Abstract
Objective
To assess the evolution of antiepileptic drug (AED) treatment patterns and seizure outcomes in England from 2003 to 2016.
Design, setting and participants
Retrospective cohort study of electronic medical records from Clinical Practice Research Datalink and National Health Service Digital Hospital Episode Statistics databases. Patients newly diagnosed with epilepsy were identified and followed until end of data availability. Three eras were defined starting 1 April 2003 (first National Institute for Health and Care Excellence (NICE) guideline); 1 September 2007 (Standard and New Antiepileptic Drugs publication); and 1 January 2012 (second NICE guideline).
Outcome measures
Time from diagnosis to first AED; AED sequence; time from first AED to first 1-year remission period (no new AED attempts and no seizure-related healthcare events); time from first AED to refractoriness (third AED attempt regardless of reason); Kaplan-Meier analysis of time-to-event variables.
Results
4388 patients were included (mean follow-up: 6.8, 4.2 and 1.7 years by era). 84.6% of adults (≥16 years), 75.5% of children (<16) and 89.1% of elderly subgroup (65+) received treatment within 1 year; rates were generally stable over time. Treatment trends included reduced use of carbamazepine (adult first line, era 1: 34.9%; era 3: 10.7%) and phenytoin, earlier line and increased use of levetiracetam (adult first line, era 1: 2.6%; era 3: 26.2%) and lamotrigine (particularly in adults and elderly subgroup), and a larger number of different AEDs used. Valproate use shifted somewhat to later lines. Rates of 1-year remission within 2 years of starting treatment increased in adults (era 1: 71.9%; era 3: 81.4%) and elderly (era 1: 76.1%; era 3: 81.7%). Overall, 55.5% of patients relapsed after achieving 1-year remission. Refractoriness rates remained stable over time (~26% of adults within 5 years).
Conclusion
Treatment trends often were not aligned with era-relevant guidance. However, our results suggest a slight improvement in epilepsy treatment outcomes over the 13-year period.
Keywords: drug resistance, refractoriness, remission, epilepsy, seizure freedom, treatment patterns
Strengths and limitations of this study.
Use of the Clinical Practice Research Datalink and National Health Service Digital Hospital Episode Statistics databases allowed access to a large national pool of patients for identification of those newly diagnosed with epilepsy.
Treatment eras were delineated by epilepsy guideline updates to allow capture of changes in antiepileptic drug treatment practice.
The stringency of diagnostic criteria may limit the generalisability of the data.
The nature of the data is prone to incomplete or incorrect medical records and coding, lack of specificity, and captures prescriptions but not prescription fills.
The definition of remission was based on healthcare consultations, with 1 year possibly too short to be considered for remission; and drug resistance was based on switching antiepileptic drugs without taking into account the reasons for treatment changes, which were unknown but likely driven by lack of effect and poor tolerability.
Introduction
The introduction of new antiepileptic drugs (AED) since 2003 has been accompanied by studies of the comparative efficacy, safety and tolerability of older and newer AEDs,1–3 as well as by evolving clinical practice guidelines that incorporate newer medications into recommendations for epilepsy treatment.4–6 Treatment patterns would be expected to reflect the latest guidance for individual AEDs in epilepsy management, but scant information is available to assess alignment in clinical practice. A number of studies have reported an increase in the use of newer AEDs prescribed for first-line treatment in new-onset epilepsy in UK primary care settings7 8 and across European Union countries.9
Use of newer AEDs with reported similar or improved efficacy and better tolerability than older AEDs would be expected to benefit overall epilepsy treatment success and patient outcomes. However, literature suggests that there has been no meaningful improvement in epilepsy treatment-related outcomes1 10–12 and a notable portion of patients still fail first-line AED therapy.13 14 The objective of this study was to evaluate AED treatment patterns and seizure outcomes in England over three time periods from 2003 to 2016, using electronic medical record (EMR) data, to provide further insights into the management of patients newly diagnosed with epilepsy.
Methods
Study design
This was an exploratory, retrospective cohort analysis of primary care EMRs from the UK Clinical Practice Research Datalink (CPRD) and secondary care claims data from the National Health Service (NHS) Digital Hospital Episode Statistics (HES) databases. The CPRD contained over 4 million active patient records (and more than 11 million overall) drawn from 674 UK primary care practices, representing approximately 7% of the UK population, in 201515; these numbers have since grown.16 Data that may be captured in the CPRD primary care data include general practitioner (GP) prescriptions, diagnoses, procedures, and referrals, coded as Read codes (a system of clinical terms used in UK primary care EMRs),17–19 as well as UK Quality and Outcomes Framework (QOF) indicators, which were designed to reward quality care by GPs and may offer additional clinical information (in the case of epilepsy, the indicators concern seizure frequency). The HES database contains details of all secondary care admissions, outpatient appointments and accident and emergency attendances at NHS hospitals in England.20 Within this database, diagnoses are coded using the International Classification of Diseases, Tenth Edition (ICD-10) and procedures are coded using the Office of Population, Censuses and Surveys Classification of Surgical Operations and Procedures, Fourth Revision.
AED treatment patterns and seizure outcomes in England were assessed over three 4.5-year eras. Era 1 (first guideline era) included dates from 1 April 2003 to 31 August 2007 and encompassed the publication of the first National Institute for Health and Care Excellence (NICE) epilepsy guidance.4 21 22 These guidelines recommended carbamazepine (CBZ) or sodium valproate (VPA) as first-line treatment for focal (partial-onset) and generalised seizures. Era 2 (intermediate era) was defined as 1 September 2007 to 31 December 2011, and captured updated guidance that recommended lamotrigine (LTG) or CBZ as first line for focal (partial-onset) seizures and VPA for generalised seizures based on a large randomised pragmatic trial (Standard and New Antiepileptic Drugs (SANAD)).1 Era 3 (newer guideline era) spanned the time frame from 1 January 2012 to 31 May 2016, with the second NICE epilepsy guidance recommending CBZ or LTG as first-line treatment for focal seizures, VPA for generalised seizures and advice to be given to women of childbearing potential regarding foetal risks of malformation and neurodevelopmental impairments with VPA.6 23 A 2015 update warned against prescribing VPA to pregnant women and those of childbearing potential unless other AEDs were ineffective or not tolerated (in 2018 guidelines, VPA is contraindicated in girls and women of childbearing potential).24
Cohort selection
Patients with epilepsy newly diagnosed between 1 April 2003 and 31 May 2016 (date of last available CPRD practice data) were included in the study. Diagnosis was operationalised as an incident epilepsy diagnosis code (online supplementary table S1), with evidence of a neurologist visit on the same date or in the preceding 3 months, as assessed in HES data or primary care referral data using June 2016 data sets, and constituted the index date. Patients were assigned to a treatment era based on their index date (ie, the index date fell within one of the three defined treatment guideline eras). Those who had started an AED attempt less than 3 months prior to a diagnosis were included. An AED attempt was defined on the start date of an AED prescription that a patient had never used before, and maintained for at least 31 days, as identified in primary care records. A preindex period of at least 2 years was required with their practice’s data flagged as up to standard. Patients were excluded if they had an epilepsy diagnosis at any time before the index date, or AED treatment during the 2-year preindex period. Included patients were followed until data were no longer available, owing either to death or to leaving their GP practice or date of last CPRD data (31 May 2016).
bmjopen-2019-032551supp001.pdf (116.7KB, pdf)
Three age cohorts were considered: adults aged ≥16 years, children ≥2 to <16 years of age, and the elderly, ≥65 years of age (a subset of adult patients).
Outcome measures
The primary outcome was time to 1-year remission from seizures for all treated patients, starting from the time of first AED attempt until the first 1-year period of remission. One-year remission was defined as having no new AED attempts, and the absence of all seizure-related healthcare events (ie, seizure-related hospitalisation or seizure-related GP or outpatient visit; for instance, a GP visit with a diagnosis recorded as ‘1B64.00—had a convulsion’), QOF data and Read codes (online supplementary table S2) indicating a seizure at any time for at least 1 year. A subsequent occurrence of any of these events is defined as a relapse. Outcomes also included time from diagnosis to first AED prescription (all patients); treatment patterns by era, age and sex cohort; and time from first AED prescription to refractoriness (treated patients only), which was defined as a third distinct AED attempt as identified in primary care records. The end of AED exposure and treatment as poly/monotherapy were not assessed.
Statistical analysis
Descriptive statistics were used to summarise both continuous variables and categorical variables such as mean, SD, median and percentages. Analyses were conducted on unmatched cohorts and reported results are unadjusted. Outcomes were evaluated using Kaplan-Meier analysis.
Patient involvement statement
This research was conducted without patient involvement in the design or interpretation of this study, or in the writing and editing of this document.
Results
Study participants
Overall, of 137 267 patients with an epilepsy diagnosis code in the data, 4388 (adults n=3861; children n=527) met the study inclusion criteria and were available for analysis. Mean follow-up was 6.8 years (era 1), 4.2 years (era 2) and 1.7 years (era 3). Baseline characteristics were largely as expected for diagnosed patients (overall population: mean age at diagnosis, 41.4 years; 85.0% of patients with ≥1 AED treatment; 78.4% with unspecified epilepsy) (table 1). There appeared to be higher rates of comorbidities, reflected by epilepsy-specific comorbidity index scores, and lower rates of unspecified epilepsy diagnosis in era 3 compared with era 1. There were minor changes in the regional make-up of CPRD data; for example, in England, the number of practices participating in CPRD generally decreased in eastern regions and increased in London and southern regions over time (ie, in the study data set, London-based practices represent 13.7% of practices contributing data for 2005 whereas they represent 19.0% of those contributing for 2015); other regions remained relatively stable.
Table 1.
Baseline characteristics of the sample population
| Adults (≥16 years) n=3861 |
Children (<16 years) n=527 |
Elderly subgroup* (≥65 years) n=876 |
|
| Age at diagnosis (years), mean (SD) | 45.9 (20.4) | 8.5 (4.0) | 74.7 (7.0) |
| Female, n (%) | 1845 (47.8) | 244 (46.3) | 372 (42.5) |
| Number diagnosed | |||
| Era 1 | 1276 (33.0) | 133 (25.2) | 260 (29.7) |
| Era 2 | 1452 (37.6) | 248 (47.1) | 322 (36.8) |
| Era 3 | 1133 (29.3) | 146 (27.7) | 294 (33.6) |
| Germaine-Smith epilepsy-specific comorbidity index, mean (SD) | 0.8 (1.9) | 0.3 (1.0) | 2.1 (2.5) |
| Era 1 | 0.6 (1.5) | 0.3 (1.0) | 1.4 (2.3) |
| Era 2 | 0.8 (1.8) | 0.2 (0.7) | 2.0 (2.3) |
| Era 3 | 1.1 (2.4) | 0.4 (1.2) | 2.7 (2.9) |
| Epilepsy type | |||
| Generalised, n (%) | 324 (8.4) | 72 (13.7) | 65 (7.4) |
| Era 1, n (% of era) | 91 (7.1) | 15 (11.3) | 15 (5.8) |
| Era 2, n (% of era) | 119 (8.2) | 44 (17.7) | 22 (6.8) |
| Era 3, n (% of era) | 114 (10.1) | 13 (8.9) | 28 (9.5) |
| Focal (partial onset), n (%) | 475 (12.3) | 76 (14.4) | 114 (13.0) |
| Era 1, n (% of era) | 162 (12.7) | 15 (11.3) | 46 (17.7) |
| Era 2, n (% of era) | 153 (10.5) | 31 (12.5) | 29 (9.0) |
| Era 3, n (% of era) | 160 (14.1) | 30 (20.5) | 39 (13.3) |
| Unspecified | 3062 (79.3) | 379 (71.9) | 697 (79.6) |
| Era 1, n (% of era) | 1023 (80.2) | 103 (77.4) | 199 (76.5) |
| Era 2, n (% of era) | 1180 (81.3) | 173 (69.8) | 271 (84.2) |
| Era 3, n (% of era) | 859 (75.8) | 103 (70.5) | 227 (77.2) |
| At least one AED treatment, n (%) | 3313 (85.8) | 417 (79.1) | 783 (89.4) |
| Total follow-up (patient-years)† | 16 483.92 | 2363.94 | 3257.28 |
| Mean follow-up (years) | 4.3 | 4.5 | 3.7 |
| Era 1 | 6.7 | 7.3 | 6.0 |
| Era 2 | 4.1 | 4.6 | 3.7 |
| Era 3 | 1.7 | 1.8 | 1.7 |
Era 1: 1 April 2003 to 31 August 2007 (first NICE guidance); era 2: 1 September 2007 to 31 December 2011 (SANAD); era 3: 1 January 2012 to 31 May 2016 (second NICE guidance).
*Elderly patients are a subset of the adult patient population.
†Total follow-up, patient-years: calculated by adding the follow-up time for all patients.
AED, antiepileptic drug; NICE, National Institute for Health and Care Excellence; SANAD, Standard and New Antiepileptic Drugs.
Many patients (n=4456) who met inclusion criteria were excluded owing to prediagnosis AED use, primarily with AEDs that have multiple indications (eg, VPA, CBZ, LTG). Because of the required 2-year baseline period, no 0 or 1-year-old children were included in the sample.
Time from diagnosis to first AED prescription
Kaplan-Meier estimates revealed that 84.6% of adults, 75.5% of children and 89.1% of the elderly subgroup received AED treatment within 1 year of index date (figure 1). Treatment rates from era 1 to era 3 appeared to increase slightly in adults (from 82.3% to 86.6%) and the elderly subgroup (from 86.3% to 90.8%), and to decrease slightly in children (from 77.4% to 69.2%).
Figure 1.
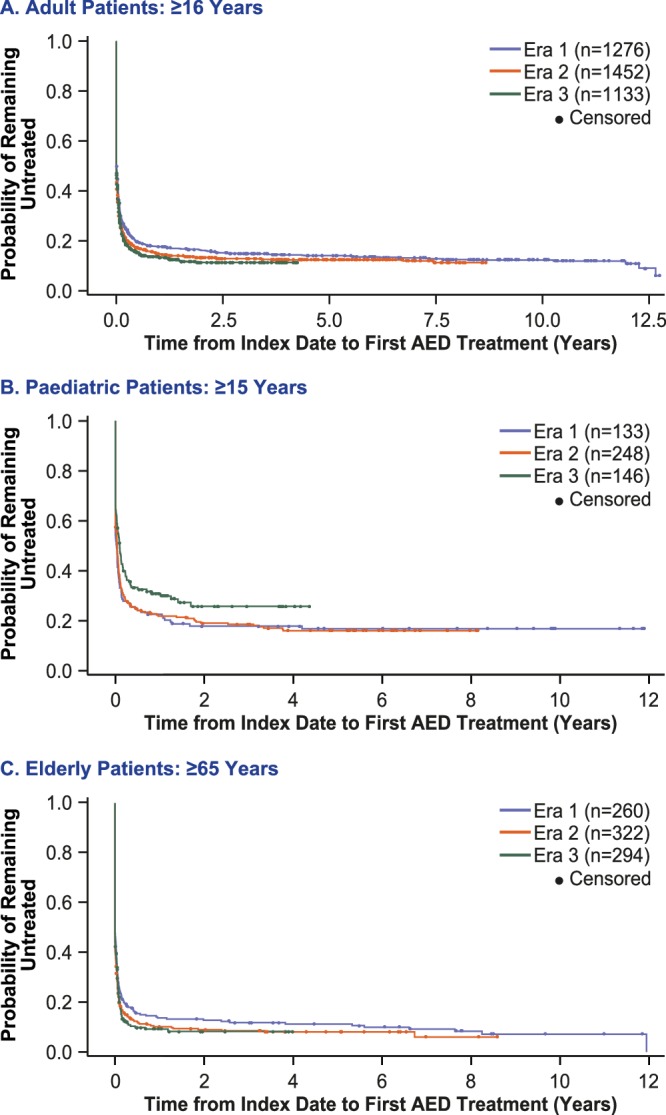
Time from first diagnosis to first AED prescription in (A) adults (n=3861), (B) children (n=527) and C) elderly subgroup (n=876). AED, antiepileptic drug.
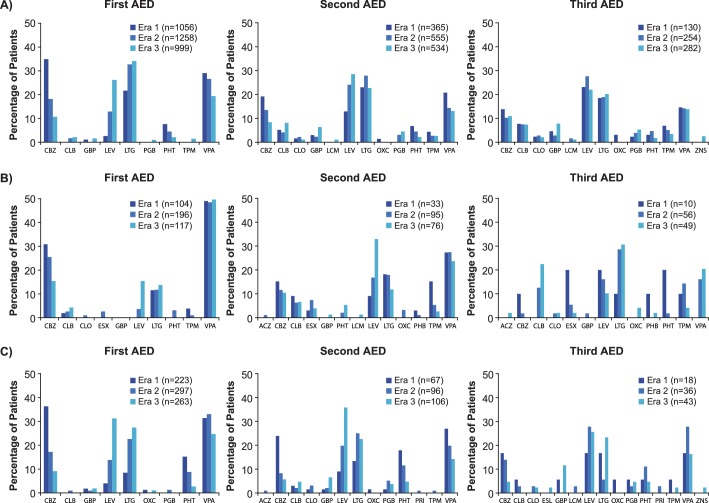
Treatment patterns over time
Analysis of treatment patterns over time in adults shows a large shift away from CBZ (adult first-line share, era 1: 34.9%; era 3: 10.7%) and phenytoin (PHT; adult first-line share, era 1: 7.6%; era 3: 2.1%), and towards earlier and increased use of levetiracetam (LEV; adult first-line share, era 1: 2.6%; era 3: 26.2%) (figure 2A). The use of first-line VPA remained relatively stable over eras 1 and 2, and decreased in era 3. AED treatment patterns appeared to be far more stable in children than adults, with use of first-line VPA remaining high over time (first-line share: in children, era 1: 49.0%; era 3: 49.6%; in adults, era 1: 29.0%; era 3: 19.4%; figure 2B). Nevertheless, the use of CBZ decreased in favour of earlier use of LEV (CBZ first-line share, era 1: 30.8%; era 3: 15.4%; LEV first-line share, era 1: 0%; era 3: 15.4%). These AED patterns were consistent for second-line treatment in children. Treatment pattern changes in the elderly subgroup were similar but more pronounced than in the overall adult patient population. In the elderly subgroup, CBZ use fell in all treatment lines (first-line share, era 1: 36.3%; era 3: 9.1%; second-line share, era 1: 23.9%; era 3: 5.7%; third-line share, era 1: 16.7%; era 3: 4.7%) in favour of earlier and increased use of LTG and LEV (LTG first-line share, era 1: 8.5%; era 3: 27.4%; LEV first-line share, era 1: 4.0%; era 3: 31.2%; figure 2C).
Figure 2.
Treatment patterns by AED attempt in (A) adults, (B) children and (C) elderly subgroup (AEDs accounting for ≥1% of attempts in any patient group in any era). ACZ, acetazolamide; AED, antiepileptic drug; CBZ, carbamazepine; CLB, clobazam; CLO, clonazepam; ESL, eslicarbazepine; ESX, ethosuximide; GBP, gabapentin; LCM, lacosamide; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PGB, pregabalin; PHB, phenobarbital; PHT, phenytoin; PRI, primidone; TPM, topiramate; VPA, valproate; ZNS, zonisamide.
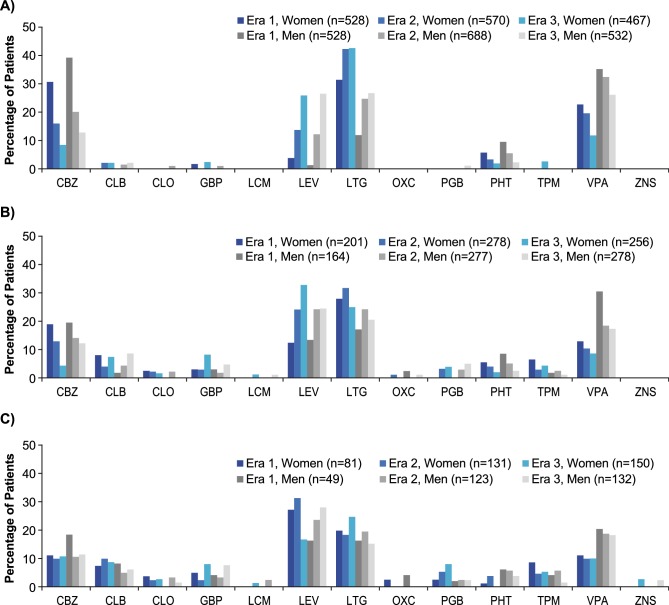
Analysis of treatment patterns by sex indicated that in adults, women were prescribed first-line VPA and CBZ less often and LTG more often than men—a finding that remained stable over time (figure 3). The trends in VPA and LTG use were mostly intact through third-line AED (figure 3). In children, generally LEV and LTG were more common and VPA less common for girls than boys across eras as well as through second-line AED (online supplementary figure S1). In the elderly subgroup, first-line treatment patterns were comparable between men and women (online supplementary figure S2).
Figure 3.
Treatment patterns in adult men and women for (A) first-line, (B) second-line and (C) third-line treatments (AEDs accounting for ≥1% of attempts in any patient group in any era). For first-line treatment, the proportions of women versus men receiving AEDs were: for era 1: VPA, 22.7% vs 35.2%; CBZ, 30.7% vs 39.2%; LTG, 31.4% vs 11.9%; and for era 3: VPA, 11.8% vs 26.1%; CBZ, 8.4% vs 12.8%; LTG, 42.6% vs 26.7%. AED, antiepileptic drug; CBZ, carbamazepine; CLB, clobazam; CLO, clonazepam; GBP, gabapentin; LCM, lacosamide; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PGB, pregabalin; PHT, phenytoin; TPM, topiramate; VPA, valproate; ZNS, zonisamide.
One-year remission rates
One-year remission rates, within 1 or 2 years of treatment initiation, increased somewhat over time in adults and the elderly subgroup, but were more variable in children (table 2). The percentage of patients achieving a 1-year period of remission within 1 year of starting treatment increased from era 1 to era 3 in all three age cohorts. The most substantial increase (from era 1 to era 3) was observed in the elderly subgroup (from 31.5% to 47.3%; a 50.3% increase from era 1). The percentage of adults and the elderly subgroup achieving 1-year remission within 2 years of starting treatment was higher than within 1 year and increased over time (era 1 to era 3: 71.9% to 81.4% adults; 76.1% to 81.7% elderly). In children, there was a slight decrease in remission rates within 2 years of treatment from era 1 to era 3 (table 2). Overall, 55.5% of patients relapsed after achieving 1-year remission.
Table 2.
Treatment outcomes by study population and era
| Adults (≥16 years) n=3313 |
Children (<16 years) n=417 |
Elderly subgroup* (≥65 years) n=783 |
|
| Rate of 1-year remission within 1 or 2 years of treatment | |||
| Patients with at least one period of 1-year remission†, n (%) |
2430 (73.3) | 317 (76.0) | 536 (68.5) |
| Of these patients, at least one relapse†, n (%) | 1362 (56.0) | 163 (51.4) | 310 (57.8) |
| 1-year period of remission 1 year from treatment start (KM estimate) | 35.2% | 40.1% | 36.3% |
| Era 1‡ | 31.6% | 36.4% | 31.5% |
| Era 2‡ | 34.7% | 41.9% | 32.8% |
| Era 3‡ | 42.0% | 40.8% | 47.3% |
| 1-year period of remission within 2 years of treatment start (KM estimate) | 75.3% | 75.9% | 78.4% |
| Era 1‡ | 71.9% | 73.0% | 76.1% |
| Era 2‡ | 75.3% | 78.3% | 78.2% |
| Era 3‡ | 81.4% | 72.8% | 81.7% |
| Rate of refractoriness within 3 years of starting first AED treatment | |||
| Patients refractory 3 years from start of treatment (KM estimate) | 17.5% | 23.8% | 11.9% |
| Era 1‡ | 17.3% | 20.8% | 11.1% |
| Era 2‡ | 17.4% | 24.3% | 13.4% |
| Era 3‡ | 17.6% | (n<10) | 11.2% |
Era 1: 1 April 2003 to 31 August 2007 (first NICE guidance); era 2: 1 September 2007 to 31 December 2011 (SANAD); era 3, 1 January 2012 to 31 May 2016 (second NICE guidance).
*Elderly patients are a subset of the adult patient population.
†Raw figures, not adjusted for differential follow-up between eras.
‡Number of patients diagnosed in eras 1, 2 and 3, respectively, and received treatment: adults, n=1097, 1254 and 962; children, n=110, 204 and 103; elderly subgroup, n=234, 291 and 258.
AED, antiepileptic drug; KM, Kaplan-Meier estimate; NICE, National Institute for Health and Care Excellence; SANAD, Standard and New Antiepileptic Drugs.
Time from first AED treatment to refractoriness
Overall, a similar percentage (about 17%–18%) of adult patients became refractory within 3 years of first starting treatment across the different eras (table 2). Approximately 25%–26% of adults were treatment refractory after 5 years (data not shown). The subgroup of elderly patients was less likely to become treatment refractory than all adults or children (table 2).
Discussion
The results of this analysis suggest that there has been some improvement in epilepsy outcomes, reflected by shorter times to 1-year remission (no new AED attempts or seizure-related healthcare events for at least 1 year) over the 13-year period. Various reasons may contribute to this observation, including improved diagnosis of epilepsy and differential diagnosis of non-epilepsy disorders, more active epilepsy management with personalised treatment and wider use of newer, better tolerated AEDs, particularly to replace enzyme-inducing drugs in the elderly subgroup who are most susceptible to risks associated with enzyme induction.25 Although our study assessed treatment patterns by age group and sex, it did not assess whether prescribing is targeted based on other patient characteristics.
The decreasing adherence of prescribing to treatment guidelines over the time course of this study suggests guidelines are not keeping up with clinical practice and a possible issue in guideline writing. The nature of evidence that contributes to guidelines might play a role here. In epilepsy, the reliance of guidelines primarily on randomised controlled trials, which are scarce, may have led to relevant information being ignored. The observed major changes in prescription trends during era 3 are at odds with NICE guidelines, which suggest prescribing CBZ or LTG as first-line treatment in children and adults with newly diagnosed focal seizures, and VPA for those newly diagnosed with generalised seizures.6 Analysis of first-line treatment patterns over time showed a reduction in VPA use in era 3, a large shift away from CBZ and PHT in all treatment lines and a trend towards earlier and increased usage of LEV in adults, particularly the elderly subgroup.6 Although the sharp decrease in PHT aligns with 2012 NICE guidelines,6 possibly reflecting a change in AEDs used in acute settings or a shift to non-enzyme active AEDs (nEAAEDs) in older patients, the decrease in CBZ and increase in LEV do not align with treatment guidelines.
For elderly patients, 2012 NICE guidelines recommend CBZ as an extended release formulation.6 Preferential use of CBZ in older patients was reported in a study of CPRD data from 2001 to 2010, which found that patients receiving enzyme-inducing AEDs (EIAEDs: CBZ, 63.3%; PHT, 35.3%) were older and had more comorbid illness; the study also reported the use of EIAEDs resulted in higher healthcare costs compared with nEAAEDs.25 Our findings also show that CBZ was most often prescribed for the elderly subgroup during era 1 (2003–2007). Given the higher susceptibility to risks associated with enzyme induction, as well as increased costs, a shift to nEAAEDs would appear a rational change that is reflected in era 3 prescribing trends.
In children, treatment patterns were more stable, which may reflect the situation that fewer new AEDs have become available for this population. Limited choices may be related to the more stringent criteria necessary for drug approval, with more complex trial designs and challenges in recruitment. The stability of VPA use in children may be related to its broad spectrum activity when diagnosis is uncertain.
In women, use of VPA greatly diminished across eras, lending support to a database study in the USA that reported decreased VPA use among adult women.26 These findings are perhaps not surprising given the teratogenic profile of VPA and increased warnings associated with VPA in girls and women of childbearing potential. In 2018, the Medicines and Healthcare products Regulatory Agency advised against the use of VPA in girls and women of childbearing potential27; thus, one might anticipate further declines in its use going forward.
Our study shows that comorbidity burden increased across eras, indicating that patients newly diagnosed in era 3 were sicker than patients newly diagnosed in era 1 or 2. The reasons for this trend are not discerned, but may reflect a changing make-up of practices contributing CPRD data, societal changes in levels of physical inactivity and diet, improved diagnosis by healthcare providers and increased treatment-seeking behaviour by patients. A similar observed increase in comorbidity burden from 2004 to 2014 has been reported in a UK population with cardiovascular disease.28
A longitudinal cohort study describing seizure freedom rates over 30 years reported a virtually unchanged seizure-freedom rate, and a decrease in the probability of achieving seizure freedom with each unsuccessful AED regimen prescribed.11 The study reported 61%–64% of patients achieved 1-year seizure freedom over time.11 Study authors concluded that despite changing treatment patterns and greater use of newer AEDs, as observed in the present study, no meaningful improvements in long-term outcomes had occurred. In contrast, our study found an improvement in outcomes, with a higher proportion of patients entering remission, nearly half of whom subsequently relapsed. Across eras, an increasing proportion of patients achieved 1-year remission (eg, 71.9% to 81.4% for adult subgroup). There are a number of differences that may explain the discrepancy in results between these studies. First, the prior study assessed 1-year seizure freedom before study end (thus excluding those who were seizure free for ≥12 months who relapsed before study end), whereas our study assessed 1-year remission from AED initiation. Because patients with longer follow-up also have more time and opportunity to relapse, in the prior study 1-year seizure freedom favours patients with shorter follow-up (ie, those diagnosed in later eras), which is not the case in the current study. By using Kaplan-Meier methods to adjust for this differential follow-up time between eras, we found increasing remission rates over time in adult and elderly subgroups. Other notable differences include time periods of the study cohorts (1982–2012 vs 2003–2012), available AEDs, reported study population (overall vs age-specific subgroups), settings (single epilepsy centre in Glasgow vs GP practices across England) and data source (medical records and notes vs structured EMRs). It is possible that reporting outcomes for an overall population may have masked trends in the adult population, as our study found treatment patterns and remission rates in children were relatively stable over time periods.
The proportion of patients who were refractory within 3 years of first starting AED treatment was similar across eras, although percentages varied somewhat according to age group. The trend for increasing remission rates and stable refractoriness rates would appear to be a contradiction. A possible explanation may be that patients progress through three AED treatments via more active management. Although these patients would meet the definition for refractory epilepsy, the AEDs may represent more specifically chosen, better tolerated treatments that lead to improved remission rates. Further, improved tolerability of newer AEDs may permit higher dosing, which may lead to improved efficacy outcomes. Thus, the increasing rates of remission over time may reflect the improved tolerability and efficacy of newer AEDs used in the later eras.
Study interpretation is limited by a number of factors. The conservative, stringent diagnostic criteria may have limited the generalisability of the data, as the study selection rate was approximately 25%–30% of the expected incidence rate.29 The accuracy of EMR data is limiting, as instances whereby a seizure or epilepsy code was used after a non-seizure event could be present. The requirement for a neurologist visit as part of our epilepsy diagnosis criteria was intended to maximise the accuracy of the diagnosis. Additionally, the epilepsy type was not usually discernible from medical record recording practices in our study, with 70%–80% of patients having been classified as having an unspecified epilepsy diagnosis. Because patient characteristics by AED were not assessed, the accuracy of selected AED(s) is not known (all prescriptions reflect data from GPs).
Outcome definitions may also contribute to limitations. Our definition of remission was based on healthcare consultations, and it could be argued that the 1-year time period was insufficient to be considered as ‘remission’,30 or that basing remission on healthcare encounters rather than information about seizure frequency may be unspecific. This proxy was designed to use as much information as is captured in the databases. No Read or ICD-10 codes record seizure frequency. The QOF data, which are intended to record seizure frequency, were found to be very poorly populated. EMR free text is not available from CPRD. Further, the definition of seizure freedom has evolved, with the International League Against Epilepsy (ILAE) proposing a ‘rule of three’, including the absence of seizures for at least the previous 12 months OR for three times the longest pretreatment interval between seizures, whichever is greater.31 In our study, drug resistance (refractoriness) was based on switching AEDs and did not take into account the reasons for treatment changes, which are not explicitly recorded in CPRD (eg, adverse events/tolerability, pregnancy) but likely were predominantly driven by a combination of lack of efficacy and poor tolerability. This definition differs from that proposed by the ILAE in 2010: ‘Drug resistant epilepsy may be defined as failure of adequate trials of two tolerated and appropriately chosen and used AED schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.’31
Our study is also limited by incomplete information regarding prescribing data from GPs, particularly lack of information regarding patient adherence and the appropriateness of AED selection based on patient characteristics. Indeed, database studies are subject to miscoding and missing or incomplete information. In our description of the data, we found that epilepsy is often coded with no more specificity than simply ‘epilepsy’, perhaps the result of the unwillingness or lack of necessity to code more specifically, on the part of physicians. Shifts in the make-up of practices supplying data to CPRD (eg, reflected by capture of higher rates of comorbidities and specified epilepsy diagnosis, and shifts in the regional make-up of CPRD data, which may be associated with regional treatment practices) over time may have introduced unmeasured bias in patient baseline characteristics. Changes to data availability and accuracy over eras may have affected results, with a prior study noting the improved accuracy of administrative or registry data in later years.32 A crucial limitation is that cohorts are unmatched and analyses unadjusted; thus, there is no statistical basis for comparisons between outcomes. As such, our findings are exploratory in nature and should be interpreted with caution.
Despite these potential limitations, our study suggests an evolution of AED treatment patterns and AED effectiveness over a 15-year period in clinical practice in England. Major changes in treatment patterns, particularly a reduction in CBZ and PHT use in favour of earlier and increased use of LEV, were observed. Although our study did not assess the use of particular AEDs based on patient characteristics or appropriateness, we generally found a reduction in the use of EIAEDs in the elderly subgroup and VPA in women, in keeping with newer treatment recommendations. In contrast to other studies reporting no meaningful improvement in the overall epilepsy population,10 11 we found an increase in 1-year remission rates following AED initiation in adults, which, given the limitations of the current study, will need to be further studied. Overcoming the limitations of the current study would require a data source that captures relevant diagnosis and outcomes data, particularly reasons for treatment change and remission, in more depth; while also still being generalisable and with a sufficient sample size. The lack of availability of such data is currently a major hurdle to comparative effectiveness research and real-world evidence in epilepsy.
Although some improvement in epilepsy treatment outcomes was observed, a sizeable proportion of patients with epilepsy remain uncontrolled on first and second-line treatments, indicating a continued need for innovations for patients living with poorly controlled epilepsy.
Supplementary Material
Acknowledgments
The authors acknowledge Cheryl Hudson (UCB Pharma, Slough, UK) for publication coordination, and Lynne Isbell, PhD, CMPP, and Richard Fay, PhD, CMPP (Evidence Scientific Solutions, Philadelphia, PA, USA) for writing assistance, which was funded by UCB Pharma.
Footnotes
Contributors: GP and SB conceived the study and had a role in study design. JL and VK conducted data collection, extraction and analysis of data. GP, SB and JL contributed to data interpretation. All authors contributed to the review and revision of the manuscript and approved the final manuscript for publication.
Funding: This work was supported by UCB Pharma. Contributions from UCB Pharma include study design and interpretation (SB, as an employee), data collection and analysis (JL and VK, as paid consultants), and review, revision and approval of the final manuscript (SB, JL and VK).
Disclaimer: This study is based in part on data from the General Practice Research Database obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this report are those of the authors alone.
Competing interests: GP is a paid medical consultant for UCB Pharma. JL and VK are paid consultants for UCB Pharma. SB is an employee of UCB Pharma.
Patient consent for publication: Not required.
Ethics approval: The protocol for this study was reviewed and approved by the Independent Scientific Advisory Committee, the CPRD Scientific/Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available.
References
- 1. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet 2007;369:1000–15. 10.1016/S0140-6736(07)60460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brodie MJ, Mintzer S, Pack AM, et al. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia 2013;54:11–27. 10.1111/j.1528-1167.2012.03671.x [DOI] [PubMed] [Google Scholar]
- 3. Johannessen Landmark C, Patsalos PN. Drug interactions involving the new second- and third-generation antiepileptic drugs. Expert Rev Neurother 2010;10:119–40. 10.1586/ern.09.136 [DOI] [PubMed] [Google Scholar]
- 4. Stokes T, Shaw EJ, Juarez-Garcia A, et al. The diagnosis and management of the epilepsies in adults and children in primary and secondary care. London: Royal College of General Practitioners, 2004. [Google Scholar]
- 5. Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2013;54:551–63. 10.1111/epi.12074 [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence Epilepsies: diagnosis and management, CG137, 2012. Available: https://www.nice.org.uk/guidance/cg137 [Accessed 15 Jan 2019].
- 7. Pickrell WO, Lacey AS, Thomas RH, et al. Trends in the first antiepileptic drug prescribed for epilepsy between 2000 and 2010. Seizure 2014;23:77–80. 10.1016/j.seizure.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 8. Nicholas JM, Ridsdale L, Richardson MP, et al. Trends in antiepileptic drug utilisation in UK primary care 1993-2008: cohort study using the General Practice Research Database. Seizure 2012;21:466–70. 10.1016/j.seizure.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 9. de Groot MCH, Schuerch M, de Vries F, et al. Antiepileptic drug use in seven electronic health record databases in Europe: a methodologic comparison. Epilepsia 2014;55:666–73. 10.1111/epi.12557 [DOI] [PubMed] [Google Scholar]
- 10. Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 2011;52:657–78. 10.1111/j.1528-1167.2011.03024.x [DOI] [PubMed] [Google Scholar]
- 11. Chen Z, Brodie MJ, Liew D, et al. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol 2018;75:279–86. 10.1001/jamaneurol.2017.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwan P, Brodie MJ. Clinical trials of antiepileptic medications in newly diagnosed patients with epilepsy. Neurology 2003;60:S2–12. 10.1212/WNL.60.11_suppl_4.S2 [DOI] [PubMed] [Google Scholar]
- 13. Karlsson L, Wettermark B, Tomson T. Drug treatment in patients with newly diagnosed unprovoked seizures/epilepsy. Epilepsy Res 2014;108:902–8. 10.1016/j.eplepsyres.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 14. Bonnett LJ, Tudur Smith C, Donegan S, et al. Treatment outcome after failure of a first antiepileptic drug. Neurology 2014;83:552–60. 10.1212/WNL.0000000000000673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clinical Practice Resarch Datalink Data, 2019. Available: https://cprd.com/Data [Accessed 9 Apr 2019].
- 17. Booth N. What are the read codes? Health Libr Rev 1994;11:177–82. [DOI] [PubMed] [Google Scholar]
- 18. Bradley SH, Lawrence NR, Carder P. Using primary care data for health research in England - an overview. Future Healthc J 2018;5:207–12. 10.7861/futurehosp.5-3-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Springate DA, Kontopantelis E, Ashcroft DM, et al. ClinicalCodes: an online clinical codes repository to improve the validity and reproducibility of research using electronic medical records. PLoS One 2014;9:e99825 10.1371/journal.pone.0099825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. NHS Digital Hospital episode statistics (HES), 2019. Available: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics [Accessed 9 Apr 2019].
- 21. National Institute for Health and Care Excellence The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care, CG20, 2004. Available: https://www.nice.org.uk/guidance/cg20 [Accessed 15 Jan 2019].
- 22. Perucca E. NICE guidance on newer drugs for epilepsy in adults. BMJ 2004;328:1273–4. 10.1136/bmj.328.7451.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nunes VD, Sawyer L, Neilson J, et al. Diagnosis and management of the epilepsies in adults and children: summary of updated NICE guidance. BMJ 2012;344:e281 10.1136/bmj.e281 [DOI] [PubMed] [Google Scholar]
- 24. Medicines and Healthcare products Regulatory Agency Medicines related to valproate: risk of abnormal pregnancy outcomes, 2015. Available: https://www.gov.uk/drug-safety-update/medicines-related-to-valproate-risk-of-abnormal-pregnancy-outcomes [Accessed 6 Feb 2019].
- 25. Borghs S, Thieffry S, Noack-Rink M, et al. Health care cost associated with the use of enzyme-inducing and non-enzyme-active antiepileptic drugs in the UK: a long-term retrospective matched cohort study. BMC Neurol 2017;17:59 10.1186/s12883-017-0837-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thurman D, Faught E, Kim H, et al. Antiepileptic drug treatment patterns in women of childbearing age with epilepsy: a US database analysis [abstract]. American Epilepsy Socity 71st Annual Meeting; 1–5 Dec 2017; Washington DC. Available: https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/342678
- 27. Medicines and Healthcare products Regulatory Agency Valproate medicines (Epilim, Depakote): contraindicated in women and girls of childbearing potential unless conditions of Pregnancy Prevention Programme are met, 2018. Available: https://www.gov.uk/drug-safety-update/valproate-medicines-epilim-depakote-contraindicated-in-women-and-girls-of-childbearing-potential-unless-conditions-of-pregnancy-prevention-programme-are-met [Accessed 13 Feb 2019].
- 28. Tran J, Norton R, Conrad N, et al. Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: a population-based cohort study. PLoS Med 2018;15:e1002513 10.1371/journal.pmed.1002513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joint Epilepsy Council of the UK and Ireland Epilepsy prevalence, incidence and other statistics, 2011. Available: http://www.epilepsyscotland.org.uk/pdf/Joint_Epilepsy_Council_Prevalence_and_Incidence_September_11_(3).pdf [Accessed 15 Jan 2019].
- 30. Sillanpää M, Schmidt D, Saarinen MM, et al. Remission in epilepsy: how long is enough? Epilepsia 2017;58:901–6. 10.1111/epi.13732 [DOI] [PubMed] [Google Scholar]
- 31. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–77. 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- 32. Burns EM, Rigby E, Mamidanna R, et al. Systematic review of discharge coding accuracy. J Public Health 2012;34:138–48. 10.1093/pubmed/fdr054 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-032551supp001.pdf (116.7KB, pdf)