Abstract
Background
Ischaemia-reperfusion injury in kidney transplantation leads to delayed graft function (DGF), which is associated with reduced long term graft function. Remote ischaemic conditioning (RIC) improved early kidney graft function in a porcine model of donation after brain death and was associated with improved long-term cardiac outcome after myocardial ischaemia. This randomised, double-blinded trial evaluated the effect of RIC on kidney graft outcome in the first year, and examined the predictive value of a new measure of initial kidney graft function, i.e. the estimated time to a 50% reduction in plasma creatinine post-transplantation (tCr50).
Methods
A total of 225 patients undergoing deceased donor kidney transplantation were randomised to RIC or a sham procedure performed prior to kidney reperfusion. Up to four repetitive cycles of five minutes of leg ischaemia and five minutes of reperfusion were given. GFR, plasma creatinine, cystatin C and neutrophil gelatinase associated lipocalin (NGAL) were measured at three and twelve months and estimated GFR was calculated using four different equations. Other secondary outcomes were identified from patient files.
Results
RIC did not affect GFR or other outcomes when compared to the sham procedure at three or twelve months. tCr50 correlated with one year graft function (p<0.0001 for both mGFR and eGFR estimates). In contrast, DGF i.e. “need of dialysis the first week” did not correlate significantly with one year GFR.
Conclusion
RIC during deceased donor kidney transplantation did not improve one year outcome. However, tCr50 may be a relevant marker for studies aiming to improve graft onset.
Trial registration
www.ClinicalTrials.gov Identifier: NCT01395719.
Introduction
Delayed graft function (DGF) is a consequence of ischaemia-reperfusion injury and a common complication after deceased donor kidney transplantation [1,2]. It is associated with an increased incidence of post-transplant complications, acute rejection, reduced long-term graft outcome and graft loss [3–5].
Remote ischaemic conditioning (RIC) protects against ischaemia-reperfusion injury in various tissues in both experimental and some clinical studies, particularly in the heart [6,7], but also in the transplanted kidney in our porcine model [8]. It is believed that RIC sends a protective signal to a remote organ or tissue that is transferred via humoral, neuronal or other systemic pathway to the cells in the target organ. RIC applied during myocardial infarction has been associated with a better long-term outcome when evaluated at a mean of 3.8 years follow-up [9]. Indeed, in our porcine donation after brain death transplantation model, RIC improved early glomerular filtration rate (GFR) and renal plasma perfusion [8].
The multi-centre, randomised, controlled clinical study CONTEXT [10] evaluated the effect of recipient RIC in deceased donor kidney transplantation. Many definitions of delayed graft function have been proposed based on reduction of creatinine level or need for dialysis [11]. As previously published, RIC had no effect on early kidney graft function measured as the estimated time to a 50% reduction in plasma (P-) creatinine (tCr50) [12] or the incidence of DGF defined as the need for dialyses within the first week after transplantation [13]. In this follow up of the CONTEXT study, we evaluated the effect of RIC on kidney graft function, rejection rate, new onset diabetes after transplantation (NODAT), patient survival, and the kidney injury marker neutrophil gelatinase associated lipocalin (NGAL) [14,15] at three and twelve months post-transplantation. We further examined whether tCr50 predicted GFR at twelve months. GFR was measured and estimated based on P-creatinine and P-cystatin C.
Materials and methods
Study design
The study design of CONTEXT (www.ClinicalTrials.gov Identifier: NCT01395719) was previously described in detail [10]. A total of 225 adult patients undergoing deceased donor kidney transplantation were included at four centres: Aarhus, Denmark; Gothenburg, Sweden; and Groningen and Rotterdam in the Netherlands [13]. All patients provided written informed consent. The study was approved by the National Committee on Health Research Ethics (Denmark), Regional Ethical Board (Sweden) and METCUMCG (the Netherlands).
Inclusion criteria were deceased donor kidney transplantation and age ≥ 18 years. Exclusion criteria were double kidney transplant, risk of lower limb ischaemia during RIC, and AV-fistula in the leg where RIC was performed.
Patients were randomised to receive either RIC or sham-RIC during surgery. The RIC procedure consisted of up to four cycles of inflation and deflation of a tourniquet around the thigh contralateral to the transplantation side. This resulted in cycles of five minutes ischaemia and five minutes reperfusion performed just prior to reperfusion of the kidney. Kidney graft function was evaluated at three and twelve months post-transplantation. Additional information on patient and graft survival, the need for dialysis, rejection episodes and NODAT was obtained from patient records.
Blood and urine
EDTA-blood and urine samples were collected, centrifuged at 2800G at 4°C for ten minutes and stored at -80°C until further analysed. P-NGAL was measured using a particle-enhanced turbidimetric immunoassay (®BioPorto A/S, Hellerup, Denmark). P-cystatin C was measured using a turbidimetric immunoassay (Siemens Healthcare A/S, Ballerup, Denmark). P-creatinine, U-creatinine and U-albumin were measured using automated, standard clinical assays at the Department of Clinical Biochemistry, Aarhus University Hospital. tCr50, which was the primary end point of the CONTEXT study, was calculated from post-transplant changes in P-creatinine as previously described [13] and DGF was defined as the need for dialysis within the first week after transplantation.
Glomerular filtration rate
Measured GFR (mGFR) was determined using 51chrome-ethylenediamine tetraacetic acid [16] (51Cr-EDTA) or iodothalamate plasma clearance. Estimated GFR (eGFR) was calculated using the following equations without correction for race (>90% of the patients were Caucasian): a) the simplified MDRD creatinine based formula [17]; b) the CKD-EPI creatinine based formula (eGFRCr) [18]; c) the CKD-EPI cystatin C based formula (eGFRCys) [19]; and d) the CKD-EPI creatinine and cystatin C based formula (eGFRCr-Cys) [19].
Statistical analyses
The mGFR, eGFR estimates, and P-NGAL at three and twelve months were analysed using repeated measurement analysis of variance or linear regression. A linear mixed regression effects model was used to compare log transformed outcomes between the RIC and sham-RIC groups, with RIC and centre as fixed effects, and donor as random effect. Data are presented as n (%), mean (SD), median (IQR) or estimated median (95% CI). The Chi-square test or Fisher’s exact test was used to examine the significance of categorical outcomes. Continuous variables were correlated using simple linear regression. Data were analysed in Stata/IC 12.1 for Mac (Copyright 1985–2011 Stata-Corp LP, College Station, TX).
Results
Inclusion
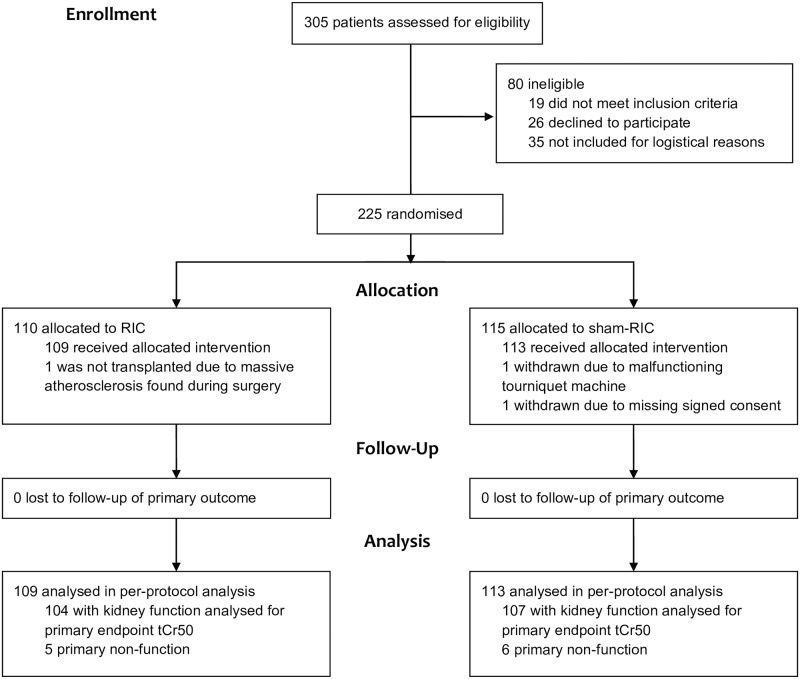
225 patients were included from June 12, 2011, to December 28, 2014. Three patients were withdrawn from the study during the transplant surgery, leaving a total of 222 patients for the per-protocol analysis (Fig 1).
Fig 1. CONSORT flow diagram of inclusion and randomisation.
RIC did not affect GFR or other outcomes at three months and one year
As previously reported, baseline data, tCr50 and the incidence of DGF were similar in the RIC and sham-RIC groups (Table 1) [13].
Table 1. Recipient and donor characteristics.
Baseline characteristics, tCr50 and the incidence of DGF. Data are mean (standard deviation), n (%), or median (interquartile range). No statistically significant differences were identified between the groups. tCr50 = estimated time to a 50% reduction in plasma creatinine post-transplantation. DGF = delayed graft function defined as need of dialysis first week post-transplantation. DBD = donation after brain death. DCD = donation after circulatory death. Remuzzi score on biopsies taken 30 min after reperfusion of the graft (baseline-biopsy). + score if including only biopsies with minimum six or ten glomeruli in the analysis.
| Recipient | RIC (n = 109) | Sham-RIC (n = 113) |
|---|---|---|
| Gender, male | 65 (60%) | 69 (61%) |
| Age (years) | 58.1 (49.5–65.0) | 61.4 (49.4–66.6) |
| Preemptive transplantation | 17 (16%) | 23 (20%) |
| First transplantation | 95 (87%) | 98 (87%) |
| Diabetes mellitus | 13 (12%) | 13 (12%) |
| Total HLA-A, B, DR mismatches | ||
| 0 | 6 (5.5%) | 6 (5.3%) |
| 1–2 | 17 (16%) | 24 (21%) |
| 3–4 | 70 (64%) | 58 (51%) |
| 5–6 | 16 (15%) | 25 (22%) |
| Immunosuppression at discharge | ||
| Tacrolimus | 98 (90%) | 106 (94%) |
| Mycophenolate mofetil | 105 (96%) | 111 (98%) |
| Corticosteroids | 101 (93%) | 109 (96%) |
| Cyclosporine | 7 (6.4%) | 5 (4.4%) |
| None (graft loss) | 4 (3.7%) | 2 (1.8%) |
| Donor | ||
| Gender, male | 60 (55%) | 61 (54%) |
| Age (years) | 58 (52–66) | 58 (52–65) |
| DBD | 98 (90%) | 102 (90%) |
| DCD | 11 (10%) | 11 (10%) |
| Cause of death, DBD (n = 98) | ||
| Cerebrovascular insult | 64 (65%) | 70 (69%) |
| Cerebral anoxia | 22 (22%) | 20 (20%) |
| Trauma | 11 (11%) | 12 (12%) |
| Benign cerebral neoplasm | 1 (1.0%) | 0 |
| Warm ischemia time DCD donor (min) | 20 (15–21) | 14 (13–18) |
| Missing data | 0 | 1 |
| Cold ischemia time (hours), DBD+DCD | 13.3 (4.0) | 13.6 (4.8) |
| n > 24h | 3 (2.8%) | 4 (3.5) |
| Missing data | 2 (1.9%) | 1 (0.9%) |
| Remuzzi score (baseline-biopsy) | ||
| Score all biopsies (n = 91&94) | 2 (1–3) | 2 (1–4) |
| Score 6 glom+ (n = 80&82) | 2 (1–3.5) | 2 (1–4) |
| Score 10 glom+ (n = 53&63) | 3 (1–4) | 3 (1–4) |
| Missing dataa | 18 (17%) | 19 (17%) |
| Post-transplantation | ||
| tCr50 (hours) (n = 104&107) | 122 (98–151) | 112 (91–139) |
| DGF | 36 (33%) | 40 (35%) |
aBiopsies were either not performed (n = 22) or insufficient (n = 15).
This extended analysis showed that both the three month and one-year graft function were also similar, with no significant differences either in mGFR or eGFR between RIC and sham-RIC (Table 2). Also, no differences between groups were observed with respect to mortality, need for dialysis, rejections, NODAT or the renal injury biomarker P-NGAL. Analyses including only the youngest 50% of the transplant recipients [20] also did not reveal any tendencies on effects of RIC regarding tCr50 (RIC: mean 129 hrs, 95% confidence interval (CI) 96–172, n = 60; vs. sham-RIC: 92 hrs, 95% CI 63–135, n = 47; p = 0.16) or mGFR at one year (RIC: 56 ml/min/1.73m2 95% CI 49–63, n = 40; vs. sham-RIC: 52 ml/min/1.73m2, 95% CI 46–59, n = 32; p = 0.43).
Table 2. Outcomes at three and twelve months.
Recipient and renal graft outcomes at three and twelve months depending on RIC or sham procedure. Values are n (%), estimated median (95% CI) or mean (95% CI). mGFR and eGFR values are ml/min/1.73m2. mGFR, measured GFR; eGFR, estimated GFR.
| Three months | RIC (n = 109) | Sham-RIC (n = 113) | p | ||
|---|---|---|---|---|---|
| Deaths | 1 (0.9%) | 0 | 0.49 | ||
| On dialysis | 7/109 (6.4%) | 7/113 (6.2%) | 0.94 | ||
| Rejectiona | 10 (9.2%) | 9 (8.0%) | 0.74 | ||
| NODATb | 8 (7.3%) | 12 (10.1%) | 0.39 | ||
| P-creatinine, μmol/L | 136 (127–146) | n = 101 | 140 (131–149) | n = 106 | 0.60 |
| P-NGAL, μg/L | 163 (147–180) | n = 83 | 176 (160–192) | n = 90 | 0.25 |
| P-cystatin C, mg/L | 2.00 (1.88–2.13) | n = 83 | 2.17 (2.01–2.34) | n = 90 | 0.11 |
| mGFR | 41 (37–44) | n = 78 | 43 (39–47) | n = 70 | 0.35 |
| eGFR, MDRDc | 42 (39–46) | n = 101 | 40 (38–44) | n = 106 | 0.34 |
| eGFRCrd | 45 (41–48) | n = 101 | 42 (39–46) | n = 106 | 0.30 |
| eGFRCyse | 30 (28–33) | n = 83 | 27 (25–30) | n = 90 | 0.09 |
| eGFRCr-Cysf | 36 (33–39) | n = 83 | 33 (31–36) | n = 90 | 0.21 |
| Twelve months | |||||
| Deaths | 2 (1.8%) | 2 (1.8%) | 1.00 | ||
| On dialysis | 8/109 (7.3%) | 10/113 (8.8%) | 0.68 | ||
| Rejectiona | 17 (15.6%) | 14 (12.4%) | 0.49 | ||
| NODATb | 11 (10.1%) | 14 (12.4%) | 0.59 | ||
| P-creatinine, μmol/L | 135 (125–145) | n = 99 | 134 (124–144) | n = 101 | 0.89 |
| P-NGAL, μg/L | 179 (163–197) | n = 79 | 181 (166–197) | n = 79 | 0.87 |
| P-cystatin C, mg/L | 1.85 (1.72–1.99) | n = 79 | 1.92 (1.78–2.07) | n = 78 | 0.48 |
| mGFR | 46 (41–51) | n = 67 | 44 (39–48) | n = 74 | 0.44 |
| eGFR, MDRDc | 43 (39–46) | n = 99 | 42 (39–45) | n = 101 | 0.77 |
| eGFRCrd | 45 (41–49) | n = 99 | 44 (40–48) | n = 101 | 0.75 |
| eGFRCyse | 34 (31–37) | n = 80 | 33 (30–36) | n = 77 | 0.56 |
| eGFRCr-Cysf | 38 (35–42) | n = 80 | 38 (34–41) | n = 77 | 0.85 |
| U-albumin/creatinine, mg/g | 52 (37–73 | n = 68 | 46 (32–66) | n = 68 | 0.63 |
aTotal number of patients with one or more rejections at three and twelve months.
bNODAT: total number of patients with NODAT (new-onset diabetes after transplantation) at three and twelve months.
cThe simplified MDRD creatinine based formula.
dThe CKD-EPI creatinine based formula (eGFRCr).
eThe CKD-EPI cystatin C based formula (eGFRCys).
fThe CKD-EPI creatinine and cystatin C based formula (eGFRCr-Cys).
tCr50 as predictor of GFR at twelve months
The tCr50, which is a previously developed marker of early graft function for the CONTEXT study [13], correlated weakly, but highly significantly, with both mGFR and eGFR at twelve months (Table 3), i.e. a longer tCr50 is associated with a lower GFR at one year. In contrast, the presence or absence of DGF, i.e. need of dialysis first week post-transplant, did not correlate significantly with mGFR (DGF: 48 ml/min/1.73m2 95% CI 44–52, n = 106; vs. no DGF 52 ml/min/1.73m2 95% CI 45–58, n = 35; p = 0.39) at twelve months.
Table 3. tCr50 and kidney graft function at twelve months.
Linear regression identifying a negative correlation between tCr50 and kidney graft function at twelve months. tCr50 = the estimated time to a 50% reduction in P-creatinine. mGFR = measured GFR.
| tCr50 | ||||
|---|---|---|---|---|
| n | p | r | r2adj. | |
| mGFR | 141 | <0.0001 | -0.39 | 0.15 |
| eGFR, MDRDa | 200 | <0.0001 | -0.30 | 0.09 |
| eGFRCrb | 200 | <0.0001 | -0.30 | 0.09 |
| eGFRCysc | 157 | <0.0001 | -0.34 | 0.11 |
| eGFRCr-Cysd | 157 | <0.0001 | -0.36 | 0.13 |
aThe simplified MDRD creatinine based formula.
bThe CKD-EPI creatinine based formula (eGFRCr).
cThe CKD-EPI cystatin C based formula (eGFRCys).
dThe CKD-EPI creatinine and cystatin C based formula (eGFRCr-Cys).
Discussion
In this randomised clinical CONTEXT trial, RIC did not improve GFR at one year after renal transplantation. Also, RIC did not affect the risks of graft loss, death, rejection or NODAT. This suggests that RIC does not offer any long-term protection in deceased donor kidney transplantation. At the same time tCr50, the primary endpoint of the CONTEXT study, was shown to correlate significantly with GFR at one year.
To our knowledge, the CONTEXT study is the largest investigation to date for RIC in deceased donor kidney transplantation using a multicentre approach. The results were further strengthened by the randomised double blinded design as well as the use of both mGFR and P-cystatin C which are considered to provide more accurate estimates of GFR compared to eGFR based on P-creatinine alone.
Transplantation from deceased donors is accompanied by more profound ischaemia reperfusion injury and an increased risk of DGF compared to living donor kidney transplantation. Still, our results are clear and in concordance with findings in living donor transplantation reported in the REPAIR study [21], which investigated the effect of early and late phase RIC applied to both the donor and recipient at different time points prior to transplantation. The REPAIR study also failed to demonstrate any significant effect of RIC on kidney function at one year. However, a trend towards higher GFR (mGFR 58.3 vs. 55.9 mL/min/1.73m2, p = 0.13) was observed in the group receiving RIC with a similar timing to the one used in the CONTEXT study, when compared to the control group. Other and smaller studies have also not been able to demonstrate a beneficial effect of RIC in renal transplantation either on patient or kidney graft outcomes nor on various renal biomarkers [22–25].
Thus, the positive short term effects of RIC reported by our group in a porcine donation after brain death transplant model [8] were not reproduced in patients in respect of either the early graft function [10] or GFR at one year. Although animal studies have resulted in promising results to ameliorate renal ischaemia reperfusion injury, more research is required to understand the mechanisms involved as little of the positive animal research data can be replicated in the clinic nor can it be shown to improve transplant outcome [26]. A number of factors, such as uraemia, co-morbidity including diabetes, age [20], anaesthesia [27] or other yet unidentified factors might explain this lack of effect in the clinical setting and they may also blur potential beneficial effects of RIC in patients. Most animal studies are performed in healthy animals not receiving immunosuppressants or other drugs that may potentially influence outcomes. It is possible that such medications may either block the protective mechanism induced by the RIC or could have yielded other anti-ischaemic effects [28–30], thus blunting or obliterating any beneficial effect of RIC. In addition to recipient-related and potentially confounding factors, there are additional donor-related variables that may influence and attenuate the effect of RIC such as organ injury and inflammatory activation during brain death [31]. In most animal models organs are retrieved shortly after brain death, e.g. after four hours in our porcine model [8], while in humans retrieval is often delayed. The protective mechanisms induced by RIC (if any) were unfortunately insufficient to result in meaningful clinical effects.
It is possible that another explanation as to why our study failed to show a protective effect of RIC may be the timing of application of the RIC stimulus which could be important for the protective effects. Transplantation is a unique setting for the simple reason that the organ is not connected to the recipient until reperfusion. It is believed that RIC may provide a first window of protection conferred by humoral factors released from the conditioned tissue. It is possible that such factors were no longer in circulation at the time of reperfusion although this was only 38 (IQR 19–54) minutes after the end of the RIC procedures last cuff release [13]. The suggested humoral factors have short plasma half-lives [32]. Also, since the transplanted kidney is denervated, any neuronal pathway for RIC effects is excluded.
In addition our study demonstrated a weak, but highly statistically significant, correlation between tCr50 and both mGFR and eGFR at twelve months. No such correlation was demonstrated when comparing the incidence of DGF with GFR at twelve months. This may suggest that tCr50 is a more sensitive marker of the impact of early graft function on long term GFR than the commonly used definition of DGF, i.e. the need for dialysis within the first week post-transplantation. Thus, tCr50 although not simple to calculate [13] could be of use in future studies on interventions to improve early and long term graft function. In addition to tCr50’s correlation with one-year GFR, it has the advantage of being a continuous variable allowing all patients to be included in the analysis except for the few who never obtain a 50% reduction in P-creatinine.
In conclusion, our results indicate that RIC of the organ recipient does not offer any advantages in kidney transplantation either on short or one year outcomes. tCr50 is negatively associated with GFR at one year and may be used as a marker of early graft function with an impact on long term outcome.
Supporting information
(DOC)
Abbreviations
- 51Cr-EDTA
51chrome-ethylenediamine tetraacetic acid
- DBD
donation after brain death
- DCD
donation after circulatory death
- DGF
delayed graft function
- eGFR
estimated glomerular filtration rate
- eGFR
glomerular filtration rate
- eGFRCr
the CKD-EPI creatinine based GFR formula
- eGFRCr-CYS
the CKD-EPI creatinine and cystatin C based GFR formula
- eGFRCys
the CKD-EPI cystatin C based GFR formula
- mGFR
measured glomerular filtration rate
- NGAL
neutrophil gelatinase associated lipocalin
- NODAT
new onset diabetes mellitus after transplantation
- P-
plasma
- RIC
remote ischaemic conditioning
- tCr50
the estimated time to a 50% reduction in plasma creatinine
Data Availability
Data available on request to the CONTEXT Data Access Committee, Bende Ingvorsen, Aarhus University Hospital, (bendingv@rm.dk). It is not possible to anonymise data sufficient for public access according to Danish Data Protection Regulations.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Marie B. Nielsen received a research scholarship from The Danish Council for Independent Research.
References
- 1.Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant 2009. March;24(3):1039–1047. 10.1093/ndt/gfn667 [DOI] [PubMed] [Google Scholar]
- 2.Summers DM, Johnson RJ, Allen J, Fuggle SV, Collett D, Watson CJ, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 2010. October 16;376(9749):1303–1311. 10.1016/S0140-6736(10)60827-6 [DOI] [PubMed] [Google Scholar]
- 3.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet 2004. November 13–19;364(9447):1814–1827. 10.1016/S0140-6736(04)17406-0 [DOI] [PubMed] [Google Scholar]
- 4.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 1997. April 15;63(7):968–974. 10.1097/00007890-199704150-00011 [DOI] [PubMed] [Google Scholar]
- 5.Miglinas M, Supranaviciene L, Mateikaite K, Skebas K, Kubiliene A. Delayed graft function: risk factors and the effects of early function and graft survival. Transplant Proc 2013. May;45(4):1363–1367. 10.1016/j.transproceed.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 6.Candilio L, Malik A, Hausenloy DJ. Protection of organs other than the heart by remote ischemic conditioning. J Cardiovasc Med (Hagerstown) 2013. March;14(3):193–205. [DOI] [PubMed] [Google Scholar]
- 7.Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol 2015. January 20;65(2):177–195. 10.1016/j.jacc.2014.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soendergaard P, Krogstrup NV, Secher NG, Ravlo K, Keller AK, Toennesen E, et al. Improved GFR and renal plasma perfusion following remote ischaemic conditioning in a porcine kidney transplantation model. Transpl Int 2012. September;25(9):1002–1012. 10.1111/j.1432-2277.2012.01522.x [DOI] [PubMed] [Google Scholar]
- 9.Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 2014. January;35(3):168–175. 10.1093/eurheartj/eht369 [DOI] [PubMed] [Google Scholar]
- 10.Krogstrup NV, Oltean M, Bibby BM, Nieuwenhuijs-Moeke GJ, Dor FJ, Birn H, et al. Remote ischaemic conditioning on recipients of deceased renal transplants, effect on immediate and extended kidney graft function: a multicentre, randomised controlled trial protocol (CONTEXT). BMJ Open 2015. August 20;5(8):e007941-2015-007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant 2008. September;23(9):2995–3003. 10.1093/ndt/gfn158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krogstrup NV, Bibby BM, Aulbjerg C, Jespersen B, Birn H. A new method of modelling early plasma creatinine changes predicts 1-year graft function after kidney transplantation. Scand J Clin Lab Invest 2016. July;76(4):319–323. 10.3109/00365513.2016.1161233 [DOI] [PubMed] [Google Scholar]
- 13.Krogstrup NV, Oltean M, Nieuwenhuijs-Moeke GJ, Dor FJ, Moldrup U, Krag SP, et al. Remote Ischemic Conditioning on Recipients of Deceased Renal Transplants Does Not Improve Early Graft Function: A Multicenter Randomized, Controlled Clinical Trial. Am J Transplant 2017. April;17(4):1042–1049. 10.1111/ajt.14075 [DOI] [PubMed] [Google Scholar]
- 14.Ramirez-Sandoval JC, Herrington W, Morales-Buenrostro LE. Neutrophil gelatinase-associated lipocalin in kidney transplantation: A review. Transplant Rev (Orlando) 2015. July;29(3):139–144. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen MB, Krogstrup NV, Nieuwenhuijs-Moeke GJ, Oltean M, Dor FJMF, Jespersen B, et al. P-NGAL Day 1 predicts early but not one year graft function following deceased donor kidney transplantation—The CONTEXT study. PLoS One 2019. February 28;14(2):e0212676 10.1371/journal.pone.0212676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medeiros FS, Sapienza MT, Prado ES, Agena F, Shimizu MH, Lemos FB, et al. Validation of plasma clearance of 51Cr-EDTA in adult renal transplant recipients: comparison with inulin renal clearance. Transpl Int 2009. March;22(3):323–331. 10.1111/j.1432-2277.2008.00799.x [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. Am Soc Nephrol 2000;11(155A). [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009. May 5;150(9):604–612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012. July 5;367(1):20–29. 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinen A, Behmenburg F, Aytulun A, Dierkes M, Zerbin L, Kaisers W, et al. The release of cardioprotective humoral factors after remote ischemic preconditioning in humans is age- and sex-dependent. J Transl Med 2018. April 27;16(1):112-018-1480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacAllister R, Clayton T, Knight R, Robertson S, Nicholas J, Motwani M, et al. REmote preconditioning for Protection Against Ischaemia–Reperfusion in renal transplantation (REPAIR): a multicentre, multinational, double-blind, factorial designed randomised controlled trial. 2015 May(No. 2.3.). [PubMed]
- 22.Wu J, Feng X, Huang H, Shou Z, Zhang X, Wang R, et al. Remote ischemic conditioning enhanced the early recovery of renal function in recipients after kidney transplantation: a randomized controlled trial. J Surg Res 2014. May 1;188(1):303–308. 10.1016/j.jss.2013.06.058 [DOI] [PubMed] [Google Scholar]
- 23.Nicholson ML, Pattenden CJ, Barlow AD, Hunter JP, Lee G, Hosgood SA. A Double Blind Randomized Clinical Trial of Remote Ischemic Conditioning in Live Donor Renal Transplantation. Medicine (Baltimore) 2015. August;94(31):e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Zheng H, Wang X, Zhou Z, Luo A, Tian Y. Remote ischemic preconditioning fails to improve early renal function of patients undergoing living-donor renal transplantation: a randomized controlled trial. Transplantation 2013. January 27;95(2):e4–6. 10.1097/TP.0b013e3182782f3a [DOI] [PubMed] [Google Scholar]
- 25.van den Akker EK, Hesselink DA, Manintveld OC, Lafranca JA, de Bruin RW, Weimar W, et al. Ischemic postconditioning in human DCD kidney transplantation is feasible and appears safe. Transpl Int 2014. February;27(2):226–234. 10.1111/tri.12242 [DOI] [PubMed] [Google Scholar]
- 26.Saat TC, van den Akker EK, IJzermans JN, Dor FJ, de Bruin RW. Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? J Transl Med 2016. January 20;14:20-016-0767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunte S, Behmenburg F, Eckelskemper F, Mohr F, Stroethoff M, Raupach A, et al. Cardioprotection by Humoral Factors Released After Remote Ischemic Preconditioning Depends on Anesthetic Regimen. Crit Care Med 2019. January 2. [DOI] [PubMed] [Google Scholar]
- 28.Dagenais F, Cartier R, Hollmann C, Buluran J. Calcium-channel blockers preserve coronary endothelial reactivity after ischemia-reperfusion. Ann Thorac Surg 1997. April;63(4):1050–1056. 10.1016/s0003-4975(96)01278-7 [DOI] [PubMed] [Google Scholar]
- 29.Lemoine S, Pillot B, Rognant N, Augeul L, Rayberin M, Varennes A, et al. Postconditioning with cyclosporine a reduces early renal dysfunction by inhibiting mitochondrial permeability transition. Transplantation 2015. April;99(4):717–723. 10.1097/TP.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 30.Tuuminen R, Holmstrom E, Raissadati A, Saharinen P, Rouvinen E, Krebs R, et al. Simvastatin pretreatment reduces caspase-9 and RIPK1 protein activity in rat cardiac allograft ischemia-reperfusion. Transpl Immunol 2016. July;37:40–45. 10.1016/j.trim.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 31.Morariu AM, Schuurs TA, Leuvenink HG, van Oeveren W, Rakhorst G, Ploeg RJ. Early events in kidney donation: progression of endothelial activation, oxidative stress and tubular injury after brain death. Am J Transplant 2008. May;8(5):933–941. 10.1111/j.1600-6143.2008.02166.x [DOI] [PubMed] [Google Scholar]
- 32.Kierulf-Lassen C, Nieuwenhuijs-Moeke GJ, Krogstrup NV, Oltean M, Jespersen B, Dor FJ. Molecular Mechanisms of Renal Ischemic Conditioning Strategies. Eur Surg Res 2015. September 2;55(3):151–183. 10.1159/000437352 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
Data available on request to the CONTEXT Data Access Committee, Bende Ingvorsen, Aarhus University Hospital, (bendingv@rm.dk). It is not possible to anonymise data sufficient for public access according to Danish Data Protection Regulations.