Abstract
Objectives
Wearable motion sensors are used with increasing frequency in the evaluation of gait, function and physical activity within orthopaedics and sports medicine. The integration of wearable technology into the clinical pathway offers the ability to improve post-operative patient assessment beyond the scope of current, questionnaire-based patient-reported outcome measures. This scoping review assesses the current methodology and clinical application of accelerometers and inertial measurement units for the evaluation of patient activity and functional recovery following knee arthroplasty.
Design
This is a systematically conducted scoping review following Joanna Briggs Institute methodology for scoping reviews and reported consulting the Preferred Reporting Items for Systematic Review and Meta-Analyses extension for scoping reviews. A protocol for this review is registered with the Open Science Framework (https://osf.io/rzg9q).
Data sources
CINAHL, EMBASE, MEDLINE and Web of Science databases were searched for manuscripts published between 2008 and 2019.
Eligibility criteria
We included clinical studies reporting the use of any combination of accelerometers, pedometers or inertial measurement units for patient assessment at any time point following knee arthroplasty.
Data extraction and synthesis
Data extracted from manuscripts included patient demographics, sensor technology, testing protocol and sensor-based outcome variables.
Results
45 studies were identified, including 2076 knee arthroplasty patients, 620 patients with end-stage osteoarthritis and 449 healthy controls. Primary aims of the identified studies included functional assessment, physical activity monitoring and evaluation of knee instability. Methodology varied widely between studies, with inconsistency in reported sensor configuration, testing protocol and output variables.
Conclusions
The use of wearable sensors in evaluation of knee arthroplasty procedures is becoming increasingly common and offers the potential to improve clinical understanding of recovery and rehabilitation. While current studies lack consistency, significant opportunity exists for the development of standardised measures and protocols for function and physical activity evaluation.
Keywords: accelerometry, gait analysis, systematic review, knee arthroplasty, physical activity
Strengths and limitations of this study.
This is a comprehensive, systematic literature review following Joanna Briggs Institute methodology for scoping reviews.
The Preferred Reporting Items for Systematic Review and Meta-Analyses Extension for Scoping Reviews guidelines were followed for reporting clarity.
The use of wearable sensors was evaluated in subcategories based on the assessment functional analysis, physical activity and joint stability. Validation studies not directly addressing patient outcome were excluded from review based on the authors’ discretion.
Introduction
Patient-reported outcome measures (PROMs) are routinely used to evaluate the efficacy of knee arthroplasty procedures in terms of post-operative patient satisfaction, function and pain.1 2 PROMs are commonly implemented pre-operatively through patient questionnaires to establish a baseline measurement, and then again at 6 months post-operatively.3 Various forms of validated PROMs have been used throughout orthopaedic clinical practice and are effective in demonstrating overall patient improvement following surgical intervention.2 The effectiveness of PROMs, however, can be limited in some instances as a result of inherent ceiling effects1 4 as well as inaccurate and non-repeatable patient-reported post-operative activity.5 6 The Osteoarthritis Research Society International and Outcome Measures in Rheumatology and Clinical Trials advocate the use of functional outcomes for all randomised controlled trials of interventions after knee replacement.7 Traditional assessment has come through timed tests such as the Timed Up and Go or 6 min walk test; however, these are burdensome for participants and do not replicate their normal activity. Some researchers have suggested that PROMs incorporating patient function and activity measures may be the way forward in improved understanding of patient outcome and performance following unicompartmental knee arthroplasty (UKA) or total knee arthroplasty (TKA).8 9
Within the healthcare field, wearable technology is the application of data-recording transducers onto a person’s body or clothing to monitor measurable health indicators. Wearable technology is increasingly being used to perform gait analysis and to assess patient’s mobility and activity levels in orthopaedic and sports medicine, with wearable motion sensors generally consisting of an accelerometer measuring accelerations in 1, 2 or 3 degrees of freedom (df) to quantify limb or whole-body movement. More complex movement data can be collected from inertial measurement units (IMUs), sensors pairing accelerometers with gyroscopes and magnetometers to provide detailed analysis of limb movement and orientation within a spatial reference frame.
Several methodological considerations must be made during the implementation of wearable motion sensors in the study of surgical outcome. Body location, sampling rate, wear time and testing protocols are key aspects of sensor-based research which may affect the quality and reliability of data being collected.10–13 The manner in which these sensors have been deployed in knee arthroplasty research and clinical care has not been fully examined. This scoping review aims to assess the current methodology and clinical application of wearable sensors to evaluate patient recovery and functional outcomes following knee arthroplasty.
Methods
We conducted a scoping review following the Joanna Briggs Institute Methodology for JBI Scoping Reviews14 and consulted the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Extension for Scoping Reviews checklist for reporting.15 The protocol is registered within the Open Science Framework (https://osf.io/rzg9q/). For inclusion within this review, manuscripts must report the use of wearable sensor technology for monitoring patient function, mobility, surgery effectiveness or joint stability, or to serve as the basis for the assessment of a questionnaire or other measure of physical activity and function. Included studies could be conducted in a hospital or home setting and must include post-operative data collection following any total or partial knee arthroplasty for end-stage osteoarthritis (OA). Explicit validation studies of wearable sensor technology and studies implementing the use of IMUs for robotic-assisted rehabilitation or surgical navigation were excluded, as those studies do not meet the primary aims of evaluating basic patient outcome. Full inclusion and exclusion screening criteria are presented in table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|
|
IMUs, inertial measurement units.
To identify relevant studies, an electronic database literature search was conducted with assistance from outreach librarian Nia Roberts. Databases searched were CINAHL (1982 to present), EMBASE (1974 to present), MEDLINE (1950 to present) and Web of Science Core Collection (1945 to present) (see online supplementary table S1 for details on the strategies). Since wearable sensor technology is an emerging field benefiting from developments in miniaturisation and wireless data transfer, the literature search was designed to primarily capture the current use of modern wearable instrumentation. Thus, manuscripts published between January 2008 and June 2019 were considered. Additionally, reference lists of relevant articles were searched for additional appropriate inclusions. Systematic reviews were not included in the review but were also checked for potentially relevant references. On completion of the comprehensive searches, duplicate entries were removed through manual verification within Mendeley reference management software.
bmjopen-2019-033832supp001.pdf (63.1KB, pdf)
Screening of manuscript titles and abstracts were conducted by two independent, blinded reviewers (SRS and GSB) through the use of the Rayyan systematic review web app.16 Prior to screening, reviewers discussed inclusion and exclusion criteria, while working through a calibration exercise of example titles and abstracts to ensure consistency between individuals. Following title and abstract review, SRS and GSB evaluated the subsequent full-text manuscripts for inclusion in the final review. Any disagreements following full-text assessment were discussed between reviewers. A third reviewer was available for consultation if no consensus could be reached.
Following the screening process, data from all included full-text manuscripts were collected within a data extraction spreadsheet by the first author (SRS) and then verified by GSB. Any inconsistencies were resolved by consensus. Data extracted from the manuscripts were categorised by patient demographics, technology, testing protocol and sensor-based outcome variables. Specifically, data were extracted to include the number of male and female participants in knee arthroplasty, end-stage OA or healthy control populations. The type of wearable sensor was recorded, including number of axes of motion recorded, as well as the use of accelerometers, gyroscopes, magnetometers and global positioning systems (GPS), and the reported data sampling rate for all sensor measurements. The number of sensors and body location was also recorded. For data charting purposes, manuscripts were divided into three distinct subgroups based on the primary aims of each study as follows: (1) studies with the primary aim of assessing functional or gait parameters, (2) physical activity studies with the primary aim of quantifying change in overall activity as a result of knee arthroplasty and (3) instability studies with the primary aim of assessing post-operative joint stability. The duration of free-living activity monitoring was extracted for all physical activity studies, as well as the pre-operative and post-operative time points when data were collected. Finally, sensor-based outcome variables were recorded to assess the specific metrics reported in each study.
Patient and public involvement
Patients and the public were not involved in any phase of this study.
Results
A PRISMA flow diagram17 detailing the results of the literature search and review is presented in figure 1. The searches yielded 346 records for title and abstract screening, of which 69 were assessed as full texts. Of these, 45 were included in the review. The 24 excluded studies lacked knee arthroplasty patients or stratified data, included too few study participants, were protocol or validation studies or deemed unrelated to the review question. While any type of knee arthroplasty for end-stage OA was included in the search, no studies evaluating patients following bi-compartmental, patellofemoral or other partial knee arthroplasty were identified.
Figure 1.
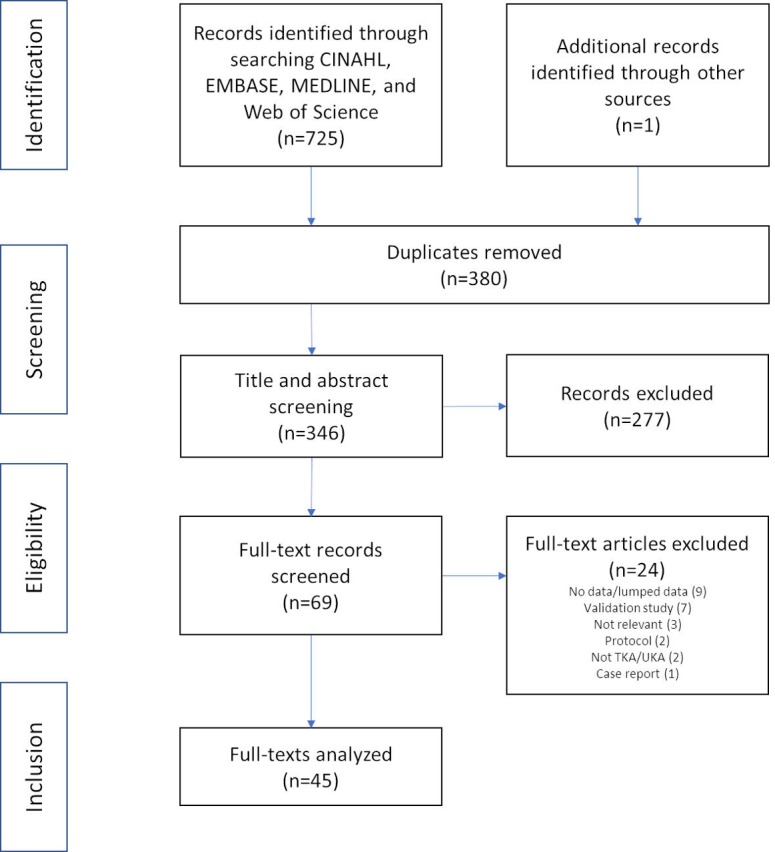
Preferred Reporting Items for Systematic Review and Meta-Analyses literature review flow diagram.17
The use of wearable technology to assess outcome and effectiveness of TKA and UKA has increased over the period evaluated in this review. As shown in figure 2, an average of just 1–2 studies per year were published between 2008 and 2012. More recently there has been continued growth in the number of studies implementing wearable motion sensors entering the public record, with 10 wearable sensor-based studies focused on knee replacement in 2018. A full list of studies along with model and manufacturer of devices used can be found in online supplementary tables S2-S4.
Figure 2.
The number of publications within this review by publication year (*2019 through June).
Functional assessment
Sixteen studies were included in the functional assessment subgroup (tables 2 and 3), comprising data collection on 451 TKA patients (57% female), mirroring the 43:57 male-to-female ratio within primary TKA across the UK.18 A single study included functional comparisons with 29 end-stage OA patients without knee replacement,19 while eight studies included comparative assessments with a total of 141 healthy control populations.8 19–25
Table 2.
Population and methods summary for functional assessment studies
| Population | Testing protocol | Time points | |||||||||||||
| TKA/UKA | TKA/UKA % female | Healthy control | Control % female | Dynaport ADL protocol34 | 10–20 m walkway | Outdoor course | Stair stepping | Other | Pre-operative | 0–6 weeks post-operative | 7–12 weeks post-operative | 24–26 weeks post-operative | 1+ year post-operative | Unclear | |
| Ali et al20 | 5 | 40 | 10 | 30 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| Bolink et al26 | 20 | 65 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| Chapman et al25 | 20 | 41 | 10 | 50 | ✔ | ✔ | ✔ | ✔ | |||||||
| Chiang et al27 | 18 | ✔ | ✔ | ✔ | |||||||||||
| Christiansen et al21 | 24 | 54 | 19 | 53 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| Hiyama et al28 | 57 | 61 | ✔ | ✔ | ✔ | ✔ | |||||||||
| Kluge et al29 | 24 | 67 | ✔ | ✔ | ✔ | ||||||||||
| Kwasnicki et al22 | 14 | 57 | 15 | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| Rahman et al19 | 45 | 29 | 59 | ✔ | ✔ | ✔ | ✔ | ||||||||
| Ramkumar et al30 | 25 | 56 | ✔ | ✔ | ✔ | ✔ | |||||||||
| Senden et al8 | 24 | 54 | 24 | 54 | ✔ | ✔ | ✔ | ✔ | |||||||
| Storey et al 201323 | 16 | 22 | ✔ | ✔ | |||||||||||
| van Hemert et al32 | 53 | ✔ | ✔ | ✔ | |||||||||||
| van Hemert et al33 | 76 | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| Youn et al31 | 18 | 50 | ✔ | ✔ | |||||||||||
| Zhang et al24 | 12 | 58 | 12 | 50 | ✔ | ✔ | ✔ | ✔ | |||||||
TKA, total knee arthroplasty; UKA, unicompartmental knee arthroplasty.
Table 3.
Sensor summary for functional assessment studies
| Technology | Sampling rate | Body location | Sensor metric | |||||||||||||||||||||
| 1-axis accelerometer(s) | 3-axis accelerometer(s) | 3-axis gyroscope(s) | 3-axis magnetometer(s) | GPS | Total no of sensors | Not reported | ≤50 Hz | 100–200 Hz | >200 Hz | Ear | Sternum | Arm/wrist | Lower back | Hip/waist | Thigh | Proximal tibia | Foot/ankle | Acceleration magnitude | Activity transitions | Gait symmetry/variability | Multivariate sensor-based function score | Range of motion | Spatiotemporal gait parameters | |
| Ali et al20 | ✓ | 1 | ✓ | ✓ | ✓ | |||||||||||||||||||
| Bolink et al26 | ✓ | ✓ | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Chapman et al25 | ✓ | ✓ | ✓ | 2 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
| Chiang et al27 | ✓ | ✓ | 2 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||
| Christiansen et al21 | ✓ | 2 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Hiyama et al28 | ✓ | 1 | ✓ | ✓ | ||||||||||||||||||||
| Kluge et al29 | ✓ | ✓ | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
| Kwasnicki et al22 | ✓ | 1 | ✓ | ✓ | ✓ | |||||||||||||||||||
| Rahman et al19 | ✓ | ✓ | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
| Ramkumar et al30 | ✓ | ✓ | ✓ | 2 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
| Senden et al8 | ✓ | 1 | ✓ | ✓ | ✓ | |||||||||||||||||||
| Storey et al23 | ✓ | ✓ | 3 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||
| van Hemert et al32 | ✓ | ✓ | 7 | ✓ | * | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| van Hemert et al33 | * | 6* | ✓ | * | * | * | * | * | * | ✓ | ||||||||||||||
| Youn et al31 | ✓ | 2 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Zhang et al24 | ✓ | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
*Dynaport ADL consists of six uniaxial accelerometers recording at 32 Hz.34
GPS, global positioning systems.
A triaxial accelerometer was the most commonly used sensor incorporated in the functional assessment subgroup, with 15 of 16 studies (94%)8 19–32 using the sensor alone or in combination with other instrumentation. Seven of the functional studies exclusively used triaxial accelerometers as the means for data collection during functional analysis.8 20–22 24 28 31 Three studies used IMUs with an integrated triaxial accelerometer and triaxial gyroscope,19 27 29 while three others recorded gait with a 9 df IMU integrating triaxial accelerometer, gyroscope and magnetometer.25 26 30 Storey et al23 used a triaxial accelerometer for indoor gait analysis, but included two GPS watches for outdoor walking tests. Two studies by van Hemmert et al32 33 incorporated the DynaPort ADL sensor system consisting of six uniaxial accelerometers, with one of those studies supplementing the DynaPort system with an additional triaxial accelerometer.32 Sensor sampling rate for data recording was varied across studies, ranging from below 50 Hz to 500 Hz and not directly reported in a third of studies.19 24 30 32 33 Body location for sensor placement was equally varied, with no more than seven studies sharing any one specific sensor placement.
Testing protocols within the function studies were generally consistent and primarily comprised gait analysis, with patients walking on a 10–20 m walkway or treadmill. Four studies20 22 32 33 used the DynaPort knee test protocol34 which includes a series of walking, stepping and lifting movements to mimic activities of daily living. Bolink et al26 tested patients through a sit-to-stand protocol, while both Bolink et al and Christiansen et al21 tested patients in stepping exercises. Most studies (13 studies, 81%)8 19–22 24–30 33 included pre-operative data collection with repeated tests at one or more time points up to 1-year post-operatively, most frequently up to 6 weeks after surgery. Three studies collected data at an unspecified point any time greater than 6 months post-operatively.23 31 32
The evaluation of gait symmetry and variability was the most common sensor-based outcome metric (eight studies, 50%),8 21 24 26 28 29 31 32 while dynamic range of motion was also commonly reported (six studies, 38%).19 25–27 29 30 A wide variety of spatiotemporal gait parameters were also reported, including varied combinations of walking speed,8 23 24 26 29 32 cadence,8 26 32 stride length,8 23 24 26 29 32 stride time,8 19 29 stance time,24 29 vertical displacement,8 32 swing power24 and foot fall.24 Three studies reported functional performance primarily based on a stand-alone function score.22 32 33 Other studies reported on gait function through a variety of other parameters including acceleration magnitudes,21 26 31 activity transitions,20 multiple aspects of dynamic range of motion19 26 27 29 and varied measures of gait symmetry, inefficiency and variability.8 21 24 26 28 29 31 32
Physical activity studies
Twenty-six studies were identified as primarily focusing on physical activity following knee arthroplasty (tables 4 and 5), including a total of 1490 patients (58% women). Five of those studies included comparisons with a total of 620 end-stage OA patients without a knee replacement,35–38 though 458 of those patients came from a single report.37 Three studies compared physical activity levels of knee replacement patients with a total of 272 healthy controls.35 39 40 Four studies also included physical activity from measures from total hip replacement patients for comparison with knee patients.41–44
Table 4.
Population and methods summary for physical activity studies
| Population | Session length | Wear time | Time points | ||||||||||||||||
| TKA/UKA | TKA/UKA % female | End-stage OA | OA (% female) | Healthy control | Control % female | <7 days | =7 days | >7 days | Waking hours only | 24 hours/day | Not reported | Pre-op | 0–6 weeks post-op | 7–12 weeks post-op | 6 months post-op | 1 year post-op | >1 year post-op | Unspecified post-op | |
| Agarwal et al45 | 32 | 47 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Bolszak et al46 | 50 | 50 | ✓ | ✓ | |||||||||||||||
| Brandes et al58 | 53 | 64 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Christiansen et al47 | 43 | 53 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Cooper et al39 | 62 | 58 | 62 | 42 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Daugaard et al35 | 52 | 50 | 54 | 54 | 171 | 44 | ✓ | ✓ | ✓ | ||||||||||
| de Groot et al41 | 44 | 55 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Fenten et al48 | 80 | 54 | ✓ | ✓ | ✓ | ||||||||||||||
| Frimpong et al49 | 45 | 93 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Harding et al42 | 44 | 64 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Hayashi et al43 | 40 | 85 | ✓ | ✓ | ✓ | ||||||||||||||
| Hayes et al59 | 52 | 52 | 13 | 54 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Kahn and Schwarzkopf36 | 60 | 50 | 63 | 49 | ✓ | ✓ | ✓ | ||||||||||||
| Kahn and Schwarzkopf37 | 60 | 50 | 458 | 51 | ✓ | ✓ | ✓ | ||||||||||||
| Losina et al50 | 150 | 57 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Luna et al51 | 41 | 59 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Lützner et al40 | 97 | 46 | 39 | 59 | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Lützner et al52 | 221 | 57 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Pellegrini et al53 | 16 | 69 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Schotanus et al54 | 20 | 35 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Taniguchi et al55 | 81 | 90 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Tsuji et al56 | 20 | 70 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Twiggs et al57 | 91 | 51 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Vissers et al60 | 44 | 55 | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Vissers et al44 | 14 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Webber et al38 | 38 | 58 | 32 | ✓ | ✓ | ✓ | |||||||||||||
OA, osteoarthritis; TKA, total knee arthroplasty; UKA, unicompartmental knee arthroplasty.
Table 5.
Technology summary for physical activity studies
| Technology | Sampling rate | Body location | Sensor metric | ||||||||||||||||||
| 1-axis accelerometer(s) | 3-axis accelerometer(s) | Not reported/unclear | Total no of sensors | 10 Hz | 30–32 Hz | 100 Hz | Not reported/unclear | Sternum | Arm/wrist | Hip/waist | Thigh | Anterior tibia/lower leg | Foot/ankle | Not reported | Activity intensity/time | Active/sedentary time | Counts/METS/energy | Steps/gait cycles | Sit-to-stand transfers | Walking bouts | |
| Agarwal et al45 | ✓ | 1 | ✓ | ✓ | ✓ | ||||||||||||||||
| Bolszak et al46 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Brandes et al58 | ✓ | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Christiansen et al47 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Cooper et al39 | ✓ | 1 | ✓ | ✓ | ✓ | ||||||||||||||||
| Daugaard et al35 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| de Groot et al41 | ✓ | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Fenten et al48 | ✓ | 1 | ✓ | ✓ | ✓ | ||||||||||||||||
| Frimpong et al49 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Harding et al42 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Hayashi et al43 | ✓ | 1 | ✓ | ✓ | ✓ | ||||||||||||||||
| Hayes et al59 | ✓ | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Kahn and Schwarzkopf36 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Kahn and Schwarzkopf37 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Losina et al50 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Luna et al51 | ✓ | 1 | ✓ | ✓ | ✓ | ||||||||||||||||
| Lützner et al40 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Lützner et al52 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Pellegrini et al53 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Schotanus et al54 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Taniguchi et al55 | ✓ | 1 | ✓ | ✓ | ✓ | ||||||||||||||||
| Tsuji et al56 | ✓ | 1 | ✓ | ✓ | ✓ | ||||||||||||||||
| Twiggs et al57 | ✓ | 1 | ✓ | ✓ | ✓ | ||||||||||||||||
| Vissers et al60 | ✓ | 4 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Vissers et al44 | ✓ | 4 | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Webber et al38 | ✓ | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
Physical activity studies were consistent in the number of sensors implemented, with 21 studies (81%) using a single sensor for activity measurements.35–40 42 43 45–57 As technology and availability of wearable accelerometers has progressed, the type of sensor used has also changed. In studies published between 2008 and 2016, nine studies (69%)36 37 40 42 44 45 58–60 reported using a uniaxial accelerometer versus just three studies (23%)46 55 56 using a triaxial accelerometer. Conversely, in studies published in 2017–2019, eight studies (62%)35 38 47 49 50 53 54 57 reported using a triaxial accelerometer, while only three (23%)39 43 45 used a uniaxial accelerometer. Overall, three studies did not report the number of axes recorded,41 48 51 and most studies within this subgroup (73%)36 37 40–45 47 48 50–55 57 60 did not report the sampling rate at which data were collected. No standard frequency was observed within studies reporting sampling rate, as two studies collected data at 10 Hz,39 56 two at or near 30 Hz49 58 and three at 100 Hz.35 38 46 Accelerometers were mounted at hip or waist level, most commonly with an elastic strap, in half of the included physical activity studies.36–38 42 43 45–47 49 50 53 56 58 Seven studies positioned sensors on the thigh,35 39 41 44 48 58 60 four on the sternum,41 44 59 60 four on the lower leg,40 52 54 59 one on the foot59 and two on the wrist.51 57
All studies within this subgroup quantified physical activity based on free-living activity. Most commonly, free-living data was collected in 7-day sessions (13 studies, 50%),36–39 42 45–47 49 50 53 57 58 with 10 studies (43%)35 40 41 44 48 52 54 58–60 collecting data over shorter sessions (eg, 2–4 days). Four studies reported longitudinal activity metrics for longer time periods, with Hayashi et al43 and Tsuji et al56 reporting physical activity during post-operative hospitalisation for 8 and 14 days, respectively, while Luna et al51 reported basic accelerometer counts for 20 days post-operatively and Taniguchi et al55 asked patients maintain a daily step count log for 6 months following surgery. Two primary methodologies exist for sensor wear time. Fifteen studies (58%)35–38 42 43 45–48 53–55 58 59 instructed patients to wear the accelerometers during waking hours only, while 10 studies (43%)39–41 44 49 51 52 56 57 60 instructed patients to wear the accelerometers 24 hours/day.
Physical activity was measured at different time points primarily dependent on the type of studies being conducted. The longitudinal studies reviewed most frequently assessed physical activity within 1–2 weeks pre-operatively, and then subsequently near 6 weeks and 6 months post-operatively. Three longitudinal studies assessed physical activity up to 1 year post-operatively,47 52 58 while Vissers et al44 reported physical activity up to 4 years follow-up. Cross-sectional studies were more varied, with some reporting at exact post-operative time points, while others evaluated patient activity at non-specific or broadly defined time points.36 37 45 46 The most commonly reported time points for activity analysis across all physical activity studies were pre-operatively (18 studies, 69%)25 39–42 44 45 49–54 56–60 and at 6 months post-operatively (12 studies, 46%).39 41 42 44 47 49 50 53 55 58–60
Sensor-based outcome variables within the physical activity studies varied considerably between studies, though not as widely as in the functional assessment subgroup. Reporting of step counts is the most commonly used measure of physical activity within the knee arthroplasty population and has been increasingly common within these studies, included in 9 of 12 (75%) studies35 38 39 43 47 49 50 54 57 from 2017 to 2019, compared with just 4 of 13 (31%) studies40 52 55 58 from 2008 to 2016. The amount of active and sedentary time (12 studies, 46%),38 40–42 44 48 49 52–54 59 60 quantification of time spent at different activity intensity levels (12 studies, 46%),38 40–42 44 48 49 52–54 59 60 and approximations of energy expenditure (10 studies, 38%)35–37 46 49 51 53 54 56 59 are also commonly used measures which provide another level of detail beyond basic step counting techniques. Three studies reported transitions between activities and bouts of activities.35 41 54
Instability studies
Three studies from the literature review used wearable sensors affixed to the operative tibia to examine overall joint instability following TKA.61–63 A total of 135 patients (69% women) were examined (table 6) with two studies including comparisons with a total of 36 healthy control patients.61 62 For instability measurement, Khan et al61 and Soeno et al63 used triaxial accelerometers, while Roberts et al62 used an integrated IMU with triaxial gyroscope and triaxial magnetometer. Both Khan et al61 and Roberts et al62 recorded joint motion in patients at a minimum of 6 months post-operatively at a sampling rate of 100 Hz while patients performed stepping, sit-to-stand and walking protocols. Similarly, both studies compared joint stability between TKA patients and healthy controls. Khan et al61 reported instability based on mean acceleration and distribution of acceleration frequencies, while Roberts et al62 quantified means and ranges of acceleration and jerk parameters. Soeno et al63 collected data on patients more than 1 year post-operatively at a sampling rate of 250 Hz while quantifying instability directional root mean square acceleration, comparing patients with and without self-reported subjective instability.
Table 6.
Population and technology summary for instability studies
| Population | Technology | Sampling rate | Sensor location | Testing protocol | Time points | |||||||||||
| TKA patients | TKA % female | Healthy controls | Control % female | 3-axis accelerometer(s) | 3-axis gyroscope(s) | 3-axis magnetometer(s) | Total no of sensors | 100 Hz | 250 Hz | Proximal tibia | Walk | Sit-to-stand | Block/stair step | Minimum 6 months post-operative | >1 year post-operative | |
| Khan et al61 | 38 | 59 | 18 | 61 | ✓ | 1–2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Roberts et al62 | 27 | 59 | 18 | 81 | ✓ | ✓ | ✓ | 1–2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Soeno et al63 | 70 | 77 | ✓ | 1–2 | ✓ | ✓ | ✓ | ✓ | ||||||||
TKA, total knee arthroplasty.
Discussion
Wearable motion sensors are increasingly being used to assess patient function, physical activity and joint stability following knee arthroplasty. The use of IMUs and accelerometers allows clinicians and researchers the ability to track a broad scope of outcome measures as they relate to free-living activities outside of the clinical setting, with greater objectivity when compared with activity questionnaires. Currently, wearable technology has been most commonly used for pre-operative and post-operative comparisons at 6–12 months of follow-up, with less focus on early recovery and rehabilitation. No standard outcome measure or testing methodology has been established in wearable-based gait analysis or physical activity monitoring.
The use of wearable motion sensors for gait analysis is an attractive alternative to optical gait analysis for functional assessment of knee arthroplasty patients. Whereas optical gait analysis requires significant overhead with dedicated equipment and facilities, IMU gait analysis offers the potential of quick setup and data capture in a wide variety of gait protocols and environments. As is evident within the context of this review, however, current knee arthroplasty literature lacks consensus for testing method and performance measures of spatiotemporal gait parameters using wearable technology. Within optical gait analysis, standard reflective marker positions and testing protocols are well established in TKA research.64 65 Similar progress is needed in the implementation of standard measurements and performance-based tests for characterisation of patient function and recovery in sensor-based analysis. Testing protocols incorporating multiple activities, including those in which patients with knee OA find more challenging, may be more indicative measures of patients’ ability to return to participation in normal activities and overall function.66 In the authors’ clinical experience, many knee patients find activities such as navigating through a crowd of people to be one of the more difficult daily activities. Evaluation of more complex, real-world activities is perhaps where the benefit to integrating IMU-based gait analysis may be most valuable.
Functional assessment apart from gait analysis through simple clinical measures (eg, range of motion) can also be derived using IMUs. Ramkumar et al30 recently reported a longitudinal study using two IMUs for monitoring knee flexion during daily exercise sessions up to 12 weeks post-operatively. Their method requires the patient to set up one IMU on the thigh and another on the proximal tibia across the operative joint, whereupon the patient performs daily regimented movements. Take-home IMU-based systems for functional assessment, as described by Ramkumar et al,30 are less prevalent than accelerometer-based systems for general activity monitoring as gyroscopes in 9 df IMUs have substantial power and data storage requirements.67 As a result, longitudinal functional assessment via IMU can be logistically difficult and requires a level of active interaction by the patient or caregiver for battery charging and initiation of data collection and transfer. Nevertheless, Ramkumar et al30 demonstrate the feasibility of implementing longitudinal functional assessment of simple parameters, such as knee range of motion, into remote patient monitoring schemes.
Physical activity monitoring through accelerometry is a more established technique than IMU-based gait analysis. Accelerometers are less battery and memory intensive than IMUs and offer the possibility of extended data collection without active involvement by the patient or caregiver. To date, sensors have been typically deployed near the patient’s centre of mass for activity analysis, at the hip or back, to most accurately monitor patients’ overall movement.12 One of the more surprising findings of this review is the lack of research using wrist-worn activity monitors for evaluation of patients’ physical activity. While networks of IMUs may provide improved data quality when performing gait analysis with wearable sensors, a single wrist-based triaxial accelerometer is the most common method of personal, at-home activity tracking. Based on current market research, wrist-based monitors and smart watches account for 95% of commercial wearables sales.68 Outside of orthopaedics, a movement towards wrist-based sensors for physical activity monitoring has developed.12 This is likely to be a result of widespread public adoption of commercial fitness trackers and smartwatches,68 69 concerns of patient compliance with hip-based monitors,12 70 71 improved accuracy in wrist-based accelerometry72–75 and development of techniques for analysis of raw accelerometer data output.12 76 The largest deployment of population-based physical activity monitoring was the assessment of over 100 000 volunteers enrolled within the UK Biobank.77 The study used a triaxial accelerometer (AX3, Axivity) mounted on the participant’s dominant wrist to collect activity data continuously for 7 days.77 Presently, no studies have analysed physical activity from knee arthroplasty patients within the UK Biobank; however, significant research potential exists across a variety of fields and disciplines.
Within this review, most studies using wearable sensors in longitudinal cohort studies report incremental changes in physical activity from a pre-operative baseline to 6 weeks, 6 months or 12 months post-operatively. Within these studies, there has been conflicting evidence as to the level of improvement in physical activity levels of knee arthroplasty patients beyond their pre-operative baseline.49 78 Conversely, limited research has been conducted regarding the pattern of recovery based on physical activity and how the trajectory towards return to participation may inform clinicians of an individual patient’s early rehabilitation. Mapping early patient recovery may be a particularly valuable clinical application of activity monitoring after TKA, as patient questionnaires and self-reported patient function may not accurately represent true functional abilities in near-term follow-up.58 79 80 We identified only three studies employing a method for monitoring patients over a continuous period for more than 2 weeks following surgery. Recently orthopaedic manufacturer Zimmer Biomet along with corporate partner Apple began the largest prospective study to date involving wearable technology and joint arthroplasty, in the rollout of their Apple Watch myMobility platform.81 This multicentre prospective longitudinal cohort study will rely on iPhone and Apple Watch integration to continuously track patient step count, while exercise coaching following TKA, UKA and total hip arthroplasty in an aim will provide motivation during the recovery process. The primary and secondary outcomes of this study are based on 90-day post-operative questionnaire responses, incidence of manipulation under anaesthesia, standard function tests, satisfaction and healthcare costs, and not on patient activity measures. Nevertheless, this study demonstrates the capacity for activity monitoring and feedback through near-continuous data collection, as well as the continued emergence of wrist-worn devices as a preferred data collection site in post-operative activity studies.
As Thompson et al82 have noted, opportunity exists for physical activity monitors to provide multidimensional metrics to fine-tune activity guidelines for specific patient populations. Current literature lacks depth as to the type of sensor-based outcome metric best suited for evaluation of post-operative recovery, and how to best provide feedback to the patient. Since physical activity is multidimensional, improved monitoring during early rehabilitation can be used to explore the rate of recovery, overall mobility and patient satisfaction based not only on the amount of activity achieved, but also on the nature and timing of that activity within early post-operative period. The establishment of an evidence-based target recovery curve could be used as a baseline during the entire rehabilitation process from which to identify positively or negatively trending patient outcomes. Feedback mechanisms could be established to encourage more, or less, of a specific type of activity based on the expected activity levels at the specific patient’s post-operative time. Recently, Panda et al reported the ability to differentiate oncological patients suffering post-operative complications through passive physical activity monitoring.83 As they have demonstrated, real-time monitoring of physical activity by means of a sensor paired with a data network could direct the clinical care team to patients exhibiting a suboptimal recovery pattern who might benefit from additional evaluation or follow-up. As Ramkumar et al30 explored in their recent pilot study, remote monitoring of patient recovery also has the potential to reduce un-necessary in-person clinical follow-up visits if the clinical care team can remotely identify patients’ tracking along an established recovery trajectory.30
The use of accelerometers in instability studies seems to be a niche research area, but one that demonstrates the versatility of wearable motion sensors within post-operative care and research. The development of research techniques, such as the quantitative measurement of the subjective patient experience of joint instability, could be a valuable tool in the refinement of overall evaluation of surgical intervention. The application of wearable technology in the assessment of two competing implant designs, as Soeno et al63 have done, adds another tool available in the effort to improve outcomes and overall patient satisfaction.
The authors acknowledge limitations to this scoping review. The use of wearable sensors for the assessment of knee arthroplasty patients is accelerating. Subsequently, several studies will likely be published between the completion and publication of this review. Since the use of wearable technology continues to be an emerging technique in knee arthroplasty patient assessment, particularly within the field of gait analysis, exclusion of validation studies based on their level of clinical inquiry was subject to the authors’ best judgement. However, this review focuses on usage of sensor-based patient care and reflects emerging techniques practically implemented into current clinical practice.
The goal of this review was to investigate key trends in wearable sensor research within the scope of TKA and UKA. The use of wearable sensors in knee arthroplasty research is increasing, with gait analysis and physical activity the two primary modes of investigation. Wearable sensors offer potential for the advancement of patient care to include bespoke rehabilitation and follow-up schedules based on remotely collected ambulatory monitoring of physical activity, range of motion and gait parameters within the patient’s free-living environment. In practice, this could result in fewer clinical visits for patients with on-target recovery markers and opportunities for early intervention in patients following a suboptimal recovery curve. The technology also offers a mechanism for patient coaching and encouragement for patients to reach targeted goals during rehabilitation. To fully realise the clinical potential of wearable technology for knee replacement patients, this review highlights the need for increased focus on early recovery as well as increased inter-study consistency through improved technical reporting and standardisation of sensor-based outcome metrics.
Supplementary Material
Acknowledgments
The authors would like to thank Nia Roberts and Shona Kirtley for their support developing literature review and data extraction procedures, and Aiden Doherty for support in manuscript review.
Footnotes
Contributors: All authors contributed to this review and approved the final manuscript. SRS designed and conducted the search, reviewed articles and wrote the manuscript. GSB reviewed articles and edited the manuscript. SK supervised the study process and edited the manuscript. KB supervised the study process and edited the manuscript. MT provided systematic review training to SRS, supervised the study process and edited the manuscript. AJP conceived the study, supervised the study process and edited the manuscript.
Funding: Student funding for both SS and GB was provided by the Clarendon Fund from the University of Oxford and Oxford University Press.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Harris K, Dawson J, Gibbons E, et al. Systematic review of measurement properties of patient-reported outcome measures used in patients undergoing hip and knee arthroplasty. Patient Relat Outcome Meas 2016;7:101–8. 10.2147/PROM.S97774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramkumar PN, Harris JD, Noble PC. Patient-reported outcome measures after total knee arthroplasty. Bone Joint Res 2015;4:120–7. 10.1302/2046-3758.47.2000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. NHS Digit Proms methodologies, 2018. Available: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/patient-reported-outcome-measures-proms/proms-methodologies [Accessed 10 Apr 2019].
- 4. Dowsey MM, Choong PFM. The utility of outcome measures in total knee replacement surgery. Int J Rheumatol 2013;2013:1–8. 10.1155/2013/506518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vaughn NH, Dunklebarger MF, Mason MW. Individual patient-reported activity levels before and after joint arthroplasty are neither accurate nor reproducible. Clin Orthop Relat Res 2019;477:536–44. 10.1097/CORR.0000000000000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terwee CB, van der Slikke RMA, van Lummel RC, et al. Self-reported physical functioning was more influenced by pain than performance-based physical functioning in knee-osteoarthritis patients. J Clin Epidemiol 2006;59:724–31. 10.1016/j.jclinepi.2005.11.019 [DOI] [PubMed] [Google Scholar]
- 7. Singh JA, Dowsey MM, Dohm M, et al. Achieving consensus on total joint replacement trial outcome reporting using the OMERACT filter: endorsement of the final core domain set for total hip and total knee replacement trials for endstage arthritis. J Rheumatol 2017;44:1723–6. 10.3899/jrheum.161113 [DOI] [PubMed] [Google Scholar]
- 8. Senden R, Grimm B, Meijer K, et al. The importance to including objective functional outcomes in the clinical follow up of total knee arthroplasty patients. Knee 2011;18:306–11. 10.1016/j.knee.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 9. Bolink SAAN, Grimm B, Heyligers IC, et al. Patient-reported outcome measures versus inertial performance-based outcome measures: a prospective study in patients undergoing primary total knee arthroplasty. Knee 2015;22:618–23. 10.1016/j.knee.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 10. Brønd JC, Arvidsson D. Sampling frequency affects the processing of Actigraph RAW acceleration data to activity counts. J Appl Physiol 2016;120:362–9. 10.1152/japplphysiol.00628.2015 [DOI] [PubMed] [Google Scholar]
- 11. Godfrey A, Hetherington V, Shum H, et al. From a to Z: wearable technology explained. Maturitas 2018;113:40–7. 10.1016/j.maturitas.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 12. Clark CCT, Nobre GC, Fernandes JFT, et al. Physical activity characterization: does one site fit all? Physiol Meas 2018;39:09TR02 10.1088/1361-6579/aadad0 [DOI] [PubMed] [Google Scholar]
- 13. Matthews CE, Hagströmer M, Pober DM, et al. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc 2012;44:S68–76. 10.1249/MSS.0b013e3182399e5b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The Joanna Briggs Institute Reviewer’s Manual 2015 Methodology for JBI scoping reviews, 2015. Available: www.joannabriggs.org [Accessed 30 Nov 2018].
- 15. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 16. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile APP for systematic reviews. Syst Rev 2016;5:210 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Joint Registry for England, Wales NI and the I of M . 15Th annual report. Hertfordshire, UK, 2018. [Google Scholar]
- 19. Rahman J, Tang Q, Monda M, et al. Gait assessment as a functional outcome measure in total knee arthroplasty: a cross-sectional study. BMC Musculoskelet Disord 2015;16:66 10.1186/s12891-015-0525-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ali R, Atallah L, Lo B, et al. Detection and analysis of transitional activity in manifold space. IEEE Trans Inform Technol Biomed 2012;16:119–28. 10.1109/TITB.2011.2165320 [DOI] [PubMed] [Google Scholar]
- 21. Christiansen CL, Bade MJ, Paxton RJ, et al. Measuring movement symmetry using tibial-mounted accelerometers for people recovering from total knee arthroplasty. Clin Biomech 2015;30:732–7. 10.1016/j.clinbiomech.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwasnicki RM, Ali R, Jordan SJ, et al. A wearable mobility assessment device for total knee replacement: a longitudinal feasibility study. Int J Surg 2015;18:14–20. 10.1016/j.ijsu.2015.04.032 [DOI] [PubMed] [Google Scholar]
- 23. Storey AST, Myrah AM, Bauck RA, et al. Indoor and outdoor mobility following total knee arthroplasty. Physiotherapy Canada 2013;65:279–88. 10.3138/ptc.2012-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang H-H, Yan S-H, Fang C, et al. Clinical evaluation and gait characteristics before and after total knee arthroplasty based on a portable gait analyzer. Orthop Surg 2016;8:360–6. 10.1111/os.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chapman RM, Moschetti WE, Van Citters DW. Stance and swing phase knee flexion recover at different rates following total knee arthroplasty: an inertial measurement unit study. J Biomech 2019;84:129–37. 10.1016/j.jbiomech.2018.12.027 [DOI] [PubMed] [Google Scholar]
- 26. Bolink SAAN, Grimm B, Heyligers IC. Patient-Reported outcome measures versus inertial performance-based outcome measures: a prospective study in patients undergoing primary total knee arthroplasty. Knee 2015;22:618–23. 10.1016/j.knee.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 27. Chiang C-Y, Chen K-H, Liu K-C, et al. Data collection and analysis using wearable sensors for monitoring knee range of motion after total knee arthroplasty. Sensors 2017;17 10.3390/s17020418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hiyama Y, Asai T, Wada O, et al. Gait variability before surgery and at discharge in patients who undergo total knee arthroplasty: a cohort study. PLoS One 2015;10:e0117683 10.1371/journal.pone.0117683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kluge F, Hannink J, Pasluosta C, et al. Pre-operative sensor-based gait parameters predict functional outcome after total knee arthroplasty. Gait Posture 2018;66:194–200. 10.1016/j.gaitpost.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 30. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning–based surveillance platform. J Arthroplasty 2019;34:2253–9. 10.1016/j.arth.2019.05.021 [DOI] [PubMed] [Google Scholar]
- 31. Youn I-H, Youn J-H, Zeni J, et al. Biomechanical gait variable estimation using wearable sensors after unilateral total knee arthroplasty. Sensors 2018;18 10.3390/s18051577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Hemert WLW, Senden R, Grimm B, et al. Patella retention versus replacement in total knee arthroplasty; functional and clinimetric aspects. Arch Orthop Trauma Surg 2009;129:259–65. 10.1007/s00402-008-0640-8 [DOI] [PubMed] [Google Scholar]
- 33. van Hemert WLW, Meyers WGH, Kleijn LLA, et al. Functional outcome of knee arthroplasty is dependent upon the evaluation method employed. Eur J Orthop Surg Traumatol 2009;19:415–22. 10.1007/s00590-009-0450-x [DOI] [Google Scholar]
- 34. van den Dikkenberg N, Meijer O, van der Slikke R, et al. Measuring functional abilities of patients with knee problems: rationale and construction of the DynaPort knee test. Knee Surg Sports Traumatol Arthrosc 2002;10:204–12. 10.1007/s00167-002-0279-x [DOI] [PubMed] [Google Scholar]
- 35. Daugaard R, Tjur M, Sliepen M, et al. Are patients with knee osteoarthritis and patients with knee joint replacement as physically active as healthy persons? J Orthop Translat 2018;14:8–15. 10.1016/j.jot.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kahn TL, Schwarzkopf R. Does total knee arthroplasty affect physical activity levels? data from the osteoarthritis initiative. J Arthroplasty 2015;30:1521–5. 10.1016/j.arth.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 37. Kahn TL, Schwarzkopf R. Do total knee arthroplasty patients have a higher activity level compared to patients with osteoarthritis? Geriatr Orthop Surg Rehabil 2016;7:142–7. 10.1177/2151458516654518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Webber SC, Strachan SM, Pachu NS. Sedentary behavior, cadence, and physical activity outcomes after knee arthroplasty. Med Sci Sports Exerc 2017;49:1057–65. 10.1249/MSS.0000000000001207 [DOI] [PubMed] [Google Scholar]
- 39. Cooper NA, Rakel BA, Zimmerman B, et al. Predictors of multidimensional functional outcomes after total knee arthroplasty. J Orthop Res 2017;35:2790–8. 10.1002/jor.23596 [DOI] [PubMed] [Google Scholar]
- 40. Lützner C, Kirschner S, Lützner J. Patient activity after TKA depends on patient-specific parameters. Clin Orthop Relat Res 2014;472:3933–40. 10.1007/s11999-014-3813-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Groot IB, Bussmann HJ, Stam HJ, et al. Small increase of actual physical activity 6 months after total hip or knee arthroplasty. Clin Orthop Relat Res 2008;466:2201–8. 10.1007/s11999-008-0315-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harding P, Holland AE, Delany C, et al. Do activity levels increase after total hip and knee arthroplasty? Clin Orthop Relat Res 2014;472:1502–11. 10.1007/s11999-013-3427-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hayashi K, Kako M, Suzuki K, et al. Impact of variation in physical activity after total joint replacement. J Pain Res 2018;11:2399–406. 10.2147/JPR.S178853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vissers MM, Bussmann JB, de Groot IB, et al. Physical functioning four years after total hip and knee arthroplasty. Gait Posture 2013;38:310–5. 10.1016/j.gaitpost.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 45. Agarwal V, Smuck M, Shah NH. Quantifying the relative change in physical activity after total knee arthroplasty using accelerometer based measurements : AMIA joint Summits on translational science proceedings. AMIA joint Summits on translational science. United States, 2017: 463–72. [PMC free article] [PubMed] [Google Scholar]
- 46. Bolszak S, Casartelli NC, Impellizzeri FM, et al. Validity and reproducibility of the physical activity scale for the elderly (PASE) questionnaire for the measurement of the physical activity level in patients after total knee arthroplasty. BMC Musculoskelet Disord 2014;15:46 10.1186/1471-2474-15-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Christiansen MB, Thoma LM, Master H, et al. The feasibility and preliminary outcomes of a physical therapist‐administered physical activity intervention after total knee replacement. Arthritis Care Res 2019. 10.1002/acr.23882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fenten MGE, Bakker SMK, Scheffer GJ, et al. Femoral nerve catheter vs local infiltration for analgesia in fast track total knee arthroplasty: short-term and long-term outcomes. Br J Anaesth 2018;121:850–8. 10.1016/j.bja.2018.05.069 [DOI] [PubMed] [Google Scholar]
- 49. Frimpong E, McVeigh JA, van der Jagt D, et al. Light intensity physical activity increases and sedentary behavior decreases following total knee arthroplasty in patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2019;27:2196–205. 10.1007/s00167-018-4987-2 [DOI] [PubMed] [Google Scholar]
- 50. Losina E, Collins JE, Deshpande BR, et al. Financial incentives and health coaching to improve physical activity following total knee replacement: a randomized controlled trial. Arthritis Care Res 2018;70:732–40. 10.1002/acr.23324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luna IE, Kehlet H, Wede HR, et al. Objectively measured early physical activity after total hip or knee arthroplasty. J Clin Monit Comput 2019;33:509–22. 10.1007/s10877-018-0185-5 [DOI] [PubMed] [Google Scholar]
- 52. Lützner C, Beyer F, Kirschner S, et al. How much improvement in patient activity can be expected after TKA? Orthopedics 2016;39:S18–23. 10.3928/01477447-20160509-15 [DOI] [PubMed] [Google Scholar]
- 53. Pellegrini CA, Chang RW, Dunlop DD, et al. Comparison of a patient-centered weight loss program starting before versus after knee replacement: a pilot study. Obes Res Clin Pract 2018;12:472–8. 10.1016/j.orcp.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schotanus MGM, Bemelmans YFL, Grimm B, et al. Physical activity after outpatient surgery and enhanced recovery for total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2017;25:3366–71. 10.1007/s00167-016-4256-1 [DOI] [PubMed] [Google Scholar]
- 55. Taniguchi M, Sawano S, Kugo M, et al. Physical activity promotes gait improvement in patients with total knee arthroplasty. J Arthroplasty 2016;31:984–8. 10.1016/j.arth.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 56. Tsuji S, Tomita T, Fujii M, et al. Is minimally invasive surgery–total knee arthroplasty truly less invasive than standard total knee arthroplasty? J Arthroplasty 2010;25:970–6. 10.1016/j.arth.2009.06.016 [DOI] [PubMed] [Google Scholar]
- 57. Twiggs J, Salmon L, Kolos E, et al. Measurement of physical activity in the pre- and early post-operative period after total knee arthroplasty for osteoarthritis using a Fitbit flex device. Med Eng Phys 2018;51:31–40. 10.1016/j.medengphy.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 58. Brandes M, Ringling M, Winter C, et al. Changes in physical activity and health-related quality of life during the first year after total knee arthroplasty. Arthritis Care Res 2011;63:328–34. 10.1002/acr.20384 [DOI] [PubMed] [Google Scholar]
- 59. Hayes DA, Watts MC, Anderson LJ, et al. Knee arthroplasty: a cross-sectional study assessing energy expenditure and activity. ANZ J Surg 2011;81:371–4. 10.1111/j.1445-2197.2010.05570.x [DOI] [PubMed] [Google Scholar]
- 60. Vissers MM, de Groot IB, Reijman M, et al. Functional capacity and actual daily activity do not contribute to patient satisfaction after total knee arthroplasty. BMC Musculoskelet Disord 2010;11:121 10.1186/1471-2474-11-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khan H, Walker PS, Zuckerman JD, et al. The potential of accelerometers in the evaluation of stability of total knee arthroplasty. J Arthroplasty 2013;28:459–62. 10.1016/j.arth.2012.07.025 [DOI] [PubMed] [Google Scholar]
- 62. Roberts D, Khan H, Kim JH, et al. Acceleration-based joint stability parameters for total knee arthroplasty that correspond with patient-reported instability. Proc Inst Mech Eng H 2013;227:1104–13. 10.1177/0954411913493724 [DOI] [PubMed] [Google Scholar]
- 63. Soeno T, Mochizuki T, Tanifuji O, et al. No differences in objective dynamic instability during acceleration of the knee with or without subjective instability post-total knee arthroplasty. PLoS One 2018;13:e0194221 10.1371/journal.pone.0194221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Papagiannis GI, Triantafyllou AI, Roumpelakis IM, et al. Gait analysis methodology for the measurement of biomechanical parameters in total knee arthroplasties. A literature review. J Orthop 2018;15:181–5. 10.1016/j.jor.2018.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Davis RB, Õunpuu S, Tyburski D, et al. A gait analysis data collection and reduction technique. Hum Mov Sci 1991;10:575–87. 10.1016/0167-9457(91)90046-Z [DOI] [Google Scholar]
- 66. Terwee CB, Mokkink LB, Steultjens MPM, et al. Performance-based methods for measuring the physical function of patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Rheumatology 2006;45:890–902. 10.1093/rheumatology/kei267 [DOI] [PubMed] [Google Scholar]
- 67. Wang C, Lu W, Narayanan MR, et al. Low-Power technologies for wearable telecare and telehealth systems: a review. Biomed. Eng. Lett. 2015;5:1–9. 10.1007/s13534-015-0174-2 [DOI] [Google Scholar]
- 68. IDC Wearable device Shipments slow in Q1 2018 as consumers shift from basic Wearables to smarter devices, according to IDC. IDC media cent, 2018. Available: https://www.idc.com/getdoc.jsp?containerId=prUS43900918 [Accessed 9 Apr 2019].
- 69. Walter RT, Thompson WR. Worldwide survey of fitness trends for 2019. Am Coll Sport Med Heal Fit J 2018;22:10–17. [Google Scholar]
- 70. Lim SER, Dodds R, Bacon D, et al. Physical activity among hospitalised older people: insights from upper and lower limb accelerometry. Aging Clin Exp Res 2018;30:1363–9. 10.1007/s40520-018-0930-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Troiano RP, McClain JJ, Brychta RJ, et al. Evolution of accelerometer methods for physical activity research. Br J Sports Med 2014;48:1019–23. 10.1136/bjsports-2014-093546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Trost SG, Zheng Y, Wong W-K. Machine learning for activity recognition: hip versus wrist data. Physiol Meas 2014;35:2183–9. 10.1088/0967-3334/35/11/2183 [DOI] [PubMed] [Google Scholar]
- 73. Amiri AM, Peltier N, Goldberg C, et al. WearSense: detecting autism stereotypic behaviors through Smartwatches. Healthcare 2017;5:11 10.3390/healthcare5010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang S, Murray P, Zillmer R, et al. Activity classification using the GENEA: optimum sampling frequency and number of axes. Med Sci Sports Exerc 2012;44:2228–34. 10.1249/MSS.0b013e31825e19fd [DOI] [PubMed] [Google Scholar]
- 75. Zhang S, Rowlands AV, Murray P, et al. Physical activity classification using the GENEA wrist-worn accelerometer. Med Sci Sports Exerc 2012;44:742–8. 10.1249/MSS.0b013e31823bf95c [DOI] [PubMed] [Google Scholar]
- 76. Cleland I, Kikhia B, Nugent C, et al. Optimal placement of accelerometers for the detection of everyday activities. Sensors 2013;13:9183–200. 10.3390/s130709183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLoS One 2017;12:e0169649 10.1371/journal.pone.0169649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hammett T, Simonian A, Austin M, et al. Changes in physical activity after total hip or knee arthroplasty: a systematic review and meta-analysis of six- and twelve-month outcomes. Arthritis Care Res 2018;70:892–901. 10.1002/acr.23415 [DOI] [PubMed] [Google Scholar]
- 79. Stevens-Lapsley JE, Schenkman ML, Dayton MR. Comparison of self-reported knee injury and osteoarthritis outcome score to performance measures in patients after total knee arthroplasty. Pm R 2011;3:541–9. 10.1016/j.pmrj.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 80. Mizner RL, Petterson SC, Clements KE, et al. Measuring functional improvement after total knee arthroplasty requires both performance-based and patient-report assessments: a longitudinal analysis of outcomes. J Arthroplasty 2011;26:728–37. 10.1016/j.arth.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. A Prospective Multicenter Longitudinal Cohort Study of the Mymobility Platform—Full Text View—ClinicalTrials.gov. Available: https://clinicaltrials.gov/ct2/show/NCT03737149 [Accessed 1 Feb 2019].
- 82. Thompson D, Peacock O, Western M, et al. Multidimensional physical activity. Exerc Sport Sci Rev 2015;43:67–74. 10.1249/JES.0000000000000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Panda N, Solsky I, Huang EJ, et al. Using Smartphones to capture novel recovery metrics after cancer surgery. JAMA Surg 2019;02114:1–7. 10.1001/jamasurg.2019.4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-033832supp001.pdf (63.1KB, pdf)



