in middle- aged C57BL/6J mice.
Fig 1 :
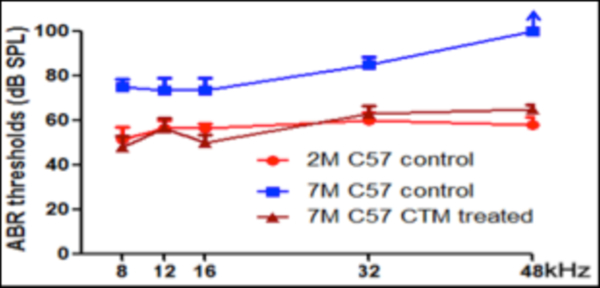
ABR thresholds in groups of mice.
Mean (+/− SEM, n=6/group) ABR thresholds in normal control group (C57BL/6J mice drank tap water till 2 months of age), experimental control group (C57BL/6J mice drank tap water till 7 months of age), and traditional Chinese medicine treated group (C57BL/6J mice drank traditional Chinese medicine till 7 months of age). Note C57Bl/6J mice drank tap water daily at 2 months of age had normal response of ABR on 8, 12, 16, 32, and 48 kHz tone bursts. In contrast to normal control group, C57BL/6J mice drank tap water daily till 7 months of age and developed a significant elevation of ABR thresholds in all tested frequencies (P < 0.05). However, no statistically significant differences in threshold means were detected between normal control group (C57BL/6J mice drinking tap water daily till 2 months of age) and traditional Chinese medicine treated group (C57BL/6J mice drinking traditional Chinese medicine daily till 7 months of age) (P> 0.05). *Significantly different from normal control group at 2 months of age (P < 0.05) and traditional Chinese medicine treated group at 7 months of age (P < 0.05).
3.2. Cochlear hair cell degeneration
To investigate whether the long-term treatment with traditional Chinese medicine can protect cochlear hair cells against age-related loss and damage in C57BL/6J mice, the cochlear surface preparations were prepared with hematoxylin staining for analysis of cochleogram.
We first confirmed that 2 month-old control C57BL/6J mice (drinking tap water daily from 1–2 months of age) showed normal cochlear hair cells throughout the whole cochlea (Figure 2A, Figure 3A), but untreated 7 month-old control C57BL/6J mice (drinking tap water daily from 1–7 months of age) displayed severe damage in their cochlear hair cells located in the basal turn of the cochlea (Figure 2B, Figure 3B). However, the hair cells were well protected in mice that had been treated with traditional Chinese medicine through daily drinking JEP from weaning to 7 months of age (Figure 2C, Figure 3C). The density of surviving cochlear hair cells in the basal region (70–100% from the apex) in the control group was 18% (Figure 3B). In contrast, the density of surviving hair cells in the TCM-treated C57BL/6J mice was 68% (Figure 3C). Statistical analysis showed a significant difference between the control group and TCM-treated groups (p<0.05, Figure 3D–F). The results indicate that long-term treatment with TCM can reduce age-related cochlear damage in C57BL/6J mice.
Fig 2:
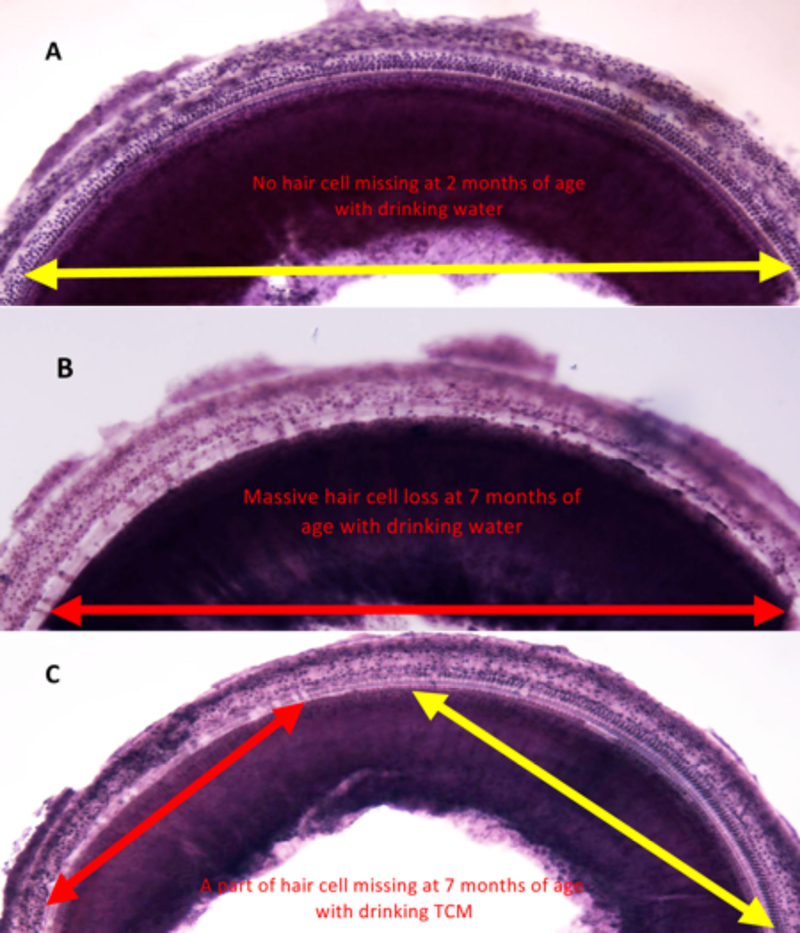
Typical examples of cochlear surface preparation at basal turn near the hook region.
Fig 3:
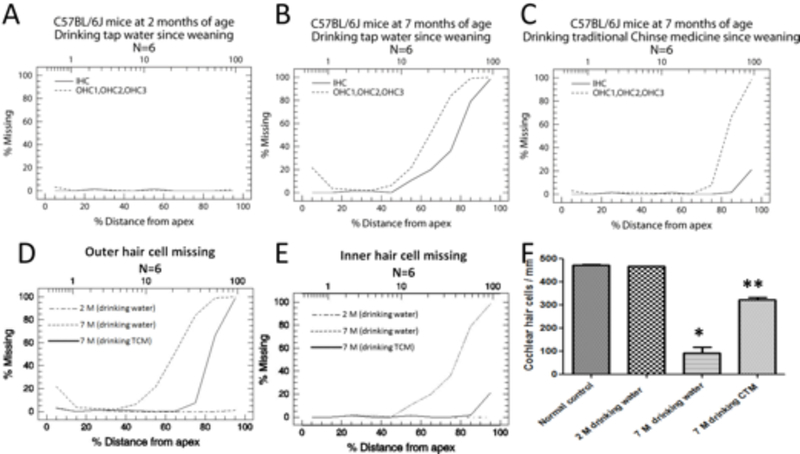
Average cochleogram and hair cell density from C57BL/6J mice.
(A) Mice at 2 months of age with drinking tap water. Note all hair cells were intact at 2 months of age. (B). Typical example of cochlear surface preparation at basal turn near hook region at 7 months of age with drinking tap water, note most hair cells were missing. (C) Typical example of cochlear surface preparation at basal turn near hook region at 7 months of age with drinking traditional Chinese medicine. Note the region and level of hair cell degeneration was effectively reduced by traditional Chinese medicine treatment (Orange arrow represents the extending range of intact hair cells. Red arrow represents the extending range of missing hair cells).
(A) Cochleogram from mice at 2 months of age with drinking tap water group; (B) Cochleogram from C57BL/6J mice at 7 months of age with drinking tap water; (C); Cochleogram from C57BL/6J mice at 7 months of age with drinking traditional Chinese medicine; (D) Comparison of outer hair cell loss among three groups; (E) Comparison of inner hair cell loss between three groups; (F) Quantification of hair cell density in the region of cochlear basal turn from 80–100% from apex. *Significantly different from normal control group at 2 months of age (P < 0.05). **Significantly different from between experimental group (drinking tap water till 7 months of age) and traditional Chinese medicine treated group (drinking traditional Chinese medicine) (P < 0.05).
3.3. Degeneration of spiral ganglion neurons
To investigate whether the long-term treatment with TCM could protect spiral ganglion neurons (SGNs) against age-induced degeneration in C57BL/6J mice, standard temporal bone sections were stained with toluidine blue or hematoxylin- eosin (HE) and observed under a light microscope.
Normal control C57BL/6J mice (drinking tap water daily from 1 to 2 months of age) displayed a high density of SGNs with a healthy morphological appearance (Figure 4A1, A2). However, a large number of SGNs in the cochlear basal turn were missing in untreated control C37BL/6J mice at 7 months of age (Figure 4B1, B2). In contrast, most of the SGNs at the same location were intact in C57BL/6J mice receiving long-term treatment with TCM (Figure 4C1, C2). Statistical analysis showed a significant difference between the control group and the TCM-treated group or the normal control group (2 months of age) (p<0.05), but there was no statistically significant difference between the normal control group (2 months of age) and the TCM-treated group (7 months of age) (p> 0.05) (Figure 4D). These results indicate that TCM had a protective effect to prevent degeneration in SGNs.
Fig 4:
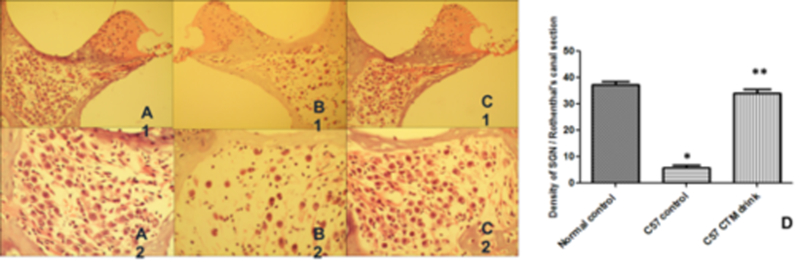
Effects on ganglion neurons.
(A1, A2) Spiral ganglion neurons were intact in the hook region in control mouse at 2 months of age with drinking tap water. (B1, B2) Massive ganglion cell loss was detected in the experimental group in the hook region at 7 months of age with drinking tap water. (C1, C2) Many spiral ganglion neurons survived after traditional Chinese medicine treatment at 7 months of age with drinking TCM. (D) Comparison of density of spiral ganglion neurons in the hook region between C57BL/6J control mice at 2 months of age with drinking tap water, 7 mouths of age with drinking tap water, and 7 months of age with drinking traditional Chinese medicine. *Significantly different from control group at 2 months of age (P < 0.05). **Significantly different between experimental group at 7 months of age with drinking tap water and 7 months of age with drinking traditional Chinese medicine (P < 0.05).
3.4. Assays for FasL, Cyt-C, caspase-3 and malondialdehyde
To investigate the mechanism of action of “Jian Er” TCM on prevention of presbycusis in the mouse model, we measured Cyt-C, FasL and caspase-3 expression in cochlear cells. The results shown in Figure 5 display a significant difference in all three measures between the 2-month control group and the 7-month control group (both drinking tap water) as the age-related disease progressed. The TCM-treated group at 7 months was significantly different from the 7 month control group in all three measures (Cyt-C, FasL, caspase-3, Figure 5), There were no significant differences between the TCM-treated group at 7 months and the normal control group at 2 months. These results suggested that compound JEP TCM could inhibit Cyt-c release in mitochondria, and FasL activation in the cell membrane both of which would lead to a reduction in apoptosis caused by caspase-3 activation.
Fig 5:
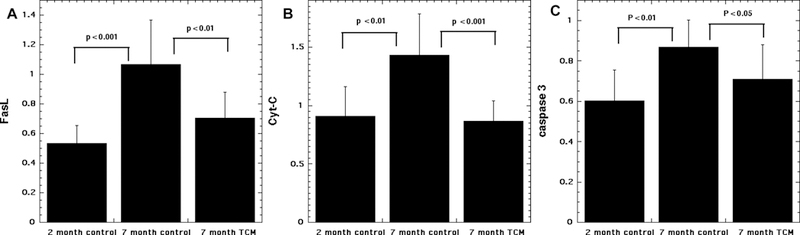
Comparisons of FasL, Cyt-C and caspase-3 mRNA expression levels.
(A) FasL; (B) Cyt-c; (C) caspase-3. Expression levels shown by RT-PCR 2-ΔΔCt values from Ct value conversion, for control group at 2 months, control group at 7 months of age (both drinking tap water) and treated group at 7 months of age (drinking water containing traditional Chinese medicine). P values calculated by one-way ANOVA and Tukey test.
We also measured the malondialdehyde content in cochlea, auditory brain cortex and hepatic tissue in mice in the 7-month control group and in the 7-month TCM group. The significant decrease in MDA concentrations in all three tissue types suggested that JEP could act as a systemic anti-oxidant and could inhibit the age-dependent increase in lipid peroxidation. If Jian Er TCM could decrease formation of reactive oxygen species (ROS) and inhibit oxidative stress, then this is a plausible mechanism for its beneficial effects on reducing apoptosis caused by caspase-3 in cochlear cells because oxidative stress is well-known to be involved in cochlear hair cell degeneration [36, 37].
MDA values shown in nmol/mg prot from cochlea, auditory cortex and hepatic tissue, between control group at 7 months of age drinking water and treated group for 7 months of age drinking water containing traditional Chinese medicine. * indicates significant differences between two groups (P < 0.05).
4. Discussion
AHL is a form of sensorineural deafness that leads to bilateral progressive loss of hearing. Its onset is unpredictable, its etiology is uncertain, and its pathogenesis remains unclear. The main pathological changes of AHL include damage to the cochlear stria vascularis (SV), progressive loss of HCs and afferent SGNs, and destruction of the central auditory pathway. A key causal factor for AHL is thought to be atherosclerosis in the blood vessels supplying the SV, which leads to reduced cochlear blood flow. The significantly decreased cochlear blood supply inhibits oxidative phosphorylation in the mitochondria, and this mitochondrial dysfunction then leads to production of ROS in the cochlea, and consequent oxidative stress. This oxidative stress induces further damage to the mitochondria including mutations or deletions in the mitochondrial DNA (mtDNA), and greater mitochondrial dysfunction, finally leading to apoptosis in the cochlear cells via the mitochondrial pathway initiated by Cyt-c release followed by caspase-3 activation [38]. A study compared specimens of human temporal bone from subjects between 10 and 85 years of age. The SV and the SGNs both showed a tendency for progressive atrophy to develop with increasing age, and atrophy in the basal cochlear turns was the most obvious sign. There was a statistically significant correlation observed between age of subjects and SV atrophy, only in the apical and basal cochlear turns. These findings were consistent with pathological findings previously reported in gerbils [39]. Therefore, it was proposed that normal cochlear blood supply was very important for maintaining the cochlear membrane potential, ensuring sufficient ion transport, and a good lymphatic function, thus maintaining a stable cochlear environment and good auditory function. SV degeneration due to a disorder of the cochlear microcirculation has been considered to be one of the important causes of AHL [39, 40]. Our results showed that, not only was damage to the HCs and the SGNs in the basal cochlear turns quite obvious in this model, but there was also serious hearing loss at all frequencies in the control group at 7 months of age. However, damage to the apical cochlear regions, and to the middle cochlear turns was not seen, as cellular morphology studies did not change by comparison with control group at 2 months of age. This indicated that age-related damage to HCs and to SGNs in C57BL/6J mice commenced from the base of the cochlea and progressed towards the apex. Hearing loss also showed a tendency for progressive development starting with high frequencies and progressing to low frequencies with increasing age. Our study proved that the compound Jian Er of TCM preparation could not only significantly protect the cochlear HCs, but could also markedly protect the cochlear SGNs against apoptosis.
Caspase (cysteinyl-aspartate specific proteinase)-mediated programmed cell death is recognized as part of the classical apoptotic pathway, originating in the mitochondria. When Cyt c is released from damaged or dysfunctional mitochondria by opening of the mitochondrial permeability transition pore, it can interact with several proteins to form “apoptosomes”. Cyt c is anchored to the anionic phospholipid, cardiolipin (CL) in the mitochondrial inner membrane. When dysfunctional mitochondria produce ROS, CL can be oxidized, breaking its association with Cyt c, which is then released out of the mitochondria [41]. The released Cyt c binds to and activates apoptotic protease activating factor-1 (Apaf-1) leading to assembly of the apoptosome. The apoptosome then activates different caspases: the upstream caspase-9 and the downstream caspases (caspase-3, caspase-6 or caspase-7) all requiring ATP hydrolysis. These active caspases degrade intracellular proteins, and lead to DNA degradation thus causing apoptosis [42]. Caspase-3 is one of the family members of ICE (interleukin 1-converting enzyme B), also known as cysteinyl-aspartate specific proteinase 32 (CPP32), which has homology with Ced-3. Caspase-3 is the main active caspase and serves as a common downstream effector for many apoptotic pathways, so it has also been named as “death executive protease” [43].
According to results of modern pharmacological studies on TCM, the herbal medicines contained in compound Jian Er preparation, possess an extensive range of different pharmacological effects that can modify the etiology and pathogenesis of AHL. The herbal constituents exhibit multifunctional properties that can lower blood lipids, can prevent arteriosclerosis, can dilate blood vessels, improving the microcirculation, enhancing the activity of SOD in histocytes and prolonging histiocytic lifetime, increasing Na+, K+-ATP enzyme activity in cell membranes, and acting as an antioxidant thus reducing oxidative stress [44]. Our research showed that compound Jian Er preparation of TCM not only could significantly decrease MDA in cochlea, auditory cortex and hepatic tissue, but also could reduce the expression of Cyt-C and caspases-3 in cochlear cells of C57BL/6J mice with AHL. MDA is an end product of lipid peroxidation, which is formed by interaction of ROS with phospholipids and lipid constituents of biological membranes, membrane-associated polyunsaturated fatty acids. MDA is a highly reactive molecule that can react with amino groups on nucleic acids and proteins due to its reactive aldehyde groups. MDA is an important marker for detecting lipid peroxidation and ROS generation. A recent report [45] described a close relationship between increased MDA levels in tissues and many diseases characteristic of aging. Besides, our earlier research also showed that compound Jian Er preparation of TCM could significantly increase brain-derived neurotrophic factor (BDNF) expression in cochlear tissue in a DBA mouse model [46]. A recent study reported that neural stem/progenitor cells (NSPCs), which had been harvested from the adult rat spinal cord, and exposed to hydrogen peroxide (H2O2) for 24 hours in vitro to induce oxidative stress, led to a marked reduction in cell survival. In contrast, pretreatment with BDNF for 48 hours attenuated the increase in intracellular ROS and enhanced survival. Moreover, it was pointed out that BDNF-induced survival was associated with a significant reduction in the number of apoptotic cells and a significant increase in the activity of the antioxidant enzymes, glutathione reductase and superoxide dismutase [47].
5. Conclusion
In conclusion, we believe that compound Jian Er preparation of TCM acts as an anti-oxidant, reducing lipid peroxidation and MDA levels, lessening mtDNA damage, and decreasing Cyt-C release, therefore inhibiting caspase-mediated apoptosis via the mitochondrial pathway, and protecting cochlear HCs and SGNs from age-related cell death. Moreover there have been no side-effects reported, or any potential adverse effects that have been observed in the clinical application of TCM over many years, and there appears to be no risks associated with long-term consumption of TCM in humans.
Fig 6:
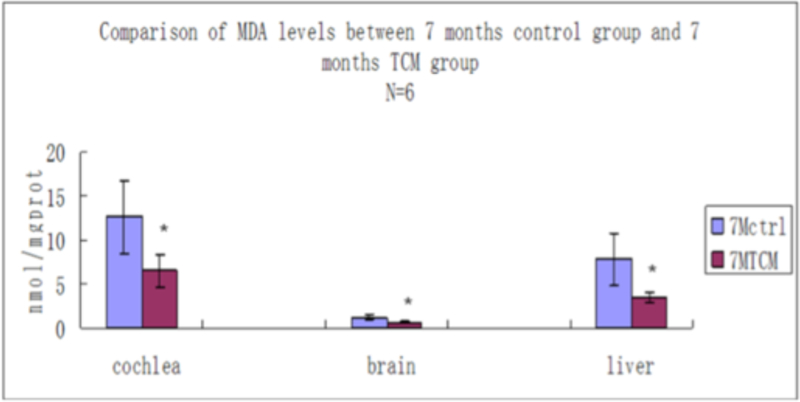
Comparisons of MDA levels.
6. Acknowledgements
This research was supported by National Natural Science Foundation of China grant numbers 81260552, 81373700, 81774374 and Guangxi Natural Science Foundation of China grant No. 2014GXNSFAA118162. MRH was supported by US NIH grants R01AI050875 and R21AI121700.
Footnotes
Conflict of interest
We wish to draw attention to the following facts, which may be considered as potential conflicts of interest. Weijun Xuan and other authors in China are inventors on the Chinese patent that was issued to cover Jian Er.
MRH declares the following potential COIs
Scientific Advisory Boards:
Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiThera Inc, Poulsbo, WA; Vielight, Toronto, Canada;
Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX
Consultant for: USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V.; Johnson & Johnson Inc, Philadelphia, PA; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Photothera Inc, Carlsbad, CA.
Contributor Information
Yi Xuan, School of Engineering, Tufts University, Medford, MA, USA.
Dalian Ding, Center for Hearing Deafness, the State University of New York at Buffalo, Buffalo, NY, USA.
Weijun Xuan, Department of Otorhinolaryngology, Head and Neck Surgery, First Clinical Medical College and Hospital, Guangxi University of Chinese Medicine,Nanning, China.
Liyi Huang, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA; Department of Dermatology, Harvard Medical School, Boston, MA, USA; Department of Infectious Diseases, First Affiliated Hospital, Guangxi Medical University, Nanning, China.
Junbo Tang, Department of Otorhinolaryngology, Head and Neck Surgery, First Clinical Medical College and Hospital, Guangxi University of Chinese Medicine, Nanning, China.
Yulong Wei, Department of Pharmaceutical Manufacturing, Ruikang Clinical Medical College, Guangxi University of Chinese Medicine, Nanning, China.
Sizhong Chen, Department of Otorhinolaryngology, Head and Neck Surgery, First Clinical Medical College and Hospital, Guangxi University of Chinese Medicine, Nanning, China.
Michael R Hamblin, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA; Department of Pharmaceutical Manufacturing, Ruikang Clinical Medical College, Guangxi University of Chinese Medicine, Nanning, China; Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA.
7. References
- 1.Sprinzl GM, Riechelmann H. Current trends in treating hearing loss in elderly people: a review of the technology and treatment options - a mini-review. Gerontology. 2010; 56:351–358. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Duan J. Hearing recovery of elder people (M). Beijing press: Beijing, 2010, 1–2, 75–132. [Google Scholar]
- 3.Xuan W A medicine for preventing and treating sensorineural deafness State intellectual property office of the people’s republic of China. In: Inquiry website of SIPO of the PRC http://epubsipogovcn Sipootpsro; China: (ed.). China, 2015, 1–6. [Google Scholar]
- 4.Xuan W, Lan Y, Wang C. Clinical observation on treatment of 40 senilis of high frequency tinnitus with combination of Chinese Drug and masking method. Chinese Journal of Otorhinolaryngology In Integrative Medicine. 2001; 9:131–133. [Google Scholar]
- 5.Xuan W Clinical observation on treatment of explosive deafness with traditional Chinese medicine. Yunnan Journal of Traditional Chinese Medicine and Materia Medica. 2003; 24:9–11. [Google Scholar]
- 6.Xuan W, Yu Y. Advance in treating sensorineural hearing loss by traditional Chinese medicine. Chinese Journal of Otorhinolaryngology In Integrative Medicine. 2003; 11:257–260. [Google Scholar]
- 7.Xuan W, He X, Tang J, Chen Y. Therapeutic effect with Chinese medicine therapy on 50 cases of sudden hearing loss based on Liver-Gallbladder theory. Chinese Journal of Otorhinolaryngology In Integrative Medicine. 2015; 23:21–23. [Google Scholar]
- 8.Xuan W, Dong MS, Dong MM. Effects of compound injection of ryrola rotundifolia L and astragalus membranaceus bge on experimental guinea pigs’gentamicin ototoxicity. Ann Otol Rhinol Laryngol. 1995; 104:374–380. [DOI] [PubMed] [Google Scholar]
- 9.Xuan W, Huang Z, Ding D. Experimental study in prevention of age-related Cochlear damage in C57 BL /6J mice using Jianer H capsule of Chinese traditional medicine. J Aud Speech Pathol. 2007; 15:47–50. [Google Scholar]
- 10.Xuan W, Zhou Z. Experiment study on the preventive effect of Bu She Jian Pi capsule on age-related cochlear hair cells apoptosis. China Journal of Traditional Chinese Medicine and Pharmacy. 2008; 23:114–117. [Google Scholar]
- 11.Ding D, Wang J, Hu B, Salvi RJ. Age-induced alterations of succinic dehydrogenase activity in the organ of Corti in mice. Journal of Clinical Otorhinolaryngology. 1998; 12:78–80. [Google Scholar]
- 12.Kane KL, Longo-Guess CM, Gagnon LH, Ding DL, Salvi RJ, Johnson KR. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hearing Research. 2012; 283:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG et al. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J Comp Neurol. 1999; 413:101–112. [PubMed] [Google Scholar]
- 14.McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999; 20:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Zheng QY, Ding DL, Yu HP, Salvi RJ, Johnson KR. A locus on distal chromosome 10 (ahl4) affecting age-related hearing loss in A/J mice. Neurobiology of Aging. 2009; 30:1693–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997; 114:83–92. [DOI] [PubMed] [Google Scholar]
- 17.Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hearing Research. 2004; 188:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Brederlow B, Bolz H, Janecke A, Cabrera AL, Rudolph G, Lorenz B et al. Identification and in vitro expression of novel CDH23 mutations of patients with Usher syndrome type 1D. Human Mutation. 2002; 19:268–273. [DOI] [PubMed] [Google Scholar]
- 19.Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A et al. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med. 2005; 352:1557–1564. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Tan H, Zheng JR, Wang F, Jiang C, He M et al. Association of cadherin CDH23 gene polymorphisms with noise induced hearing loss in Chinese workers. Wei Sheng Yan Jiu. 2006; 35:19–22. [PubMed] [Google Scholar]
- 21.Chen Q Methodology of pharmacological study in traditional Chinese medicine People’s medical publishing house: Beijing, 1993, 1103–1105. [Google Scholar]
- 22.Ding D, McFadden S, Salvi RJ. Cochlear hair cell densities and inner-ear staining techniques In: Handbook of Mouse Auditory Research. JF Willott (ed.). CRS Press: Florida, 2001, 189–204. [Google Scholar]
- 23.McFadden SL, Ding DL, Jiang HY, Woo JM, Salvi RJ. Chinchilla models of selective cochlear hair cell loss. Hearing Research. 2002; 174:230–238. [DOI] [PubMed] [Google Scholar]
- 24.Fu Y, Ding DL, Wei L, Jiang HY, Salvi R. Ouabain-Induced Apoptosis in Cochlear Hair Cells and Spiral Ganglion Neurons In Vitro. Biomed Research International, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding DWQ, Yu D, Jiang H, Han C, Katsuno K et al. NAD+ prevents mefloquine-induced neuroaxonal and hair cell degeneration through reduction of caspase-3- mediated apoptosis. PloS one. 2013; 8:e79817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding DL, He JC, Allman BL, Yu DZ, Jiang HY, Seigel GM et al. Cisplatin ototoxicity in rat cochlear organotypic cultures. Hearing Research. 2011; 282:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding DL, Wang J, Salvi R, Henderson D, Hu BH, McFadden SL et al. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann N Y Acad Sci. 1999; 884:152–170. [DOI] [PubMed] [Google Scholar]
- 28.Fu Y, Ding DL, Jiang HY, Salvi R. Ouabain-Induced Cochlear Degeneration in Rat. Neurotoxicity Research. 2012; 22:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller M, Von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005; 202:63–73. [DOI] [PubMed] [Google Scholar]
- 30.Ding DL, McFadden SL, Salvi RJ. Calpain immunoreactivity and morphological damage in chinchilla inner ears after carboplatin. Jaro. 2002; 3:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding D, Wang J, Salvi RJ. Early damage in the chinchilla vestibular sensory epithelium from carboplatin. Audiol Neurootol. 1997; 2:155–167. [DOI] [PubMed] [Google Scholar]
- 32.Ding D, Wang J, Zheng XY, Sun H, Salvi R. Quantitative analysis of hair cells, spiral ganglion neurons and nerve fibers in the same cochlea. Chinese Journal of Otorhinolaryngology-Skull Base Surgery. 1998; 4:200–204. [Google Scholar]
- 33.Ding D, Zheng XY, Wang J, Hosfstetter P, Salvi R. Quantitative analysis of nerve fibers in habenular perforata in chinchilla. Chinese Journal of Otorhinolaryngology In Integrative Medicine. 1998; 33:30–31. [Google Scholar]
- 34.Dai ZJ, Wang XJ, Li ZF, Ji ZZ, Ren HT, Tang W et al. Scutellaria barbate extract induces apoptosis of hepatoma H22 cells via the mitochondrial pathway involving caspase-3. World Journal of Gastroenterology. 2008; 14:7321–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deepa PR, Vandhana S, Krishnakumar S. Fatty Acid Synthase Inhibition Induces Differential Expression of Genes Involved in Apoptosis and Cell Proliferation in Ocular Cancer Cells. Nutrition and Cancer-an International Journal. 2013; 65:311–316. [DOI] [PubMed] [Google Scholar]
- 36.Reiss M, Reiss G. Presbyacusis: pathogenesis and treatment. Med Monatsschr Pharm. 2009; 32:221–225. [PubMed] [Google Scholar]
- 37.Menardo J, Tang Y, Ladrech S, Lenoir M, Casas F, Michel C et al. Oxidative Stress, Inflammation, and Autophagic Stress as the Key Mechanisms of Premature Age-Related Hearing Loss in SAMP8 Mouse Cochlea. Antioxidants & Redox Signaling. 2012; 16:263–274. [DOI] [PubMed] [Google Scholar]
- 38.Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, Kondo K. Current concepts in age-related hearing loss: Epidemiology and mechanistic pathways. Hearing Research. 2013; 303:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki T, Nomoto Y, Nakagawa T, Kuwahata N, Ogawa H, Suzuki Y et al. Age-dependent degeneration of the stria vascularis in human cochleae. Laryngoscope. 2006; 116:1846–1850. [DOI] [PubMed] [Google Scholar]
- 40.Kimura RS. Animal models of inner ear vascular disturbances. Am J Otolaryngol. 1986; 7:130–139. [DOI] [PubMed] [Google Scholar]
- 41.Bayir H, Fadeel B, Palladino MJ, Witasp E, Kurnikov IV, Tyurina YY et al. Apoptotic interactions of cyctochrome c: Redox flirting with anionic phospholipids within and outside of mitochondria. Biochimica Et Biophysica Acta-Bioenergetics. 2006; 1757:648–659. [DOI] [PubMed] [Google Scholar]
- 42.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997; 91:479–489. [DOI] [PubMed] [Google Scholar]
- 43.Odonkor CA, Achilefu S. Modulation of effector caspase cleavage determines response of breast and lung tumor cell lines to chemotherapy. Cancer Invest. 2009; 27:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Deng W, Xue C. Pharmacology and application of traditional Chinese medicine. People’s medical publishing house: Beijing, 2000, 833–835, 982–998, 190–209, 1146–1152. [Google Scholar]
- 45.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011; 283:65–87. [DOI] [PubMed] [Google Scholar]
- 46.Xuan W, Chu H, Xuan Y. Preliminary study of the impact from Chinese herbal compound Jian-er agent on BDNF expression in cochlear tissue of the domestic DBA / 2 J mice. China Journal of Traditional Chinese Medicine and Pharmacy. 2013; 28:2748–2751. [Google Scholar]
- 47.Hachem LD, Mothe AJ, Tator CH. Effect of BDNF and Other Potential Survival Factors in Models of In Vitro Oxidative Stress on Adult Spinal Cord-Derived Neural Stem/Progenitor Cells. Biores Open Access. 2015; 4:146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]


