Abstract
OBJECTIVE:
Several studies have established a relationship between low serum vitamin D levels and the onset of asthma in childhood. In this study, we aim to assess the relationship between vitamin D and asthma.
METHODS:
This study included 29 mild and 30 moderate persistent asthma and 38 healthy control group. Evaluation of the three groups was carried out in respect of serum vitamin D levels, Respiratory Function Test (RFT), and Exercise Provocation Test (EPT). The two asthma groups were also examined using the Asthma Control Test (ACT) and Nitric Oxide in Exhaled Breath (FeNO) level.
RESULTS:
The vitamin D levels of the mild and the moderate persistent asthma groups were determined to be lower than the vitamin D levels of the control group (p=0.007). A significant negative correlation was determined in all cases between the vitamin D levels and the broncho-reversibility percentage (p=0.0002). The negative correlation between the vitamin D levels and the broncho-reversibility percentage was more evident in the moderate persistent asthma group (p=0.0001). In the moderate persistent asthma group, a significant positive correlation was determined between the lowness of the maximum forced expiratory volume in EPT and a low vitamin D level (p=0.009). The ACT scores were lower, and the FeNO levels were higher in the moderate asthma group compared to the mild asthma group (p=0.0001).
CONCLUSION:
The findings showed that low serum vitamin D levels were observed more often in children with asthma, and there was a correlation with increased broncho-reversibility in the RFT and increased bronchial hyper-reactivity in the EPT.
Keywords: Asthma, Asthma control test, child, exercise, FeNO, vitamin D
In recent years, there has been an increase in both asthma and serum vitamin D deficiency throughout the world in general [1, 2]. This increase has encouraged research on the subject of a relationship between asthma and serum vitamin D deficiency and studies conducted by several researchers have provided valuable contributions to this issue.
In the literature, there is an increasing number of studies related to the therapeutic benefits of vitamin D in patients with asthma. It is noticeable that there is a relationship between vitamin D deficiency and poorly controlled asthma [3]. It has also been reported that there is a relationship between vitamin D deficiency and increased inflammation in asthma, asthma exacerbation and poorly controlled asthma [4]. Some studies have stated that vitamin D supplementation has reduced severe asthma attacks [5]. Other studies have reported that asthma attacks requiring systemic steroid treatment have reduced with vitamin D supplementation [6].
In investigations into the relationship between asthma and serum vitamin D deficiency, various methods have been used. One of these methods used is the respiratory function test [1, 2]. Other methods used show the relationship between vitamin D and the exercise provocation test [7, 8] and the measurement of nitric oxide in exhaled breath [7–9]. Researchers have also examined the relationship of the serum vitamin D level with asthma using the Asthma Control Test [10, 11].
This study aims to evaluate the relationship of serum vitamin D levels with mild and moderate persistent asthma in paediatric patients using measurements of broncho-reversibility in the respiratory function test, bronchial hyper-reactivity in the exercise provocation test and nitric oxide in exhaled breath. Thus, through a better understanding of the effects of serum vitamin D levels on asthma, the subject of the necessity for vitamin D supplementation in asthma treatment was discussed.
MATERIALS AND METHODS
Study Design
The study participants were selected from cases presenting at the Paediatric Immunology and Allergy Polyclinic of Gaziantep University Medical Faculty Hospital. The asthma group was formed of newly-diagnosed, treatment-naive cases. That all the asthma patients were not in an exacerbation phase, had no infectious disease, and no other chronic disease was determined from the anamnesis, and the findings of physical examination and laboratory tests. The control group was formed of healthy volunteers who presented at the hospital for various reasons and were determined with no acute or chronic disease and no allergic disease with the ISAAC questionnaire [12]. As a result of the patient selection, three groups were formed of 29 children with mild persistent asthma, 30 children with moderate persistent asthma and 38 healthy control subjects. The subjects ranged in age from 7 to 14 years old. The Ethics Committee of the Gaziantep University Medical Faculty approved this study (Code: 02-2013/76). Informed consent was obtained from each patient who was included in this study, and the study protocol was followed according to the ethical guidelines of the 2014 Declaration of Helsinki.
Laboratory Analysis and Data Collection
On the first day, all the patients with asthma were applied with the fractional exhaled nitric oxide (FeNO/NIOX, Aerocrine AB, Stockholm, Sweden) measurement and the asthma control test (ACT) questionnaire. The values of FeNO were stratified into: low (<20 ppb for children under 12 years of age and <25 ppb for the children older than 12 years), intermediate levels (20–35 ppb for children under 12 years of age and 35–50 ppb for children above 12 years of age), and high levels (>35 ppb for children under 12 years of age and >50 ppb for those older than 12 years) [13]. Then, the respiratory function test (RFT) was applied to measure broncho-reversibility and full blood count, serum IgE and serum vitamin D tests were made. The following day, the exercise provocation test (EPT) was applied to all the cases.
In this study, broncho-reversibility in the RFT, percentage change in forced expiratory volume (FEV1 change %), bronchial hyper-reactivity in EPT and the measured maximum forced expiratory volume reduction (max FEV1 reduction) were examined.
Following the basal measurement of broncho-reversibility [15 mins after inhalation of 400 mcg (4 puffs) salbutamol], the percentage change from the basal value in FEV1 was recorded (FEV1 % change in RFT).
Bronchial hyper-reactivity was applied according to the test protocol of the American Thorax Society/European Respiratory Society (ATS/ERS) [14]. After the basal measurement, the FEV1 change percentages were recorded at 5, 10, 15, 20 and 30 mins after running on a running band for 6 mins by children aged <12 years and 8 mins for those aged ≥12 years to provide 80%–90% of maximum heartbeat rate (220- age). The reductions were recorded as negative values. The highest change % was recorded as the maximum FEV1 reduction.
Due to the temporary changes caused in FeNO, spirometric procedures were applied after the FeNO measurement. Care was taken that the patients were not obese, that they fasted for one hour before the measurement, the mouth was rinsed with water, they were not exposed to cigarette smoke, and they had not recently had any upper or lower respiratory tract infection.
Statistical Analysis
One-way ANOVA and Independent Samples T-test were used to investigate a possible relationship between FeNO levels and serum vitamin D concentration and serum vitamin D concentration and serum vitamin D concentration. A Pearson Correlation Test analysis was used to investigate the possible association between RFT results and serum vitamin D concentration and EPT results and serum vitamin D concentration. P<0.05 was considered statistically significant.
RESULTS
The demographic characteristics of the study groups and the control group are summarised in Table 1. The examination results of the study groups and the control group are shown in Table 2. No statistically significant relationship was determined in the current study between the FEV1 and FVC values and serum vitamin D levels.
TABLE 1.
Demographic characteristics of the study participants
| Mild persistent asthma | Moderate persistent asthma (n=29) | Control group (n=30) | p (n=38) | |
|---|---|---|---|---|
| Age (years)* | 11.8±2.7 | 11.0±3.0 | 12.7±2.8 | 0.1311 |
| Gender (female/male) | 13/16 | 13/17 | 20/18 | 0.7102 |
| Body weight (kg)* | 46.3±3.0 | 41.8±2.9 | 45.8±1.9 | 0.3921 |
Mean±Standard deviation;
One-Way Anova Test;
Chi-square Test.
TABLE 2.
Examination results of the study participants
| Mild persistent asthma (n=29) | Moderate persistent (n=30) | Control group (n=38) | p | |
|---|---|---|---|---|
| Eosinophil perecentage (%)* | 5.8±4.1 | 6.3±3.6 | 3.1±3.2 | 0.0011 |
| Vitamin D (ng/mL)* | 17.0±4.8 | 16.4±5.6 | 20.0±4.7 | 0.0071 |
| IgE (IU/ml)* | 299.7±295.1 | 475.8±337.7 | 67.5±60.2 | 0.00011 |
| Basal FVC (%)* | 96.2±10.5 | 94.0±14.4 | 97.4±1.4 | 0.5851 |
| Basal FEV1 (%)* | 96.2±9.7 | 89.4±13.2 | 103.8±12.9 | 0.00011 |
| Basal FEF25–75 (%)* | 61.2±22.9 | 50.6±15.1 | 78.0±23.6 | 0.00011 |
| Basal FEV1/FVC* | 85.5±8.3 | 82.1±10.2 | 90.5±7.8 | 0.0011 |
| RFT FEV1 change (%)* | 7.3±4.7 | 9.6±5.1 | 1.95±2.8 | 0.00011 |
| EPT maxFEV1 reduction (%)* | -5.8±5.2 | -8.2±8.7 | -4.7±3.6 | 0.0541 |
| EPT positive number of patients | 3 (%5.1) | 9 (%15.3) | 0 | 0.0031 |
| FeNO (ppb)* | 23.6±11.0 | 47.9±14.8 | – | 0.00013 |
| FeNO level result | 0.00012 | |||
| Low | 17 | 2 | – | |
| Moderate | 8 | 8 | – | |
| High | 4 | 20 | – | |
| ACT score* | 20.3±2.8 | 14.6±4.2 | – | 0.00013 |
| ACT score result | 0.00012 | |||
| Full control | 19 | 2 | – | |
| Partial control | 10 | 18 | – | |
| Not under control | 0 | 10 | – |
Mean±Standard deviation;
One-Way Anova Test;
Chi-square Test;
Independent Samples T-Test; FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume In 1 Second; FEF: Peak Expiratory Flow; RFT: Respiratory function test; EPT: Exercise provocation test; FeNO: Fractional exhaled nitric oxide; ACT: Asthma control test.
Vitamin D Results
A statistically significant difference was determined between the control group and the mild and moderate persistent asthma groups in respect of vitamin D levels (p=0.007). The vitamin D levels of the mild persistent asthma group and the moderate persistent asthma group were lower than those of the control group (respectively p=0.013, p=0.005) (Fig. 1).
FIGURE 1.
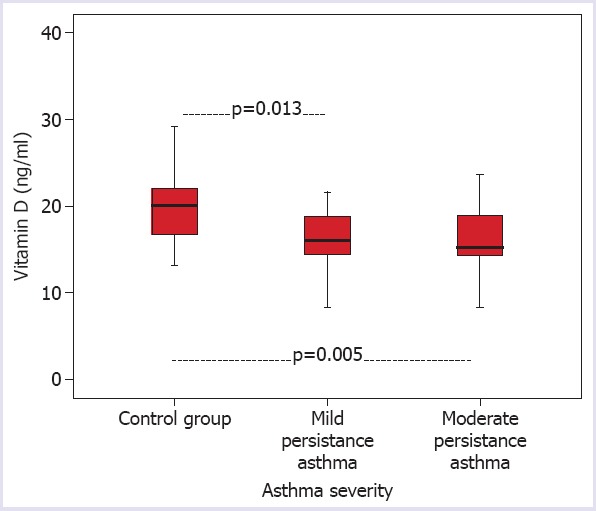
Distribution of vitamin D levels according to the severity of asthma.
RFT RESULTS
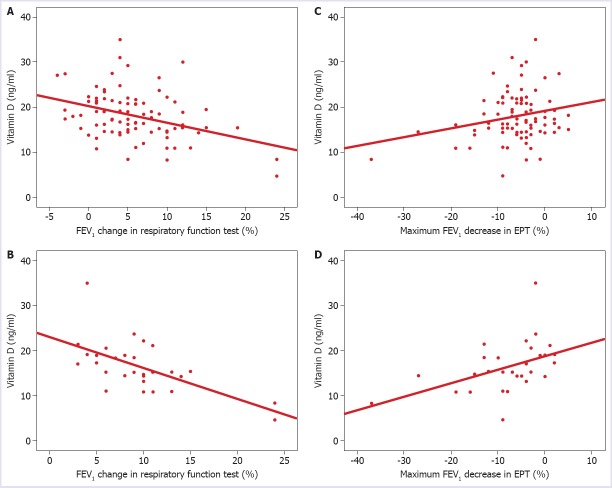
In 13 (22.1%) of the asthma patients, the FEV1 change percentage was ≥12%. Of these patients, 7 (11.9%) of them were in the mild persistent asthma group, and 6 (10.2%) of them were in the moderate persistent asthma group. In all the cases, a significant negative correlation was determined between the vitamin D levels and the FEV1 % change (broncho-reversibility) (p=0.0002, r=-0.372, n=98) (Fig. 2A). The negative correlation between the FEV1 % change and serum vitamin D levels was more evident in the moderate persistent asthma group (p=0.0001, r=-0.623, n=30) (Fig. 2B).
FIGURE 2.
The relationship between FEV1 % change in RFT and vitamin D levels in all cases (A). The relationship between FEV1 % change in RFT and vitamin D levels in patients with moderate persistent asthma (B). The relationship between maximum FEV1 reduction in EPT and serum vitamin D levels in all cases (C). The relationship between maximum FEV1 reduction in EPT and serum vitamin D levels in the moderate persistent asthma group (D).
EPT Results
The EPT was positive in 12 (20.7%) of the asthma patients, and 3 (5.1%) of them were in the mild persistent asthma group and 9 (15.3%) of them were in the moderate persistent asthma group.
A significantly positive correlation was determined between the max FEV1 reduction and serum vitamin D levels in all cases (p=0.023, r=0.231, n=98) (Fig. 2C).
The positive correlation between the maximum FEV1 reduction in the exercise test and serum vitamin D levels was more evident in the moderate persistent asthma group (p=0.009, r=0.468, n=30) (Fig. 2D).
FeNO Results
The FeNO levels of the asthma patients were determined as low in 19 (32.2%) patients, moderate in 16 (27.1%) patients and high in 24 (40.7%) patients. The FeNO levels of the moderate persistent asthma group (47.9±14.8) were determined to be higher than patients in the mild persistent asthma group (23.6±11.0) (p=0.0001) (Fig. 3A).
FIGURE 3.
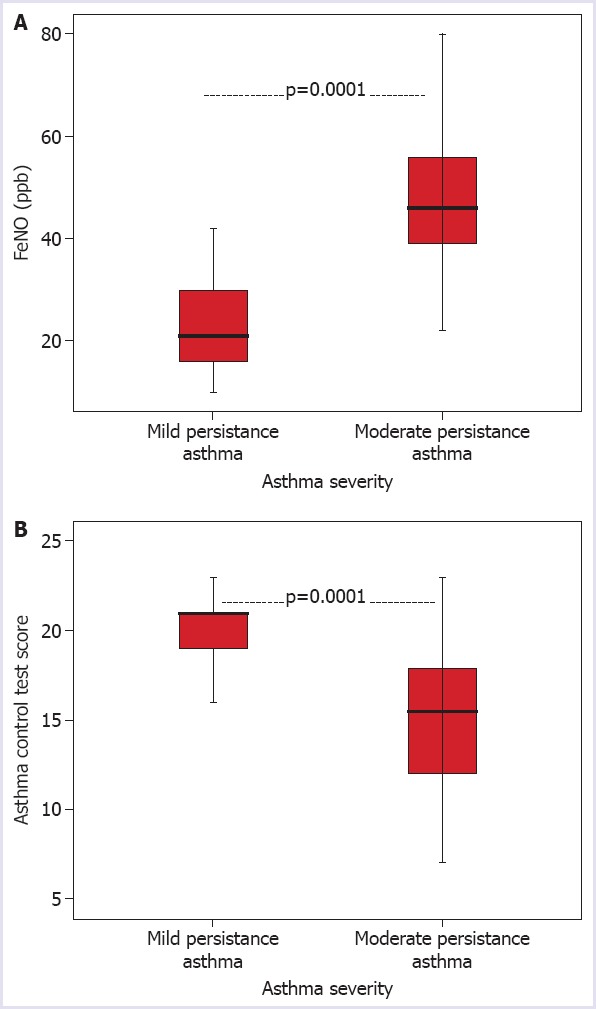
Distribution of the FeNO levels according to the severity of asthma (A). Distribution of the ACT scores according to the severity of asthma (B).
No correlation was determined between the FeNO levels and the serum vitamin D levels.
ACT Results
According to the ACT results, 19 (32.2%) patients were evaluated as good, 29 (49.2%) as moderate and 11 (18.6%) as poor. The ACT scores of the moderate persistent asthma group (14.6±4.2) were lower than those of the mild persistent asthma group (20.3±2.8) (p=0.0001) (Fig. 3B).
No correlation was determined between the ACT scores and the serum vitamin D levels.
DISCUSSION
Several studies have established an association between serum vitamin D levels and asthma incidence [4]. The results of the current study showed that low serum vitamin D levels were observed more frequently in children with asthma. Moreover, a relationship was shown between increased broncho-reversibility in pulmonary functions and increased bronchial hyper-reactivity in the exercise provocation test and serum vitamin D levels.
No statistically significant relationship was determined in the current study between the FEV1 and FVC values and serum vitamin D levels. In a previous cross-sectional study of adults, a relationship was shown between FEV1 and FVC values and serum vitamin D levels [15]. However, Tolppanen et al. did not determine a relationship between FEV1 and FVC values and serum vitamin D levels in a study, including paediatric age groups [16]. There could be two different reasons for these different results. First is that the patients of the current study were children, and the second is that a compatible correlation may not always be seen between the RFT and the symptoms of asthma.
In the current study, a significantly positive correlation was determined between the reduction in maxFEV1 values and serum vitamin D levels, and this correlation was more evident in the moderate persistent asthma group. A study conducted in asthmatic children in Costa Rica found a relationship between increased airway sensitivity in the methacholine test and serum vitamin D levels [17]. There has not yet been full clarification of the relationship between bronchial hyper-reactivity associated with exercise and low serum vitamin D levels. A previous study determined an increase in the number of mast cells in connective tissues in patients with low serum vitamin D levels [18]. In another study, vitamin D was reported to increase apoptosis and inhibit the maturation of precursor mast cells of bone marrow origin in mice, and inhibit the expression of histamine from mast cells [19]. Other studies showed that IL-13 expression was reduced by vitamin D analogues [20] and there was a relationship between bronchial hyper-reactivity associated with exercise and IL-13 polymorphism in children with asthma [21]. These studies suggest that the inflammatory mediators in cases giving a response of bronchoconstriction to exercise were of mast cell origin and/or are mediated by IL-13. In addition, many studies showed that the primary controller of natural and acquired immunity is vitamin D [22]. The clinical severity of the disease, the number of exacerbations and the systemic glucocorticoid need were related to vitamin D level [23].
The results of the current study showed that the FeNO levels of the moderate persistent asthma group were higher than those of the mild persistent asthma group and no correlation was determined between the FeNo levels and the serum vitamin D levels. Similarly, in a study by Bar et al. [7], no relationship was shown between FeNO and serum vitamin D in asthma. Dabbah et al. [8] also showed no relationship between FeNO and vitamin D in asthma, and Tenero et al. [9] reported that the administration of vitamin D to asthma patients had no effect on the FeNO levels.
In both asthma groups of the current study, no correlation was determined between the ACT scores and serum vitamin D levels. In a study by Jolliffe et al. [11], no relationship was seen between the ACT scores and serum vitamin D levels. However, Boonpiyathad et al. [10] stated that a correlation was found between vitamin D and ACT. Similarly, Havan et al. [24] also reported a relationship between serum vitamin D levels and ACT.
Due to these noticeably conflicting results in previous studies, there is a clear need for further studies to examine the relationship between vitamin D and asthma.
CONCLUSIONS
In conclusion, the results of the current study showed that low serum vitamin D levels were observed more frequently in children with asthma, and there was a relationship between increased broncho-reversibility in RFT and increased bronchial hyper-reactivity in EPT and serum vitamin D levels.
Footnotes
Ethics Committee Approval: Ethics Committee of the Gaziantep University Medical Faculty approved this study (Code: 02-2013/76).
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – M.Y.O., O.K., M.A.; Design – M.Y.O., O.K., M.A.; Supervision – O.K., E.K., M.K.; Materials – M.Y.O., O.K., M.A.; Data collection and/or processing – M.Y.O., O.K., M.A., O.B.; Analysis and/or interpretation – M.Y.O., O.K., M.A.; Writing – M.Y.O., O.K., M.A.; Critical review – M.K., E.K.
REFERENCES
- 1.Solidoro P, Bellocchia M, Aredano I, Mattei A, Pivetta E, Patrucco F, et al. Asthmatic Patients with Vitamin D Deficiency have Decreased Exacerbations afterVitamin Replacement. Nutrients. 2017;9:ii, 1234. doi: 10.3390/nu9111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babar MZM, Hussain M, Majeed SA. Vitamin D supplementation improves FEV1 in patients of Bronchial Asthma. Pak J Med Sci. 2017;33:1144–7. doi: 10.12669/pjms.335.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeffer PE, Hawrylowicz CM. Vitamin D in Asthma:Mechanisms of Action and Considerations for Clinical Trials. Chest. 2018;153:1229–39. doi: 10.1016/j.chest.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Hall SC, Agrawal DK. Vitamin D and Bronchial Asthma:An Overview of Data From the Past 5 Years. Clin Ther. 2017;3:917–29. doi: 10.1016/j.clinthera.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511. doi: 10.1002/14651858.CD011511.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA, Jr, Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations:a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881–90. doi: 10.1016/S2213-2600(17)30306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar Yoseph R, Livnat G, Schnapp Z, Hakim F, Dabbah H, Goldbart A, et al. The effect of vitamin D on airway reactivity and inflammation in asthmatic children:A double-blind placebo-controlled trial. Pediatr Pulmonol. 2015;50:747–53. doi: 10.1002/ppul.23076. [DOI] [PubMed] [Google Scholar]
- 8.Dabbah H, Bar Yoseph R, Livnat G, Hakim F, Bentur L. Bronchial Reactivity, Inflammatory and Allergic Parameters, and Vitamin D Levels in Children With Asthma. Respir Care. 2015;60:1157–63. doi: 10.4187/respcare.03763. [DOI] [PubMed] [Google Scholar]
- 9.Tenero L, Piazza M, Zanoni L, Bodini A, Peroni D, Piacentini GL. Antioxidant supplementation and exhaled nitric oxide in children with asthma. Allergy Asthma Proc. 2016;37:8, 13. doi: 10.2500/aap.2016.37.3920. [DOI] [PubMed] [Google Scholar]
- 10.Boonpiyathad T, Chantveerawong T, Pradubpongsa P, Sangasapaviliya A. Serum Vitamin D Levels and Vitamin D Supplement in Adult Patients with Asthma Exacerbation. J Allergy (Cairo) 2016. 2016 doi: 10.1155/2016/4070635. 4070635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolliffe DA, Kilpin K, MacLaughlin BD, Greiller CL, Hooper RL, Barnes NC, et al. Prevalence, determinants and clinical correlates of vitamin D deficiency in adults with inhaled corticosteroid-treated asthma in London, UK. J Steroid Biochem Mol Biol. 2018;175:88–96. doi: 10.1016/j.jsbmb.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Asher M. World Wide variations in the prevalance asthma symptoms:The International Study of Asthma and Allergies in Childhood (ISAAC) Eur Respir J. 1998;12:315–35. doi: 10.1183/09031936.98.12020315. [DOI] [PubMed] [Google Scholar]
- 13.Salviano LDDS, Taglia-Ferre KD, Lisboa S, Costa ACCD, Campos HDS, March MFP. Association between fraction of exhaled nitric oxide and spirometry data and clinical control of asthma in children and adolescents. Rev Paul Pediatr. 2018;36:8. doi: 10.1590/1984-0462/;2018;36;1;00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999 This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 15.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 16.Tolppanen AM, Sayers A, Granell R, Fraser WD, Henderson J, Lawlor DA. Prospective association of 25-hydroxyvitamin d3 and d2 with childhood lung function, asthma, wheezing, and flexural dermatitis. Epidemiology. 2013;24:310–9. doi: 10.1097/EDE.obo13e318280dd5e. [DOI] [PubMed] [Google Scholar]
- 17.Brehm JM, Celedo´n JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baroni E, Biffi M, Benigni F, Monno A, Carlucci D, Carmeliet G, et al. VDR-dependent regulation of mast cell maturation mediated by 1,25-dihydroxyvitamin D3. J Leukoc Biol. 2007;81:250–62. doi: 10.1189/jlb.0506322. [DOI] [PubMed] [Google Scholar]
- 19.Toyota N, Sakai H, Takahashi H, Hashimoto Y, Lizuka H. Inhibitory effect of 1a,25- dihydroxyvitamin D3 on mast cell proliferation and A23187- induced histamine release, also accompanied by a decreased c-kit receptor. Arch Dermatol Res. 1996;288:709–15. doi: 10.1007/BF02505282. [DOI] [PubMed] [Google Scholar]
- 20.Benigni F, Baroni E, Zecevic M, Zvara P, Streng T, Hedlund P, et al. Oral treatment with a vitamin D3 analogue (BXL628) has anti-inflammatory effects in rodent model of interstitial cystitis. BJU Int. 2006;97:617–24. doi: 10.1111/j.1464-410X.2006.05971.x. [DOI] [PubMed] [Google Scholar]
- 21.Kang MJ, Lee SY, Kim HB, Yu J, Kim BJ, Choi WA, et al. Association of IL-13 polymorphisms with leukotriene receptor antagonist drug responsiveness in Korean children with exercise-induced bronchoconstriction. Pharmacogenet Genomics. 2008;18:551–58. doi: 10.1097/FPC.0b013e3282fe94c5. [DOI] [PubMed] [Google Scholar]
- 22.Adams JS, Hewison M. Unexpected actions of vitamin D:new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dogru M, Kırmızıbekmez H, Yesiltepe-Mutlu RG, Aktas A, Ozturkmen S. Clinical Effects of Vitamin D in Children with Asthma. Int Arch Allergy Immunol. 2014;164:319–25. doi: 10.1159/000366279. [DOI] [PubMed] [Google Scholar]
- 24.Havan M, Razi CH, Bulus AD, Köksal AO, Andıran N. Effects of 25 hydroxy vitamin D levels on the severity and asthma control in school age asthma patients. Arch Argent Pediatr. 2017;115:336–42. doi: 10.5546/aap.2017.eng.336. [DOI] [PubMed] [Google Scholar]