Abstract
Background
The Anxiety Symptoms Questionnaire (ASQ) is a brief self-report questionnaire which measures frequency and intensity of symptoms and was developed to improve assessment of anxiety symptoms in a clinical setting. We examined the reliability and validity of the ASQ in patients with anxiety disorders and/or depression, non-clinical control subjects and college students.
Methods
240 outpatients with generalised anxiety disorder, social anxiety disorder, panic disorder or major depressive disorder were administered the ASQ and additional questionnaires measuring depression and anxiety, as were 111 non-clinical control subjects and 487 college students. Factor analysis, Pearson’s correlation coefficients and logistic regression were used to assess reliability and validity. Test–retest reliability of the ASQ was measured using a subset who were re-administered the ASQ after 4 weeks.
Results
Factor analysis revealed measurement of a single dimension by the ASQ. Internal consistency and test–retest reliability were strong. The ASQ total score also significantly distinguished patients with an anxiety disorder from the clinical controls above and beyond the clinician-rated Hamilton Anxiety Scale.
Conclusions
The ASQ is a valid, reliable and effective self-rated measure of anxiety and may be a useful tool for screening and assessing anxiety symptoms in psychiatric as well as college settings.
Keywords: anxiety, depression, outcome and process assessment (healthcare), psychiatry and psychology, psychiatric status rating scales
Collectively, anxiety disorders are the most common mental health problem in the USA, affecting approximately 18% of adults in the general population per year, and 29% of adults at some point during their life-time.1 Anxiety itself is an adaptive and universal human reaction to stressful situations. Determining when anxiety reaches the level of clinical interference with daily activities or the level of diagnosing an anxiety disorder is guided by (1) intensity (the level of distress experienced by the person) and (2) frequency/duration (whether the anxiety occurs often and persists for longer than would be expected under the circumstances). For the diagnosis of an anxiety disorder according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) to be made, the presence of a specific symptom profile associated with significant distress or impairment is required.2 Therefore, it is clinically important to be able to reliably measure both the intensity and frequency of anxiety symptoms during a specified period of time, and to measure how that changes over time.
Anxiety symptoms manifest in physical, affective, cognitive and behavioural domains. Physical symptoms of anxiety typically reflect autonomic arousal, such as shortness of breath, chest tightness, racing heartbeat, upset stomach, dizziness, trembling and numbness/tingling. Emotional symptoms of anxiety range from feelings of nervousness and edginess to terror and panic. Cognitive symptoms of anxiety include worry, apprehension, trouble concentrating and negative thoughts regarding possible threat. Behavioural symptoms of anxiety are typically aimed at diminishing or preventing the perceived threat or distress through avoidance, escape and safety-seeking behaviours; both behavioural and cognitive symptoms of anxiety often lead to poor functioning at home, work or in social situations.
Over the last 20 years, research has advanced the development, validation and dissemination of evidence-based psychological and pharmacological treatments for anxiety disorders.3 4 However, until recently, evidence-based approaches to assessing anxiety and anxiety disorders have received much less attention in the literature. Moreover, the current approach to assessing anxiety commonly remains disorder-based, therefore not capturing the nosological research that suggests a more dimensional approach to understanding and classifying anxiety and other psychiatric disorders.5 6 The most common measure used to assess anxiety in treatment outcome studies is the Hamilton Anxiety Scale (HAM-A),7 8 which is a primary measure for generalised anxiety disorder (GAD) and is often used to assess general anxiety symptoms across conditions. Although the HAM-A is a valid and reliable measure, it is clinician-administered, requires extensive training to use and is time consuming. The HAM-A has also been criticised for focusing too heavily on somatic symptoms, although this is understandable given that it was developed before the separation of GAD and panic disorder.9 Researchers have also pointed out that the HAM-A does not adequately measure the central symptoms of GAD, and that the majority of symptoms assessed by the HAM-A (eg, cardiovascular, respiratory and gastrointestinal symptoms) are not among the associated symptoms of GAD in the DSM, fourth edition, text revision ( DSM-IV-TR) or DSM-5.10 These issues have resulted in a lack of sensitivity of the HAM-A to symptom change, as observed in a clinical trial in which worry symptoms decreased significantly, but HAM-A scores did not.11 Another potential problem with the HAM-A scale is that it does not provide guidance on how to balance severity/intensity and frequency/duration when rating a given symptom, thus limiting its reliability. The HAM-A also has been shown to have only moderate discriminant validity between symptoms of anxiety and depression.8
Alternatives to the HAMA-A include self-report measures of anxiety, the most commonly used being the Beck Anxiety Inventory (BAI).12 The BAI is a self-report scale, but research has suggested that the BAI is not a broad measure of anxiety as a whole, but rather of physical symptoms of anxiety only.13 Significantly higher BAI scores have been observed in individuals with panic disorder than those with other anxiety disorders.14 In addition to the BAI, the GAD-715 is another ultra-brief self-report measure being used mostly in primary care settings to screen and assess GAD but has not been widely adopted for use by the research community. The GAD-7 is an excellent tool for measuring presence/absence of anxiety dysfunction and as an initial screener, but assesses only frequency but not intensity of symptoms.
As a possible alternative to the HAM-A and BAI, our research team from the Massachusetts General Hospital developed a new measure of anxiety—the Anxiety Symptoms Questionnaire (ASQ). The ASQ is a brief and practical self-report assessment for evaluating the severity of anxiety symptoms over the past week. The ASQ contains 17 items, selected by a panel of experts in anxiety disorders, that measure the frequency and intensity of anxiety symptoms on a 0–10 Likert-type scale, for example, “How intense or bothersome have the symptom(s) been in the past week and how frequently have you experienced the symptom(s) in the past week?” (see the Appendix for the full scale). The ASQ was designed to be a global measure of anxiety (ie, measuring more than just physical symptoms), and to assess a range of symptoms central to anxiety, including nervousness, worrying, irritability, trouble relaxing, insomnia, lack of energy, difficulty concentrating, somatic symptoms and impairment in functioning due to anxiety. For each anxiety symptom, the individual reports both frequency and intensity of that symptom, yielding two subscales (ASQ frequency and ASQ intensity, with scores obtained by summing the 17 questions within that subscale). The ASQ total score is obtained by summing the 17 frequency and 17 intensity scores together, with no reverse scoring used. The ASQ takes an average of 2–3 min to complete, and as such, is briefer than the BAI, with fewer items and a broader range of answer choices (0–10 rather than 0–3).
gpsych-2019-100144supp001.pdf (28.2KB, pdf)
The present analysis examines the reliability and validity of the ASQ in both a clinical sample (patients and healthy controls seen at the Massachusetts General Hospital Center for Anxiety and Traumatic Stress Disorders, and a college student sample (students from two Boston area colleges with various levels of undiagnosed anxiety and depression). We also sought to demonstrate the ability of the ASQ to detect symptoms of anxiety independent of symptoms of depression, and investigated demographic predictors of ASQ total score.
Method
Analysis population
A total of 240 outpatients with GAD (n=70), social anxiety disorder (SAD, n=57), major depressive disorder (MDD, n=88) or panic disorder (n=25) according to DSM-IV were administered the ASQ and additional questionnaires measuring depression and anxiety (see figure 1 for flow chart). The ASQ was also administered to 111 non-clinical, healthy control subjects. Subjects in the clinical sample were recruited and provided written consent. Analyses of reliability and internal consistency were based on those 152 patients with GAD, SAD or panic disorder (ie, excluded those with MDD and healthy controls). Demographic characteristics of these patients and controls are summarised in table 1.
Figure 1.
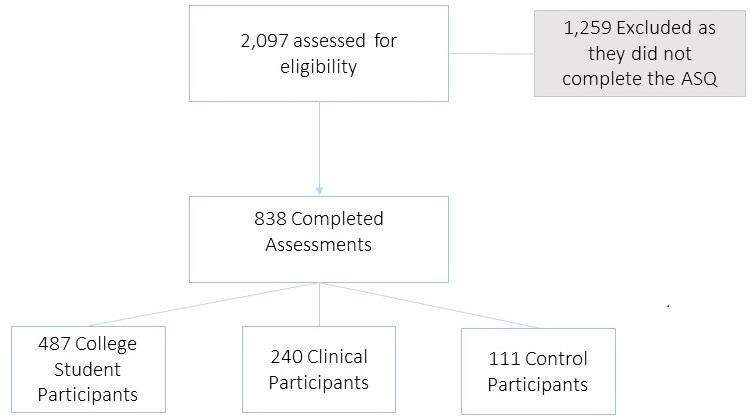
Study Flow Diagram. ASQ, Anxiety Symptoms Questionnaire.
Table 1.
Demographic and clinical characteristics of the analysis population
| Characteristic | Clinical sample—excluding controls (n=240) | Clinical sample—controls (n=111) | College sample (n=487) |
|||
| N | % | N | % | N | % | |
| Age (years), mean (SD) | 37.9 | (14.3) | 41.2 | (13.1) | 19.6 | (1.5) |
| Gender | ||||||
| Female | 111 | 46 | 62 | 56 | 329 | 68 |
| Race* White | 201 | 84 | 78 | 70 | 358 | 74 |
| Black/African-American | 18 | 8 | 16 | 14 | 16 | 3 |
| Asian | 8 | 3 | 7 | 6 | 70 | 14 |
| Native American/Alaska Native | 1 | <1 | 0 | 0 | 4 | <1 |
| Other | 12 | 5 | 10 | 9 | 19 | 4 |
| Ethnicity | ||||||
| Non-Hispanic | 222 | 93 | 100 | 91 | – | |
| Hispanic | 18 | 8 | 11 | 10 | – | – |
| Any comorbidity† | 124 | 52 | 0 | 0 | 59 | 12% |
| ASQ total score, mean (SD) | 101.8 | (76.6) | 14.2 | (16.6) | 72.7 | (54.3) |
| HAM-A total score, mean (SD) | 13.6 | (9.6) | – | – | ||
| BAI total score, mean (SD) | – | – | 7.9 | (8.6) | ||
Non-Hispanic Black, American Indian/Alaskan Native, Asian/Pacific Islander, other.
*In the college sample, race/ethnicity were captured using the categories: non-Hispanic white,.
†College students were asked if they had any chronic medical conditions.
ASQ, Anxiety Symptoms Questionnaire; BAI, Beck Anxiety Inventory; HAM-A, Hamilton Anxiety Scale.
The college sample comprised a total of 487 college students recruited from two local colleges in Boston for participation in an on-campus mental health screening (see figure 1 for flow chart). All participants received a description of the parent study’s aims and screening procedures and provided written informed consent to participate in the screening. A detailed description of the parent study can be found in the study by Farabaugh et al. 16 Demographic characteristics of the college sample, including self-identified race and ethnicity, are described in table 1.
Procedures
All participants in the clinical sample completed the ASQ in a clinical research setting. A subset of patients who were participating in either a study of mindfulness-based stress reduction for the treatment of GAD17 or a double-blind randomised placebo-controlled trial of three potential pharmacological treatment strategies for patients with treatment refractory SAD18 completed the ASQ again after 4 weeks (allowing enough time to not have a memory of previous answers), and were also re-administered questionnaires measuring depression and anxiety.
Data from the college student sample were collected during the screening portion of a larger study assessing depression and suicide risk in college students.16 Participants completed self-report measures described below in the assessment section. Data were collected at baseline, and for a subset of students at one of the colleges, measures were repeated after 4 weeks.
Assessment
Diagnosis and clinical severity
Anxiety
The ASQ (see online supplementary appendix) is a 17-item self-report inventory designed to measure symptoms of anxiety. The intensity (how intense or bothersome the symptom has been) and the frequency of each symptom (within the past week) are rated on a 10-point scale with anchors. Intensity anchors include none (0), mild (1–3), moderate (4–6), severe (7–9) and extreme (10) distress. Frequency anchors include never (0), occasionally (1–3), often (4–6), usually (7–9) and all the time (10). When clusters of symptoms are presented in a single item (eg, gastrointestinal symptoms of stomach upset, nausea, constipation, diarrhoea or irritable bowels), the participant is instructed to rate the intensity and severity for the most troublesome symptom. Total ASQ scores range from 0 to 340, and intensity and frequency subtotals range from 0 to 170. Constructs assessed include anxiety, nervousness, worry, irritability, muscle tension, trouble relaxing, restlessness or being on edge, concentration and memory difficulties, anticipatory anxiety, four clusters of somatic symptoms, and functional impairment due to anxiety.
The HAM-A is a 14-item clinician-rated scale measuring anxiety severity. Each item is defined by a series of symptoms and is rated on a 5-point scale ranging from 0 (no symptoms or absent) to 4 (very severe). Total HAM-A scores range from 0 to 56.7 Only the clinical sample was administered the HAM-A. The BAI12 is a 21-item self-report measure of intensity of anxiety. Each item is scored from 0 to 3, with higher scores indicative of greater anxiety severity. Prior studies suggest that the BAI comprises between two to four factors, which often are made up of one cognitive and at least one somatic factor.19–21 Only participants in the college sample were administered the BAI.
Depression
The Montgomery Asberg Depression Rating Scale (MADRS) is a 10-item clinician-rated measure of MDD symptom severity. Each item is scored from 0 to 6; total MADRS scores range from 0 to 60. Higher MADRS score indicates more severe depression.22 Only the clinical sample was administered the MADRS. The Beck Depression Inventory (BDI-II)23 is a 21-item self-rated assessment of depression that measures both psychological symptoms and physical symptoms. Each item is scored from 0 to 3, with higher scores indicative of greater depression severity. Prior research has supported the reliability and validity of the BDI for use in various populations.24–26 Only participants in the college sample were administered the BDI-II.
Analysis overview
The clinical analyses are based on 240 patients’ and 111 controls’ responses to the ASQ, HAM-A, MADRS and Clinical Global Impression-Severity (CGI-S).27 In most analyses, the 152 patients with GAD, SAD and panic disorder were combined to form a group with any anxiety disorder. The college analyses are based on 487 college students’ responses to the ASQ, BAI and BDI. College students were not administered diagnostic assessments, but we examined ASQ results by presence or absence of high anxiety symptoms (n=71, defined a priori as BAI total ≥16) and/or high depressive symptoms (n=74, defined a priori as BDI total ≥14).
Below, in the results section, we detail the methods and results of our examination of the reliability and validity of the ASQ in clinical and college samples, along with demographic and clinical predictors of ASQ total score. The square root transformation was applied to the ASQ, HAM-A, BAI and MADRS total scores measures to improve or achieve normality. All analyses were conducted using SAS V.9.3 (SAS Institute, Cary, North Carolina, USA) and employed a two-sided α=0.05 for significance.
Unidimensionality of the ASQ
Statistical methods
To examine the construct validity of the ASQ, we conducted an exploratory factor analysis with a four-factor model without any factor rotation. We examined eigenvalues, factor loadings, the proportion of the total variance explained and Cattell’s scree plot. In the clinical sample, the analysis was based on 152 patients with an anxiety disorder (GAD/SAD/panic disorder), but was repeated by diagnosis as well.
Results
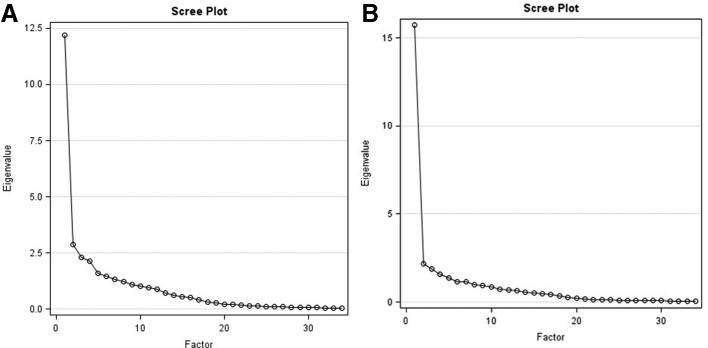
Table 2 shows the results of the exploratory factor analyses, and figure 2A and B show Cattell’s scree plots in the clinical/college samples. The magnitude of difference between the eigenvalues for factor 2 vs factors 2, 3 or 4, along with the presence of a single elbow after factor 1, supports the unidimensionality of the ASQ. Factor 1 accounted for 62%/74% of the total variance in the clinical/college samples, respectively, and each of the remaining factors accounted for <15% of the total variance. Items generally loaded strongly onto factor 1 and weakly onto the remaining factors, with the exception of question 7 (Trouble falling or staying asleep) intensity and frequency in the clinical sample, and question 10 (Trouble remembering things) intensity in the college sample, which loaded almost equally onto two different factors. The evidence for unidimensionality of the ASQ remained strong in factor analyses conducted by diagnosis within the clinical sample.
Table 2.
Exploratory factor analysis results (without rotation)
| ASQ scale question | Clinical sample | College sample | ||||||
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
| A1 | 0.62 | 0.10 | – 0.43 | 0.00 | 0.76 | –0.21 | – 0.32 | –0.09 |
| A2 | 0.64 | 0.01 | – 0.39 | 0.05 | 0.75 | –0.20 | – 0.31 | –0.13 |
| A3 | 0.68 | –0.10 | –0.26 | –0.02 | 0.73 | –0.28 | – 0.31 | –0.12 |
| A4 | 0.57 | –0.08 | –0.09 | 0.05 | 0.61 | –0.18 | 0.11 | 0.19 |
| A5 | 0.52 | –0.06 | 0.48 | –0.25 | 0.59 | 0.17 | 0.07 | 0.34 |
| A6 | 0.67 | –0.01 | 0.05 | – 0.43 | 0.77 | 0.01 | –0.18 | 0.25 |
| A7 | 0.35 | 0.06 | 0.41 | – 0.47 | 0.63 | 0.09 | –0.01 | 0.44 |
| A8 | 0.62 | –0.38 | 0.10 | 0.30 | 0.71 | –0.21 | 0.14 | 0.23 |
| A9 | 0.57 | –0.59 | 0.09 | 0.36 | 0.69 | – 0.40 | 0.31 | –0.06 |
| A10 | 0.51 | – 0.31 | 0.41 | 0.38 | 0.56 | – 0.33 | 0.58 | –0.20 |
| A11 | 0.52 | 0.47 | 0.03 | 0.24 | 0.65 | 0.33 | 0.15 | –0.20 |
| A12 | 0.43 | 0.39 | 0.07 | 0.25 | 0.62 | 0.36 | –0.05 | –0.01 |
| A13 | 0.57 | 0.47 | 0.14 | 0.08 | 0.68 | 0.28 | 0.12 | 0.07 |
| A14 | 0.55 | 0.52 | 0.10 | 0.11 | 0.61 | 0.48 | 0.19 | – 0.31 |
| A15 | 0.76 | –0.06 | –0.02 | 0.04 | 0.75 | 0.04 | –0.02 | 0.03 |
| A16 | 0.71 | 0.12 | –0.11 | 0.07 | 0.69 | 0.09 | –0.22 | –0.21 |
| A17 | 0.64 | –0.04 | – 0.31 | –0.06 | 0.74 | –0.04 | –0.09 | –0.22 |
| B1 | 0.72 | –0.08 | – 0.35 | –0.20 | 0.74 | –0.21 | – 0.33 | –0.11 |
| B2 | 0.7 | –0.09 | – 0.43 | –0.13 | 0.74 | –0.20 | –0.29 | –0.18 |
| B3 | 0.68 | –0.17 | –0.27 | –0.19 | 0.74 | –0.27 | –0.3 | –0.17 |
| B4 | 0.67 | –0.10 | –0.08 | –0.08 | 0.65 | –0.10 | 0.09 | 0.17 |
| B5 | 0.49 | –0.11 | 0.45 | – 0.35 | 0.6 | 0.20 | 0.09 | 0.33 |
| B6 | 0.66 | –0.01 | 0.09 | – 0.54 | 0.76 | 0.01 | –0.16 | 0.29 |
| B7 | 0.34 | 0.05 | 0.42 | – 0.52 | 0.63 | 0.10 | –0.02 | 0.42 |
| B8 | 0.60 | – 0.33 | 0.17 | 0.23 | 0.69 | –0.21 | 0.12 | 0.28 |
| B9 | 0.60 | – 0.57 | 0.14 | 0.22 | 0.65 | – 0.39 | 0.35 | –0.01 |
| B10 | 0.50 | –0.30 | 0.42 | 0.28 | 0.52 | – 0.32 | 0.60 | –0.18 |
| B11 | 0.57 | 0.39 | 0.09 | 0.29 | 0.67 | 0.33 | 0.17 | –0.19 |
| B12 | 0.43 | 0.37 | 0.11 | 0.22 | 0.61 | 0.35 | –0.02 | 0.03 |
| B13 | 0.55 | 0.47 | 0.17 | 0.15 | 0.68 | 0.24 | 0.11 | 0.12 |
| B14 | 0.52 | 0.51 | 0.15 | 0.11 | 0.61 | 0.48 | 0.20 | –0.3 |
| B15 | 0.76 | –0.06 | 0.06 | –0.01 | 0.78 | 0.07 | 0.02 | –0.02 |
| B16 | 0.70 | 0.07 | –0.07 | –0.02 | 0.69 | 0.15 | –0.18 | –0.18 |
| B17 | 0.63 | –0.1 | –0.29 | –0.17 | 0.73 | –0.04 | –0.04 | –0.23 |
| Eigenvalues | 12.19 | 2.88 | 2.31 | 2.14 | 15.73 | 2.16 | 1.87 | 1.58 |
| % of variance | 62% | 15% | 12% | 11% | 74% | 10% | 9% | 7% |
Factor loadings with an absolute value >0.30 are bolded.
ASQ, Anxiety Symptoms Questionnaire.
Figure 2.
(A) Cattell's Scree Plot for the ASQ - Clinical Sample. (B) Cattell's Scree Plot for the ASQ - College Sample.
Reliability of the ASQ
Statistical methods
To examine the internal consistency of the ASQ, Cronbach’s α was calculated for the entire ASQ scale, and separately for the intensity and frequency subscales in the clinical and college samples. We also examined whether reliability changed within subgroups of gender, race/ethnicity, and comorbidity.
Test–retest reliability of the ASQ total score was measured using Pearson’s correlation between week 0 and week 4. In the clinical sample, we restricted the test–retest analysis to a subset of GAD/SAD (n=43) patients who had no change in global severity (as measured by the CGI-S) between week 0 and 4, to ensure that any differences observed in the ASQ total score were unlikely to be explained by improvement or progression of the patients’ anxiety symptoms. This was done as to not have confounding results between reliability and clinical change over time. Instead, we isolated participants who were known to have no substantial change in clinical symptoms, which was operationalised as no change in score on the CGI-S scale (eg, CGI-S=4 at week 0 and CGI-S=4 at week 4). These 43 patients also had either no change in treatment or decreased their medication between weeks 0 and 4. In the college sample, the test–retest analysis was based on a subset of students with longitudinal data at one of the colleges (n=27), with no further restrictions made. We compared the ASQ test–retest reliability with that of the HAM-A/BAI in the clinical/college sample, respectively, using two-sided 95% CIs around the correlation coefficients.
Results
Reliability of the ASQ was extremely high in the clinical/college samples, respectively, with a Cronbach’s α of 0.94/0.96 for the scale overall, 0.89/0.93 for the intensity subscale and 0.90/0.93 for the frequency subscale. Reliability remained high by disorder and presence of comorbidity/chronic medical conditions (table 3), as well as within categories of gender and race/ethnicity (results not shown). Each question was positively and strongly correlated with the total. While question 7 (Trouble falling or staying asleep) had the lowest correlation with other questions with respect to both intensity (r=0.35) and frequency (r=0.33) in the clinical sample, removing this question did not improve the Cronbach’s α. Similarly, while question 10 (Trouble remembering things) had the lowest correlation with the total with respect to both intensity (r=0.54) and frequency (r=0.50) in the college sample, reliability did not improve with its removal.
Table 3.
Reliability of the Anxiety Symptoms Questionnaire (ASQ)
| ASQ measure | Diagnosis | n | Cronbach’s α |
| Clinical sample | |||
| Overall | Anxiety disorder (GAD/SAD/panic) | 152 | 0.94 |
| With any comorbidity | 75 | 0.94 | |
| Without comorbidity | 77 | 0.94 | |
| By diagnosis: | |||
| GAD | 70 | 0.93 | |
| SAD | 57 | 0.92 | |
| Panic | 25 | 0.97 | |
| MDD | 88 | 0.96 | |
| Control | 111 | 0.92 | |
| Intensity subscale | Anxiety disorder (GAD/SAD/Panic) | 152 | 0.89 |
| Frequency subscale | Anxiety disorder (GAD/SAD/panic) | 152 | 0.9 |
| College sample | |||
| Overall | All students | 487 | 0.96 |
| With ≥1 chronic medical condition | 59 | 0.96 | |
| Without chronic medical conditions | 427 | 0.96 | |
| By anxiety: | |||
| BAI ≥16 | 71 | 0.92 | |
| BAI <16 | 416 | 0.94 | |
| By depression: | |||
| BDI ≥14 | 74 | 0.94 | |
| BDI <14 | 413 | 0.95 | |
| Intensity subscale | All students | 487 | 0.93 |
| Frequency subscale | All students | 487 | 0.93 |
BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; GAD, generalised anxiety disorder; MDD, major depressive disorder ; SAD, social anxiety disorder.
In the clinical sample, test–retest reliability of the ASQ was high and not significantly different from that of the HAM-A among the GAD/SAD patients with no change in CGI severity between weeks 0 and 4. Pearson’s correlation for the ASQ total between week 0 and week 4 was 0.72 (95% CI 0.53 to 0.84, p<0.001), while that of the HAM-A total was 0.75 (95% CI 0.57 to 0.85, p<0.001). The test–retest reliability of the ASQ total was also high in the college sample, and did not significantly differ from that of the BAI total. Pearson’s correlation for the ASQ total between week 0 and week 4 was 0.77 (95% CI 0.55 to 0.89, p<0.001), while that of the BAI total was 0.80 (95% CI 0.59 to 0.91], p<0.001).
Concurrence with existing measures of anxiety
Statistical methods
To examine the concurrent validity of the ASQ with the HAM-A/BAI (clinical/college samples), we calculated Pearson’s partial correlations between the ASQ total and HAM-A total in the clinical sample (controlling for presence of any comorbidity), and between the ASQ total and BAI total in the college sample (controlling for presence of a chronic medical condition).
Results
The partial correlation (95% CI) between the ASQ total and HAM-A total, controlling for comorbidity, was 0.68 (0.58 to 0.75) among patients with an anxiety disorder, 0.59 (0.43 to 0.71) among patients with MDD and 0.43 (0.27 to 0.57) among controls. In patients with an anxiety disorder, the correlation with the HAM-A was strongest among those with panic disorder (r=0.80), followed by those with GAD (r=0.61) and those with SAD (r=0.48). In the college sample, the partial correlation (95% CI) between the ASQ total and BAI total, controlling for chronic medical conditions, was 0.79 (0.75 to 0.82). Thus, concurrent validity with existing measures of anxiety was high in both samples, and in the clinical sample was highest among patients with panic disorder, which is unsurprising given the HAM-A focus on somatic symptoms.
Differentiation between anxiety and depression
Statistical methods
In the clinical sample, we examined the ability of the ASQ to discriminate between patients with an anxiety disorder and healthy controls, above and beyond the discriminant ability of the HAM-A. To do so, we generated a multivariate logistic regression model with diagnostic group (GAD/SAD/panic vs controls) as the outcome, ASQ total score as the covariate of interest and controlling for HAM-A total score. Below, in the results section, we report the resulting OR and 95% CI associated with a 10-point increase in ASQ total score (each question on the ASQ is scored on a scale of 0–10). We also examined the ability of the ASQ to discriminate between patients with an anxiety disorder diagnosis and patients with an MDD diagnosis, restricting the analysis to those with no comorbid diagnoses.
In the college sample, we were unable to examine whether the ASQ predicted anxiety diagnosis above and beyond the BAI, since the presence of high anxiety was defined using the BAI total score rather than diagnosis (as diagnostic assessments were not undertaken for the student sample). Instead, we examined the ability of the ASQ to discriminate between those with and without high anxiety, as well as between those with high anxiety but low depression versus those with high depression but low anxiety (ie, akin to examining patients with no comorbidity).
Results
The ASQ total significantly discriminated between those with an anxiety disorder and healthy controls, both univariately (χ2=30.63, p<0.0001) and after accounting for the discriminant ability of the HAM-A (χ2=12.42, p=0.0004). A 10-point increase in ASQ total score resulted in double the likelihood of being classified as having an anxiety disorder versus being a control (OR, 95% CI 2.2, 1.4 to 3.3), after controlling for the HAM-A.
Of the 152 patients diagnosed with an anxiety disorder, 75 had one or more comorbid disorders, as did 49 of the 88 patients diagnosed with MDD. When we examined the ability of the ASQ to differentiate between anxiety and depression among patients with no comorbidity, we found that the ASQ total score was a significant univariate predictor of diagnosis (χ2=6.83, p=0.0090), although the effect size associated with a 10-point increase in total score was small (OR, 95% CI 1.1, 1.03 to 1.2). Once we controlled for the HAM-A, the ASQ total was no longer a significant predictor of anxiety only versus depression (χ2=2.31, p=0.13), although power may have been limited for this analysis.
In the college sample, the ASQ total was able to significantly distinguish between patients with high versus low anxiety (based on a BAI total score cut-off of 16). Results were similar when we controlled for the presence of high or low depression. We also found that the ASQ total was a borderline significant predictor of high anxiety/low depression versus high depression/low anxiety (p=0.092; OR, 95% CI 1.1, 0.98 to 1.2 associated with a 10-unit increase in ASQ total score).
To better dismantle the detection of depression by the ASQ and HAM-A in the clinical sample, we examined partial Pearson’s correlations (and their 95% CIs) between the ASQ/HAM-A and the MADRS, controlling for presence of comorbidity and separately by diagnosis. As seen in table 4, the ASQ total was less strongly correlated with the MADRS overall than was the HAM-A, although differences were not statistically significant. Because the MADRS measures both anxiety and depression constructs, we examined the correlations with only those MADRS items that targeted depression by summing question 1 (apparent sadness) and question 2 (reported sadness). We found that the magnitude of the correlation coefficients substantially decreased when correlating the ASQ with the MADRS q1+q2 rather than the MADRS total score and that among patients with MDD, the correlation with MADRS q1+q2 was virtually zero.
Table 4.
Correlation of the ASQ vs HAM-A with depression—clinical sample
| Diagnosis | Pearson’s partial correlation (95% CI), controlling for presence of comorbidity | |||
| ASQ vs MADRS total | ASQ vs MADRS q1+q2 | HAM-A vs MADRS total | HAM-A vs MADRS q1+q2 | |
| GAD/SAD/Panic | r=0.55 (0.43 to 0.65) | r=0.32 (0.17 to 0.46) | r=0.62 (0.51 to 0.71) | r=0.38 (0.24 to 0.51) |
| GAD | r=0.50 (0.30 to 0.66) | r=0.25 (0.02 to 0.46) | r=0.49 (0.29 to 0.65) | r=0.33 (0.11 to 0.53) |
| SAD | r=0.30 (0.04 to 0.52) | r=0.20 (–0.07 to 0.44) | r=0.29 (0.03 to 0.52) | r=0.29 (0.03 to 0.52) |
| Panic | r=0.70 (0.41 to 0.86) | r=0.44 (0.05 to 0.72) | r=0.79 (0.56 to 0.90) | r=0.45 (0.06 to 0.73) |
| MDD | r=0.41 (0.22 to 0.57) | r=0.05 (-0.16 to 0.26) | r=0.44 (0.25 to 0.60) | r=0.03 (–0.19 to 0.23) |
| Control | r=0.48 (0.32 to 0.61) | r=0.30 (0.12 to 0.46) | r=0.61 (0.48 to 0.71) | r=0.21 (0.03 to 0.38) |
ASQ, Anxiety Symptoms Questionnaire; GAD, generalised anxiety disorder; MADRS, Montgomery Asberg Depression Ratting Scale; MDD, major depressive disorder; SAD, social anxiety disorder.
Thus, it appears that the ASQ has high discriminant validity when comparing patients with an anxiety disorder versus healthy controls, and that the ASQ captures aspects of anxiety not captured by the HAM-A such as the explicit frequency and intensity of symptoms. The ASQ was also able to distinguish between anxiety and depression.
Predictors of ASQ total score
Statistical methods
Covariates examined as predictors of the ASQ total score included diagnosis (clinical sample only), gender, age (years), presence of a comorbidity/chronic medical condition (clinical/college sample), race (white, black/African-American, Native American/Alaska Native, Asian, other), ethnicity (Hispanic/Latino, non-Hispanic/Latino) and MADRS/BDI total score (clinical/college sample). In the college sample, race/ethnicity were collected in combined fashion as non-Hispanic black, Hispanic, non-Hispanic white, American Indian/Alaskan Native, Asian/Pacific Islander, other. Covariates were first tested individually, and significant individual predictors were then entered into a multivariate linear regression model. In the clinical sample, we controlled for diagnosis in all models. The assumption of linearity of each continuous covariate with the outcome was verified. For comparison, we also examined predictors of the HAM-A/BAI (clinical/college samples).
Results
After controlling for diagnosis (which was a significant univariate predictor), gender, age, presence of comorbidity and MADRS total score were all significant predictors of the ASQ total score individually in the clinical sample. Correlations between these covariates were generally small, indicating that collinearity was not of great concern (the largest correlation, between MADRS total and presence of comorbidity, was r=0.50). In the final multivariate model in the clinical sample (table 5), significance of each covariate was maintained, with the exception that comorbidity became borderline significant (χ2=3.68, p=0.055). Patients with the highest ASQ total scores were those with a primary diagnosis of GAD, female patients, younger patients, those with one or more comorbid diagnoses and patients with higher MADRS total scores. The significance of gender and age as predictors of ASQ total score persisted even among healthy controls. For comparison, we found that patients with the highest HAM-A total scores were those with a primary diagnosis of GAD (β (SE)=3.1 (0.13), t=614.33, p<0.001 vs controls), female patients (β (SE)=0.2 (0.085), t=8.11, p=0.004) and those with one or more comorbid diagnoses (β (SE)=0.3 (0.10), t=8.97, p=0.003). Unlike with the ASQ, HAM-A total scores did not significantly differ by age or MADRS total score, nor were age or gender significant predictors of HAM-A total score among controls.
Table 5.
Multivariate predictors of ASQ total—clinical sample
| Covariate | Clinical | |||
| β estimate | SE | Wald χ2 | P value | |
| Diagnosis (vs healthy control) | 711.8 | <0.0001 | ||
| GAD | 5.1 | 0.51 | 100.3 | <0.0001 |
| SAD | 4.5 | 0.47 | 92 | <0.0001 |
| Panic | 5.2 | 0.56 | 85.9 | <0.0001 |
| MDD | 2.8 | 0.59 | 21.9 | <0.0001 |
| Gender (F vs M) | 0.4 | 0.23 | 3.9 | 0.049 |
| Age (years) | –0.04 | 0.0079 | 22.3 | <0.0001 |
| Comorbidity (Y vs N) | 0.5 | 0.27 | 3.68 | 0.055 |
| MADRS total | 1.3 | 0.076 | 99.8 | <0.0001 |
| BDI total | – | – | – | – |
ASQ, Anxiety Symptoms Questionnaire; BDI, Beck Depression Inventory; F, female; GAD, generalised anxiety disorder; M, male; MADRS, Montgomery Asberg Depression Ratting Scale; MDD, major depressive disorder; N, no; Y, yes.
Significant univariate predictors of the ASQ total score in the college sample included presence of a chronic medical condition (χ2=6.16, p=0.013) and BDI total score (χ2=573.81, p<0.001). The correlation between BDI total score and chronic medical condition was statistically significant, but low in magnitude (r=0.11, p=0.013). In a multivariate model, chronic medical condition was no longer significant (χ2=0.89, p=0.34) after controlling for BDI total score. Students with the highest ASQ total scores were those with higher BDI total scores (β (SE)=1.7 (0.073), χ2=573.8, p<0.0001), and once depression was controlled for, the ASQ total score did not significantly vary by any other demographic covariates examined. For comparison, students with the highest BAI scores also tended to have higher BDI total scores (β (SE)=0.7 (0.038), χ2=345.5, p<0.001), but we also found that BAI total scores were significantly higher among females (β (SE)=0.4 (0.11), χ2=10.4, p=0.001).
Discussion
The results of this validation study of the ASQ suggest that it is a highly reliable, consistent and valid self-rated anxiety measure that is comparable, and in some cases superior, to the gold-standard measure frequently used in treatment outcome studies—the more time consuming, clinician-rated HAM-A. In both clinical and college student samples, we found that the reliability of the ASQ was extremely high, with Cronbach’s α ranging from 0.89 to 0.96 both for the entire measure and the frequency and intensity subscales. Factor analyses upheld construct validity by confirming a single dimension in patients with an anxiety diagnosis in the clinical sample and among college students overall, supporting its relevance for identifying those with a core set of anxiety symptoms that occur concurrently. The evidence for unidimensionality of the ASQ remained strong when examined by diagnosis as well (based on the eigenvalue plots). This offers a significant advantage in using the ASQ over the HAM-A which is not unidimensional. Additionally, the test–retest reliability of the ASQ was high and comparable to that of the HAM-A (r=0.72).
The ASQ also discriminated well between patients with and without anxiety disorders, and between anxiety and depression symptoms in patients without comorbid disorders. However, it was unable to significantly distinguish between patients with an anxiety disorder and MDD when comorbidity existed. When we examined correlations between the ASQ/HAM-A and apparent and reported sadness (items 1 and 2 of the MADRS), our findings revealed that the correlation between the MADRS and the ASQ is largely due to the items more closely tied to anxiety symptoms rather than those of depression symptoms. These findings align with previous research suggesting a significant overlap between GAD and depression,5 and the current research domain criteria at the National Institute of Mental Health focusing on better elucidating a core set of negative valence symptoms that may not align with traditional diagnosis of depression and anxiety disorders.28 They also provide further evidence that the ASQ picks up fewer features of depression than does the HAM-A.
As a brief, self-report assessment for measuring symptoms of anxiety, the ASQ has multiple advantages over other such scales, and can provide clinicians and researchers with a more efficient means of evaluating anxiety symptom severity. Moreover, as the ASQ measures the frequency and intensity associated with the wide range of physical, emotional, cognitive and behavioural symptoms experienced by patients struggling with anxiety over time, this scale offers a significant advantage over the BAI, which focuses predominantly on physical symptoms. While literature has been mixed, some studies have suggested that the BAI is more a measure of symptoms of panic than of general anxiety,13 given its somatic focus. In studies of the BAI, individuals with panic disorder endorse significantly higher scores on the BAI than those with other anxiety disorders (eg, Beck and Steer14). The ASQ, on the other hand, appears to be a more global measure of anxiety, with implications both for the clinical and research-oriented assessment of anxiety.
ASQ and HAM-A total scores differed significantly by demographic and clinical characteristics. The magnitude and direction of effects of predictors were similar for the ASQ and HAM-A, with the main differences being that whereas ASQ total scores were higher among females than males and among younger versus older patients, no significant differences in HAM-A total score were observed by gender or age. However, the magnitude of the effects were small, with female gender and each decade decrease in age associated with only a 0.4-point increase in the square root of the ASQ total score. Our findings in the college sample that higher ASQ and BAI total scores were observed among those with higher BDI total scores may be due to comorbidity, and are limited by the relatively low level of symptom severity and lack of diagnostic assessments.
Future studies should examine whether the ASQ can discriminate between other psychopathological groups in clinical populations with anxiety; the present study was limited to samples with anxiety and/or depression. Validation in racially and culturally diverse populations is also needed, as the present study was largely comprised of white participants. Additionally, research on the ASQ’s sensitivity to clinical change (ie, treatment gains) as well as validation against other measures of anxiety (eg, the State-Trait Anxiety Inventory,29 the Depression Anxiety Stress Scales)30 is needed. Our reliance on CGI score to determine lack of clinical change is a limitation of this evaluation of test–retest reliability. Additionally, having self-administered questionnaires in the college sample and clinician administered questionnaires in the clinical sample is a potential confounding factor that should be eliminated in future studies. Although the 10-point Likert scale may offer added sensitivity to therapeutic effects, it may also be useful in future studies to see if the scale can be collapsed to fewer anchors by reducing the Likert scale for increased ease of use. To further establish the ASQ as an empirically supported assessment, studies with larger samples of clinical groups as well as studies correlating the ASQ with physiological and behavioural indices of anxiety (eg, startle response, fear-conditioning, etc) would be useful. Also, examining how the ASQ relates to other aspects of anxiety such as worry or experiential avoidance would be beneficial. These future directions would further solidify the clinical utility as well as utility in reliably detecting change in clinical trials of the ASQ.
Conclusion
Taken together, our results show that the ASQ is a valid and reliable measure across age and gender groups, correlating highly with existing anxiety measures, yet able to capture symptoms of anxiety not captured by the HAM-A. The ASQ allows for specific reporting of the frequency and intensity of symptoms, and offers the additional advantages of being self-rated in format, and brief in administration.31 It is thus a good candidate for use in outcome studies for GAD and other anxiety disorders, and its strong test–retest reliability supports its utility in longitudinal clinical assessments. Finally, the ASQ provides a global assessment of general anxiety that parallels the mental health field’s paradigm shift towards a more dimensional approach to assessing psychological disorders.
Biography
 Dr. Amanda Baker received her Ph.D. from Boston University in 2013 and completed her pre-doctoral internship and post-doctoral training at Massachusetts General Hospital/Harvard Medical School. She is currently a research and clinical psychologist at the Center for Anxiety and Traumatic Stress Disorders in the Psychiatry Department at Massachusetts General Hospital and assistant professor at Harvard Medical School where she has worked since 2013. Dr. Baker is an active member of several professional societies such as the Anxiety and Depression Association of America where she is vice-chair of the Early Career Special Interest Group and the Association for Behavioral and Cognitive Therapies. Her main research interests include the development, validation, and dissemination of empirically based psychosocial treatments for anxiety and related disorders.
Dr. Amanda Baker received her Ph.D. from Boston University in 2013 and completed her pre-doctoral internship and post-doctoral training at Massachusetts General Hospital/Harvard Medical School. She is currently a research and clinical psychologist at the Center for Anxiety and Traumatic Stress Disorders in the Psychiatry Department at Massachusetts General Hospital and assistant professor at Harvard Medical School where she has worked since 2013. Dr. Baker is an active member of several professional societies such as the Anxiety and Depression Association of America where she is vice-chair of the Early Career Special Interest Group and the Association for Behavioral and Cognitive Therapies. Her main research interests include the development, validation, and dissemination of empirically based psychosocial treatments for anxiety and related disorders.
Footnotes
Contributors: NS, AF, TD, EH, MF and MPP conceived of the project and designed the study and contributed to the writing of the manuscript. NS, AF, TD, JJW and EH collected the data. AK and AB designed the analyses and conducted the statistical plan. NS and AB wrote the initial draft of the manuscript.
Funding: Jed Foundation and Highland Street Foundation.
Competing interests: NS has the following disclosures to declare: Research Grants: Department of Defense, PCORI, Highland Street Foundation, NIH; Speaking/CME/Consulting: MGH Psychiatry Academy, Axovant Sciences, Springworks, Praxis Therapeutics, Aptinyx, Genomind; Equity: Spouse: G1 Therapeutics; Royalty/patent: Wolters Kluwer (royalty). AB has the following disclosures to declare: Research Grants: NARSAD; Speaking/CME/Consulting: MGH Psychiatry Academy; Royalty/patent: New Harbinger Publications; MF has the following disclosures to declare: Research Support: Abbott Laboratories; Acadia Pharmaceuticals; Alkermes, Inc.; American Cyanamid; Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; AXSOME Therapeutics; Biohaven; BioResearch; BrainCells, Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clarus Funds; Clexio Biosciences; Clintara, LLC; Covance; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; FORUM Pharmaceuticals; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; Indivior; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck, Inc.; Marinus Pharmaceuticals; MedAvante; Methylation Sciences, Inc; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Coordinating Center for Integrated Medicine (NiiCM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; NeuroRx; Novartis AG; Organon Pharmaceuticals; Otsuka Pharmaceutical Development, Inc.; PamLab, LLC.; Pfizer, Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates, Inc.; Pharmavite LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shenox Pharmaceuticals, LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceuticals; Tal Medical; VistaGen; Wyeth-Ayerst Laboratories. Advisory Board/Consultant: Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma, Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; BioXcel Therapeutics; Biovail Corporation; Boehringer Ingelheim; Boston Pharmaceuticals; BrainCells, Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; Clexio Biosciences; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co., Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai, Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; Indivior; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm, Inc.; Lorex Pharmaceuticals; Lundbeck, Inc.; Marinus Pharmaceuticals; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Navitor Pharmaceuticals, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC; Perception Neuroscience; Pfizer, Inc.; PharmaStar; Pharmavite LLC; PharmoRx Therapeutics; Polaris Partners; Praxis Precision Medicines; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; PPD; PThera, LLC; Purdue Pharma; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Relmada Therapeutics, Inc.; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Shenox Pharmaceuticals, LLC; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex; Teva Pharmaceuticals; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Usona Institute, Inc.; Vanda Pharmaceuticals, Inc.; Versant Venture Management, LLC; VistaGen. Speaking/Publishing: Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer, Inc.; PharmaStar; United BioSource, Corp.; Wyeth-Ayerst Laboratories. Stock/Other Financial Options: Equity Holdings: Compellis; PsyBrain, Inc. Royalty/Patent, Other Income: Patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD) (US_7840419, US_7647235, US_7983936, US_8145504, US_8145505) and patent application for a combination of Ketamine plus Scopolamine in major depressive disorder (MDD), licensed by MGH to Biohaven. Patents for pharmacogenomics of Depression Treatment with Folate (US_9546401, US_9540691). Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ) and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte. Ltd.
Patient consent for publication: Not required.
Ethics approval: This study was approved by Massachusetts General Hospital Institutional Review Board (approval numbers: 2003P-000051 and 2004P001522).
Provenance and peer review: Commissioned; externally peer reviewed.
Data availability statement: Data are available upon request
References
- 1. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National comorbidity survey replication. Arch Gen Psychiatry 2005;62:617–27. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association Diagnostic and statistical manual of mental disorders. 5th edn, 2013. [Google Scholar]
- 3. McLean PD, Woody SR. Anxiety disorders in adults: an evidence-based approach to psychological treatment. Oxford University Press, 2001. [Google Scholar]
- 4. Stein DJ. Evidence-Based treatment of anxiety disorders. Int J Psychiatry Clin Pract 2006;10 Suppl 1:16–21. 10.1080/13651500600552487 [DOI] [PubMed] [Google Scholar]
- 5. Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM-IV anxiety and mood disorders: implications for assessment and treatment. Psychol Assess 2009;21:256–71. 10.1037/a0016608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frances AJ, Widiger T. Psychiatric diagnosis: lessons from the DSM-IV past and cautions for the DSM-5 future. Annu Rev Clin Psychol 2012;8:109–30. 10.1146/annurev-clinpsy-032511-143102 [DOI] [PubMed] [Google Scholar]
- 7. Hamilton M. Diagnosis and rating of anxiety. Br J Psychiatry 1969;3:76–9. [Google Scholar]
- 8. Maier W, Buller R, Philipp M, et al. The Hamilton anxiety scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord 1988;14:61–8. 10.1016/0165-0327(88)90072-9 [DOI] [PubMed] [Google Scholar]
- 9. Montgomery D, European College of Neuropsychopharmacology . ECNP consensus meeting March 2000. Guidelines for investigating efficacy in GAD. Eur Neuropsychopharmacol 2002;12:81–7. 10.1016/s0924-977x(01)00147-x [DOI] [PubMed] [Google Scholar]
- 10. Koerner N, Antony MM, Dugas MJ. Limitations of the Hamilton anxiety rating scale as a primary outcome measure in randomized, controlled trials of treatments for generalized anxiety disorder. Am J Psychiatry 2010;167:103–4. 10.1176/appi.ajp.2009.09091264 [DOI] [PubMed] [Google Scholar]
- 11. Leichsenring F, Salzer S, Jaeger U, et al. Short-term psychodynamic psychotherapy and cognitive-behavioral therapy in generalized anxiety disorder: a randomized, controlled trial. Am J Psychiatry 2009;166:875–81. 10.1176/appi.ajp.2009.09030441 [DOI] [PubMed] [Google Scholar]
- 12. Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893–7. 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- 13. Cox BJ, Cohen E, Direnfeld DM, et al. Does the Beck anxiety inventory measure anything beyond panic attack symptoms? Behav Res Ther 1996;34:949–54. 10.1016/S0005-7967(96)00037-X [DOI] [PubMed] [Google Scholar]
- 14. Beck AT, Steer RA. Relationship between the Beck anxiety inventory and the Hamilton anxiety rating scale with anxious outpatients. J Anxiety Disord 1991;5:213–23. 10.1016/0887-6185(91)90002-B [DOI] [Google Scholar]
- 15. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 16. Farabaugh A, Bitran S, Nyer M, et al. Depression and suicidal ideation in college students. Psychopathology 2012;45:228–34. 10.1159/000331598 [DOI] [PubMed] [Google Scholar]
- 17. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry 2013;74:786–92. 10.4088/JCP.12m08083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollack MH, Van Ameringen M, Simon NM, et al. A double-blind randomized controlled trial of augmentation and switch strategies for refractory social anxiety disorder. Am J Psychiatry 2014;171:44–53. 10.1176/appi.ajp.2013.12101353 [DOI] [PubMed] [Google Scholar]
- 19. Hewitt PL, Norton GR. The Beck anxiety inventory: a psychometric analysis. Psychol Assess 1993;5:408–12. 10.1037/1040-3590.5.4.408 [DOI] [Google Scholar]
- 20. Osman A, Kopper BA, Barrios FX, et al. The Beck anxiety inventory: reexamination of factor structure and psychometric properties. J Clin Psychol 1997;53:7–14. [DOI] [PubMed] [Google Scholar]
- 21. Wetherell JL, Areán PA. Psychometric evaluation of the Beck anxiety inventory with older medical patients. Psychol Assess 1997;9:136–44. 10.1037/1040-3590.9.2.136 [DOI] [Google Scholar]
- 22. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9. 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 23. Beck AT, Steer RA, Ball R, et al. Comparison of Beck depression Inventories-IA and-II in psychiatric outpatients. J Pers Assess 1996;67:588–97. 10.1207/s15327752jpa6703_13 [DOI] [PubMed] [Google Scholar]
- 24. Bumberry W, Oliver JM, McClure JN. Validation of the Beck depression inventory in a university population using psychiatric estimate as the criterion. J Consult Clin Psychol 1978;46:150–5. 10.1037/0022-006X.46.1.150 [DOI] [Google Scholar]
- 25. Lester D, Beck AT. Suicidal wishes and depression in suicidal ideators: a comparison with attempted suicides. J Clin Psychol 1977;33:92–4. [DOI] [PubMed] [Google Scholar]
- 26. Shaw JA, Donley P, Morgan DW, et al. Treatment of depression in alcoholics. Am J Psychiatry 1975;132:641–4. 10.1176/ajp.132.6.641 [DOI] [PubMed] [Google Scholar]
- 27. Guy W. ECDEU assessment manual for psychopharmacology. US Department of Health, and Welfarea, 1976: 534–7. [Google Scholar]
- 28. Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 2013;11:126 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spielberger CD, Gorssuch RL, Lushene PR, et al. Manual for the State-Trait anxiety inventory. Consulting Psychologists Press, Inc, 1983. [Google Scholar]
- 30. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the Beck depression and anxiety inventories. Behav Res Ther 1995;33:335–43. 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- 31. Nyer M, Mischoulon D, Alpert JE, et al. College students with depressive symptoms with and without fatigue: differences in functioning, suicidality, anxiety, and depressive severity. Ann Clin Psychiatry 2015;27:100–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gpsych-2019-100144supp001.pdf (28.2KB, pdf)