Abstract
Context
Individuals with type 1 diabetes mellitus (T1DM) have alterations in brain activity that have been postulated to contribute to the adverse neurocognitive consequences of T1DM; however, the impact of T1DM and hypoglycemic unawareness on the brain’s resting state activity remains unclear.
Objective
To determine whether individuals with T1DM and hypoglycemia unawareness (T1DM-Unaware) had changes in the brain resting state functional connectivity compared to healthy controls (HC) and those with T1DM and hypoglycemia awareness (T1DM-Aware).
Design
Observational study.
Setting
Academic medical center.
Participants
27 individuals with T1DM and 12 HC volunteers participated in the study.
Intervention
All participants underwent blood oxygenation level dependent (BOLD) resting state functional magnetic brain imaging during a 2-step hyperinsulinemic euglycemic (90 mg/dL)–hypoglycemic (60 mg/dL) clamp.
Outcome
Changes in resting state functional connectivity.
Results
Using 2 separate methods of functional connectivity analysis, we identified distinct differences in the resting state brain responses to mild hypoglycemia between HC, T1DM-Aware, and T1DM-Unaware participants, particularly in the angular gyrus, an integral component of the default mode network (DMN). Furthermore, changes in angular gyrus connectivity also correlated with greater symptoms of hypoglycemia (r = 0.461, P = 0.003) as well as higher scores of perceived stress (r = 0.531, P = 0.016).
Conclusion
These findings provide evidence that individuals with T1DM have changes in the brain’s resting state connectivity patterns, which may be further associated with differences in awareness to hypoglycemia. These changes in connectivity may be associated with alterations in functional outcomes among individuals with T1DM.
Type 1 diabetes mellitus (T1DM) is associated with a host of changes in brain function including cognitive deficits (1–4) as well as neuropsychological changes such as increased risk of depression and anxiety (5–7). The mechanisms underlying these associations are incompletely understood but may involve both structural cerebral changes as well as functional changes in brain activity (4). It has been postulated that many of these changes in cerebral function are secondary to exposure to not only chronic hyperglycemia but also frequent episodes of hypoglycemia.
While normally the body has multiple layers of defense mechanisms to prevent hypoglycemia, patients with T1DM are reliant on exogenous insulin and have deficits in counterregulatory hormonal responses to prevent hypoglycemia. Patients with T1DM who have frequent episodes of hypoglycemia often develop hypoglycemia unawareness, which is characterized by an inability to mount the normal symptomatic and counterregulatory hormonal responses required to restore plasma glucose levels. Individuals with T1DM also have different responses in regional brain activity in response to working memory tasks (8) as well as visual food cues (9) during mild hypoglycemia. Moreover, these responses are further modulated by the presence or absence of hypoglycemia awareness (9). These studies suggest that brain responses to specific tasks are altered by T1DM and hypoglycemia unawareness.
However, over the past 2 decades, there has been a growing understanding that the vast majority of the brain’s energy is devoted to intrinsic neuronal signaling, that is, activity not related to any tasks or in response to environmental stimuli. This activity has been called resting state functional connectivity because different regions of the brain that are functionally coordinated will fluctuate in a synchronous pattern “at rest” or in the absence of stimuli. In fact, it has been suggested that nearly 95% of the brain’s energy budget is utilized during intrinsic neuronal signaling (10, 11). Furthermore, a growing body of work has revealed compelling relationships between alterations in resting state functional connectivity and numerous neurologic and psychiatric diseases including anxiety and depression (12) as well as metabolic diseases such as obesity and type 2 diabetes (13). In addition, children and adults with T1DM appear to have changes in functional connectivity as well. In a study using resting state functional magnetic resonance imaging (rsfMRI), children with T1DM had compensatory hyperconnectivity compared to children without diabetes in the dorsal and ventral attention networks (14). Adults with well-controlled T1DM patients and no microangiopathy had increased connectivity in the visual and sensorimotor networks compared to healthy control (HC) subjects (15). In contrast, among adult T1DM patients with microangiopathy, functional connectivity was reported to be decreased (15). While these studies have laid the groundwork for subsequent investigations of connectivity changes in T1DM, a limitation of many of them was that participants had a wide range of plasma glucose levels ranging from 70 to 300 mg/dL (14) and 72 to 270 mg/dL (15). To our knowledge, only 1 prior study has investigated the impact of hypoglycemia on resting state functional connectivity among individuals with T1DM. In this study by Bolo and colleagues (16), T1DM participants had increased functional connectivity in the prefrontal cortex network compared to control subjects.
However, no studies have investigated whether individuals with T1DM and hypoglycemia unawareness have alterations in resting state functional connectivity. Thus, in this study, we utilized a 2-step euglycemia-mild hypoglycemia insulin clamp to assess resting state functional connectivity among HC subjects as well as T1DM individuals with and without hypoglycemia unawareness.
Research Design and Methods
Participants
The participants in this study were part of a larger cohort of participants in a previously published study to investigate the impact of hypoglycemia unawareness on brain responses to visual food tasks (9). Thirty-nine participants from that study also completed rsfMRI scanning and were included in this analysis. Participants were categorized into the following three groups: 12 HC participants, 15 participants with T1DM and hypoglycemia awareness (T1DM-Aware), and 12 participants with T1DM and hypoglycemia unawareness (T1DM-Unaware). The Clarke score (17) was used to differentiate participants with hypoglycemia awareness versus unawareness. If the Clarke score was not classifiable (ie, when individuals reported a score of 3 R) then the Gold (18) method was used to determine whether they had impaired hypoglycemia awareness.
All participants underwent a screening history, physical examination, and laboratory testing at the Yale New Haven Hospital Research Unit (HRU). Exclusion criteria included inability to enter the magnetic resonance imaging (MRI); smoking, illegal drug, or recent steroid use; known psychiatric or neurological disorders; active infection; malignancy; abnormal thyroid function; cerebrovascular disease; cardiovascular disease; hepatobiliary disease; or weight change in the last 3 months. The protocol was approved by the Yale University School of Medicine Human Investigation Committee. All subjects provided informed, written consent before participation.
Study protocol
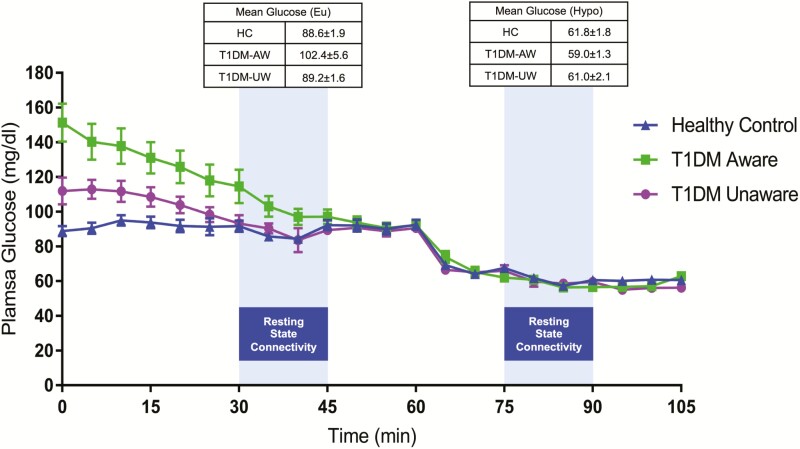
All participants with T1DM were asked to wear a continuous glucose monitor (Dexcom G4) 1 week prior to their scheduled MRI visit to monitor for antecedent hypoglycemia. If participants had any episodes of hypoglycemia (glucose < 70 mg/dl or a symptomatic episode requiring assistance) in the 5 days prior to MRI scanning, then the scans were postponed to a later date. On the day of the MRI, participants arrived to the HRU at 9:00 am. All participants were instructed to eat breakfast as usual prior to arrival and those with diabetes were further instructed to bolus insulin as usual for breakfast. At 10 am, all participants were provided with a standardized snack consisting of 41 grams of carbohydrate (turkey sandwich, apple, diet ginger ale) to neutralize feeling of hunger as previously described (19). Participants with diabetes were instructed to inject a bolus of insulin as per their home insulin to carbohydrate ratio. Intravenous catheters were placed in antecubital veins bilaterally: one for blood sampling and the other for insulin and glucose infusion. Scanning began in the MRI center at 12:00 pm simultaneously with initiation of an insulin infusion at 2 milliunits/kg/hr. Euglycemia (~90 mg/dL) that was maintained for the first phase of the study, after which glucose was decreased into the mild hypoglycemia range (~60 mg/dL) (Fig. 1A). Glycemic state was not counterbalanced out of concern that antecedent hypoglycemia may alter cerebral energetics thereby effecting resting state connectivity patterns during euglycemia. rsfMRI data were acquired during euglycemia (between time 30–45 minutes) and hypoglycemia (between time 75–90 minutes) sessions. Throughout the MRI scan, blood was periodically sampled for glucose and counterregulatory hormones (epinephrine, norepinephrine, glucagon and cortisol).
Figure 1.
Study design of 2-step hyperinsulinemic euglycemic-hypoglycemia clamp during rsfMRI BOLD scanning. Plasma glucose levels time courses (mean±standard error of the mean) during the study.
Hypoglycemia symptom assessments.
Participants were asked to verbally rate their sensation of hypoglycemic symptoms (unable to concentrate, blurry vision, anxiety, confusion, difficulty speaking, double vision, drowsiness, tiredness, hunger, weakness, sweating, trembling, warmness, heart racing) on a 7-point Likert scale (1 indicating “not at all” and 7 indicating “a lot”) based upon the Edinburgh hypoglycemia symptom score (20) at 3 separate time points during the study: baseline (prior to entering the scanner) and then at euglycemia (time 30 minutes) and hypoglycemia (at time 75 minutes).
Biochemical analysis.
Plasma glucose was measured enzymatically using glucose oxidase (YSI, Yellow Springs, OH, US). Plasma free insulin and glucagon were measured by double antibody radioimmunoassay (Millipore, Burlington, MA). Double-antibody radioimmunoassay was used to measure plasma cortisol (MP Biomedicals, Solon, OH, US). Plasma epinephrine and norepinephrine were measured by high-performance liquid chromatography (ESA, Cincinnati, OH).
Statistical analysis.
One-way analysis of variance was used to determine whether there were statistical differences between the three groups for all normally distributed hormones and variables followed by Fisher’s least significant difference test for pairwise comparisons. Analyses of every repeatedly measured variable, including plasma glucose and hormone levels, were performed using the mixed-effects regression model method, taking into account both between-subject and within-subject correlations of repeated measures using a combination of prespecified compound symmetry covariance matrix and an autoregressive covariance matrix. Age, gender, and body mass index (BMI) were adjusted as covariates (ie, as fixed effects). All analyses were performed using SAS, version 9.4 (SAS, Cary, NC) and SPSS, version 24 (SPSS, Armonk, NY). Unless otherwise stated, data are presented as mean ± standard error.
rsfMRI analysis
Image acquisition.
Participants were scanned in a Siemens 3-T Tim Trio scanner. After structural images were acquired, acquisition of functional data began in the same slice locations as the axial-oblique T1-weighted data. Resting state functional images were collected using an echo-planar image gradient echo pulse sequence (repetition time = 956 ms, echo time = 30 ms, field of vision = 210 mm, matrix size = 84×84, slice thickness = 2.5 mm, flip angle = 62°, bandwidth = 2289 Hz/pixel with 51 slices) with 274 volumes per run.
Preprocessing.
The digital data (Digital Imaging and Communication in Medicine ) were converted to NlfTI using dcm2nii (21) and then the first 3 images were discarded from each functional run to enable the signal to achieve steady-state equilibrium between radio-frequency pulsing and relaxation leaving 284 images per run for analysis. The data were motion-corrected using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8), and they were discarded if linear motion was greater than 1.5 mm or rotation was greater than 2 degrees. To account for motion confounds, images were iteratively smoothed until the smoothness for any image had a full width half maximum of approximately 6 mm (22, 23). All further analysis was performed using BioImage Suite (24) unless otherwise specified. Several covariates of no interest were regressed from the data including linear and quadratic drift, a 24 parameter model of motion, mean cerebral spinal fluid (CSF) signal, mean white matter signal, and overall global signal. The white matter and CSF areas were defined on the MNI brain, eroded to ensure only white matter or CSF signal would be included and warped to individual-participant space using a series of transformations described in the following text. Finally, the data were temporally smoothed with a 0 mean unit variance Gaussian filter (approximate cutoff frequency = 0.11 Hz).
Intrinsic connectivity analyses.
After preprocessing, the three resting state runs were concatenated and the functional connectivity of each voxel, as measured by the intrinsic connectivity distribution (ICD), was then calculated in each subject’s individual space (25). First, a gray matter mask was applied to the data so only voxels in the gray matter were considered in the calculation of connectivity. The gray matter mask was defined on the MNI brain and warped to the individual subject’s space using a series of inverse transformations (described in the following text). ICD involves correlating the time course for any given gray-matter voxel with the time course of every other gray-matter voxel in the brain and then summarizing these correlations with a network theory metric. Specifically, ICD models the entire distribution of the network measure of degree, therefore eliminating the need to specify a connection threshold. Because the removal of the global mean makes the signs of the correlations ambiguous, we only focus on the positive correlations (26). A histogram of these positive correlations was constructed to estimate the distribution of connections to the voxel in question. This distribution of connections was converted to a survival function and the survival function was fitted with a stretched exponential with unknown variance, α. As α controls the spread of the distribution of connections, a larger α indicates a greater number of high correlation connections. Finally, this process is repeated for all voxels in the gray matter resulting in a parametric image of the alpha parameter for each subject.
Seed connectivity analyses.
A reference region in the right posterior cingulate cortex (PCC) was defined on the MNI reference brain as a 9-mm cube surrounding the MNI coordinates of (−6,−43, 38) and transformed back (via the inverse transform described in the following text) into individual subject space. The time-course of the reference region in a given subject was then computed as the average time-course across all pixels in the reference region. This time-course was correlated (using Pearson correlation) with the time-courses of all other voxels in the brain to create a map of r values. These r values were transformed to z-values using Fisher’s transform and averaged across scans in each subject to yield one map for each subject representing the strength of correlation to the PCC in terms of Gaussian variables.
Motion analysis.
As group differences in motion have been shown to confound connectivity studies, we calculated the average frame-to-frame displacement for each subject’s data. In line with current reports, no subject had an average frame-to-frame displacement greater than 0.20 mm. There were no significant differences for motion between the T1DM-Unaware and HC groups (P = 0.06), between T1DM-Unaware and T1DM-Aware groups (P = 0.06), and T1DM-Aware and HC groups (P = 0.42).
Image registration.
Single-subject data were warped to a common MNI template through the concatenation of a series of linear and nonlinear registrations. The functional series were linearly registered to the T1 axial-oblique (2D anatomical) image. The 2D anatomical image was linearly registered to the magnetization-prepared rapid acquisition with gradient echo (MPRAGE; 3D anatomical) image. The 3D anatomical image was nonlinearly registered to the template MNI brain using a previously validated algorithm (27). All transformation pairs were calculated independently and combined into a single transform warping the single participant results into common space. It was then inverted to create the reverse transformation from the template MNI brain to the space of the participant. This single transformation allows the single participant images to be transformed with only one transformation, reducing interpolation error.
Group analysis.
Voxel-wise t tests were used to compare the connectivity data between the study groups. Imaging results are shown at a cluster-level threshold of P < 0.05 familywise error (FWE) correction as determined by AFNI’s 3dClustSim program (version 16.0.09) using a cluster-forming threshold of P = 0.001, 10 000 iterations, a gray matter mask, and a smoothness estimated from the residuals using 3dFWHMx with the “–ACF” option. This stringent cluster-forming threshold was used to avoid the problems of false positives associated with less stringent cluster-forming thresholds and is based on current best practices (28, 29).
Results
Participant characteristics
Twelve HC subjects and 27 individuals with T1DM participated in this study. Using the Clarke score (17), individuals with T1DM were further categorized as those with hypoglycemia awareness (T1DM Aware, N = 15) and those with hypoglycemia unawareness (T1DM Unaware, N = 12). Of note, the participants for this study include 39 of 41 subjects from a larger previously published study investigating the impact of hypoglycemia unawareness on brain responses to visual food cues (9).
Demographic characteristics are presented in Table 1. Compared to HC subjects, T1DM Aware individuals were similar in gender, age, BMI, and education level. Compared to T1DM-Aware individuals, T1DM-Unaware individuals were older (P = 0.008), had a higher BMI (P = 0.007), and longer duration of T1DM (P = 0.002). There were no differences in hemoglobin A1c (HbA1c) percentage levels between T1DM-Aware and T1DM-Unaware groups (P = 0.26). As expected, T1DM-Unaware individuals had a significantly higher self-reported number of severe hypoglycemic episodes in the preceding year (P = 0.03).
Table 1.
Participant Characteristics
| Groups | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Control | T1DM-Aware | T1DM-Unaware | ANOVA | HC vs AW | AW vs UW | HC vs UW | |
| (N = 12) | (N = 15) | (N = 12) | |||||
| Number of subjects (F/M) | 7/5 | 12/3 | 7/5 | 0.38 | |||
| Age (years) | 32.0 ± 3.1 | 30.0±2.0 | 41.7±3.4 | 0.01 | 0.61 | 0.005 | 0.024 |
| Education (years) | 17.1 ± 0.8 | 17.0±0.9 | 16.3±0.9 | 0.76 | 0.94 | 0.532 | 0.50 |
| BMI (kg/m2) | 23.8 ± 0.8 | 23.3±0.9 | 27.0±0.9 | 0.009 | 0.72 | 0.004 | 0.01 |
| HbA1c (%) | 5.0 ± 0.1 | 7.0±0.2 | 6.9±0.2 | <0.001 | <0.001 | 0.51 | <0.001 |
| Duration diabetes (years) | — | 12.7±2.7 | 25.4±2.3 | — | — | 0.002 | — |
| C-peptide (ng/ml) | 1.5 ± 0.3 | 0.26±0.1 | 0.1±0.0 | <0.001 | <0.001 | 0.61 | <0.001 |
| Severe hypoglycemic episodes last year (n) | — | 0.1±0.1 | 1.0±0.4 | — | — | 0.03 | — |
| Clarke score | — | 0.8±0.2 | 5.1±0.3 | — | — | <0.001 | — |
| Gold | — | 2.4±0.2 | 4.6±0.3 | — | — | 0.001 | — |
Abbreviations: ANOVA, analysis of variance; AW, aware; BMI, body mass index; F/M, female/male; HC, healthy controls; T1DM, type 1 diabetes mellitus; UW, unaware.
Two-step hyperinsulinemic euglycemic-hypoglycemic clamp
All participants underwent a 2-stepped hyperinsulinemic euglycemic-hypoglycemic clamp (Fig. 1). A mixed-model linear regression finds a difference in the plasma glucose levels during the clamp across the 3 groups (P = 0.01), which was driven by modestly higher glucose levels during euglycemia amongst the T1DM-Aware group. Averaged plasma glucose levels at the time of rsfMRI acquisitions at euglycemia (between time 30–45 minutes) were 102.4 ± 5.6 mg/dL for T1DM-Aware individuals, 89.2 ± 1.6 mg/dL for T1DM-Unaware individuals, and 88.6 ± 1.9 mg/dL for HC subjects (F[2,36] = 4.1, P = 0.03). During rsfMRI acquisitions at hypoglycemia (time 75–90 minutes), mean plasma glucose levels were nearly identical across the 3 groups (59.0 ± 1.3 mg/dL for T1DM-Aware individuals, 61.0 ± 2.1 mg/dL T1DM-Unaware individuals, and 61.8 ± 1.8 mg/dL for HC subjects (F[2,36] = 0.78, P = 0.47). There were no differences in plasma insulin levels across the 3 groups (mixed model linear regression, P = 0.50).
Responses to hypoglycemia
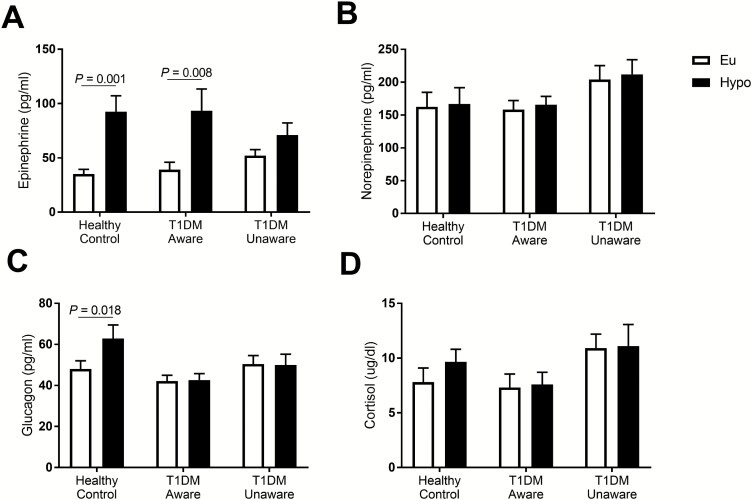
Counterregulatory hormonal responses to hypoglycemia are presented in Fig. 2. As expected, T1DM participants had no hypoglycemia-induced increase in glucagon levels. Furthermore, while HC and T1DM-Aware participants had a significant increase in epinephrine levels in response to mild hypoglycemia, T1DM-Unaware participants had no change in epinephrine levels.
Figure 2.
Hormonal responses to mild hypoglycemia: (A) epinephrine, (B) norepinephrine, (C) glucagon, and (D) cortisol. Open bars denote euglycemia, black bars denote hypoglycemia. Euglycemia values were averaged from time 30 to 45 minutes of clamp. Hypoglycemia values were averaged from time 75 to 90 minutes of clamp. Data presented as mean±standard error of the mean, paired samples t test, *P < 0.05.
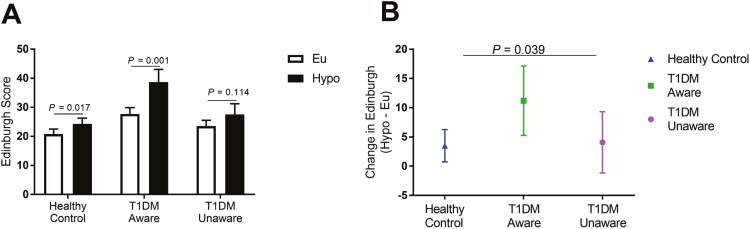
At both euglycemia and hypoglycemia (times 30 and 75 minutes, respectively), participants were asked to rate their symptoms of hypoglycemia using the Edinburgh hypoglycemia symptom score (20). At euglycemia, mean symptom scores were similar across all 3 groups (HC: 20.8 ± 1.8; T1DM-Aware: 27.7 ± 2.2; T1DM-Unaware: 23.5 ± 2.0, F[2,36] = 2.9, P = 0.07) although T1DM-Aware individuals tended to have higher baseline ratings. At hypoglycemia, symptoms scores were significantly different across groups (HC: 24.3 ± 2.0; T1DM-Aware: 38.9 ± 4.3; T1DM-Unaware: 27.6 ± 3.6, F[2,36] = 4.6, P = 0.02). While HC subjects and T1DM-Aware subjects had significant increases in hypoglycemia symptoms during mild hypoglycemia, T1DM-Unaware individuals had no differences in symptoms scores (Fig. 3A). Furthermore, T1DM-Aware individuals also had a more robust symptom response to mild hypoglycemia (Fig. 3B).
Figure 3.
Hypoglycemia symptom scores. (A) Symptoms of hypoglycemia from the Edinburgh Hypoglycemia Symptom score were administered on a Likert scale (1–7) and results were summed. Open bars denote euglycemia, and black bars denote hypoglycemia. (B) Change in Edinburgh symptom scores. Data presented as mean±standard error of the mean, paired samples t test, * P < 0.05.
rsfMRI responses to mild hypoglycemia
Several approaches to assessing resting state functional connectivity have been developed. In this study, we chose to use 2 different approaches: ICD analysis and seed-based analysis.
ICD analysis.
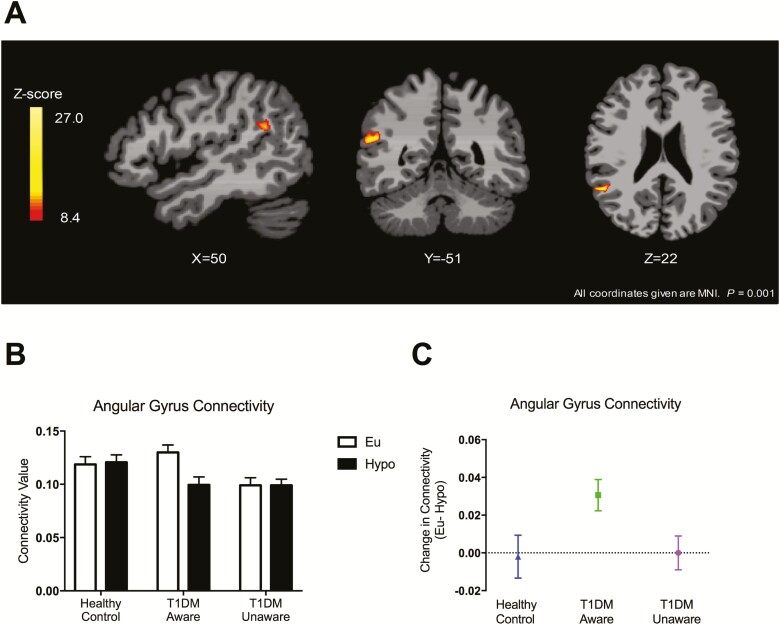
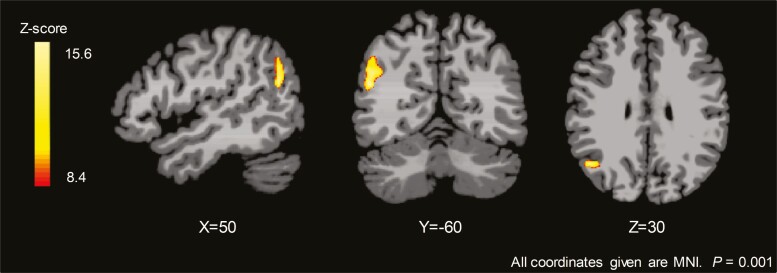
Although prior work by our group and others have identified numerous distinct brain regions that appear to be altered with T1DM and hypoglycemia unawareness (8, 9, 15), there is a great deal of heterogeneity in which brain regions are different across studies. Thus, to avoid arbitrarily limiting our analysis using predefined regions of interest, we sought to survey potential differences in functional connectivity across the 3 groups. To do this, we measured the ICD, which is a voxel-based method of rsfMRI analysis that models the distribution of all the connections to any voxel. This method has the advantage of allowing assessments of the entire brain and not requiring defined a priori regions of interest. Furthermore, ICD analysis takes into account the entire distribution of connections for each voxel and thus does not set arbitrary thresholds to determine connectivity between 2 regions (25, 30). Across all 3 groups, there was a significant difference between euglycemia and hypoglycemia (Group × Condition Interaction) in the angular gyrus, even after adjusting for age, BMI, and plasma glucose levels (Fig. 4A, initial threshold of P < 0.001, whole brain, FWE corrected at P < 0.05). Connectivity values were extracted from the significant cluster in the angular gyrus and the differences appeared to be driven by the T1DM-Aware group who had a greater change in connectivity between euglycemia and hypoglycemia compared to the other 2 groups (Fig. 4B,C). Of note, 2 T1DM-Aware individuals had plasma glucose levels >150 mg/dL at the start of rsfMRI scanning. ICD analysis was also performed without these 2 individuals and revealed nearly identical findings in the angular gyrus, and thus these individuals were included in the analysis (31). In addition, to assess whether duration of diabetes was driving our findings, we also performed a subanalysis among only the individuals with T1DM with and without covarying for duration of diabetes and found no differences (31).
Figure 4.
Intrinsic connectivity distribution analysis: Group × Glycemia Effects. (A) Brain response to mild hypoglycemia compared to euglycemia across all three groups (covaried for age, body mass index, and plasma glucose levels); initial threshold of P < 0.001, 2-tailed, family-wise error whole brain corrected at P < 0.05). (B) Averaged connectivity values extracted from each participant (region of interest identified from significant cluster in right angular gyrus). Data expressed as mean ± standard error of the mean. (C) Change in connectivity values. Data expressed as mean ± 95% confidence interval.
Seed-based connectivity analysis (PCC as a seed).
Because the angular gyrus has been shown in numerous studies to be consistently deactivated at rest as part of the DMN (31–34), we next sought to investigate changes in the DMN across groups. The DMN is an important network of discrete brain regions that consistently decrease activity during attention-demanding tasks (35, 36). One standard method to assess the DMN is by using the PCC as a seed region because it directly interacts with the other nodes in the DMN and is not lateralized (36, 37). We again identified a significant Group × Condition interaction in the right angular gyrus (Fig. 5).
Figure 5.
Seed based analysis: Group × Glycemia Effects. Brain response to mild hypoglycemia compared to euglycemia across all 3 groups (covaried for age, body mass index, and plasma glucose levels); initial threshold of P < 0.001, 2-tailed, familywise error whole brain corrected at P < 0.05.
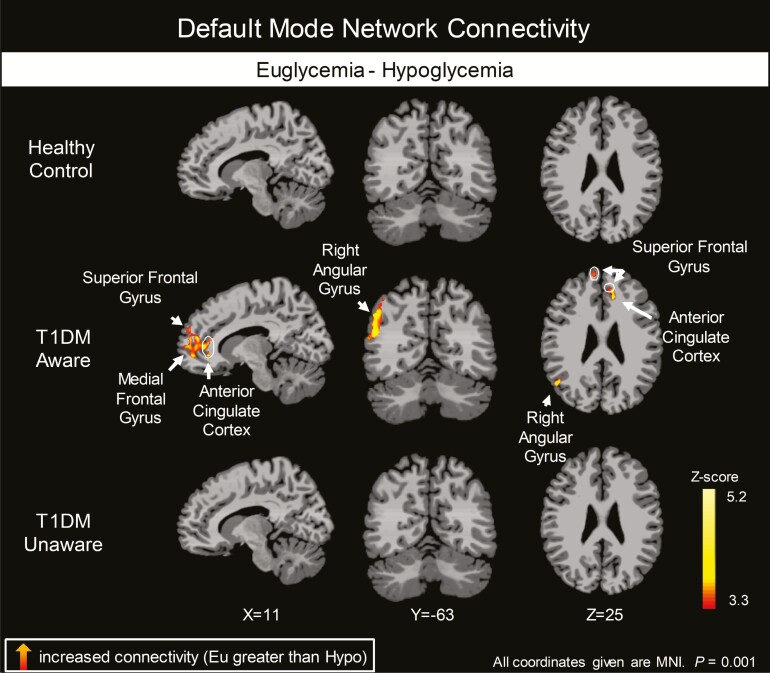
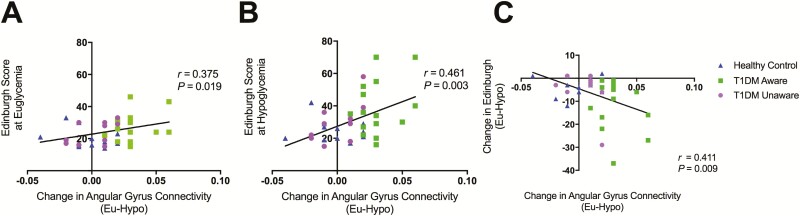
To further investigate which groups were driving this significant overall interaction, we then examined each group’s resting state functional connectivity patterns separately. The t3 groups had very different responses to mild hypoglycemia (Fig. 6 and digital research materials repository (31)). Specifically, while mild hypoglycemia did not elicit changes in the DMN in HC subjects, T1DM-Aware individuals had a greater change in resting state connectivity in the superior frontal gyrus, medial frontal gyrus, and anterior cingulate cortex, as well as angular gyrus. In contrast, T1DM-Unaware individuals also showed no changes in DMN in response to mild hypoglycemia. Moreover, the change in connectivity value in the angular gyrus correlated positively and significantly with the Edinburgh hypoglycemia symptoms scores at both euglycemia and hypoglycemia (Fig. 7).
Figure 6.
Differences in regional brain responses between mild hypoglycemia and euglycemia conditions. Healthy control, type 1 diabetes mellitus (T1DM)-aware and T1DM-unaware subjects showing differences in brain responses to mild hypoglycemia (Hypo-Eu); covaried by age, body mass index, and plasma glucose levels. Initial threshold of P < 0.001, 2-tailed, familywise error whole brain cluster corrected at α < 0.05).
Figure 7.
Relationship between change in angular gyrus connectivity values and Edinburgh hypoglycemia symptom scores at euglycemia (A), hypoglycemia (B), and euglycemia-hypoglycemia symptom scores (C).
Next, we assessed whether the observed changes in resting state connectivity could be driven by changes in cerebral perfusion between groups. Across all groups, mild hypoglycemia did elicit changes in cerebral perfusion, as expected; however, there were no significant differences between groups using P < 0.001 or P < 0.05 (31).
Relationships between functional connectivity and psychological outcomes
Finally, in an exploratory analysis to begin investigating the relationship between resting state functional connectivity and psychological outcomes in T1DM, we administered a battery of psychologic assessments for anxiety and depression. T1DM-Aware individuals were noted to have higher scores on the Perceived Stress Scale (PSS) (38) compared to T1DM-Unaware individuals (P = 0.011) (31). Furthermore, PSS scores correlated positively and significantly with angular gyrus connectivity values (from the ICD analysis) at euglycemia (r = 0.531, P = 0.016) but not at hypoglycemia (r = 0.35, P = 0.129).
Discussion
Using 2 different methods of functional connectivity analysis, we identified distinct differences in the resting state brain responses to mild hypoglycemia between HC, T1DM-Aware, and T1DM-Unaware participants. In particular, both ICD and seed-based analysis show changes in angular gyrus connectivity that were consistently different across the groups. Furthermore, changes in angular gyrus connectivity also correlated with greater symptoms of hypoglycemia as well as higher scores of perceived stress. These findings provide evidence that T1DM is associated with changes in the brain’s resting state connectivity patterns and that this may also be associated with awareness of hypoglycemia and with alterations in functional outcomes amongst individuals with T1DM.
Anatomically, the angular gyrus sits at the junction of the occipital, temporal, and parietal lobes and is believed to play an important role in the integration of inputs from these brain regions (39). It is involved in theory of mind (34), processing of concrete and abstract concepts (40), and memory (41), as well as attention (42). The angular gyrus is also part of the DMN, a network of discrete brain regions that consistently decrease activity during attention-demanding tasks. In the absence of tasks and when the brain is at wakeful rest, activity in the DMN is believed to reflect the underlying organizational structure of the brain’s intrinsic activity (35). Using the currently recommended thresholds for statistical significance (P < 0.001), we observed that mild hypoglycemia did not elicit changes in functional connectivity HC subjects when using either the ICD analysis approach or a seed-based approach. However, in contrast, T1DM-Aware individuals had greater deactivation of the angular gyrus (Fig. 4B,C) as well as other components of the DMN in response to mild hypoglycemia compared to HC subjects (Fig. 6). Moreover, this difference appears to be driven by relatively higher connectivity values among T1DM-Aware subjects at euglycemia. The T1DM-Unaware cohort did not display this increased connectivity at euglycemia. While the reason for this difference remains unknown, one possible explanation is that the increased connectivity at euglycemia in the T1DM-Aware population reflects a functional reorganization of the brain or an early compensatory hyperconnectivity (15). This is consistent with a magnetoencephalography study that found that patients with T1DM and no microvascular complications had higher DMN connectivity compared to HC subjects (43) as well as a recent fMRI study of children with T1DM, which found compensatory hyperconnectivity at baseline among children with T1DM (14). In addition, van Duinkerken et al (44) showed that patients with T1D without microangiopathy have increased functional connectivity in regions involving in motor and visual processing compared to patients with T1D with microangiopathy. The latter cohort had an earlier age of onset of T1D and a longer duration of diabetes. While our study was not designed to assess markers of cerebral microvascular disease, taken together, these results and ours suggest that perhaps the initial compensatory hyperconnectivity changes are eventually lost with disease progression. This type of pattern has not only been observed in T1D, but in multiple sclerosis patients as well, suggesting that it may be a typical biphasic brain response to certain chronic disease states (45). Future studies will be needed to clarify what factors drive the alteration in functional connectivity over time.
Because deactivation of the DMN occurs normally when the brain is engaged in attention-demanding tasks (35, 36), another possible interpretation of our findings could be that T1DM-Aware individuals respond to mild hypoglycemia with greater attention or a heightened awareness of hypoglycemia. That is, T1DM-Aware individuals are more responsive to small changes in plasma glucose levels and that the physiological stress associated with mild hypoglycemia in this group mirrors the effects of a task-oriented cognitive load, which deactivates the DMN and brings the individual out of the wakeful rest, mind-wandering state. This interpretation is supported by our findings that T1DM-Aware individuals have a more robust symptom response to mild hypoglycemia even though they have similar degree of change in counterregulatory hormone responses such as epinephrine compared to HC subjects. Unlike the T1DM-Aware individuals, T1DM-Unaware participants showed no changes in DMN connectivity in response to mild hypoglycemia. Of note, T1DM-Unaware individuals showed no changes in response to mild hypoglycemia, in a similar manner to HC subjects. Whether this is due to adaptive mechanisms to augment glucose and fuel exposure in the brain during hypoglycemia remains to be determined. Frequent exposure to hypoglycemia has been associated with increased cerebral glucose transport in some (46, 47), but not all studies (48).
While the pattern of brain connectivity changes appeared similar between T1DM-Unaware and HC participants, they had very different counterregulatory and symptom responses suggesting that there may be T1DM-specific mechanisms driving the differences in brain and peripheral responses to hypoglycemia.
To our knowledge, there is only 1 other study that used a hyperinsulinemic euglycemic-hypoglycemic clamp and rsfMRI scanning to investigate the impact of declining glucose levels on functional connectivity. In that study, Bolo and colleagues (16) studied T1DM individuals (but did not distinguish between hypoglycemia aware or unaware) and HC subjects as plasma glucose levels fell from ~90 mg/dL to ~50 mg/dL. They observed that T1DM individuals had an attenuated connectivity response during induction of hypoglycemia compared to HC subjects. Because changes in brain activity are dynamic, it is possible that the change in connectivity we observed among our T1DM-Aware individuals could reflect differences that occur later in time as our participants were scanned after they had been at stable levels of mild hypoglycemia. Moreover, differences in hypoglycemia targets (50 mg/dL vs. 60 mg/dL) or differences in fMRI analysis (ie, different choice of seed regions) may also have contributed to some of the variability in responses.
Finally, we observed significant relationships between changes in brain connectivity values (as well as at euglycemia and hypoglycemia) in the angular gyrus and symptom responses to hypoglycemia. Moreover, measures of stress and anxiety, which have been associated with poor glycemic control and less frequent blood glucose monitoring (49), were also associated with angular gyrus connectivity values at euglycemia (but not hypoglycemia). These exploratory findings would be consistent with reports that individuals with hypoglycemia unawareness have low fear of hypoglycemia (50) and individuals with preserved hypoglycemia awareness have high fear of hypoglycemia (51), suggesting that these individuals not only fail to sense hypoglycemia, but also have developed inappropriate emotional valence associated with potential hypoglycemia. Taken together, our findings raise the possibility that functional connectivity in the DMN and specifically the angular gyrus may play a role in modulating these behavioral outcomes and contribute to the inability to take corrective action to prevent or treat hypoglycemia.
There are, however, some limitations to the current study. First, our study is limited by the small sample size; however, current recommendations for sample size (52, 53) for fMRI studies were not devised for mechanistic human studies where participants are studies under carefully controlled conditions and compared to themselves at 2 clearly defined states. Another limitation to our study is the modest differences (~10 mg/dL) in plasma glucose levels during the euglycemia phase of the study across the 3 groups. Nevertheless, it is unlikely that this small difference in absolute (or rate of change) of plasma glucose levels would have had a significant impact on our findings given that we observed no differences in results when we performed the analysis while covarying for plasma glucose levels as well as with and without individuals who had plasma glucose levels >150 mg/dL. Furthermore, our findings are consistent with other rsfMRI studies that had a wide range of plasma glucose levels (up to 300 mg/dL) (14, 15). Moreover, although our T1DM participants were matched for HbA1c, there may have been other differences between our groups, which could have played a role in modulating our brain findings. In particular, our T1DM-Unaware participants were slightly older and had longer duration of diabetes compared to the T1DM-Aware participants. Thus, further studies may be needed to clarify the complex interplay between duration of diabetes, glycemic control, and age in the development of hypoglycemia unawareness. In our current study design, glycemic state was not counterbalanced across the 3 groups due to concern that antecedent hypoglycemia may alter cerebral energetics and effect resting state connectivity during euglycemia. Counterbalancing could have been achieved if we conducted a euglycemic and hypoglycemic clamp on 2separate study days for each participant. However, this type of study design was not logistically feasible. Finally, although our participants had well-controlled T1DM, were relatively young (mean age 30–40s), and had no reported history of cerebrovascular disease, occult microvascular disease could also have impacted our findings. However, because individual participants were compared to themselves at euglycemia and hypoglycemia, this would have minimized the impact of within-person variability and would decrease any between-group effects of occult cerebrovascular disease.
In conclusion, our data demonstrate that T1DM-Aware individuals have a heightened responsiveness to mild hypoglycemia leading to deactivation of the DMN including the angular gyrus compared to HC subjects. This response is lost among T1DM individuals who have developed hypoglycemia unawareness. These findings may have implications for understanding the neurocognitive changes that occur during insulin treatment among patients with T1DM.
Acknowledgments
We gratefully acknowledge the participants of this study as well as the assistance of the Yale Core lab staff (Mikhail Smolgovsky, Irene Chernyak, Ralph Jacob, Doreen Nemeth, Maria Batsu, Codruta Todeasa), the Yale HRU nurses and staff (Joanne Caprio-Adams, Gina Solomon, Anne O’Connor, Catherine Parmelee, Mary Scanlon, Lynda Knaggs, Carmen Galarza, Elizabeth O’Neal, Joyce Russell, Gayle Pietrogallo, Cynthia Smith), and the Yale Magnetic Resonance Research Center staff (Terry Hickey, Hedy Sarofin, Karen Martin).
Financial Support: This study was supported in part by grants from the NIH R01DK020495 and P30 DK045735 (Sherwin), R01DK099039 (Sinha), K23DK109284 (Hwang), K08AA023545 (Seo), K23DK098286 (Belfort- DeAguiar), the Yale Center for Clinical Investigation supported by the Clinical Translational Science Award (UL1 RR024139).
Clinical Trial Information: Clinical trials registration number: NCT 00580710.
Author Contributions: LP, RoS, and JJH had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JJH, DS, RTC, RaS, and RoS. Acquisition of data: LP, DS, MH, RBD, and JJH. Analysis and interpretation of data: all authors. Writing of manuscript: all authors. Statistical analysis: CL, DG, JJH, and FD.
Additional Information
Disclosure Summary: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. The authors have declared that no conflicts of interest exist. Dr. Sinha serves on the Scientific Advisory Board of Embera Neurotherapeutics. Dr. Hwang has received research funding from Regeneron and General Electric for research studies unrelated to this work.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–735. [DOI] [PubMed] [Google Scholar]
- 2. Brands AM, Kessels RP, Hoogma RP, Henselmans JM, van der Beek Boter JW, Kappelle LJ, de Haan EH, Biessels GJ. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes. 2006;55:1800–1806. [DOI] [PubMed] [Google Scholar]
- 3. Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J; Diabetes C, Complications Trial/Epidemiology of Diabetes I, Complications Study Research G . Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocrine Rev. 2008;29:494–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castellano-Guerrero AM, Guerrero R, Relimpio F, Losada F, Mangas MA, Pumar A, Martinez-Brocca MA. Prevalence and predictors of depression and anxiety in adult patients with type 1 diabetes in tertiary care setting. Acta Diabetologica. 2018;55:943–953. [DOI] [PubMed] [Google Scholar]
- 6. Rees G, Xie J, Fenwick EK, Sturrock BA, Finger R, Rogers SL, Lim L, Lamoureux EL. Association between diabetes-related eye complications and symptoms of anxiety and depression. JAMA Ophthalmol. 2016;134:1007–1014. [DOI] [PubMed] [Google Scholar]
- 7. Peyrot M, Rubin RR. Levels and risks of depression and anxiety symptomatology among diabetic adults. Diabetes Care. 1997;20:585–590. [DOI] [PubMed] [Google Scholar]
- 8. Bolo NR, Musen G, Jacobson AM, Weinger K, McCartney RL, Flores V, Renshaw PF, Simonson DC. Brain activation during working memory is altered in patients with type 1 diabetes during hypoglycemia. Diabetes. 2011;60:3256–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hwang JJ, Parikh L, Lacadie C, Seo D, Lam W, Hamza M, Schmidt C, Dai F, Sejling AS, Belfort-DeAguiar R, Constable RT, Sinha R, Sherwin R. Hypoglycemia unawareness in type 1 diabetes suppresses brain responses to hypoglycemia. J Clin Invest. 2018;128:1485–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. [DOI] [PubMed] [Google Scholar]
- 11. Raichle ME, Mintun MA. Brain work and brain imaging. Ann Rev Neurosci. 2006;29:449–476. [DOI] [PubMed] [Google Scholar]
- 12. Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. [DOI] [PubMed] [Google Scholar]
- 13. Liu D, Duan S, Zhou C, Wei P, Chen L, Yin X, Zhang J, Wang J. Altered Brain functional hubs and connectivity in type 2 diabetes mellitus patients: a resting-state fMRI study. Front Aging Neurosci. 2018;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saggar M, Tsalikian E, Mauras N, Mazaika P, White NH, Weinzimer S, Buckingham B, Hershey T, Reiss AL, Diabetes Research in Children N . Compensatory hyperconnectivity in developing brains of young children with type 1 diabetes. Diabetes. 2017;66:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Duinkerken E, Schoonheim MM, Sanz-Arigita EJ, RG IJ, Moll AC, Snoek FJ, Ryan CM, Klein M, Diamant M, Barkhof F. Resting-state brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes. 2012;61:1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bolo NR, Musen G, Simonson DC, Nickerson LD, Flores VL, Siracusa T, Hager B, Lyoo IK, Renshaw PF, Jacobson AM. Functional connectivity of insula, basal ganglia, and prefrontal executive control networks during hypoglycemia in type 1 diabetes. J Neurosci. 2015;35:11012–11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517–522. [DOI] [PubMed] [Google Scholar]
- 18. Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17:697–703. [DOI] [PubMed] [Google Scholar]
- 19. Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, Sinha R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121:4161–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deary IJ, Hepburn DA, MacLeod KM, Frier BM. Partitioning the symptoms of hypoglycaemia using multi-sample confirmatory factor analysis. Diabetologia. 1993;36:771–777. [DOI] [PubMed] [Google Scholar]
- 21. Li X, Morgan PS, Ashburner J, Smith J, Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods. 2016;264:47–56. [DOI] [PubMed] [Google Scholar]
- 22. Friedman L, Glover GH, Fbirn C. Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. NeuroImage. 2006;33:471–481. [DOI] [PubMed] [Google Scholar]
- 23. Scheinost D, Papademetris X, Constable RT. The impact of image smoothness on intrinsic functional connectivity and head motion confounds. NeuroImage. 2014;95:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joshi A, Scheinost D, Okuda H, Belhachemi D, Murphy I, Staib LH, Papademetris X. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics. 2011;9:69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scheinost D, Benjamin J, Lacadie CM, Vohr B, Schneider KC, Ment LR, Papademetris X, Constable RT. The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity. NeuroImage. 2012;62:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scheinost D, Kwon SH, Lacadie C, Vohr BR, Schneider KC, Papademetris X, Constable RT, Ment LR. Alterations in anatomical covariance in the prematurely born. Cerebral Cortex. 2017;27:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, Kriegeskorte N, Milham MP, Poldrack RA, Poline JB, Proal E, Thirion B, Van Essen DC, White T, Yeo BT. Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci. 2017;20:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheinost D, Shen X, Finn E, Sinha R, Constable RT, Papademetris X. Coupled intrinsic connectivity distribution analysis: a method for exploratory connectivity analysis of paired FMRI data. PLoS One. 2014;9:e93544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29:14496–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 2001;54:287–298. [DOI] [PubMed] [Google Scholar]
- 33. Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. [DOI] [PubMed] [Google Scholar]
- 34. Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. [DOI] [PubMed] [Google Scholar]
- 35. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. [DOI] [PubMed] [Google Scholar]
- 36. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage. 2008;42:1178–1184. [DOI] [PubMed] [Google Scholar]
- 38. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 39. Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang J, Conder JA, Blitzer DN, Shinkareva SV. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Hum Brain Mapp. 2010;31:1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. NeuroImage. 2010;50:1648–1657. [DOI] [PubMed] [Google Scholar]
- 42. Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. [DOI] [PubMed] [Google Scholar]
- 43. Demuru M, van Duinkerken E, Fraschini M, Marrosu F, Snoek FJ, Barkhof F, Klein M, Diamant M, Hillebrand A. Changes in MEG resting-state networks are related to cognitive decline in type 1 diabetes mellitus patients. Neuroimage Clin. 2014;5:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Duinkerken E, Klein M, Schoonenboom NS, Hoogma RP, Moll AC, Snoek FJ, Stam CJ, Diamant M. Functional brain connectivity and neurocognitive functioning in patients with long-standing type 1 diabetes with and without microvascular complications: a magnetoencephalography study. Diabetes. 2009;58:2335–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roosendaal SD, Schoonheim MM, Hulst HE, Sanz-Arigita EJ, Smith SM, Geurts JJ, Barkhof F. Resting state networks change in clinically isolated syndrome. Brain. 2010;133:1612–1621. [DOI] [PubMed] [Google Scholar]
- 46. Boyle PJ, Kempers SF, O’Connor AM, Nagy RJ. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:1726–1731. [DOI] [PubMed] [Google Scholar]
- 47. Boyle PJ, Nagy RJ, O’Connor AM, Kempers SF, Yeo RA, Qualls C. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci U S A. 1994;91:9352–9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seaquist ER, Moheet A, Kumar A, Deelchand DK, Terpstra M, Kubisiak K, Eberly LE, Henry PG, Joers JM, Oz G. Hypothalamic glucose transport in humans during experimentally induced hypoglycemia-associated autonomic failure. J Clin Endocrinol Metab. 2017;102:3571–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Herzer M, Hood KK. Anxiety symptoms in adolescents with type 1 diabetes: association with blood glucose monitoring and glycemic control. J Pediatr Psychol. 2010;35:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rogers HA, de Zoysa N, Amiel SA. Patient experience of hypoglycaemia unawareness in Type 1 diabetes: are patients appropriately concerned? Diabet Med. 2012;29:321–327. [DOI] [PubMed] [Google Scholar]
- 51. Anderbro T, Gonder-Frederick L, Bolinder J, Lins PE, Wredling R, Moberg E, Lisspers J, Johansson UB. Fear of hypoglycemia: relationship to hypoglycemic risk and psychological factors. Acta Diabetologica. 2015;52:581–589. [DOI] [PubMed] [Google Scholar]
- 52. Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol Sci. 2011;22:1359–1366. [DOI] [PubMed] [Google Scholar]
- 53. Pajula J, Tohka J. How many is enough? Effect of sample size in inter-subject correlation analysis of fMRI. Comput Intell Neurosci. 2016;2016:2094601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.