Abstract
Morphogenesis of the brain and face is intrinsically linked by a number of factors. These include: origins of tissues, adjacency allowing their physical interactions, and molecular crosstalk controlling growth. Neural crest cells that form the facial primordia originate on the dorsal neural tube. In the caudal pharyngeal arches, a Homeobox code regulates arch identity. In anterior regions, positional information is acquired locally. Second, the brain is a structural platform that influences positioning of the facial primordia, and brain growth influences the timing of primordia fusion. Third, the brain helps induce a signaling center, the Frontonasal Ectodermal Zone (FEZ), in the ectoderm, which participates in patterned growth of the upper jaw. Similarly, signals from neural crest cells regulate expression of Fibroblast growth factor 8 in the anterior neural ridge, which controls growth of the anterior forebrain. Disruptions to these interactions have significant consequences for normal development of the craniofacial complex, leading to structural malformations and birth defects.
Keywords: Brain-face interactions, Facial morphogenesis, Signaling, Physical forces, cleft lip and palate, Holoprosencephaly
1.0. Introduction
Clinicians have long recognized that facial malformations are often associated with defects in the underlying brain. In 1964, DeMeyer coined the phrase “the face predicts the brain” after studying fetuses with Holoprosencephaly (HPE; ((DeMyer 1964))), a common and severe malformation that affects both the brain and the face. Notably, DeMeyer observed that HPE has a phenotypic spectrum ranging from cyclopia to mid-facial hypoplasia, a missing central incisor, or even normal phenotype, and these malformations are accompanied by underlying brain anomalies that likewise range from failure of the forebrain to separate into right and left lobes to normal septation (Ming and Muenke 1998, Nanni, Schelper et al. 2000, Heussler, Suri et al. 2002). These observations highlight a critical feature of brain and facial morphogenesis: the developmental processes that control their formation are highly integrated, meaning that changes in one often have downstream effects on the other. These effects can be divided into three general categories. First, the brain and face have a common origin. Notably, the neural crest cells that give rise to the facial skeleton originate from the dorsal neural tube, and information about their spatial patterning is based on their anterior-posterior level of origin. Second, the brain serves as a structural platform, and as such imparts physical forces on adjacent tissues that help shape the location and directional growth of facial primordia. Third, the brain and facial tissues “talk to each other” via molecular interactions that control the cellular mechanisms responsible for facial morphogenesis. These three types of interactions suggest that the brain exerts multiple effects on facial morphogenesis, and these are both intrinsic to brain development itself and the paracrine interactions between the brain and adjacent tissues. Thus, the development of the face is highly contingent on normal brain development, and consequently, events and disruptions to the processes that regulate the brain often produce facial malformations. These interactions, which are both physical and molecular, form the basis of this chapter. We describe each of these in more detail below.
2.1. Origins of the Brain and Face
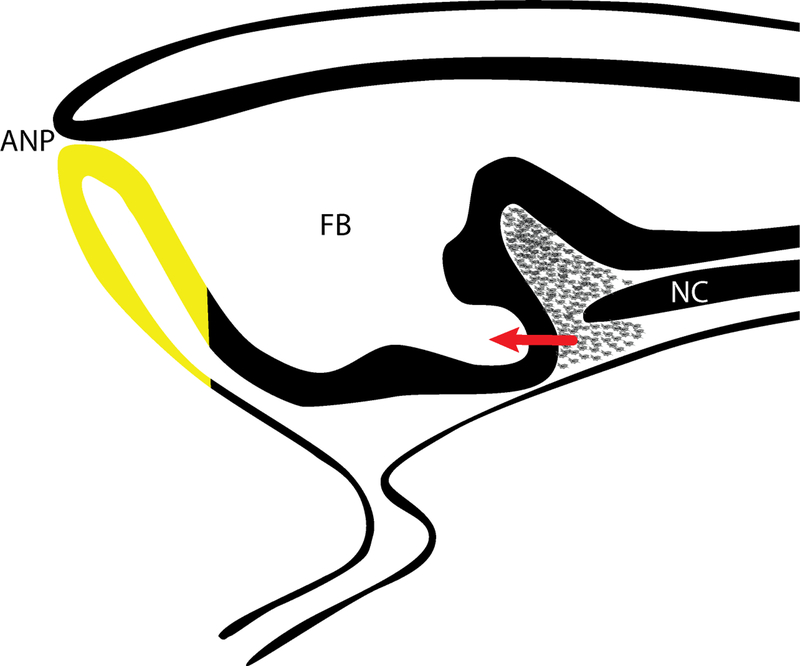
The brain and face begin to take shape during the earliest stages of development. After gastrulation, the anterior neural plate undergoes neurulation, morphogenetic movements that turn the sheet of neuro-epithelial cells into a tube and physically separate it from the surface ectoderm. In the anterior-most region of the neural tube, the anterior-neural pore forms (Fig. 1). At this region the neural and surface ectoderm are continuous, and this tissue expresses Fibroblast Growth Factor 8 (Fgf8). After the anterior neural pore closes, the neural and surface ectoderm are segregated, but Fgf8 expression is maintained in both ectodermal compartments ((Ohkubo, Chiang et al. 2002) and see Fig. 1). In this way a domain of gene expression that was initially continuous through the neural and surface ectoderm is segregated into two discrete expression domains by closure of the anterior neural pore. The Fgf8-expressing cells in the forebrain will form the anterior neural ridge, and the Fgf8-expressing cells in the surface ectoderm will form the dorsal aspect of the Frontonasal Ectodermal Zone (FEZ), a signaling center that regulates patterned growth of the upper jaw anlagen (Hu, Marcucio et al. 2003).
Figure 1. Shared Expression Domains between the Brain and Face.
Closure of the anterior neural pore segregates anterior neural and surface ectoderm. At this time the Fgf8 domain (yellow) that spans the anterior neural plate and the anterior surface ectoderm becomes two distinct domains. (ANP = anterior neuropore, FB = Forebrain, red arrow = signaling from mesendoderm to forebrain)
Similarly, the neural crest cells, which are the connective tissue progenitor cells that form the facial skeleton, originate at the border of the neural and surface ectoderm, and their location of origin along the anterior-posterior axis of the brain plays a large role in the fate of the neural crest cells. Homeobox genes (HOX) along the dorsal axis of the neural tube exhibit unique anterior-posterior patterns of expression (Fienberg, Utset et al. 1987, Holland and Hogan 1988, McGinnis and Krumlauf 1992). The pattern of HOX gene expression, or HOX “code”, is imparted on the neural crest cells while they are part of the dorsal neuroepithelium and this code is carried into the developing facial primordia, with significant implications for facial development (Rijli, Mark et al. 1993, Minoux and Rijli 2010). The anterior-most region of the neural tube, including the prosencephalon and mesenchepalon, does not express members of classic Hox gene family. Consequently, the neural crest cells derived from these regions do not have a HOX code, so when they arrive in the FNP, Maxillary Process and Mandibular Process, these cell populations are interchangeable with each other (Noden 1983, Noden 1984, Noden 1986). In contrast, the hindbrain expresses HOX genes and neural crest cells contain a HOX code, which they carry with them to the facial primordia (Minoux and Rijli 2010). When neural crest progenitors derived from HOX-free regions of the neural tube are transplanted into posterior regions, where there normally is a HOX code, development is disrupted. In a classic series of experiments, Noden transplanted crest destined for the first pharyngeal arch in place of neural crest cells destined for the second arch, and the resulting skeletal structure resembled the first arch (Noden 1983). Similarly, ablation of HOXA2 in mice transformed the second arch skeleton into a first arch skeleton (Rijli, Mark et al. 1993), and work in avian embryos demonstrated that this patterning mechanism is conserved in birds (Grammatopoulos, Bell et al. 2000). Thus, in parts of the head skeleton, the brain imparts patterning information on the connective tissue progenitors as a molecular program within the neural crest cells, but this is significantly influenced by environmental signals that neural crest cells encounter (Trainor and Krumlauf 2000, Trainor, Ariza-McNaughton et al. 2002).
However, not all neural crest cells in the developing head contain an intrinsic Hox code. In the developing lower and upper jaw anlagen, the neural crest cells do not express Hox genes, and these cells receive patterning information from their environment. This has been well described in the maxillary and mandibular processes, where a nested pattern of Dlx gene expression within the pharyngeal arch mesenchyme confers arch identity to the developing facial primordia (Depew, Simpson et al. 2005). Results of this work led to the proposal of the “hinge and caps” model of jaw development in which signaling centers located at the proximal (hinge) and distal (caps) regions of the upper and lower jaw act to control development of these two structures in an integrated fashion (Depew and Simpson 2006, Depew and Compagnucci 2008).
After the neural crest cells have completed their migration into the distinct facial primordia, the facial morphogenesis begins through a process of growth and patterning. The Frontonasal Process (FNP) and paired Maxillary Processes (MxP) grow out and fuse to form the upper jaw and midface, the paired Mandibular Processes (MnP) form the lower jaw, and the more posterior pharyngeal arches give rise to the more posterior skeletal structures. Neural crest cells are a transient mesenchymal population of progenitor cells that are generated in the dorsal neural tube at the junction between the neuroepithelium and the surface ectoderm (Bronner and LeDouarin 2012), and migrate laterally and ventrally to occupy the rostral region of the developing head to form these primordia de novo (Chambers and McGonnell 2002, Noden and Trainor 2005, Minoux and Rijli 2010). In the upper jaw the neural crest mesenchyme is encased by epithelia derived from neural and surface ectoderm, while in the lower jaw pharyngeal endoderm and surface ectoderm encase the mandibular processes. The interactions among the distinct tissue types that comprise each of these primordia direct the growth and patterning of the distinct regions of the developing face (Reviewed in: (Richman and Lee 2003, Graham, Okabe et al. 2005, Chai and Maxson 2006, Szabo-Rogers, Smithers et al. 2009)). In the lower jaw signals among the pharyngeal endoderm, surface ectoderm, and neural crest mesenchyme control patterning and growth (Graham and Smith 2001, Graham, Okabe et al. 2005, Brito, Teillet et al. 2006, Swartz, Nguyen et al. 2012), while in the upper jaw physical and molecular interactions among the forebrain, surface cephalic ectoderm, and neural crest mesenchyme control patterned growth (Marcucio, Young et al. 2011).
2.2. Physical Interactions between the Brain and the Face
The brain is the foundation upon which the face is built. In addition to sharing embryonic origins of tissues, the brain and face share a common space in the developing embryo. As the brain undergoes its own morphogenesis, the developing face is shaped in response. Both the pattern and the rate of growth of the brain provide unique influences on facial morphogenesis.
During early stages of development the physical influences of the brain on the face are easily appreciated. The brain is essentially a large tube surrounded by mesenchymal cells of mesodermal and neural crest origin. As described above, during development of the upper jaw and mid-face, neural crest cells delaminate from the posterior forebrain and anterior midbrain and migrate along the surface of the brain between it and the surface ectoderm. This population of cells is initially small compared to the expanding neural tube, but once the neural crest cells arrive at their final destination, the population expands rapidly to form the facial primordia. At the same time, the brain continues to grow, changing both its size and shape. A series of studies by Diewert and colleagues showed that this process directly impacts the positioning of the facial primordia relative to the eyes and the brain (Diewert and Lozanoff 1993, Diewert, Lozanoff et al. 1993).
The rate of brain growth also influences these early stages of facial development. As the brain expands, it displaces the adjacent facial tissues, which must themselves compensate with their own growth to maintain their relative position to each other (Fig. 2). Variation in the coordination of the growth rates of the brain and face can have significant effects. For example, the brain in different strains of mice can exhibit different growth rates, and the growth rate of the facial tissues appears to be largely independent of the rate of brain growth. This independent growth likely results from distinct signaling centers located in the brain and face that contribute to regulating growth of each tissue. As the rate of brain growth slows among strains of mice, the face appears older at any given time point, such that slower brain growth produces a longer, more mature looking face (Boughner, Wat et al. 2008). This occurs because when the brain is smaller relative to the facial prominences, then they will be more prognathic and more closely opposed. This effect results from the spatial constraints imposed by the brain on the developing face and it illustrates that the brain acts as a foundation to shape the face. Thus, a small brain may lead to a relatively larger and more prognathic face. Conversely, a larger brain will lead to a flattened face. We have conjectured based on this relationship that a larger brain relative to the facial prominences may narrow the timing window of opportunity for facial prominence fusion and thus increase the likelihood of clefting (Boughner, Wat et al. 2008, Marcucio, Young et al. 2011). This builds on similar arguments that were made earlier by Diewert and collaborators (Wang K.Y. 1992, Diewert and Lozanoff 1993, Wang, Juriloff et al. 1995)
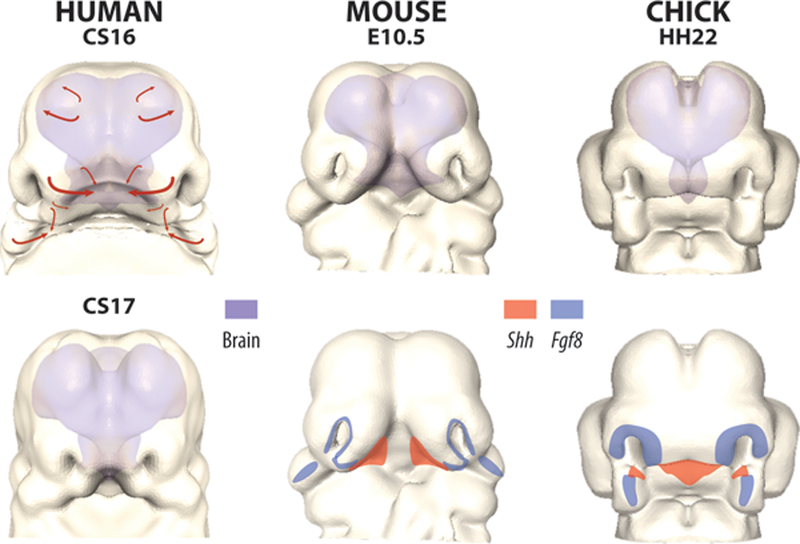
Figure 2. Growth of the brain and face.
Relationship of the brain (shaded) to the face during facial morphogenesis, shown at comparable stages in human (CS16) (A), mouse (e10.5) (B) and chicken (hh22) (C). Facial prominences grow towards each other (arrows, A), converging at the midline (D), while the brain becomes relatively wider and divides into two distinct lobes.
Recent work has shown that the brain controls some of the temporal aspects of facial development. The effect of the brain on the timing of facial development can be observed by unilaterally transplanting the brain from the faster developing duck into the slower developing chick embryo. In this case, neural crest migration into the anterior regions of the face appears to be slowed, fusion of the primary palate is delayed, and development of the scleral papillae on the eye is delayed on the transplanted side compared to the control side (Hu, Young et al. 2015). At this point, these data are phenomenological, and the molecular mechanism(s) underlying these delays are not known. Presumably, the temporal pattern of gene expression within the brain transmits some type of timing information to the adjacent tissues, but the nature of these signals, if they even exist, remains completely hypothetical.
These early influences of the brain on the face may be partially erased during later stages of development (Hallgrímsson 2009). Some processes may exert different influences at different times and may have greater or lesser overall roles in development of these tissues. For example, while the brain has a tremendous influence during early stages of normal facial development, the contribution of the brain to the final form may not be as important as other processes intrinsic to the face. As the cartilages and bones form within the facial skeleton, the morphogenetic processes that shape the skeletal elements have a profound influence on facial shape and are largely responsible for sculpting the final form of the face (Diewert 1983, Diewert 1985, Aldridge, Kane et al. 2005, Richtsmeier, Aldridge et al. 2006, Richtsmeier and Deleon 2009). Nonetheless, it is easy to see how facial dysmorphology may arise from aberrant physical interactions between the brain and the face, for example due to a growth rate that is too rapid, leading to a larger platform for the face to grow on and leaving the face relatively small. This configuration may lead to structural defects for which later developmental processes cannot fully compensate (discussed further below).
2.3. Molecular interactions between the brain and face
In addition to the direct physical forces that the brain imparts on the developing face, there are also molecular interactions between the brain and the face that regulate morphogenesis of the upper jaw (Marcucio, Young et al. 2011). These interactions begin during the earliest stages of embryonic development and continue throughout the period of patterned growth of the facial primordia that comprise the mid-face and upper jaw.
The prechordal plate (mesendoderm) is located beneath the prospective forebrain epithelium, and signals from the mesendoderm establish the basal plate of the forebrain (Fig. 1; (Muller, Albert et al. 2000, Aoto, Shikata et al. 2009)). This signaling interaction is essential for proper development of the mid-face. SHH signaling from the mesendoderm to the neuroepithelium induces expression of Nk2.1 and Shh in the basal forebrain (Aoto, Shikata et al. 2009). This domain eventually becomes the basal diencephalon. Then a signaling relay mechanism induces Nkx2.1 and Shh expression in the more anteriorly located basal telencephalon (Marcucio, Cordero et al. 2005). Disrupting this signal relay by blocking SHH signaling in the brain disrupts the induction of Shh in the telencephalon and produces severe malformations of the face (Marcucio, Cordero et al. 2005). These early interactions are essential for establishing the basis for the molecular interactions that will occur during later periods of development.
Within the developing midface, a signaling center known as the Frontonasal Ectodermal Zone (FEZ) regulates growth and patterning of the upper jaw (Hu, Marcucio et al. 2003). The FEZ, first identified in the chicken and later in mammals (Hu and Marcucio 2009, Young, Hu et al. 2014), forms as a boundary in the surface cephalic ectoderm between cells expressing Fibroblast Growth Factor 8 (Fgf8) and Shh (Fig.2). The boundary between these two gene expression domains marks the distal tip of the upper jaw, and the ectoderm flanking the boundary corresponds to the dorsal and ventral aspect of the upper jaw. Subsequent research has shown that a series of signaling molecules including Wnts and bone morphogenetic protein (BMP) family members are also expressed in the FEZ (Barlow and Francis-West 1997, Francis-West, Ladher et al. 1998, Ashique, Fu et al. 2002, Lan 2006, Geetha-Loganathan, Nimmagadda et al. 2009, Reid, Yang et al. 2011), and these signaling pathways are very likely to mediate aspects of FEZ function. The FEZ signals to the adjacent mesenchymal tissues to regulate proliferation to control directed growth of the upper jaw anlage. Shh is a very important component of FEZ function. Conditionally removing Smoothened from the neural crest cells leads to severe defects of the upper jaw (Jeong, Mao et al. 2004), illustrating the importance of SHH signaling for development of this part of the face.
Formation and patterning of the FEZ appears to require signals from the brain. Research has shown that SHH signaling within the brain is responsible for inducing the pattern of Shh expression observed in the ventral forebrain. These experiments showed that blocking SHH signaling in the brain prevented Shh expression in the FEZ (Marcucio, Cordero et al. 2005). The absence of Shh expression in the FEZ led to a truncation of the upper jaw due to a failure of outgrowth. This appears to be a direct effect of SHH on the facial ectoderm itself as removing Smoothened from the neural crest cells does not appear to affect Shh expression in the FEZ (Chong, Young et al. 2011).
While SHH signaling to the cephalic ectoderm is required for Shh expression in the FEZ, the actual role that SHH signaling plays is not entirely clear. Neural crest cells are also required for induction of Shh in the FEZ. In experiments that led to large amounts of apoptosis of neural crest cells, Shh expression in the FEZ was never initiated, but Shh expression could be restored by transplantation of neural crest cells into the FNP (Hu 2012). Furthermore, blocking signaling by BMPs within the neural crest cells also prevented Shh expression in the FEZ (Foppiano, Hu et al. 2007), and this could not be restored by addition of SHH back to the tissue (Chong, Young et al. 2011). Together, these data suggest that a two-step process may function whereby SHH induces a “competence” in the presumptive FEZ ectoderm and the neural crest cells induce the expression of Shh in a BMP-dependent manner. However, the exact molecular mechanisms that underlie this induction process are unknown.
2.4. Signaling from the Face to the Brain
Up to this point, we have described signals as emanating from the brain to control the face. However, evidence has emerged that signaling is not unidirectional, but rather that the neural crest cells also play a role in shaping the brain (Etchevers, Couly et al. 1999, Tucker, Segall et al. 2008). Just as the brain regulates facial development, it is not surprising that there is reciprocal signaling from the facial tissues that contribute to brain development, since the brain and facial skeleton co-evolved (Gans and Northcutt 1983). Nonetheless, the nature of these molecular interactions is just being discovered (Le Douarin, Couly et al. 2012).
As mentioned above, the neural crest cells that form the skeleton of the upper jaw do not express Hox genes, and misexpression of Hox genes causes severe craniofacial malformations and brain defects (Creuzet, Couly et al. 2002). This work has shed light on the molecular mechanisms by which the face contributes to brain development by uncovering a signaling network involving Six-family transcription factors, Bmp signaling and Fgf8 expression in the anterior neural ridge. It appears that in the presence of HoxA2, the transcription factors Six1, Six2, and Six4, which are normally expressed in the neural crest mesenchyme in this part of the face, are down-regulated. This down-regulation is accompanied by a decrease in expression of Bmp antagonists in the neural crest mesenchyme and a decrease in Fgf8 expression in the anterior neural ridge. These results, along with other work by these investigators, have led to the idea that expression of Bmp antagonists, such as Gremlin and Noggin, are regulated by the Six family of transcription factors, which in turn contribute to regulation of Fgf8 expression levels in the anterior neural ridge (Creuzet, Martinez et al. 2006, Aguiar, Sghari et al. 2014). This work is likely to lead to further discoveries that show the importance of signaling by neural crest cells to the developing brain, such as the importance of Wnt antagonism for expression of Foxg1 in the developing telencephalon (Garcez, Le Douarin et al. 2014).
3.0. Implications for Structural Diseases of the Face
Altering the interactions between the brain and the face can produce variable morphologies that natural selection may have acted on to produce the diversity of facial form that we see among vertebrates. Hence, it is very likely that altering these interactions may also produce disease phenotypes in the craniofacial complex. Indeed, there are many examples that illustrate this point.
3.1. Holopresencephaly
One of the most commonly studied diseases in this regard is holoprosencephaly (HPE). HPE is a structural disease of the brain that affects facial development. In HPE the telencephalon undergoes varying degrees of septation, and in its most severe presentation, patients with HPE have cyclopia, a single median eye located beneath the proboscis. However, phenotypes range from this most severe form through midfacial hypoplasia, clefting, and hypotelorism with a single central upper incisor, and some individuals with causative mutations may appear unaffected (Ming and Muenke 1998, Nanni, Schelper et al. 2000, Heussler, Suri et al. 2002).
The molecular etiology of HPE has been largely attributed to alterations in signaling by the Hedgehog pathway during brain development (Belloni, Muenke et al. 1996, Roessler, Belloni et al. 1996, Roessler, Belloni et al. 1997, Brown, Warburton et al. 1998, Ming and Muenke 1998, Roessler and Muenke 1998, Roessler and Muenke 1999, Wallis and Muenke 1999, Wallis, Roessler et al. 1999, Gripp, Wotton et al. 2000, Muenke and Beachy 2000, Nanni, Ming et al. 2001, Ming, Kaupas et al. 2002, Schell-Apacik, Rivero et al. 2003, Jeong, El-Jaick et al. 2006, Jeong, Leskow et al. 2008, Roessler, El-Jaick et al. 2009, Roessler, Lacbawan et al. 2009, Roessler, Ma et al. 2009, Solomon, Lacbawan et al. 2009, Roessler and Muenke 2010). In utero exposure to alcohol or other teratogens also causes malformations in the face, and these resemble HPE (Sulik, Cook et al. 1988, Sulik 2005, Welch, Panter et al. 2009, Hong and Krauss 2012). However, the cause of the variable phenotypic presentation is not known. Mice homozygous for a null mutation in Shh have a very severe phenotype that includes cyclopia or anencephaly of the anterior neural tube (Chiang, Litingtung et al. 1996), but these animals do not display the less severe forms observed in patients. How this spectrum of outcomes is observed in patients has been under intense investigation for a long time, and the work in mice suggests that thresholds of SHH signaling may be an important factor in phenotypic outcome. In the SHH heterozygotes, the level of SHH signaling is above a threshold that causes disease, but why does haploinsufficiency in people cause the range of phenotypes that are observed? One suggested possibility is that individuals with more severe forms of HPE have a second genetic alteration that modifies the primary mutation to produce a more severe phenotype (Ming and Muenke 2002). This second-hit model has received wide attention and has been observed in a set of HPE patients (Mercier, Dubourg et al. 2011). Further, experimental evidence supports this idea in mice. Mutations in the Shh co-receptor Cdo (Tenzen, Allen et al. 2006) lead to HPE in some strains of mice, but not in others. However, mouse strains that are normally resistant to HPE have a phenotype if they also have a mutation in the related protein Boc (Tenzen, Allen et al. 2006, Zhang, Hong et al. 2011). Further, mutations can sensitize animals to teratogenic insults. Homozygous null mutations in the putative SHH co-receptor Cdon does not produce HPE in 129S6 mice, but it does sensitize mice to other perturbations that by themselves would not produce disease. For example, in elegant work, when sub-teratogenic doses of alcohol were administered to these mice, a range of phenotypes was produced that reflected the spectrum of HPE phenotypes seen in patients, suggesting that a sensitizing mutation synergize with, in this case, a sub-teratogenic environmental insult to produce HPE (Hong and Krauss 2012), and this can be rescued by reducing levels of the negative regulator of Shh signaling Ptch1 (Hong and Krauss 2013). The extent to which the two-hit method of modifying phenotypic severity occurs in a large number of HPE patients is unknown, but this is a very intriguing possibility.
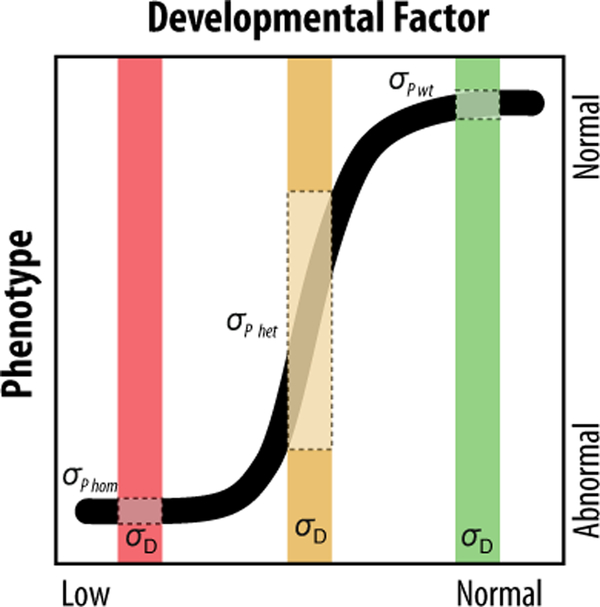
An alternative model may also help explain the variation in phenotype observed in patients; variation can be produced as a result of the function of signaling pathways in general. Modeling experiments have shown that in individual cells, the Shh pathway appears to be a binary switch (Lai, Robertson et al. 2004). A cell has either responded to Shh or it has not, and the probability that a cell has responded is related to the concentration of Shh ligand. At low concentrations cells are unlikely to respond, while at high concentrations cells are very likely to respond. However, at intermediate concentrations of Shh ligand, large amounts of variation in responsiveness can be observed. This leads to non-linear response of cells to various ligand concentrations (Fig. 3). Using a dose-response experiment, avian embryos were subjected to varying degrees of Shh signal blockade in the developing brain. A similar non-linear relationship was observed between the level of blockade and facial shape (Young, Chong et al. 2010). This indicates that for small changes in ligand concentration, large changes in facial shape can result. Hence, non-linear relationships arising as a result of the function of signaling pathways may also contribute to phenotypic variation in patients.
Figure 3. Nonlinearity in the relationship between development and phenotypic outcomes.
In a nonlinear relationship, in this case represented as an S-shaped curve, there is a continuous relationship between a genetic or developmental factor and the phenotypic outcome, however the variance in phenotypic outcomes differs for the same developmental variance. In this example, at normal levels of a developmental factor, phenotypic outcomes tend to be similar. Likewise, when the developmental factor is completely removed, phenotypic outcomes are also similar, such as the case in a highly penetrant genetic knockout. In contrast, when the developmental factor is reduced, such as in a heterozygote animal, the variance in phenotypic outcomes is much greater for the same developmental variance.
3.2. Cleft lip/palate
In addition to HPE, which produces a combined brain and face malformation, other structural malformations of the face may result from disruptions to the interactions of the brain and face during development. One particularly important area of exploration is related to non-syndromic cleft lip and palate. These forms of clefting occur in isolation from other malformations and often have no known underlying genetic cause. However, it seems apparent that alterations in the rate of brain growth could potentially lead to clefting malformations due to the influence that the brain has on positioning of the facial primordia during development in some individuals (Diewert and Lozanoff 1993, Diewert, Lozanoff et al. 1993, Jiang, Bush et al. 2006).
The developing facial primordia must exhibit a concerted pattern of growth that brings them in apposition with one another in order to fuse and form the primary and secondary palates. Failure to meet at the appropriate times in development leads to fusion failure and orofacial clefting. If the growth rate of the brain is more rapid, the facial primordia may be positioned farther apart. Hence, the primordia may have reduced likelihood of achieving apposition at the times required for proper fusion.
A significant relationship between brain shape and incidence of orofacial clefting has been observed in some studies, suggesting that alterations in the brain may contribute to the incidence and/or severity of non-syndromic clefting in the face. Non-pathological changes in the volume of various brain regions have been observed in some patients with non-syndromic cleft lip and palate (Nopoulos, Berg et al. 2002). These Individuals exhibited an enlarged cerebrum and changes in the shape of the corpus callosum, among other changes, suggesting that a change in the brain may play a role in clefting (Weinberg, Andreasen et al. 2009). Alterations to midline development of the brain may be a common event associated with facial malformations. However, the causal relationships between these brain alterations and cleft lip and palate remain unexplored, and the extent to which these changes are observed in larger cohorts of affected individuals is not known.
3.3. Shape correlations with brain pathology
While most work focuses on the structural relationships between the developing brain and face, there also appears to be a relationship between brain pathologies and facial shape. For example, recent investigations have revealed significant differences in the shape of the faces of people with psychological disorders. In patients with bipolar disorder, significant differences in facial shape were observed. The changes were primarily found in the frontonasal region, and consisted of facial widening, vertical shortening, and alterations to the nose and mouth (Hennessy, Baldwin et al. 2007, Hennessy, Baldwin et al. 2010). Interestingly, these same general changes were observed with individuals diagnosed with schizophrenia. While the exact mechanisms underlying these facial changes are unknown, it is worthy to note that the changes in facial shape occur in regions that are highly influenced by molecular signaling from the brain to the face. Whether these signaling interactions are altered in these patients is not known, but deserves consideration.
4. The Role of Brain-Face Interactions in Generating Evolutionary Variation
Given the impact of the brain on the face, variation in these interactions is likely to contribute to evolutionary differences in morphology. Indeed, the FEZ is present in all assayed amniotes (Odent, Atti-Bitach et al. 1999, Hu and Marcucio 2009, Young, Hu et al. 2014), and its spatial organization appears to vary with differences in facial morphology. For example, in mice, Shh expression is divided into medial and lateral domains, while in birds Shh expression is observed as a single domain that spans the primordial upper jaw (Hu and Marcucio 2009, Reid, Yang et al. 2011). Altering SHH signaling within the forebrain leads to changes in the spatial organization of the Shh expression domain in the FEZ. Activating SHH signaling in the forebrain of early-stage chick embryos superimposes a mammalian pattern of Shh expression in the FEZ and similarly alters the morphology of the developing upper jaw (Hu and Marcucio 2009). This result suggests that SHH signaling within the brain controls the spatial organization of Shh expression in the FEZ, and altering this signaling system may generate variation in natural systems.
The differences in the patterns of Shh expression in the FEZ of birds and mice are dramatic and obvious. However, more subtle differences in the shape of the Shh expression domains in the FEZ among closely related animals are closely related to the morphology of the animal’s face. Novel methods to quantify the shapes of Shh expression domains have revealed that differences in the shapes of the Shh expression domains in the FEZ of chicken, quail, and duck embryos are detectable, and that the shapes of these domains are highly correlated with the facial shapes of the developing embryos (Hu, Young et al. 2015).
The exact mechanisms by which these different patterns are generated are not known. Experimental evidence indicates that the brain plays some role in this process, possibly due to subtle differences in the Shh expression patterns within the brain of each embryo. In the basal forebrain of duck embryos, the Shh expression domain is wider compared to that in chick embryos, and transplanting the basal forebrain from the duck to the chick embryo shifted the morphology of the chimera in the direction of the duck. This was accompanied by a shift in the shape of the Shh expression domain in the chimera (Hu, Young et al. 2015). However, it must be noted that the changes in the shape of the Shh expression domain in the FEZ were not statistically significant, but there was likely experimental variability that precluded detecting these differences. Nonetheless, the shapes were shifted in the right direction in the experimental embryos, and importantly, the shape of the Shh expression domain in the FEZ was highly correlated with the shape of the developing midface. Together, these data indicate that the brain plays some, as yet undefined, role in patterning the shape of the Shh expression domain, and the shape of the Shh expression domain contributes to the pattern of morphogenesis observed in the developing midface.
5. Conclusions:
Studies performed in a variety of animal models and on human patients have uncovered a series of interactions between the brain and the face that regulate the coordinated development of these structures. The connective tissue precursors of the facial skeleton are derived from the brain during early stages of development. The brain exerts an epigenetic influence on the developing face through the effect of its expansion on position and subsequent growth of the facial anlagen, and these epigenetic influences may have profound consequences on the incidence and severity of structural malformations, such as cleft lip and palate, of the developing face. Finally, a series of molecular signaling interactions between the brain and facial tissues appear to coordinate the growth of the brain and the face. Understanding these interactions will ultimately lead to a greater understanding of developmental mechanisms and the etiologies of birth defects that affect the brain and the face. Ultimately, this understanding may lead to improved patient care through a better appreciation of the human condition, improved diagnostic tools, and/or development of new therapeutic regimens to directly treat patients.
References Cited:
- Aguiar DP, Sghari S and Creuzet S (2014). “The facial neural crest controls fore- and midbrain patterning by regulating Foxg1 expression through Smad1 activity.” Development 141(12): 2494–2505. [DOI] [PubMed] [Google Scholar]
- Aldridge K, Kane AA, Marsh JL, Yan P, Govier D and Richtsmeier JT (2005). “Relationship of brain and skull in pre- and postoperative sagittal synostosis.” J Anat 206(4): 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto K, Shikata Y, Imai H, Matsumaru D, Tokunaga T, Shioda S, Yamada G and Motoyama J (2009). “Mouse Shh is required for prechordal plate maintenance during brain and craniofacial morphogenesis.” Dev Biol 327(1): 106–120. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K and Richman JM (2002). “Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion.” Development 129(19): 4647–4660. [DOI] [PubMed] [Google Scholar]
- Barlow AJ and Francis-West PH (1997). “Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia.” Development 124(2): 391–398. [DOI] [PubMed] [Google Scholar]
- Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HH, Koop B, Martindale D, Rommens JM, Tsui LC and Scherer SW (1996). “Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly.” Nat Genet 14(3): 353–356. [DOI] [PubMed] [Google Scholar]
- Boughner JC, Wat S, Diewert VM, Young NM, Browder LW and Hallgrimsson B (2008). “Short-faced mice and developmental interactions between the brain and the face.” J Anat 213(6): 646–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito JM, Teillet MA and Le Douarin NM (2006). “An early role for sonic hedgehog from foregut endoderm in jaw development: ensuring neural crest cell survival.” Proc Natl Acad Sci U S A 103(31): 11607–11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner ME and LeDouarin NM (2012). “Development and evolution of the neural crest: an overview.” Dev Biol 366(1): 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Warburton D, Brown LY, Yu CY, Roeder ER, Stengel-Rutkowski S, Hennekam RC and Muenke M (1998). “Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired.” Nature Genetics 20(2): 180–183. [DOI] [PubMed] [Google Scholar]
- Chai Y and Maxson RE Jr. (2006). “Recent advances in craniofacial morphogenesis.” Dev Dyn 235(9): 2353–2375. [DOI] [PubMed] [Google Scholar]
- Chambers D and McGonnell IM (2002). “Neural crest: facing the facts of head development.” Trends Genet 18(8): 381–384. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H and Beachy PA (1996). “Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function.” Nature 383(6599): 407–413. [DOI] [PubMed] [Google Scholar]
- Chong HJ, Young NM, Hu D, Jeong J, McMahon AP, Hallgrimsson B and Marcucio RS (2011). “Signaling by SHH rescues facial defects following blockade in the brain.” Developmental dynamics : an official publication of the American Association of Anatomists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Vincent C and Le Douarin NM (2002). “Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton.” Development 129(18): 4301–4313. [DOI] [PubMed] [Google Scholar]
- Creuzet SE, Martinez S and Le Douarin NM (2006). “The cephalic neural crest exerts a critical effect on forebrain and midbrain development.” Proc Natl Acad Sci U S A 103(38): 14033–14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMyer W (1964). “The face predicts the brain: diagnostic significance of median facial anomialies for holoprosencephaly (arhinencephay).” Pediatrics August: 256–263. [PubMed] [Google Scholar]
- Depew MJ and Compagnucci C (2008). “Tweaking the hinge and caps: testing a model of the organization of jaws.” J Exp Zoolog B Mol Dev Evol 310(4): 315–335. [DOI] [PubMed] [Google Scholar]
- Depew MJ and Simpson CA (2006). “21st century neontology and the comparative development of the vertebrate skull.” Dev Dyn 235(5): 1256–1291. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA, Morasso M and Rubenstein JL (2005). “Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development.” J Anat 207(5): 501–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diewert VM (1983). “A morphometric analysis of craniofacial growth and changes in spatial relations during secondary palatal development in human embryos and fetuses.” Am J Anat 167(4): 495–522. [DOI] [PubMed] [Google Scholar]
- Diewert VM (1985). “Growth movements during prenatal development of human facial morphology.” Prog Clin Biol Res 187: 57–66. [PubMed] [Google Scholar]
- Diewert VM and Lozanoff S (1993). “Growth and morphogenesis of the human embryonic midface during primary palate formation analyzed in frontal sections.” J Craniofac Genet Dev Biol 13(3): 162–183. [PubMed] [Google Scholar]
- Diewert VM and Lozanoff S (1993). “A morphometric analysis of human embryonic craniofacial growth in the median plane during primary palate formation.” Journal of Craniofacial Genetics and Developmental Biology 13(3): 147–161. [PubMed] [Google Scholar]
- Diewert VM, Lozanoff S and Choy V (1993). “Computer reconstructions of human embryonic craniofacial morphology showing changes in relations between the face and brain during primary palate formation.” Journal of Craniofacial Genetics and Developmental Biology 13(3): 193–201. [PubMed] [Google Scholar]
- Etchevers HC, Couly G, Vincent C and Le Douarin NM (1999). “Anterior cephalic neural crest is required for forebrain viability.” Development 126(16): 3533–3543. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Utset MF, Bogarad LD, Hart CP, Awgulewitsch A, Ferguson-Smith A, Fainsod A, Rabin M and Ruddle FH (1987). “Homeo box genes in murine development.” Curr Top Dev Biol 23: 233–256. [DOI] [PubMed] [Google Scholar]
- Foppiano S, Hu D and Marcucio RS (2007). “Signaling by bone morphogenetic proteins directs formation of an ectodermal signaling center that regulates craniofacial development.” Dev Biol 312(1): 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis-West P, Ladher R, Barlow A and Graveson A (1998). “Signalling interactions during facial development.” Mechanisms of Development 75(1–2): 3–28. [DOI] [PubMed] [Google Scholar]
- Gans C and Northcutt R (1983). “Neural Crest and the Origin of Vertebrates: A New Head.” Science 220: 268–274. [DOI] [PubMed] [Google Scholar]
- Garcez RC, Le Douarin NM and Creuzet SE (2014). “Combinatorial activity of Six1-2-4 genes in cephalic neural crest cells controls craniofacial and brain development.” Cell Mol Life Sci 71(11): 2149–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Antoni L, Fu K, Whiting CJ, Francis-West P and Richman JM (2009). “Expression of WNT signalling pathway genes during chicken craniofacial development.” Dev Dyn 238(5): 1150–1165. [DOI] [PubMed] [Google Scholar]
- Graham A, Okabe M and Quinlan R (2005). “The role of the endoderm in the development and evolution of the pharyngeal arches.” Journal of Anatomy 207(5): 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A and Smith A (2001). “Patterning the pharyngeal arches.” Bioessays 23(1): 54–61. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos GA, Bell E, Toole L, Lumsden A and Tucker AS (2000). “Homeotic transformation of branchial arch identity after Hoxa2 overexpression.” Development 127(24): 5355–5365. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Wotton D, Edwards MC, Roessler E, Ades L, Meinecke P, Richieri-Costa A, Zackai EH, Massague J, Muenke M and Elledge SJ (2000). “Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination.” Nat Genet 25(2): 205–208. [DOI] [PubMed] [Google Scholar]
- Hallgrímsson B, Jamniczky H, Young NM, Rolian C, Parsons TE, Boughner JC, Marcucio RS (2009). “Deciphering the palimpsest: Studying the relationship between morphological integration and phenotypic covariation.” Evolutionary Biology 36(4): 355–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy RJ, Baldwin PA, Browne DJ, Kinsella A and Waddington JL (2007). “Three-dimensional laser surface imaging and geometric morphometrics resolve frontonasal dysmorphology in schizophrenia.” Biol Psychiatry 61(10): 1187–1194. [DOI] [PubMed] [Google Scholar]
- Hennessy RJ, Baldwin PA, Browne DJ, Kinsella A and Waddington JL (2010). “Frontonasal dysmorphology in bipolar disorder by 3D laser surface imaging and geometric morphometrics: comparisons with schizophrenia.” Schizophr Res 122(1–3): 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussler HS, Suri M, Young ID and Muenke M (2002). “Extreme variability of expression of a Sonic Hedgehog mutation: attention difficulties and holoprosencephaly.” Arch Dis Child 86(4): 293–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PW and Hogan BL (1988). “Spatially restricted patterns of expression of the homeobox-containing gene Hox 2.1. during mouse embryogenesis.” Development 102(1): 159–174. [DOI] [PubMed] [Google Scholar]
- Hong M and Krauss RS (2012). “Cdon mutation and fetal ethanol exposure synergize to produce midline signaling defects and holoprosencephaly spectrum disorders in mice.” PLoS Genet 8(10): e1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M and Krauss RS (2013). “Rescue of holoprosencephaly in fetal alcohol-exposed Cdon mutant mice by reduced gene dosage of Ptch1.” PLoS One 8(11): e79269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio R and Helms JA (2003). “A zone of frontonasal ectoderm regulates patterning and growth in the face.” Development 130(9): 1749–1758. [DOI] [PubMed] [Google Scholar]
- Hu D and Marcucio RS (2009). “A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm.” Development 136(1): 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D and Marcucio RS (2009). “Unique organization of the frontonasal ectodermal zone in birds and mammals.” Dev Biol 325(1): 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS (2012). “Neural crest cells pattern the surface cephalic ectoderm during FEZ formation “ Developmental Dynamics 241(4): 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Young NM, Xu Q, Jamniczky H, Green RM, Mio W, Marcucio RS and Hallgrimsson B (2015). “Signals from the brain induce variation in avian facial shape.” Dev Dyn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH and McMahon AP (2004). “Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia.” Genes Dev. 18(8): 937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, El-Jaick K, Roessler E, Muenke M and Epstein DJ (2006). “A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers.” Development 133(4): 761–772. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Leskow FC, El-Jaick K, Roessler E, Muenke M, Yocum A, Dubourg C, Li X, Geng X, Oliver G and Epstein DJ (2008). “Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein.” Nat Genet 40(11): 1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Bush JO and Lidral AC (2006). “Development of the upper lip: morphogenetic and molecular mechanisms.” Developmental dynamics : an official publication of the American Association of Anatomists 235(5): 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K, Robertson MJ and Schaffer DV (2004). “The sonic hedgehog signaling system as a bistable genetic switch.” Biophys J 86(5): 2748–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Ryan RC, Zhang Z, Bullard SA, Bush JO, Maltby KM, Lidral AC, Jiang R (2006). “Expression of Wnt9b and Activation of Canonical Wnt Signaling During Midfacial Morphogenesis in Mice.” Dev Dyn 235: 1448–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Couly G and Creuzet SE (2012). “The neural crest is a powerful regulator of pre-otic brain development.” Dev Biol 366(1): 74–82. [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D and Helms JA (2005). “Molecular interactions coordinating the development of the forebrain and face.” Dev Biol 284(1): 48–61. [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Young NM, Hu D and Hallgrimsson B (2011). “Mechanisms that underlie co-variation of the brain and face.” Genesis 49(4): 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W and Krumlauf R (1992). “Homeobox Genes and Axial Patterning.” Cell 68: 283–302. [DOI] [PubMed] [Google Scholar]
- Mercier S, Dubourg C, Garcelon N, Campillo-Gimenez B, Gicquel I, Belleguic M, Ratie L, Pasquier L, Loget P, Bendavid C, Jaillard S, Rochard L, Quelin C, Dupe V, David V and Odent S (2011). “New findings for phenotype-genotype correlations in a large European series of holoprosencephaly cases.” J Med Genet 48(11): 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming JE, Kaupas ME, Roessler E, Brunner HG, Golabi M, Tekin M, Stratton RF, Sujansky E, Bale SJ and Muenke M (2002). “Mutations in PATCHED-1, the receptor for SONIC HEDGEHOG, are associated with holoprosencephaly.” Hum Genet 110(4): 297–301. [DOI] [PubMed] [Google Scholar]
- Ming JE and Muenke M (1998). “Holoprosencephaly: from Homer to Hedgehog.” Clin Genet 53(3): 155–163. [DOI] [PubMed] [Google Scholar]
- Ming JE and Muenke M (2002). “Multiple hits during early embryonic development: digenic diseases and holoprosencephaly.” Am J Hum Genet 71(5): 1017–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoux M and Rijli FM (2010). “Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development.” Development 137(16): 2605–2621. [DOI] [PubMed] [Google Scholar]
- Muenke M and Beachy PA (2000). “Genetics of ventral forebrain development and holoprosencephaly.” Current Opinion in Genetics and Development 10(3): 262–269. [DOI] [PubMed] [Google Scholar]
- Muller F, Albert S, Blader P, Fischer N, Hallonet M and Strahle U (2000). “Direct action of the nodal-related signal cyclops in induction of sonic hedgehog in the ventral midline of the CNS.” Development 127(18): 3889–3897. [DOI] [PubMed] [Google Scholar]
- Nanni L, Ming JE, Du Y, Hall RK, Aldred M, Bankier A and Muenke M (2001). “SHH mutation is associated with solitary median maxillary central incisor: a study of 13 patients and review of the literature.” Am J Med Genet 102(1): 1–10. [DOI] [PubMed] [Google Scholar]
- Nanni L, Schelper RL and Muenke MT (2000). “Molecular genetics of holoprosencephaly.” Frontiers in Bioscience 5(8): D334–342. [DOI] [PubMed] [Google Scholar]
- Noden DM (1983). “The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues.” Dev Biol 96(1): 144–165. [DOI] [PubMed] [Google Scholar]
- Noden DM (1984). “Craniofacial development: New views on old problems.” Anatomical Records 208: 1–13. [DOI] [PubMed] [Google Scholar]
- Noden DM (1986). “Origins and Patterning of Craniofacial Mesenchymal Tissues.” Journal of Craniofacial Genetics and Developmental Biology 2: 15–31. [PubMed] [Google Scholar]
- Noden DM and Trainor PA (2005). “Relations and interactions between cranial mesoderm and neural crest populations.” J Anat 207(5): 575–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, Canady J, Richman L, Van Demark D and Andreasen NC (2002). “Structural brain abnormalities in adult males with clefts of the lip and/or palate.” Genet Med 4(1): 1–9. [DOI] [PubMed] [Google Scholar]
- Odent S, Atti-Bitach T, Blayau M, Mathieu M, Aug J, Delezo de AL, Gall JY, Le Marec B, Munnich A, David V and Vekemans M (1999). “Expression of the Sonic hedgehog (SHH ) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly.” Hum Mol Genet 8(9): 1683–1689. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Chiang C and Rubenstein JL (2002). “Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles.” Neuroscience 111(1): 1–17. [DOI] [PubMed] [Google Scholar]
- Reid BS, Yang H, Melvin VS, Taketo MM and Williams T (2011). “Ectodermal Wnt/beta-catenin signaling shapes the mouse face.” Developmental Biology 349(2): 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JM and Lee SH (2003). “About face: signals and genes controlling jaw patterning and identity in vertebrates.” Bioessays 25(6): 554–568. [DOI] [PubMed] [Google Scholar]
- Richtsmeier JT, Aldridge K, DeLeon VB, Panchal J, Kane AA, Marsh JL, Yan P and Cole TM 3rd (2006). “Phenotypic integration of neurocranium and brain.” J Exp Zool B Mol Dev Evol 306(4): 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtsmeier JT and Deleon VB (2009). “Morphological integration of the skull in craniofacial anomalies.” Orthod Craniofac Res 12(3): 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, Dierich A, Dolle P and Chambon P (1993). “A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene.” Cell 75(7): 1333–1349. [DOI] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC and Muenke M (1996). “Mutations in the human Sonic Hedgehog gene cause holoprosencephaly.” Nat Genet 14(3): 357–360. [DOI] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Vargas F, Scherer SW, Tsui LC and Muenke M (1997). “Mutations in the C-terminal domain of Sonic Hedgehog cause holoprosencephaly.” Hum Mol Genet 6(11): 1847–1853. [DOI] [PubMed] [Google Scholar]
- Roessler E, El-Jaick KB, Dubourg C, Velez JI, Solomon BD, Pineda-Alvarez DE, Lacbawan F, Zhou N, Ouspenskaia M, Paulussen A, Smeets HJ, Hehr U, Bendavid C, Bale S, Odent S, David V and Muenke M (2009). “The mutational spectrum of holoprosencephaly-associated changes within the SHH gene in humans predicts loss-of-function through either key structural alterations of the ligand or its altered synthesis.” Hum Mutat 30(10): E921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Lacbawan F, Dubourg C, Paulussen A, Herbergs J, Hehr U, Bendavid C, Zhou N, Ouspenskaia M, Bale S, Odent S, David V and Muenke M (2009). “The full spectrum of holoprosencephaly-associated mutations within the ZIC2 gene in humans predicts loss-of-function as the predominant disease mechanism.” Hum Mutat 30(4): E541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Ma Y, Ouspenskaia MV, Lacbawan F, Bendavid C, Dubourg C, Beachy PA and Muenke M (2009). “Truncating loss-of-function mutations of DISP1 contribute to holoprosencephaly-like microform features in humans.” Hum Genet 125(4): 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E and Muenke M (1998). “Holoprosencephaly: a paradigm for the complex genetics of brain development.” Journal of Inherited Metabolic Disease 21(5): 481–497. [DOI] [PubMed] [Google Scholar]
- Roessler E and Muenke M (1999). “The molecular genetics of holoprosencephaly: a model of brain development for the next century.” Childs Nervous System 15(11–12): 646–651. [DOI] [PubMed] [Google Scholar]
- Roessler E and Muenke M (2010). “The molecular genetics of holoprosencephaly.” Am J Med Genet C Semin Med Genet 154C(1): 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell-Apacik C, Rivero M, Knepper JL, Roessler E, Muenke M and Ming JE (2003). “SONIC HEDGEHOG mutations causing human holoprosencephaly impair neural patterning activity.” Hum Genet 23: 23. [DOI] [PubMed] [Google Scholar]
- Solomon BD, Lacbawan F, Jain M, Domene S, Roessler E, Moore C, Dobyns WB and Muenke M (2009). “A novel SIX3 mutation segregates with holoprosencephaly in a large family.” Am J Med Genet A 149A(5): 919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK (2005). “Genesis of Alcohol-Induced Craniofacial Dysmorphism.” J Experimental Biology and Medicine 230(6): 366–375. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Cook CS and Webster WS (1988). “Teratogens and craniofacial malformations: relationship to cells death.” Development 103(Supplement): 213–232. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Nguyen V, McCarthy NQ and Eberhart JK (2012). “Hh signaling regulates patterning and morphogenesis of the pharyngeal arch-derived skeleton.” Dev Biol 369(1): 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Smithers LE, Yakob W and Liu KJ (2009). “New directions in craniofacial morphogenesis.” Dev Biol. [DOI] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS and McMahon AP (2006). “The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice.” Dev Cell 10(5): 647–656. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Ariza-McNaughton L and Krumlauf R (2002). “Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning.” Science 295(5558): 1288–1291. [DOI] [PubMed] [Google Scholar]
- Trainor PA and Krumlauf R (2000). “Patterning the cranial neural crest: hindbrain segmentation and Hox gene plasticity.” Nat Rev Neurosci 1(2): 116–124. [DOI] [PubMed] [Google Scholar]
- Tucker ES, Segall S, Gopalakrishna D, Wu Y, Vernon M, Polleux F and Lamantia AS (2008). “Molecular specification and patterning of progenitor cells in the lateral and medial ganglionic eminences.” J Neurosci 28(38): 9504–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis DE and Muenke M (1999). “Molecular mechanisms of holoprosencephaly.” Molecular Genetics and Metabolism 68(2): 126–138. [DOI] [PubMed] [Google Scholar]
- Wallis DE, Roessler E, Hehr U, Nanni L, Wiltshire T, Richieri-Costa A, Gillessen-Kaesbach G, Zackai EH, Rommens J and Muenke M (1999). “Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly.” Nat Genet 22(2): 196–198. [DOI] [PubMed] [Google Scholar]
- Wang KY, V. M. D. (1992). “A morphometric analyis of craniofacial growth in cleft lip and noncleft mice.” J Craniofac Genet Dev Biol 12(3): 141–154. [PubMed] [Google Scholar]
- Wang KY, Juriloff DM and Diewert VM (1995). “Deficient and delayed primary palatal fusion and mesenchymal bridge formation in cleft lip-liable strains of mice.” J Craniofac Genet Dev Biol 15(3): 99–116. [PubMed] [Google Scholar]
- Weinberg SM, Andreasen NC and Nopoulos P (2009). “Three-dimensional morphometric analysis of brain shape in nonsyndromic orofacial clefting.” J Anat 214(6): 926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch KD, Panter KE, Lee ST, Gardner DR, Stegelmeier BL and Cook D (2009). “Cyclopamine-induced synophthalmia in sheep: defining a critical window and toxicokinetic evaluation.” J Appl Toxicol 29(5): 414–421. [DOI] [PubMed] [Google Scholar]
- Young NM, Chong HJ, Hu D, Hallgrimsson B and Marcucio RS (2010). “Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape.” Development 137(20): 3405–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NM, Hu D, Lainoff AJ, Smith FJ, Diaz R, Tucker AS, Trainor PA, Schneider RA, Hallgrimsson B and Marcucio RS (2014). “Embryonic bauplans and the developmental origins of facial diversity and constraint.” Development 141(5): 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Hong M, Bae GU, Kang JS and Krauss RS (2011). “Boc modifies the holoprosencephaly spectrum of Cdo mutant mice.” Dis Model Mech 4(3): 368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]