Abstract
Objectives:
In this study, we investigated potential effects of being a menthol smoker on response to reduced nicotine content (RNC) cigarettes in smokers especially vulnerable to smoking.
Method:
Participants were 169 smokers (61 menthol and 108 non-menthol smokers) with comorbid mental illness, substance use disorder, or socioeconomic disadvantage. Participants completed a double-blind study assessing addiction potential, withdrawal/craving, and compensatory smoking across 4 research cigarettes varying in nicotine content from very low levels to commercial levels (0.4, 2.4, 5.2, 15.8mg/g of tobacco). Repeated measures analysis of variance was used to examine potential moderating effects of menthol status.
Results:
Statistically significant effects of nicotine dose were noted across measures, with higher doses producing greater economic demand and relief from withdrawal/craving. The relationships between nicotine dose and response to RNC cigarettes do not differ by menthol status.
Conclusions:
Results of this study suggest menthol does not have a differential impact on response to RNC cigarettes across measures of economic demand, withdrawal/craving, or smoking topography. These results suggest that any potential beneficial effects of RNC cigarettes should extend to menthol smokers including those especially vulnerable to smoking.
Keywords: menthol, cigarette, nicotine reduction, nicotine reduction, tobacco regulation, vulnerable populations
The United States (US) Food and Drug Administration (FDA) has proposed several product standards to address cigarette smoking, including reducing the nicotine content in cigarettes1 with the aim of reducing the public health burden of smoking.2 Controlled studies across healthy populations, and populations especially vulnerable to tobacco use and addiction, have demonstrated that reductions in nicotine content in cigarettes decrease reinforcing efficacy, dependence, and cigarettes-per-day.3–9
As new product standards are considered, it is necessary to examine impact of nicotine reduction across subgroups of smokers. One subgroup of interest is those who regularly smoke mentholated cigarettes.10 Menthol cigarette prevalence is increasing even as smoking prevalence is decreasing. Approximately 39% of all cigarettes sold are mentholated.11,12 Additionally, there is evidence that mentholation may increase the appeal of cigarettes, reduce aversive throat sensations, and is used as an indicator of strength.13,14 Currently the FDA is considering changes in menthol regulation as a characterizing flavor in cigarettes.10
Current evidence regarding the potential moderating effect of menthol smoking status on response to nicotine reduction is mixed. A variable is a moderator of a relationship between an independent and a dependent variable if it explains under what conditions the former is related to the latter, and is usually demonstrated statistically through an interaction.15 Whereas an initial examination of acute exposure to reduced nicotine content (RNC) cigarettes demonstrated a main effect of menthol in which menthol smokers had lower positive subjective effects and lower perceived value of the cigarettes than non-menthol smokers,16 a subsequent examination of acute effects found no influence of menthol smoking status on positive subjective or direct reinforcing effects when comparing very low nicotine content to normal nicotine content cigarettes.17 An examination of extended exposure to RNCs found a moderating effect of menthol smoking at one RNC dose (5.2mg/g), in which menthol smokers reported a greater reduction in cigarettes-per-day (CPD) compared to non-menthol smokers. No other moderating effects at other doses or dependent measures (ie, withdrawal, craving, compensatory smoking) were observed in that study.3 These studies were all conducted in the general population of smokers.3,16,17
One examination of populations with comorbid conditions that increase risk for tobacco use and addiction (ie, vulnerable populations) found that menthol did not moderate response to positive subjective effects of smoking RNCs or concurrent choice between varying nicotine dose cigarettes,5 similar to a report in the general population of smokers noted above.17 However, menthol’s potential moderating effects on craving, withdrawal, and compensatory smoking have yet to be examined in these vulnerable populations. Considering that vulnerable populations make up a sizeable proportion of smokers (~30% of smokers have mental illness,18,19 50%-75% are socioeconomically disadvantaged,20,21 and 20% have substance abuse22) and have considerable problems quitting,23–26 there is good reason to make a more thorough examination of any potential moderating effects of menthol in them. The aim of the current study was to expand the examination of menthol as a potential moderator of response to RNCs in smokers with comorbid conditions by examining effects on behavioral-economic demand for cigarettes, withdrawal, craving, and compensatory smoking.
METHODS
Study Sample
Participants were 169 adult daily smokers from a parent multisite (University of Vermont, Brown University, Johns Hopkins University) study5 examining 3 populations with co-morbid conditions; 56 with affective disorder as an exemplar of smokers with mental illness; 60 with opioid dependence as an exemplar of smokers with other substance use disorders; and 53 socioeconomically disadvantaged women of reproductive age as an exemplar of smokers with economic disadvantage. All provided written informed consent. Inclusion and exclusion criteria have been reported previously.5 Briefly, all participants smoked ≥5 cigarettes-per-day for at least one year, had limited use of other tobacco products, and were not currently interested in quitting smoking. Populations were combined to serve as an exemplar for vulnerable groups and categorized by menthol smoking status.
Research Cigarettes
The study used SPECTRUM research cigarettes (22nd Century Group, Clarence, NY). Participants whose usual brand was mentholated were assigned to menthol research cigarettes; all participants reported a preference for mentholated or non-mentholated cigarettes. Four nicotine doses were investigated. The average nicotine content of doses across menthol and non-menthol products was 15.8, 5.2, 2.4, 0.4 mg of nicotine per gram of tobacco (mg/g) with the 15.8mg/g nicotine dose serving as a control for commercial cigarettes. SPECTRUM mentholated research cigarettes have menthol levels in the range of commercially available products.23,24 All cigarettes were administered under double-blind conditions.
Procedure
Procedures for this study have been described previously.5 Briefly, participants completed 14 2-to-4-hour sessions in a within-subjects design organized into 3 phases. The present study focuses on Phase 1 sessions (Sessions 1–5 described below). Participants abstained from smoking for 6–8 hours operationalized as breath carbon monoxide (CO) at ≤½ study-intake CO level, a widely-used criterion in clinical laboratory smoking research.5,25,26
In Phase 1, participants were oriented to the study protocol (Session 1) and then sampled each of the 4 research cigarette doses across separate sessions (Sessions 2–5). Cigarettes were assigned arbitrary letter codes and participants smoked 1 of the 4 research cigarettes per session ad lib using a Clinical Research Support System (CReSS) device to record smoking topography.27 Following smoking, participants completed the Cigarette Purchase Task (CPT), a behavioral economic simulation task that models: (1) demand for cigarettes when unconstrained by cost (Intensity); (2) maximal amount willing to spend on daily smoking (Omax); (3) price at which smoking demand begins decreasing proportionate to increasing price (Pmax); (4) price at which one would discontinue smoking rather than incur the cost (Breakpoint); and (5) overall sensitivity of demand to price (elasticity).28 Prior to smoking and every 15 minutes for an hour following smoking, participants also completed the Minnesota Tobacco Withdrawal Scale (MTWS),29 the Questionnaire of Smoking Urges-brief (QSU-B),30 and breath CO levels were collected.
Data Analysis
Our analyses examined whether menthol status moderated the effect of dose on CPT, MTWS, QSU-brief, smoking topography, or breath CO levels by using repeated measures analysis of covariance (ANCOVA), with nicotine dose and time (when applicable) as the within-participant factors and menthol status as a fixed effect. Menthol smokers differed from non-menthol smokers on race, education, and sex (p ≤ .05), which were included as covariates. Additional fixed effects for (1) session, (2) the 3 study populations who were studied independently using parallel research designs and combined for analysis in the original and this secondary study, and (3) study site were included. Menthol-by-dose and time-by-dose interactions were included to test whether values differed by dose and to test for differential effects of menthol status by dose; when not statistically significant, interaction terms were dropped from the models. Menthol-by-population interactions were included to test whether moderating effects of menthol were present in a single population rather than the combined group. No statistically significant menthol-by-population interactions across measures were found, so these terms were dropped from the models. Significant menthol, time, dose, or interaction effects were followed by post hoc testing using Bonferroni corrections.
For the CPT, all indices were empirically quantified from observed values. Omax, Pmax, Breakpoint, and Elasticity were log10 transformed to correct for skewness. We found systematic patterns in 92.7% of CPT demand curves; therefore, no data were excluded from analyses. In cases where participants reported zero consumption across all prices (54 of 845 cases), curve fitting was not possible, so elasticity was not analyzed and other demand indices were quantified as 0. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Participants
Menthol smokers made up 36% (61/169) of the sample and were more likely to be non-white, have lower education, and female than non-menthol smokers (ps ≤ .05); they did not differ on other assessed sociodemographic or smoking characteristics (Table 1).
Table 1.
Demographic and Smoking Characteristics
| All (N = 169) | Menthol (N = 61) | Non-menthol (N = 108) | p-value | |
|---|---|---|---|---|
| Age (M ± SD) | 35.6 ± 11.4 | 35.4 ± 11.4 | 35.6 ± 11.4 | .91 |
| % Female | 71 | 80 | 66 | .05 |
| Population (%) | .66 | |||
| Low SES Women | 31 | 27 | 34 | |
| Opioid Maintained | 36 | 39 | 34 | |
| Affective Disorder | 33 | 34 | 32 | |
| Education (%) | .009 | |||
| 8th Grade or Less | 2 | 7 | 0 | |
| Some High School | 14 | 20 | 10 | |
| High School Graduate | 34 | 23 | 41 | |
| Some College | 38 | 41 | 36 | |
| 2-Year Associate’s Degree | 6 | 5 | 6 | |
| College Graduate/4-Year Degree | 4 | 5 | 3 | |
| Graduate or Professional Degree | 2 | 0 | 4 | |
| Marital Status (%) | .14 | |||
| Never Married | 61 | 72 | 55 | |
| Married | 16 | 10 | 19 | |
| Divorced/Separated | 21 | 16 | 23 | |
| Widowed | 2 | 2 | 3 | |
| Race (%) | < .0001 | |||
| Caucasian | 73 | 44 | 89 | |
| African-American | 14 | 34 | 2 | |
| Native Hawaiian/Pacific Islander | 1 | 0 | 1 | |
| Asian | 1 | 0 | 1 | |
| Other or More than 1 race | 9 | 15 | 6 | |
| Hispanic | 4 | 7 | 2 | |
| Cigarettes Per Day (M ± SD) | 15.8 ± 7.5 | 14.7 ± 8.9 | 16.4 ± 6.6 | .14 |
| Fagerstrom Test of Nicotine Dependence | 5.0 ± 2.2 | 5.1 ± 2.3 | 4.9 ± 2.1 | .73 |
| Age of First Cig (M ± SD) | 16.3 ± 4.3 | 17.1 ± 4.7 | 15.8 ± 3.9 | .06 |
| Number of Quit Attempts | 3.0 ± 8.7 | 2.2 ± 2.8 | 3.5 ± 10.6 | .37 |
Note.
Numbers may not sum to 100% due to rounding. Bolded values represent statistically significant differences between groups (p < .05).
Cigarette Purchase Task
Regarding behavioral demand, there were no statistically significant interactions of menthol status and dose in any CPT indices (Intensity: F(3,487) = 1.05; Breakpoint: F(3,486) = 0.39; Omax: F(3,487) = 0.27; Pmax: F(3,486) = 0.34, p < .001; Elasticity: F(3,444) = 1.20, p = .30 – .79). There were statistically significant effects of dose with a general pattern of more intense and persistent demand at higher nicotine doses (Intensity: F(3,487) = 6.04, p < .001; Breakpoint: F(3,486) = 14.27, p < .001; Omax: F(3,486) = 12.29, p < .001; Pmax: F(3,486) = 12.24, p < .001; Elasticity: F(3,443) = 2.81, p = .03), but no direct effects of menthol status on any CPT indices.
Measures of Withdrawal and Craving
Regarding withdrawal and craving, there was no interaction of menthol status and nicotine dose (F(1,153) = 0.24–1.65, p ≥ .28 for MTWS total scores, desire to smoke item, and QSU Factor 1; F(1, 97.5) = 0.61, p = .43 for QSU Factor 2). There was a statistically significant interaction of dose and time on MTWS total scores (F(12,2014) = 2.64, p < .01), with each of the doses decreasing MTWS scores from pre-smoking ratings with duration of effects greatest at the 15.8 mg/g dose. There were also statistically significant interactions of dose and time noted on the MTWS desire-to-smoke item (F(12,2014) = 5.86, p < .001), as well as QSU Factor 1 (F(12, 2014) = 8.92, p < .001) and Factor 2 scales (F(12,2014) = 5.22, p < .001); all doses decreased craving significantly, but the largest and longest duration effects were seen with the 15.8mg/g dose (F(3,501), p < .01).
Compensatory Smoking Measures
Regarding smoking topography, there were no statistically significant interactions of menthol status and dose. (total puff volume: F(1,154) = 0.05, p = .82; mean puff volume: F(1,152) = 0.02, p = .89; mean puff duration: F(1,143) = 0.00, p = .98; interpuff interval: F(1,153) = 1.01, p = .32; maximum flow rate: F(1,153) = 0.00, p = .95; puff number: F(1,135) = 0.24, p = .63) (see Figure 1 for an exemplar). There were statistically significant main effects of dose on 3 measures (total puff volume: F(3,488) = 4.70, p < .01, maximum flow rate: F(3,488) = 3.56, p < .01; maximum puff number: F(3,487) = 18.04, p < .0001), with greater exposure as a function of increasing nicotine dose, opposite of what is expected with compensatory smoking. There were no effects of menthol status on any smoking topography measure. The only observed change in breath CO was the expected increase following smoking and subsequent orderly decrease over time (F(3,501) = 103.44, p < .001), with no effect of menthol status (F(1,164) = 0.05, p = .82).
Figure 1.
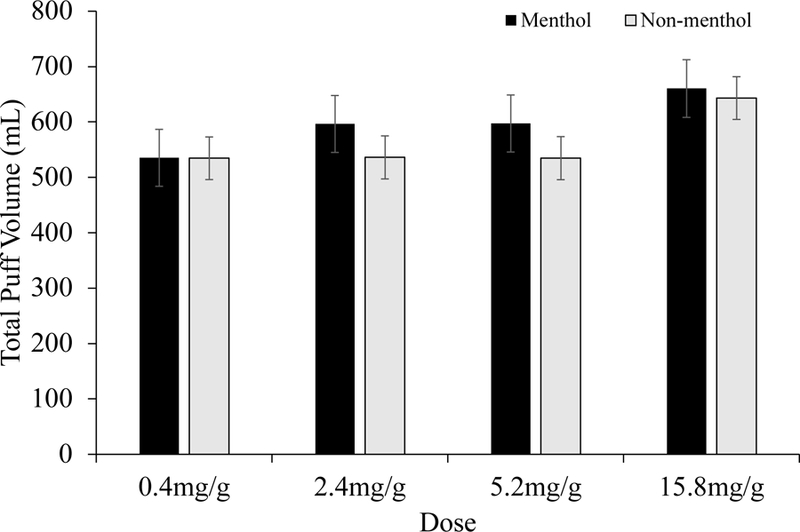
Total Puff Volume Across Cigarettes for Menthol and Non-menthol Smokers
Note.
Least square mean (+ SEM) scores for total puff volume (mL) across cigarettes smoked ad lib for the 4 nicotine doses tested across menthol (upper panel) and non-menthol (lower panel) participants. Dose effects were observed (p < .05), but no effects by menthol status were observed.
DISCUSSION
Our results show no evidence that response to RNC cigarettes across measures assessing addiction potential (ie, simulated demand for cigarettes), craving, withdrawal, or compensatory smoking in populations especially vulnerable to smoking interacts with menthol status (ie, moderation).
These findings are consistent with other studies reporting no or limited effect of menthol status on response to RNC cigarettes.3,5,17 The current work further extends the examination of this question in populations with increased vulnerability to tobacco use and addiction. Considering that menthol smokers comprise 39% of the US smoker population,11 have poorer cessation outcomes,13 and are overrepresented in populations with comorbidities,31–35 it is important to have as full of an understanding of how these groups of smokers may respond to a possible nicotine reduction policy as is practical.
Regarding study limitations, although participants were given cigarettes matching their menthol preference and SPECTRUM cigarettes have menthol levels within the range of commercial brands, nevertheless, the level may differ in amount of mentholation relative to an individual participant’s usual brand. Because the level of menthol in the cigarette affects the taste and experience of smoking,14 this may have affected the acceptability among menthol smokers.24 Additionally, menthol and non-menthol smokers self-select into these categories. We used covariates to control for observed differences between menthol and non-menthol smokers, but possible influence from unobserved differences cannot be ruled out. Lastly, the present study was a secondary analysis of a study designed with overarching aims other than examining menthol status as a moderator of the effects of nicotine dose and was not designed in a manner that might optimize discerning effects of menthol status (eg, matching menthol and non-menthol smokers on sociodemographics). It is also important to note that menthol flavoring has been used by tobacco manufacturers to increase appeal among individuals experimenting with smoking.13 Thus, whereas menthol status does not appear to alter response to reduced nicotine content cigarettes in the established smokers examined in the present and prior studies, there could be impact of menthol in how experimental smokers respond to RNC cigarettes.
IMPLICATIONS FOR TOBACCO REGULATION
The relationships between nicotine dose and cigarette smoking do not appear to differ by menthol status. These findings underscore that potential benefits of reducing the nicotine content of cigarettes to decrease the addiction potential of smoking should extend to users of menthol and non-menthol cigarettes alike, including those from populations who are especially vulnerable to smoking and the adverse health impacts of smoking.
Acknowledgements
This study was supported by the Tobacco Centers of Regulatory Science award P50DA036114 from the National Institute on Drug Abuse (NIDA) and the US Food and Drug Administration (FDA), Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences (NIGMS), and NIDA Institutional Training Grant T32DA007242.
Footnotes
Human Subjects Statement
The Institutional Review Boards at the University of Vermont, Brown University, and Johns Hopkins University School of Medicine approved the study. Clinical Trials Identifier: NCT02250534.
Conflict of Interest Statement
None declared. The content herein is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Administration.
Contributor Information
Danielle R. Davis, UVM Tobacco Center on Regulatory Science, Departments of Psychiatry and Psychological Science, University of Vermont, Burlington, VT..
Mollie E. Miller, Center for Alcohol and Addiction Studies, Brown University, Providence, RI..
Joanna M. Streck, UVM Tobacco Center on Regulatory Science, Departments of Psychiatry and Psychological Science, University of Vermont, Burlington, VT..
Cecilia L. Bergeria, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD..
Jennifer W. Tidey, Center for Alcohol and Addiction Studies, Brown University, Providence, RI..
Sarah H. Heil, UVM Tobacco Center on Regulatory Science, Departments of Psychiatry and Psychological Science, University of Vermont, Burlington, VT..
Diann E. Gaalema, UVM Tobacco Center on Regulatory Science, Departments of Psychiatry and Psychological Science, University of Vermont, Burlington, VT..
Andrea C. Villanti, UVM Tobacco Center on Regulatory Science, Departments of Psychiatry and Psychological Science, University of Vermont, Burlington, VT..
Maxine L. Stitzer, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD..
Jeff S. Priest, Department of Medical Biostatistics, University of Vermont, Burlington, VT..
Janice Y. Bunn, Department of Medical Biostatistics, University of Vermont, Burlington, VT..
Joan M. Skelly, Department of Medical Biostatistics, University of Vermont, Burlington, VT..
Valeria Diaz, Department of Psychiatry, University of Vermont, Burlington, VT..
Christopher A. Arger, Iora Health, Phoenix, AZ..
Stephen T. Higgins, Departments of Psychiatry and Psychological Science, University of Vermont, Burlington, VT..
References
- 1.US Food and Drug Adminstration. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. A Proposed Rule by the Food and Drug Adminstration HHS 2018:11818–11843. Available at: https://www.federalregister.gov/documents/2018/03/16/2018-05345/tobacco-product-standard-for-nicotine-level-of-combusted-cigarettes. Accessed December 22, 2018.
- 2.US Department of Health and Human Services (USD-HHS). The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General Atlanta, GA: USDHHS, US Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 3.Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction 2010;105(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins ST, Heil SH, Sigmon SC, et al. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tidey JW, Pacek LR, Koopmeiners JS, et al. Effects of 6-week use of reduced-nicotine content cigarettes in smokers with and without elevated depressive symptoms. Nicotine Tob Res 2017;19(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tidey JW, Rohsenow DJ, Kaplan GB, et al. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res 2013;15(1):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz NL, Dains KM, Hall SM, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev 2012;21(5):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AhnAllen CG, Bidwell LC, Tidey JW. Cognitive effects of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine Tob Res 2015;17(5):510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. Regulation of Flavors in Tobacco Products. A Proposed Rule by the Food and Drug Adminstration HHS 2018. 12294–12301. Available at: https://www.federalregister.gov/documents/2018/03/2½018-05655/regulation-of-flavors-in-tobacco-products. Accessed December 22, 2018.
- 11.Villanti AC, Mowery PD, Delnevo CD, et al. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004–2014. Tob Control 2016;25(Suppl 2):ii14–ii20. [DOI] [PubMed] [Google Scholar]
- 12.Giovino GA, Villanti AC, Mowery PD, et al. Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tob Control 2015;24(1):28–37. [DOI] [PubMed] [Google Scholar]
- 13.Villanti AC, Collins LK, Niaura RS, et al. Menthol cigarettes and the public health standard: a systematic review. BMC Public Health 2017;17(1):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreslake JM, Wayne GF, Connolly GN. The menthol smoker: tobacco industry research on consumer sensory perception of menthol cigarettes and its role in smoking behavior. Nicotine Tob Res 2008;10(4):705–715. [DOI] [PubMed] [Google Scholar]
- 15.Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur Approaches. Health Psychol 2008;27(2S):S101–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatsukami DK, Heishman SJ, Vogel RI, et al. Doseresponse effects of spectrum research cigarettes. Nicotine Tob Res 2013;15(6):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkins KA, Karelitz JL, Kunkle N. Evaluation of menthol per se on acute perceptions and behavioral choice of cigarettes differing in nicotine content. J Psychopharmacol 2018;32(3):324–331. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin RD, Zvolensky MJ, Keyes KM, Hasin DS. Mental disorders and cigarette use among adults in the United States. Am J Addict 2012;21(5):416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 2004;61(11):1107–1115. [DOI] [PubMed] [Google Scholar]
- 20.Higgins ST, Kurti AN, Redner R, et al. Co-occurring risk factors for current cigarette smoking in a U.S. nationally representative sample. Prev Med 2016;92:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levinson AH. Where the U.S. tobacco epidemic still rages: most remaining smokers have lower socioeconomic status. J Health Care Poor Underserved 2017;28(1):100–107. [DOI] [PubMed] [Google Scholar]
- 22.Stanton CA, Keith DR, Gaalema DE, et al. Trends in tobacco use among US adults with chronic health conditions: National Survey on Drug Use and Health 2005–2013. Prev Med 2016;92:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ai J, Taylor KM, Lisko JG, et al. Menthol content in US marketed cigarettes. Nicotine Tob Res 2016;18(7):1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter P, Steven PR, Bravo R, et al. Characterization of SPECTRUM variable nicotine research cigarettes. Tob Regul Sci 2016;2(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson MW, Bickel WK, Kirshenbaum AP. Substitutes for tobacco smoking: a behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes. Drug Alcohol Depend 2004;74(3):253–264. [DOI] [PubMed] [Google Scholar]
- 26.Tidey JW, O’Neill SC, Higgins ST. Effects of abstinence on cigarette smoking among outpatients with schizophrenia. Exp Clin Psychopharmacol 1999;7(4):347–353. [DOI] [PubMed] [Google Scholar]
- 27.Lee EM, Malson JL, Waters AJ, et al. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res 2003;5(5):673–679. [DOI] [PubMed] [Google Scholar]
- 28.MacKillop J, Murphy JG, Ray LA, et al. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp Clin Psychopharmacol 2008;16(1):57–65. [DOI] [PubMed] [Google Scholar]
- 29.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 30.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 31.Cohn AM, Johnson AL, Hair E, et al. Menthol tobacco use is correlated with mental health symptoms in a national sample of young adults: implications for future health risks and policy recommendations. Tob Induc Dis 2016;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence D, Rose A, Fagan P, et al. National patterns and correlates of mentholated cigarette use in the United States. Addiction 2010;105(Suppl 1):S13–S31. [DOI] [PubMed] [Google Scholar]
- 33.Young-Wolff KC, Hickman NJ 3rd, Kim R, et al. Correlates and prevalence of menthol cigarette use among adults with serious mental illness. Nicotine Tob Res 2015;17(3):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caraballo RS, Asman K. Epidemiology of menthol cigarette use in the United States. Tob Induc Dis 2011;9(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Substance Abuse and Mental Health Services Adminstration. The NSDUH Report: Recent Trends in Menthol Cigarette Use. Center for Behavioral Health Statistics and Quality 2011. Available at: https://www.samhsa.gov/data/report/nsduh-report-recent-trends-menthol-cigarette-use. Accessed December 22, 2018.


