SUMMARY
Background
Although experts have recommended testing for pretreatment drug resistance (PDR) prior to ART initiation, there is little evidence to support its implementation. Our aim was to determine whether an inexpensive point mutation assay can improve virologic suppression by identifying PDR to guide drug selection for ART in a lower-middle income country.
Methods
Investigators conducted a randomized multicenter clinical trial between May 28, 2013 and February 3, 2016 at three HIV treatment sites across Kenya: two in Nairobi and one in rural Maseno. A total of 991 HIV-infected, ART-eligible participants were enrolled and followed for twelve months after randomization. We randomized participants (1:1) to either to PDR testing by oligonucleotide ligation assay (OLA) to guide selection of ART or to standard-of-care (SOC), which did not include OLA testing. The OLA-guided therapy arm had pre-ART peripheral blood mononuclear cells evaluated for drug resistance to NNRTI at codons K103N, Y181C, G190A, and to lamivudine at M184V, and when ≥1 drug resistant codon was detected in a participant’s pre-ART specimen, clinicians were directed to prescribe protease inhibitor-based second-line ART. Those without detected resistance and those who were randomized to SOC received NNRTI-based first-line ART. The primary outcome was plasma HIV-1 RNA ≥400 copies per µL at 4, 8, or 12 months after ART initiation which defined virologic failure.
Findings
Among the 991 participants randomized to OLA-guided therapy (n=494) and SOC (n=497) the overall PDR prevalence was 9·4% (n=93) [95% confidence interval (CI), 7·7–11·4]. Virologic failure at month-12 of ART in the OLA-guided therapy arm was 8·5% (n=34) [95% CI, 6·0%–11·7%] compared to 9·7% (n=39) (95% CI, 7·0%–13·0%) in SOC and this was not a statistically significant difference (log-rank test, p=0·26). Among participants with PDR, virologic failure was lower in the OLA-guided therapy arm compared to SOC [14% (n=5) vs. 50% (n=13); p<0·01). Among those prescribed NNRTI-based ART, participants given efavirenz were less likely to experience virologic failure compared to those receiving nevirapine [Odds Ratio (OR) = 0·37; 95% CI, 0·22–0·62; p<0·01).
Interpretation
Our finding that OLA testing for PDR reduced virologic failure in only those with specific PDR mutations suggests that PDR poses less of a risk for virologic failure than that predicted by past prevalence estimates, and that the value of PDR testing to reduce virologic failure should be assessed to current antiretroviral treatment regimens.
Interpretation
National Institutes of Health
Trial Status and Registration
The study was completed and is registered with Clinicaltrials.gov #
Introduction
Antiretroviral therapy (ART) can transform the outcome of HIV infection, yet this benefit could be undermined by pretreatment drug resistance (PDR).1–4 PDR is defined by the WHO as drug resistance in HIV-infected individuals who are initiating ART and includes, but is not limited to, drug resistance that is transmitted between individuals.5 PDR has been shown to increase the risk of virologic failure of ART,1–4,6 especially to regimens that include nevirapine, a non-nucleoside reverse transcriptase inhibitor (NNRTI),7 as opposed to those that include a protease inhibitor (PI).3,7–10 Recent studies, however, suggest that only dual class PDR, not NNRTI PDR alone, pose an increased risk of virologic failure when newer NNRTI-based regimens containing efavirenz + tenofovir are administered.11
The observed association between PDR and subsequent virologic failure has led experts in high-resource settings to recommend pre-ART testing for antiretroviral drug resistance.12,13 Pre-ART testing that reveals antiretroviral-resistance guides clinicians to choose an ART regimen without cross-resistance. However, this approach has never been validated in a randomized trial, and resistance testing, generally performed by Sanger sequencing, is relatively expensive and generally unaffordable in low- and lower-middle-income countries (LMICs). Evidence regarding the value of testing for PDR is particularly relevant in LMICs where the prevalence of PDR is rising and is above 10% in many countries.14 To justify pre-ART drug testing to guide ART regimen selection in LMICs, rigorous scientific evidence using a low-cost method to detect resistance would be needed.5
The objective of this randomized trial was to determine if PDR testing using an oligonucleotide ligation assay (OLA) to guide ART regimen selection will decrease the development of virologic failure over twelve-month follow-up in Kenya.
Methods
Study Design, Setting, and Enrollment
We obtained ethical approvals from Seattle Children’s Hospital (Seattle, WA, USA) and Kenyatta National Hospital (Nairobi, Kenya). All adult participants and a parent or legal guardian of children aged younger than 18 years provided written informed consent in English or Kiswahili. This study was a multisite randomized control trial (Clinicaltrials.gov # ) conducted at the Coptic Hope Centers for Infectious Diseases in Kenya between May 28, 2013 and February 3, 2016. The Hope Centers were established by the University of Washington and the Coptic Orthodox Mission with support from the President’s Emergency Plan for AIDS Relief (PEPFAR) and includes three locations in Kenya: two in urban Nairobi and one in rural Maseno in Western Kenya.15 At the time of the trial and as per Kenyan national guidelines, newly diagnosed HIV-infected patients with CD4 count ≤350 cells per µL or WHO clinical stage III or IV disease regardless of CD4 cell count were initiated on NNRTI-based first-line ART. The first-line ART regimen included tenofovir or zidovudine, lamuvidine, and nevirapine or efavirenz. In November 2013, the national ART program began replacing nevirapine with efavirenz for individuals initiating ART. The second-line ART regimen for individuals with clinical or virologic failure to first-line ART was tenofovir or zidovudine, lamivudine, and ritonavir-boosted lopinavir.
Individuals from the Hope Center were eligible to participate if they were confirmed HIV-seropositive, ≥2 years of age, qualified for first-line ART, planned to reside in the area for more than one year, and provided informed consent. Exclusion criteria included already receiving ART from the Hope Center or plans to initiate second-line ART. We collected detailed demographic and clinical information at enrollment using standardized questionnaires, and abstracted HIV medical history and CD4 cell count data from Hope Center records. All participants had blood collected at enrollment.
Randomization and Intervention
We randomly assigned enrolled participants in a 1:1 ratio using a computer-generated randomization code to receive either OLA testing prior to ART initiation or no pre-ART OLA testing, which was standard-of-care (SOC). Randomization codes were sealed in individual envelopes labeled with an identification number. At enrollment, the study nurse opened the sealed envelope for the next ID and assigned the participant to the intervention. Study participants and investigators were not blinded to the intervention.
If a participant was randomized to OLA-guided therapy, their enrollment blood specimen was tested for HIV drug resistance. If HIV drug resistance was detected by the OLA as defined below, then the participant was initiated on second-line ART (tenofovir or zidovudine + lamuvidine + ritonavir-boosted lopinavir). Participants who tested negative for resistance by OLA as well as those randomized to SOC were prescribed first-line NNRTI-based ART (tenofovir or zidovudine + lamuvidine + nevirapine or efavirenz). We performed quality assurance of OLA genotyping by parallel tests in Seattle by OLA, Sanger consensus sequencing (CS) and Illumina “next generation sequencing” (NGS); see below. We tested SOC specimens for PDR by OLA and CS in Seattle just before each participant completed 12 months of ART.
Follow-up
Participants returned at monthly intervals to the pharmacy for pill counts and to refill their ART prescription. They returned to the study clinic at 4, 8, and 12 months after ART initiation for clinical assessment and to have their blood drawn for plasma HIV-1 RNA quantification.
Study Outcomes
The pre-defined primary outcomes of this study were (1) the difference between the OLA-guided therapy and SOC arms in rates of virologic failure, defined as plasma HIV RNA copies ≥400 copies per µL (incorrectly stated as 1,000 copies per µL in the protocol to follow WHO guidelines), during the first 12 months of ART; and (2) differences in virologic failure among participants with HIV drug-resistance in the OLA-guided therapy and SOC arms. A post-hoc outcome, introduced once Kenya switched to efavirenz-based ART, was differences in virologic failure in participants receiving nevirapine versus efavirenz-based ART.
Laboratory Methods
Peripheral blood mononuclear cells were evaluated for NNRTI and NRTI drug resistance mutations, K103N, Y181C, G190A, and K65R, and M184V, using a quantitative OLA.16,17 The OLA provides the proportion of each mutant within an individual’s viral quasispecies between 2–100%. OLA-detected mutant frequencies were categorized as follows: <2% as wild-type or “no resistance”, 2–9% as “low frequency resistance”, and ≥10% as “resistance”. We classified participants prospectively as positive for ART resistance if any one of the HIV pol codons K103N, Y181C, G190A, or M184V had the drug-resistant genotype detected by OLA at ≥10% frequency within the individual’s viral quasispecies. We evaluated K65R in all pre-ART specimens retrospectively as tenofovir became more available concomitantly with efavirenz.
NGS of peripheral blood mononuclear cells’ DNA as previously described18 confirmed resistance mutations detected by OLA at frequencies ≤25% that were missed by CS in pre-ART specimens. To rule-out “index hopping”, NGS minority variants were assessed for sequence concordance with a CS derived from the respective participant’s peripheral blood mononuclear cells’ DNA using Sequencher Program V5.0.1. GenBank accession numbers: (submitted, corrections in process)
Plasma HIV RNA was quantified (Abbott) in a Virology Quality Assurance Program compliant laboratory.
Statistical Methods
A sample size of 854 (427 per group) was expected to provide 80% power to detect a difference in primary outcome with an assumed 2-sided alpha of 0·05. Allowing for 10% loss to follow-up or death at 12 months, 949 participants would need to be enrolled (474 per group). Based on prior research,19 we anticipated that 7·5% of participants would have PDR. We estimated that without OLA, 85% of those with PDR would have virologic failure by 12 months after ART initiation, and those with no PDR at enrollment and those with PDR but receiving OLA-guided ART would have 8% virologic failure, for an overall rate of virologic failure of 14% in the SOC arm.
The primary outcome, rate of virologic failure by 12 months after ART between OLA-guided therapy and SOC arms, was assessed using a modified intent-to-treat analysis, where participants who were loss to follow-up or died were censored at their last known visit date; a sensitivity analysis categorized these as “failure”. Kaplan Meier curves were used to show time to virologic failure, and the log-rank test was used to compare the rate of virologic failure between arms. Comparisons were made using a Chi-square test for categorical variables and Mann-Whitney U test for continuous variables. The secondary outcomes were analyzed using Chi-square tests and logistic regression. Statistical analyses were conducted using STATA 13·0 (Statacorp, College Station, TX, USA).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study Population
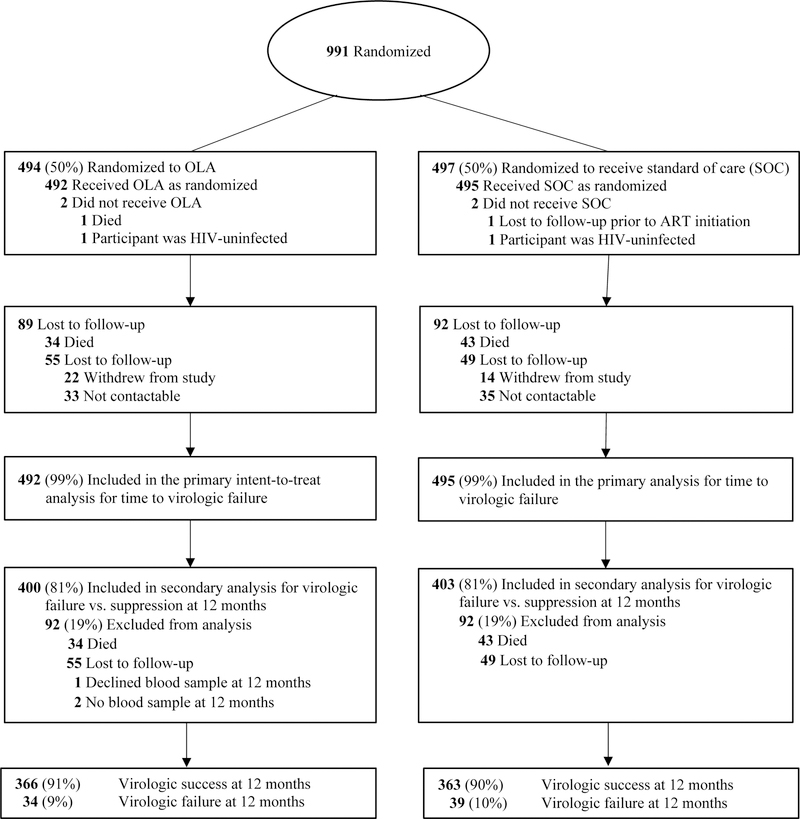
We screened a total of 1,198 participants at the Hope Centers between May 28, 2013 and November 4, 2014, and 991 were deemed eligible, consented to participate, and were enrolled in the study (Figure 1). Of these participants, we randomized 492 to OLA-guided therapy and 495 to SOC.
Figure 1:
Flow of trial participants and virologic outcomes at 12 months post-randomization
The median age of the randomized cohort at enrollment was 37 years [interquartile range (IQR), 31–45], 65% (n=641) were female, and 56% (n=533) were married (Table 1). Their median CD4 T-cell count was 231 cells per μL (IQR, 114–313) and 94% (n=923) were ART-naïve by history and pharmacy records. Baseline (i.e., enrollment) log10 transformed plasma HIV RNA levels, CD4 count, and other sociodemographic characteristics did not differ between randomization arms. Of the 939 participants who initiated ART, 60% (n=567) received an efavirenz-based regimen, 36% (n=333) nevirapine-based, and 4% (n=39) ritonavir-boosted lopinavir-based. Adherence measured by pill count did not differ between those who took efavirenz- vs nevirapine-based regimens (91% vs. 91%).
Table 1:
Enrollment characteristics of the study population by randomization arm
| OLA-guided therapy (n=492) | Standard of Care (n=495) | |
|---|---|---|
| Clinic site | ||
| Nairobi, Ngong Rd | 331 (67%) | 336 (68%) |
| Nairobi, Industrial Area | 42 (9%) | 48 (10%) |
| Maseno | 119 (24%) | 111 (22%) |
| Female | 320 (65%) | 321 (65%) |
| Age (years) | 38 (32–46) | 37 (30–45) |
| 2–24 | 33 (7%) | 53 (11%) |
| 25–34 | 152 (31%) | 162 (33%) |
| 35–49 | 224 (45%) | 215 (43%) |
| ≥50 | 83 (17%) | 65 (13%) |
| Education, adults onlya | ||
| Less than primary | 47 (10%) | 37 (8%) |
| Primary | 180 (37%) | 169 (36%) |
| Secondary | 131 (27%) | 141 (30%) |
| Some College/Technical | 123 (26%) | 123 (26%) |
| Marital status, adults onlya | ||
| Single | 91 (19%) | 73 (15%) |
| Married/Committed relationship | 263 (55%) | 270 (58%) |
| Divorced/Separated/Widowed | 127 (26%) | 127 (27%) |
| Unemployed, adults onlya | 51 (11%) | 41 (9%) |
| Sexual partners in past yearb | ||
| None or never had sex | 173 (37%) | 182 (38%) |
| 1–2 | 284 (60%) | 289 (60%) |
| ≥3 | 12 (3%) | 12 (2%) |
| ART-naïve | 460 (94%) | 463 (94%) |
| CD4 cells per uL | 233 (116–311) | 231 (113–318) |
| Plasma HIV RNA (log10 copies per µL) | 10·8 (9·2–12·1) | 10·7 (9·1–11·8) |
| % Resistance of mutation with highest frequency in individual’s HIV quasispeciesc | ||
| ≥10% | 45 (9%) | 34 (7%) |
| 2–9% | 6 (1%) | 8 (2%) |
| Wild-type | 441 (90%) | 452 (91%) |
| ART regimen post-randomizationd | ||
| Efavirenz | 267 (57%) | 300 (63%) |
| Nevirapine | 160 (34%) | 173 (37%) |
| Lopinavir/ritonavir | 39 (9%) | 0 (0%) |
Data are shown by absolute numbers, “n” (%) or median (IQR).
Adults only, OLA-guided therapy (n=481) and SOC (n=470);
Does not include 35 responses categorized as “Don’t Know” or “Refused”;
Point mutations (K103N, Y181C, G190A, M184V) detected by OLA;
ART regimen base, OLA-guided therapy (n=466) and SOC (n=473);
Abbreviations: OLA, oligonucleotide ligation assay
Pretreatment Drug Resistance (PDR)
The overall prevalence of PDR was 9·4% (n=93) [95% confidence interval (CI), 7·7–11 ·4]. PDR was 10·4% (n=51) in the OLA-guided therapy arm and 8·5% (n=42) in SOC, but this difference was not statistically significant (p=0·32). PDR was 9·3% (n=86) among ART-naïve individuals and 10·9% (n=7) among antiretroviral-experienced individuals; this difference was also not statistically significant (p=0·67). PDR was higher among women (10·8%, n=69) than men (6·9%, n=24; p=0·05), and among participants ≥18 years of age compared to those who were younger [9·7% (n=92) vs. 2·8% (n=1), p=0·16], though this was not statistically significant.
The median age among those with PDR was 35 years (IQR, 29–41), 74% (n=69) were female, 53% (n=49) were married, and the median CD4 T-cell count was 225 per µL (IQR, 80–296). Compared to those without PDR, those with PDR were more likely to be female [Odds Ratio (OR), 1·62; 95% CI, 0·99–2·63, p=0·05]. Participants with PDR versus those without PDR did not differ in antiretroviral-experience, age, CD4 count, plasma HIV-1 RNA, and randomization assignment.
Testing by OLA detected and CS and/or NGS confirmed 122 mutations in 93 participants at enrollment across both study arms: 61 participants had only mutations conferring resistance to NNRTI, 15 had NNRTI resistance mutations and M184V conferring resistance to lamivudine, and one had M184V alone. The mutant frequency in each participant’s HIV quasispecies ranged from 2% to 100% (median 68%). We collected virologic outcomes from 70 of the 93 participants with PDR. These included 31 in the SOC arm, among whom 15 (48%) experienced VF (7 had 1 mutation and 8 had 2 PDR mutations), and 39 in the OLA-guided therapy arm, among whom 6 (15%) experienced VF (4 with 1 mutation and 2 with 2 mutations).
Of the 7 participants with both lamivudine and NNRTI drug resistance in the OLA-guided therapy arm, all received PI-based ART, 5 had outcomes available and 2 of these had virologic failure. Of the 8 participants with both lamivudine and NNRTI drug resistance in the SOC arm, 6 had outcomes available and all 6 had virologic failure: 4 were on nevirapine-based ART and 2 on efavirenz-based ART.
Loss to Follow-up and Death
Two participants randomized to the OLA-guided therapy arm did not initiate ART; one died and HIV did not amplify by polymerase-chain-reaction in one specimen. Upon testing of an additional specimen from this participant, tests were negative for HIV RNA and was HIV-seronegative (i.e., participant had been misdiagnosed as HIV-infected). Two participants randomized to SOC arm were lost to follow-up prior to initiating ART. A total of 806 participants (81%), 403 in the OLA-guided therapy arm and 403 in SOC arm, completed 12 months of follow-up (Figure 1). During the study period, 73 participants (7%) died, 108 (11%) were lost to follow-up, and three participants did not have a blood draw at the 12-month visit. In a comparison of enrollment characteristics including PDR, age, gender, employment, education, and sexual behavior, there were no significant differences between those who were lost to follow-up or died and those retained in the study, except the median CD4 count was lower among the former (176 vs. 234 cells per μL; p<0·01). Of the 93 participants who had mutations detected by OLA at baseline, we could not ascertain virologic outcome for 23 participants: 10 died and 13 were lost to follow-up before viral load was measured. Of these, 11 were in the SOC arm (7 with 1 mutation; 3 with 2 mutations; 1 with 3 mutations) and 12 were in the OLA-guided therapy arm (9 with 1 mutation; 2 with 2 mutations; 1 with 3 mutations).
Virologic Failure
Overall, virologic failure occurred in 73 of 803 by 12 months after ART initiation, with a probability of 9·1% (95% CI, 7·2–11·3). There was no statistically significant difference in the probability of virologic failure between those randomized to OLA-guided therapy [8·5% (n=34); 95% CI, 6·0–11·7] versus SOC [9·7% (n=39); 95% CI, 7·0–13·0; log-rank test p=0·26] (Figure 2). Among participants with PDR, virologic failure was lower in the OLA-guided therapy arm compared to SOC [14% (n=5) vs. 50% (n=13); p<0·01]. Among participants identified as having PDR, those receiving second-line PI-based ART had 68% lower odds of virologic failure compared to those who received first-line NNRTI-based ART (OR, 0·32; 95% CI, 0·11–0·93; p=0·04) (Table 2). This relationship remained after adjustment for sex, age, and CD4 cell count at enrollment (adjusted OR, 0·25; 95% CI, 0·08–0·78; p=0·02). In sensitivity analyses, changing the plasma HIV RNA level to define virologic failure (≥400 or ≥1000 copies per µL) or adding loss to follow-up and death as a composite outcome did not alter results (Figure 3). Defining virologic failure as ≥1,000 copies per µL, 59 out of 803 (7·4%) participants experienced virologic failure (≥1,000 copies per µL) with 6·3% (95% CI, 4·1–9·1) virologic failure in the OLA-guided therapy arm versus 8·4% (95% CI, 5·9–11·6) in the SOC arm (p=0·24).
Figure 2:
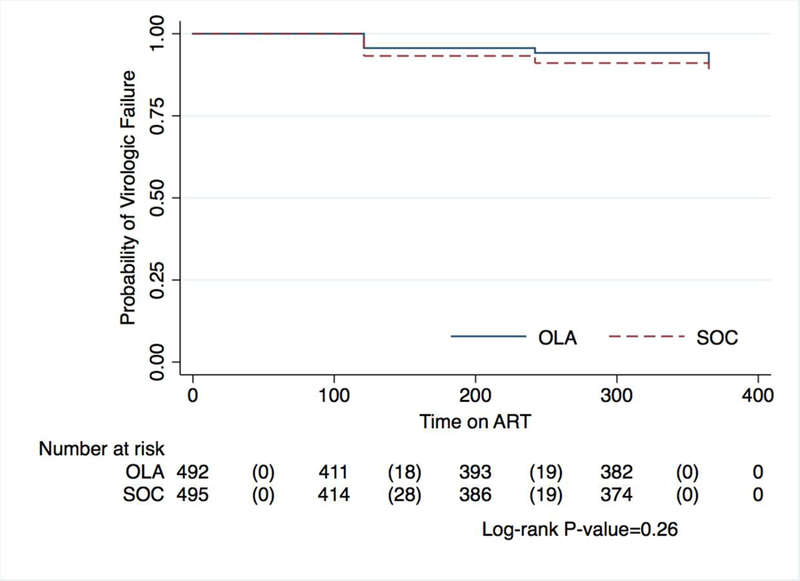
Kaplan Meier curve of primary end point comparing oligonucleotide ligation assay guided therapy versus standard of care by time to virologic failure (≥400 copies per µL)*
Abbreviations: OLA, oligonucleotide ligation assay; SOC, standard of care
*The graph indicates the number of participants at risk at each time point and the number experiencing virologic failure in parenthesis.
Table 2:
Virologic failure (≥400 copies/µL) by randomization arm and HIV drug-resistance mutation frequency
| Pretreatment drug resistance: categorization and mutant frequency | OLA-guided therapy | Standard of Care | p-value | ||
|---|---|---|---|---|---|
| N tested | n (%) VF | N tested | n (%) VF | ||
| Total | 400 | 34 (9%) | 403 | 39 (10%) | 0·56 |
| Wild-type; <2% | 361 | 28 (8%) | 372 | 24 (7%) | 0·49 |
| Low frequency resistance; 2–9% | 4* | 1 (25%) | 5* | 2 (40%) | 0·64 |
| Resistant; ≥10% | 35^ | 5 (14%) | 26* | 13 (50%) | <0·01 |
Abbreviations: OLA, oligonucleotide ligation assay; VF, virologic failure;
prescribed NNRTI-based ART;
only participants prescribed 2nd-line PI-based ART
Figure 3:
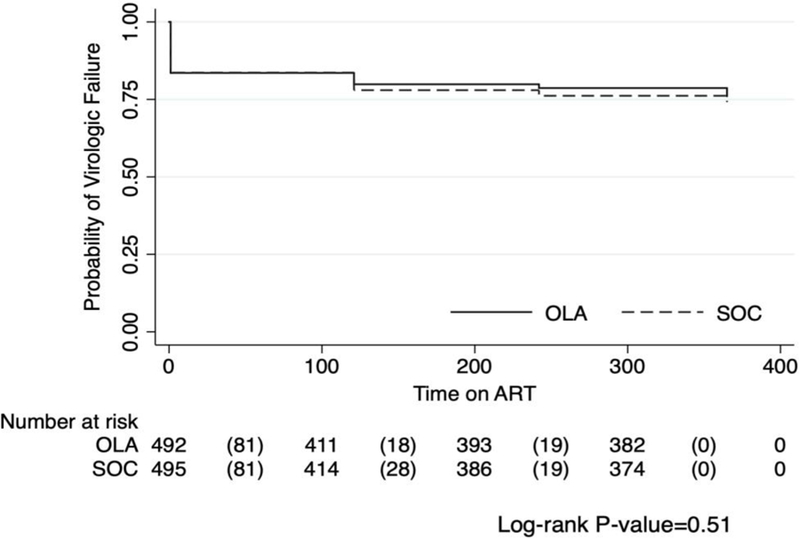
Kaplan Meier curves comparing oligonucleotide ligation assay guided therapy versus standard of care on combined end point of time to death, loss to follow-up or virologic failure*
Abbreviations: OLA, oligonucleotide ligation assay; SOC, standard of care
*The graph indicates the number of participants at risk at each time point and the number experiencing combined end point of virologic failure, death, or loss to follow-up in parenthesis.
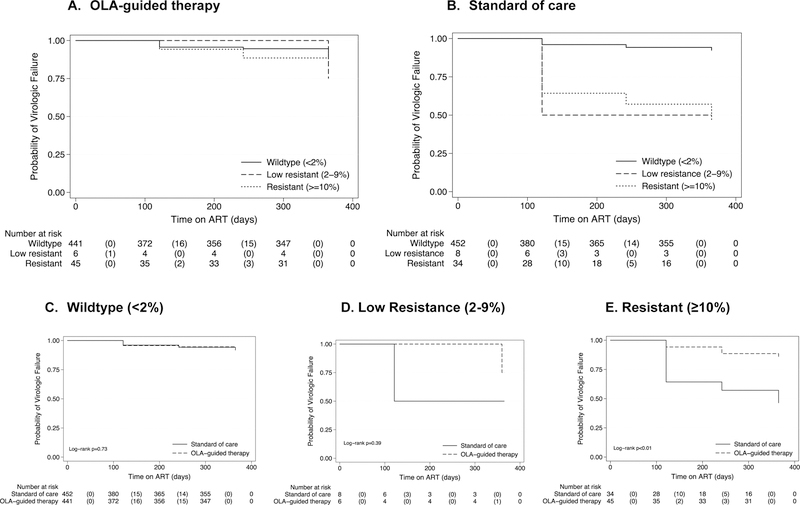
Across study arms, those prescribed efavirenz-based ART were 63% less likely to experience virologic failure compared to those given nevirapine-based ART (OR=0·37; 95% CI, 0·22–0·62; p<0·01) (Table 3). This relationship remained after adjustment for sex, age and CD4 cell count per µL (adjusted OR, 0·38; 95% CI 0·23–0·64, p<0·01). The proportion of deaths was slightly higher among subjects initiating efavirenz-compared to nevirapine-based ART (p=0.05), but there was no difference in rates of virologic failure or lost to follow-up (n=3 on efavirenz- vs. n=8 on nevirapine-based ART). Of the 68 participants receiving NNRTI-based ART who experienced virologic failure, PDR was detected at a mutant concentration ≥10% in only 13 (19·1%), all of whom were in the SOC arm (note; those with PDR mutant concentrations ≥10% in OLA-guided therapy arm are were given ritonavir-boosted lopinavir-based ART) (Table 2) (Figure 4).
Table 3:
Virologic failure (≥400 copies/µL) by nevirapine- versus efavirenz-based antiretroviral therapy and pretreatment drug resistance
| Pretreatment drug resistance: categorization and mutant frequency | Nevirapine | Efavirenz | |||
|---|---|---|---|---|---|
| N | n (%) VF | N | n (%) VF | p-value | |
| Total | 273 | 39 (14%) | 497 | 29 (6%) | <0·01 |
| Wild-type; <2% | 258 | 30 (12%) | 472 | 22 (5%) | <0·01 |
| Pretreatment drug resistance | 15 | 9 (60%) | 25 | 7 (28%) | 0·05 |
| Low frequency resistance; 2–9%* | 3 | 2 (67%) | 6 | 1 (17%) | 0·23 |
| Resistant; ≥10% | 12 | 7 (58%) | 19 | 6 (32%) | 0·14 |
Abbreviations: VF, virologic failure;
Fisher’s exact test
Figure 4:
Kaplan Meier curves of time to virologic failure (≥400 copies/µL) in the OLA-Guided Therapy (a) and SOC participants (b) by HIV drug-resistance mutation frequency, and drug resistance mutation frequencies by arm (c-e)
*The graph indicates the number of participants at risk at each time point and the number experiencing virologic failure in parenthesis.
Of the 73 participants who experienced virologic failure (68 on NNRTI- and 5 on ritonavir-boosted lopinavir-based ART), the enrollment specimen of 21 participants had 34 mutations detected by OLA. CS confirmed 21 of these enrollment mutations in 14 participants and detected 7 mutations at non-OLA codons in these individuals. CS detected an additional 7 individuals with mutations conferring drug resistance to etravirine only (E38AV; V90IV; E138A; V179T; A98AG, V179T; V179DV; and A98G), who were wild-type at OLA codons. In the virologic failure specimen of the 73 participants, OLA detected 117 mutations in 58 (79·4%) participants, including 25 of the 34 mutations detected in the 17 participants at enrollment, and CS detected 111 of the 117 mutations detected by OLA plus an additional 86 mutations at non-OLA codons in 56 (76·7%) participants.
Adverse Events
The frequency and severity of adverse events were similar between the groups (Table 4). The OLA-guided therapy arm had 39 serious non-lethal adverse events by the Division of AIDS (DAIDS) Table for Grading the Severity of Adverse Events and 34 deaths. The SOC arm had 34 severe adverse events and 43 deaths; differences that were not statistically significant. Adverse events judged by the clinicians to potentially be due to ART were few and similar between groups with 17 (16%) in the OLA-guided therapy arm and 16 (16%) in the SOC arm (p=0.90).
Table 4:
Adverse events by randomization arm
| Adverse event grades | OLA-guided therapy | Standard of Care | p-value |
|---|---|---|---|
| Grade 1 – Mild | 5 (5%) | 2 (2%) | 0.44 |
| Grade 2 - Moderate | 27 (26%) | 24 (23%) | |
| Grade 3 – Severe | 36 (34%) | 29 (28%) | |
| Grade 4 – Life Threatening | 3 (3%) | 5 (5%) | |
| Grade 5 – Death | 34 (32%) | 43 (42%) |
Abbreviations: OLA, oligonucleotide ligation assay
Discussion
In this study, the implementation of OLA-guided therapy for NNRTI and lamivudine mutations prior to the initiation of ART did not reduce the overall rate of virologic failure over 12-months of ART in this resource-limited setting. However, those with PDR who were prescribed second-line ritonavir-boosted lopinavir-based ART had less failure compared to those given NNRTI-based first-line regimen. Our finding that OLA testing diminished rates of virologic failure in those with PDR but did significantly impact rates of virologic failure between study arms in the RCT is due in part to our additional observation of significantly less virologic failure despite the presence of known NNRTI mutations in those prescribed efavirenz- compared to nevirapine-based ART.
Our study results suggest that PDR defined by the presence of an NNRTI mutation may not necessarily result in virologic failure if efavirenz is part of the first-line ART regimen, as was recently shown in South Africa.20 Participants identified as having PDR yet prescribed an efavirenz-based regimen were 60% less likely to fail compared to those prescribed nevirapine-based ART. Seventy-four percent of participants on efavirenz maintained a suppressed viral load <400 copies/µL after one year of follow-up despite being identified as having a NNRTI mutation. While efavirenz has been considered superior to nevirapine as a first-line NNRTI in general practice,21–23 this study shows it is more robust than nevirapine in the presence of known drug mutations. These findings further justify the discontinuation of nevirapine in NNRTI-based regimens and its replacement with efavirenz in fixed dose combination pills.
Our results also suggest that models predicting treatment failure based on the prevalence of PDR within a community may need to be re-examined since efavirenz has largely replaced nevirapine in most LMIC first-line regimens. While WHO has recommended that countries change their first-line regimens or institute genotypic screening if the prevalence of PDR (defined as ≥1 DR mutation) rises above 10%,5 our study findings combined with those from South Africa20 suggest that the theorized risk from PDR may be less than expected with efavirenz-based ART and that the definition of PDR that warrants public health officials to act to replace efavirenz-based ART should be modified to exclude single NNRTI mutations.
While PDR testing did not benefit our overall study population, its efficacy would likely be evident in larger populations or among those with higher PDR prevalences, such as young women and adolescent girls with PDR prevalences as high as 20% in Kenya.18 Based on our study data, 71 individuals treated with efavirenz-based ART or 30 treated with nevirapine-based ART would need to be tested to avert one virologic failure due to drug resistance using the OLA cut-off of ≥2% mutant frequency within an individual’s HIV quasispecies, and 83 individuals treated with efavirenz-based ART or 39 treated with nevirapine-based ART would need to be tested if using the cut-off of 10% mutant frequency.
PDR testing may continue to be important when dolutegravir-based ART regimens are used. Dolutegravir-based ART has been adopted by many LMICs as part of first-line therapy.24 While dolutegravir plus tenofovir and lamivudine may suppress viral replication in people without both tenofovir and lamivudine resistance, individuals with resistance to both antiretrovirals could have increased rates of virologic failure similar to dolutegravir monotherapy.25,26 While dolutegravir-based ART should lessen issues with HIV drug resistance, recent reports indicate that HIV drug resistance will not disappear.27,28
The limitations of this study include that its power to detect a difference between study arms was limited, despite the enrollment of nearly 1,000 participants. The power of this study was diminished compared to our projections due to the reduction in virologic failure associated with efavirenz-based ART, a higher rate of death and loss to follow-up (19%) than anticipated (10%) and the finding that participants with minority variants had an increased rate of virologic failure compared to individuals testing as WT by OLA; as those with minority variants in the OLA-guided therapy arm were not prescribed second-line ritonavir-boosted lopinavir-based ART. We calculate that a sample size of nearly 1,500 participants would have been needed to detect a statistically significant difference in virologic failure between OLA-guided therapy and SOC arms. Finally, outcomes were assessed during the initial 12 months of ART, and while failure associated with resistance occurs within 6 months of initiation with nevirapine-based ART, 20 additional time may be required to adequately assess risk of resistance with efavirenz-based ART.
In summary, OLA efficiently detected those with PDR and OLA-guided selection of ART reduced virologic failure among those with PDR. However, despite greater PDR rates than predicted, the use of OLA to guide therapy for all HIV-infected patients initiating therapy did not significantly reduce overall rates of virologic failure in this study because the failure rates associated with PDR were less than predicted, particularly among those prescribed efavirenz-based ART. OLA-guided therapy may be useful in targeted populations with a high prevalence of PDR, such as HIV-infected young women or among those with virologic failure switching ART regimens.
Supplementary Material
Research in context.
Evidence before this study
The observed association between pretreatment drug resistance (PDR) and subsequent virologic failure has led experts in high-resource settings to recommend testing for antiretroviral drug resistance prior to initiation of antiretroviral therapy (ART). However, this approach has never been validated in a randomized trial, and evidence is lacking to justify pre-ART drug testing to guide current first-line ART regimen selection in low- and lower-middle income countries. We searched PubMed for articles published in English from 1995 through December 2018 with the terms “pretreatment drug resistance” OR “transmitted drug resistance” AND “treatment failure” OR “virologic failure”, and reviewed data from scientific conferences. Studies reveal that the prevalence of PDR to non-nucleoside reverse transcriptase inhibitors (NNRTI) is increasing in low- and lower-middle income countries, and this PDR may increase the risk of virologic failure. These findings have led health care leaders to recommend that ART programs change their approach to first-line ART; either to employ drug resistance testing to guide selection of antiretroviral drugs, or, administer drugs without NNRTI cross-resistance for first-line ART. Oligonucleotide ligation assay (OLA) has been used successfully to detect ART resistance and, as a relatively inexpensive and simple assay, may assist clinicians in low- and lower-middle income countries to select drugs for an ART regimen to avoid virologic failure.
Added value of this study
In this randomized clinical trial of 991 HIV-infected participants we demonstrate that OLA testing is an effective method to detect PDR in a lower-middle income community. OLA-guided therapy reduced virologic failure among those with PDR at follow-up month-12. However, OLA-guided therapy did not reduce the overall rate of virologic failure (p=0·56). The lack of an effect on the population was in large part because our sample size was based on past study outcomes with nevirapine-based ART. However, during accrual to this study Kenya switched from nevirapine-to efavirenz-based ART for individuals initiating ART. Study participants prescribed efavirenz-based ART were less likely to experience virologic failure than those prescribed nevirapine-based ART, despite the presence of NNRTI mutations [Odds Ratio (OR) = 0·4; 95% CI, 0·2–0·6; p<0·001). Our study found that (1) OLA identified drug-resistance mutations associated with an increased risk of virologic failure to efavirenz- and nevirapine-based ART; (2) OLA detected HIV drug resistance similarly to sequencing (Sanger and Illumina); (3) genotypic predictions of the risk of virologic failure differ significantly between efavirenz- and nevirapine-based ART; (4) efavirenz-based ART appears to maintain efficacy despite the most prevalent K103N mutation associated with virologic failure of nevirapine-based ART; and (5) that a larger study would likely obtain population level data supporting a role for testing for PDR.
Implications of all the available evidence
We demonstrated that OLA is an effective method to detect PDR in a lower-middle income country and should be considered to detect drug resistance. PDR testing before ART initiation to avoid virologic failure may be warranted when there is a high prevalence of specific PDR mutations that limit the effectiveness of the planned ART regimen. Similarly, if supported by data, testing for HIV drug resistance when during ART plasma HIV RNA levels exceed 1000 copies per µL may be useful to guide decisions on whether to switch to a new ART regimen. Efavirenz-based ART regimens have demonstrated greater efficacy at suppressing viral loads compared to nevirapine based ART, even in the face of NNRTI mutations, and should replace nevirapine where NNRTI ART regimens are currently prescribed.
Acknowledgements:
We would like to thank the participants who joined our study, the staff who implemented the study, Coptic Hope Centers for providing the HIV care, and technical work by Jane Mwende, Steve Bii and James M. Kingoo, the Clinical and Retrovirology Research Core and the Molecular Profiling and Computational Biology Core of the UW CFAR [P30 AI027757]. In addition, we appreciate the expertise of Richard, D’Aquila, James Hughes, and Robert Shafer for participating on the study’s Data Safety and Monitoring Board and Joseph Fitzgibbon and Catherine Godfrey, NIH Program Officers, for valuable contributions to the study.
Funding Sources: National Institutes of Health awards R01 AI100037 (LMF) and R01 AI100037 Administrative Supplement (LMF). The Coptic Hope Center for Infectious Diseases is supported by the President’s Emergency Plan for AIDS Relief through a cooperative agreement [U62/CCU024512 to MHC] from the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: The authors have no conflicts of interest to declare.
Data Sharing: Data collected from the study, including individual deidentified participant data, a data dictionary defining each field in the set, study protocol, statistical analysis plan, and informed consent form will be made available to others upon request to Lisa M. Frenkel (lfrenkel@uw.edu). Data will be available with a signed data access agreement after the publication of this and three manuscripts (two in revision, one in preparation) reporting study results.
References
- 1.Hamers RL, Schuurman R, Sigaloff KC, et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis 2012; 12(4): 307–17. [DOI] [PubMed] [Google Scholar]
- 2.Kantor R, Smeaton L, Vardhanabhuti S, et al. Pretreatment HIV Drug Resistance and HIV-1 Subtype C Are Independently Associated With Virologic Failure: Results From the Multinational PEARLS (ACTG A5175) Clinical Trial. Clin Infect Dis 2015; 60(10): 1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuritzkes DR, Lalama CM, Ribaudo HJ, et al. Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis 2008; 197(6): 867–70. [DOI] [PubMed] [Google Scholar]
- 4.Boender TS, Hoenderboom BM, Sigaloff KC, et al. Pretreatment HIV drug resistance increases regimen switches in sub-Saharan Africa. Clin Infect Dis 2015; 61(11): 1749–58. [DOI] [PubMed] [Google Scholar]
- 5.Organization WH. Guidelines on the Public Health Repsonse to Pretreatment HIV Drug Resistance. Geneva, 2017. [Google Scholar]
- 6.Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med 2002; 347(6): 385–94. [DOI] [PubMed] [Google Scholar]
- 7.Li JZ, Paredes R, Ribaudo HJ, et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. JAMA 2011; 305(13): 1327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lockman S, Hughes MD, McIntyre J, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med 2010; 363(16): 1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med 2010; 363(16): 1510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halvas EK, Wiegand A, Boltz VF, et al. Low frequency nonnucleoside reverse-transcriptase inhibitor-resistant variants contribute to failure of efavirenz-containing regimens in treatment-experienced patients. J Infect Dis 2010; 201(5): 672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derache A, Iwuji CC, Baisley K, et al. Impact of Next-generation Sequencing Defined Human Immunodeficiency Virus Pretreatment Drug Resistance on Virological Outcomes in the ANRS 12249 Treatment-as-Prevention Trial. Clin Infect Dis 2019; 69(2): 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis 2008; 47(2): 266–85. [DOI] [PubMed] [Google Scholar]
- 13.Gunthard HF, Saag MS, Benson CA, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA 2016; 316(2): 191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta RK, Gregson J, Parkin N, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18(3): 346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung MH, Drake AL, Richardson BA, et al. Impact of prior HAART use on clinical outcomes in a large Kenyan HIV treatment program. Curr HIV Res 2009; 7(4): 441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck IA, Crowell C, Kittoe R, et al. Optimization of the oligonucleotide ligation assay, a rapid and inexpensive test for detection of HIV-1 drug resistance mutations, for non-North American variants. J Acquir Immune Defic Syndr 2008; 48(4): 418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner TA, Kress CM, Beck I, et al. Detection of HIV-1 drug resistance in women following administration of a single dose of nevirapine: comparison of plasma RNA to cellular DNA by consensus sequencing and by oligonucleotide ligation assay. J Clin Microbiol 2010; 48(5): 1555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverman RA, Beck IA, Kiptinness C, et al. Prevalence of Pre-antiretroviral-Treatment Drug Resistance by Gender, Age, and Other Factors in HIV-Infected Individuals Initiating Therapy in Kenya, 2013–2014. J Infect Dis 2017; 216(12): 1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milne RS, Silverman RA, Beck IA, et al. Minority and majority pre-treatment HIV-1 drug resistance associated with failure of 1st-line NNRTI ART in Kenyan women. AIDS 2019. [DOI] [PMC free article] [PubMed]
- 20.Derache A, Iwuji CC, Baisley K, et al. Impact of next generation sequencing defined HIV pre-treatment drug resistance on virological outcomes in the ANRS 12249 treatment as prevention trial. Clin Infect Dis 2018. [DOI] [PMC free article] [PubMed]
- 21.Shearer K, Brennan AT, Maskew M, et al. The relation between efavirenz versus nevirapine and virologic failure in Johannesburg, South Africa. Journal of the International AIDS Society 2014; 17: 19065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cain LE, Phillips A, Lodi S, et al. The effect of efavirenz versus nevirapine-containing regimens on immunologic, virologic and clinical outcomes in a prospective observational study. AIDS 2012; 26(13): 1691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Leth F, Phanuphak P, Ruxrungtham K, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet 2004; 363(9417): 1253–63. [DOI] [PubMed] [Google Scholar]
- 24.Organization WH. Dolutegravir (DTG) and the fixed dose combination (FDC) of tenofovir/lamivudine/dolutegravir (TLD). Geneva, 2018. [Google Scholar]
- 25.Wijting IEA, Lungu C, Rijnders BJA, et al. HIV-1 Resistance Dynamics in Patients With Virologic Failure to Dolutegravir Maintenance Monotherapy. J Infect Dis 2018; 218(5): 688–97. [DOI] [PubMed] [Google Scholar]
- 26.Blanco JL, Rojas J, Paredes R, et al. Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother 2018; 73(7): 1965–71. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen T, Fofana DB, Le MP, et al. Prevalence and clinical impact of minority resistant variants in patients failing an integrase inhibitor-based regimen by ultra-deep sequencing. J Antimicrob Chemother 2018; 73(9): 2485–92. [DOI] [PubMed] [Google Scholar]
- 28.Fulcher JA, Du Y, Zhang TH, Sun R, Landovitz RJ. Emergence of Integrase Resistance Mutations During Initial Therapy Containing Dolutegravir. Clin Infect Dis 2018; 67(5): 791–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.