Abstract
Objectives
To identify the association between the presence and severity of depressive symptoms and those of chronic knee pain.
Design
A retrospective cross-sectional study.
Participants
We used data from the sixth Korea National Health and Nutrition Examination Survey (KNHANES VI-2) performed in 2014. Overall, 7550 patients were included in the KNHANES VI-2.
Outcome measures
Participants were asked whether they had chronic knee pain, and each answer was either ‘yes’ or ‘no’. Patient Health Questionnaire-9 (PHQ-9) was used as a screening tool for depressive symptoms, and PHQ-9 scores of 10 or higher was classified as the depressed group. In total, 527 patients reported that they had pain in their knee, of whom 91 also had depressive symptoms.
Results
The prevalence of chronic knee pain in the Korean population aged over 50 years was 19.8%. Multiple logistic regression was conducted after adjustment for sex, age, smoking, alcohol drinking, education level, household income, physical activity, sleep duration and comorbidity. The analysis revealed a significant association between depressive symptoms and chronic knee pain (adjusted OR=2.333, p<0.001). In contrast, the severity of depressive symptoms was linearly correlated with the intensity of chronic knee pain (p for trend <0.001). In participants with no chronic knee pain (Numerical Rating Scale; NRS=0) or mild chronic knee pain (NRS=1–4), the prevalence of moderate and severe depressive symptoms was 3.4% and 0.6%, respectively. However, in those with severe chronic knee pain (NRS=8–10), there was a higher prevalence of moderate and severe depressive symptoms (10.1% and 5.8%, respectively) (p<0.001).
Conclusions
A strong association was observed between the presence and severity of depressive symptoms and the presence of chronic knee pain. The association became stronger with higher levels of depressive symptoms, indicating a positive correlation between depressive symptoms severity and chronic knee pain.
Keywords: chronic knee pain, depressive symptoms, Patient Health Questionnaire-9, Korea National Health and Nutrition Examination Survey
Strengths and limitations of this study.
This study is based on highly reliable data from the national survey using the Korean population-based questionnaire.
We found a strong association between the severity of depressive symptoms and the presence and intensity of chronic knee pain.
Because the questionnaire regarding chronic knee pain was only asked to a population aged over 50 years, the younger population with chronic knee pain was excluded in the analysis.
As this was a retrospective cross-sectional study, it assessed depressive symptoms and chronic knee pain using ORs without analysing the cause–effect relationship between the variables.
Response biases cannot be ruled out because self-report questionnaires, Numerical Rating Scale and Patient Health Questionnaire-9, were used to assess chronic knee pain and depressive symptoms, respectively.
Introduction
Depressive symptoms are one of the most common mental illnesses. An earlier systematic review and meta-analysis reported that a lifetime prevalence of psychotic depressive symptoms ranges from 0.35% to 1% and that the rates of psychotic depressive symptoms increase with age.1 According to a Chinese systematic review, a lifetime prevalence of major depressive disorder (MDD) varies between 1.1% and 3.3%.2 However, the prevalence of major depressive episode (MDE), which indicates the relatively mild symptoms of depression, is markedly higher. A cohort study using a sample of 29 621 people representing the general US population found that the 3-year prevalence rate was 8% for MDE.3 Additionally, a Canadian longitudinal study reported that the prevalence of depressive episode at baseline was 7.5%.4 Based on World Mental Health Survey data, a cross-sectional study identified diabetes, arthritis, asthma, chronic pulmonary disease, angina symptoms, stroke, advanced age, women, underweight and lower levels of income and education as risk factors related to depressive symptoms.5
The prevalence of knee pain is one of the most common symptoms in the ageing population around the world. According to the sixth National Health and Nutrition Survey (KNHANES VI, 2013–2015), 20.8% of Korean population aged over 50 years reported chronic knee pain.6 In an Italian cross-sectional study, 22.4% of the population aged over 65 years complained about pain in the knee.7 In a study involving the British population aged over 65 years, 32.6% of participants reported knee pain.8 As risk factors for chronic knee pain, advanced age, low education and high BMI have a cause–effect relationship with chronic knee pain.9
Emotional distress is also known to worsen physical pain, and many studies are underway in this context. The patients with temporomandibular disorders who have anxiety and depressive symptoms are more likely to develop migraine and primary headache.10 According to a systematic review, depressive symptoms and anxiety symptoms are associated with high morbidity neck pain.11
The relationship between depressive symptoms and chronic knee pain has been investigated in previous literature.12 A systematic review revealed strong evidence supporting such a relationship between depressive symptoms and chronic knee pain, with reference to five high-quality studies.13 A cohort study involving the Japanese population aged over 65 years found that the development of depressive symptoms was associated with the presence of chronic knee pain.14 Among previous studies, a cross-sectional study described the relationship between depressive symptoms and severity of chronic knee pain assessed by the Numerical Rating Scale (NRS) and the Kellgren-Lawrence Scale.15 Moreover, a recent study revealed a relationship between depressive symptom subtypes and risk for symptomatic knee pain.16
However, few studies have been conducted to identify the association between the severity of depressive symptoms and the presence and intensity of knee pain. Recently, one study revealed that participants with physical pain symptoms were significantly associated with depressive symptoms severity. According to this study, participants with pain showed higher scores of Montgomery-Asberg depressive symptoms rating scales. However, results of these studies are limited to showing the mean of depressive symptom severity score depending on the presence of pain symptoms.17 Moreover, a longitudinal analysis revealed that pain severity was a strong predictor of subsequent depressive symptom severity, and conversely, depressive symptom severity was a strong predictor of subsequent pain severity.18
In addition to previous studies regarding depressive symptoms and chronic knee pain, this study aimed to identify the association between the presence and severity of depressive symptoms and those of chronic knee pain. To our knowledge, our study is the first to explore both aspects of the severity of depressive symptoms and severity of chronic knee pain measured by Patient Health Questionnaire-9 (PHQ-9) and NRS.
Methods
Study population
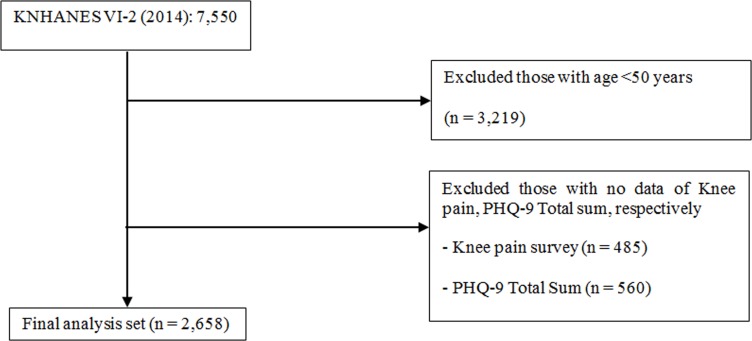
This study was based on the results of the nationally representative survey, the sixth Korea National Health and Nutrition Examination Survey (KNHANES VI-2), which was first launched in 1998 and conducted every year with population-based random samples using standardised questionnaires. This survey is intended to identify sociodemographic characteristics and health and nutritional status of the general Korean population and households, and survey responses are not tracked when the survey is completed each year. KNHANES VI-2 used the PHQ-9 as a screening instrument to diagnose depressive disorder. This questionnaire was provided to 7550 respondents. Of those administered questionnaires, 2658 questionnaires completed by Korean adults aged over 50 years were analysed with respect to the relationship between depressive symptoms and chronic knee pain (figure 1).
Figure 1.
Flow chart diagram of inclusion and exclusion of participants from the 2014 Korea National Health and Nutrition Examination Surveys (KNHANES). PHQ-9, Patient Health Questionnaire-9.
Definition of depressive symptoms and chronic knee pain
A total score of 10 or higher on the PHQ-9 was used to define the presence of depressive symptoms.19 In previous studies, the PHQ-9 questionnaire was often used to monitor depressive symptoms in patients with chronic pain.20 21 The PHQ-9 items are based on the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders and designed to ask about symptoms that relate to depression over the last 2 weeks. Each item is scored from 0 (not at all) to 3 (nearly every day). The PHQ-9, consisting of nine questions, is a reliable and valid depressive symptoms scale. As a severity measure, its score ranges from 0 to 27, with higher scores indicating higher severity of depressive symptoms.22 23
In previous studies, a PHQ-9 score ≥10 was used to determine the presence of depressive symptoms, and the reliability of this questionnaire has been proven. In this study, a PHQ-9 score ≥10 was also used to indicate the presence of depressive symptoms. The severity of depressive symptoms was divided into not depressed (0–4 scores), mild (5–9 scores), moderate (10–14), moderately severe (15–19) and severe (20–27), according to previous studies.22–24
The questionnaire used to identify the presence of chronic knee pain in the respondents aged over 50 years included the question ‘Have you had knee pain for at least 30 days during the last 3 months? Those who answered ‘yes’ to this question were classified as the chronic knee pain group, and their knee pain intensity was assessed using the NRS. Because the questionnaire regarding chronic knee pain was only asked to respondents aged over 50 years in KNHANES VI-2, the analysed data were limited to a population aged over 50 years.
Description of demographic and characteristics of the study population
Patient information, including demographic characteristics, socioeconomic background, medical history and life habits, was analysed. Body mass index (BMI) was calculated by dividing body weight in kilograms by the square of height in metres and categorised into three groups: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2) and overweight (≥25.0 kg/m2). Smoking status was classified into non-smoker, ex-smoker and current smoker. The frequency of alcohol consumption was grouped into none, once a month or less, two drinks/month to three drinks/week and more than four times a month. Occupation was classified into four groups: unemployed (student, housewife, etc); office work/sales and services; agriculture, forestry and fishery; and machine fitting and simple labour. Household income was classified into quartiles: low, low-moderate and moderate-high/high. Education level was also categorised into three groups: ≤6 years, 7–9 years and ≥10 years. Physical activity was categorised into three groups: moderate activity at least 2 hours and 30 min a week, vigorous activity at least 1 hour and 15 min a week and combination of moderate and vigorous activity for longer hours (1 min vigorous activity was considered equal to 2 min moderate activity). This study also investigated the presence of comorbidities, such as hypertension, dyslipidaemia, stroke, myocardial infarction, angina, arthritis, asthma and diabetes mellitus.
Statistical analysis
The KNHANES is a survey of nationwide sample of Koreans selected through stratified clustered sampling, and sampling weights are used for survey data. Accordingly, complex sample survey data were analysed using parameters for strata, clusters and weights, which comprise the sample design. SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) was used for statistical analysis, and p <0.05 was used as the threshold for statistical significance. Continuous variables are presented as mean and SD, and categorical variables are presented as frequency and percentage. The Rao-Scott χ2 test or t-test was performed to determine differences between groups with/without chronic knee pain. To assess the effects of depressive symptoms on chronic knee pain, logistic regression analysis with complex sampling design was performed by adjusting for covariates. As a result, ORs, as well as 95% CIs for the ORs, were generated. Additionally, this study investigated if there was an underlying trend from different levels of depressive symptoms in each model (P for trend). To verify a linear relationship between the intensity of chronic knee pain and depressive symptoms, we used the Cochran-Armitage trend test and complex samples logistic regression.
Patient and public involvement
We did not involve patients or the public in our work.
Results
Characteristics of the study population according to chronic knee pain
This study found that the overall prevalence of chronic knee pain in the Korean population aged over 50 years was 19.8%. Of those, women (77.8%) far outpaced men (22.2%). The presence of depressive symptoms, determined by PHQ-9 score ≥10, was significantly higher in the participants with chronic knee pain (17.3%), compared with those without chronic knee pain (5.2%) (p<0.001, table 1). When compared with the participants without chronic knee pain, those with chronic knee pain showed greater frequency of depressive symptoms, higher percentages of older women and unemployment, lower household income, lower levels of education and physical activity, more comorbidities (hypertension, dyslipidaemia, stroke, arthritis, asthma) and shorter sleep duration (table 1).
Table 1.
Characteristics of the study population according to chronic knee pain
| Variable | Without chronic knee pain (n=2131) | Chronic knee pain (n=527) | P value |
| PHQ-9, n (%) | |||
| <10 | 2021 (94.8) | 436 (82.7) | <0.0001 |
| ≥10 | 110 (5.2) | 91 (17.3) | |
| Levels of depressive symptom, n (%) | |||
| None (PHQ-9 ≤4) | 1780 (83.5) | 303 (57.5) | <0.0001 |
| Mild (5–9) | 241 (11.3) | 133 (25.2) | |
| Moderate (10–14) | 66 (3.1) | 51 (9.7) | |
| Moderately severe (15–19) | 33 (1.6) | 28 (5.3) | |
| Severe (≥20) | 11 (0.5) | 12 (2.3) | |
| Age, years (mean±SD) | 61.2±8.6 | 66.1±9.1 | <0.0001 |
| Age, n (%) | |||
| 50–59 years | 845 (39.7) | 111 (21.1) | <0.0001 |
| 60–69 years | 698 (32.8) | 182 (34.5) | |
| ≥80 years | 588 (27.6) | 234 (44.4) | |
| Sex, n (%) | |||
| Male | 1002 (47) | 117 (22.2) | <0.0001 |
| Female | 1129 (53) | 410 (77.8) | |
| BMI, kg/m2 (mean±SD) | 23.9±3.1 | 24.3±3.3 | 0.0533 |
| Obesity, n (%) | |||
| Underweight (≤18.5 kg/m2) | 55 (2.6) | 13 (2.5) | 0.2473 |
| Normal (18.5–24.9 kg/m2) | 1365 (64.1) | 300 (57) | |
| Obese (≥25 kg/m2) | 711 (33.4) | 213 (40.5) | |
| Smoking status, n (%) | |||
| Non-smoker | 1265 (60.4) | 383 (75) | <0.0001 |
| Ex-smoker | 496 (23.7) | 78 (15.3) | |
| Current smoker | 334 (15.9) | 50 (9.8) | |
| Alcohol consumption, n (%) | |||
| None | 805 (38.4) | 235 (45.7) | <0.0001 |
| One drink/month or less | 516 (24.6) | 156 (30.4) | |
| Two drinks/ month to three drinks/week | 585 (27.9) | 93 (18.1) | |
| Four drinks/ week or more | 193 (9.2) | 30 (5.8) | |
| Occupation, n (%) | |||
| Unemployed (student, housewife, etc) | 1003 (47.1) | 328 (62.2) | <0.0001 |
| Office work/sales and services | 463 (21.8) | 53 (10.1) | |
| Agriculture, forestry and fishery | 186 (8.7) | 50 (9.5) | |
| Machine fitting and simple labour | 477 (22.4) | 96 (18.2) | |
| Household income, n (%) | |||
| Low | 546 (25.7) | 232 (44.3) | <0.0001 |
| Low–moderate | 589 (27.7) | 136 (26) | |
| Moderate–high/high | 989 (46.6) | 156 (29.8) | |
| Educational level, n (%) | |||
| ≤6 years | 805 (37.8) | 345 (65.5) | <0.0001 |
| 7–9 years | 396 (18.6) | 73 (13.9) | |
| ≥10 years | 928 (43.6) | 109 (20.7) | |
| Aerobic physical activity, n (%) | |||
| No | 1100 (52.0) | 313 (60.5) | 0.0005 |
| Yes | 1016 (48.0) | 204 (39.5) | |
| Duration of sleep, hours | 6.7±1.4 | 6.4±1.7 | 0.0026 |
| Comorbidities, n (%) | |||
| Hypertension | 765 (35.9) | 262 (49.7) | <0.0001 |
| Dyslipidaemia | 413 (19.4) | 145 (27.5) | 0.0002 |
| Stroke | 77 (3.6) | 38 (7.2) | 0.0002 |
| Myocardial infarction | 31 (1.5) | 13 (2.5) | 0.2165 |
| Angina | 64 (3) | 17 (3.2) | 0.698 |
| Arthritis | 252 (11.8) | 285 (54.1) | <0.0001 |
| Asthma | 56 (2.6) | 33 (6.3) | <0.0001 |
| Diabetes mellitus | 304 (14.3) | 86 (16.3) | 0.7091 |
The Rao-Scott χ2 test or t-test was performed to determine differences between groups with/without chronic knee pain. Missing values/non-responses were excluded from analysis. Individuals with PHQ-9 scores >10 were considered to have depressive symptoms. Levels of depressive symptom were divided into five quartiles: none (0–4), mild (5–9), moderate (10–14), moderately severe (15–19) and severe (20–27) according to the PHQ-9 score. Continuous variables are presented as mean and SD, and categorical variables are presented as frequency and percentage.
BMI, body mass index; PHQ-9, Patient Health Questionnaire-9.
Clinical characteristics of participants with chronic knee pain according to the presence of depressive symptoms
When compared with non-depressed patients with chronic knee pain, depressed patients with chronic knee pain had lower household income, lower levels of education and aerobic physical activity, and shorter sleep duration. However, no difference between the two groups was found with respect to age, sex, BMI, obesity, alcohol consumption and occupation. In case of smoking status, the percentage of non-smokers was high in the group with coexisting depressive symptoms and chronic knee pain (table 2).
Table 2.
Characteristics of chronic knee pain population according to presence of depressive symptoms
| Variables | Non-depressive (n=436) | Depressive (n=91) | P value |
| Age, years (mean±SD) | 65.9±9.2 | 67±8.8 | 0.4956 |
| Age, n (%) | |||
| 50–59 years | 94 (21.6) | 17 (18.7) | 0.8051 |
| 60–69 years | 152 (34.9) | 30 (33) | |
| ≥70 years | 190 (43.6) | 44 (48.4) | |
| Sex, n (%) | |||
| Male | 104 (23.9) | 13 (14.3) | 0.1181 |
| Female | 332 (76.2) | 78 (85.7) | |
| BMI, kg/m2, (mean±SD) | 24.4±3.3 | 23.8±3.1 | 0.1927 |
| Obesity, n (%) | |||
| Underweight (<18.5 kg/m2) | 9 (2.1) | 4 (4.4) | 0.1711 |
| Normal (18.5–24.9 kg/m2) | 246 (56.4) | 54 (60) | |
| Obese (≥25 kg/m2) | 181 (41.5) | 32 (35.6) | |
| Smoking status, n (%) | |||
| Non-smoker | 312 (73.8) | 71 (80.7) | 0.0372 |
| Ex-smoker | 71 (16.8) | 7 (8) | |
| Current smoker | 40 (9.5) | 10 (11.4) | |
| Alcohol consumption, n (%) | |||
| None | 183 (43) | 52 (59.1) | 0.2782 |
| One drink/month or less | 136 (31.9) | 20 (22.7) | |
| Two drinks/month to three drinks/week | 83 (19.5) | 10 (11.4) | |
| Four drinks/week or more | 24 (5.6) | 6 (6.8) | |
| Occupation, n (%) | |||
| Unemployed (student, housewife, etc) | 262 (60.1) | 66 (72.5) | 0.0561 |
| Office work/sales and services | 48 (11) | 5 (5.5) | |
| Agriculture, forestry and fishery | 42 (9.6) | 8 (8.8) | |
| Machine fitting and simple labour | 84 (19.3) | 12 (13.2) | |
| Household income, n (%) | |||
| Low | 171 (39.5) | 61 (67) | <0.0001 |
| Low–moderate | 112 (25.9) | 24 (26.4) | |
| Moderate–high/high | 150 (34.6) | 6 (6.6) | |
| Educational level, n (%) | |||
| ≤6 years | 278 (63.8) | 67 (73.6) | 0.0076 |
| 7–9 years | 58 (13.3) | 15 (16.5) | |
| ≥10 years | 100 (22.9) | 9 (9.9) | |
| Aerobic physical activity, n (%) | |||
| No | 254 (59.1) | 59 (67.8) | 0.0162 |
| Yes | 176 (40.9) | 28 (32.2) | |
| Duration of sleep, hours (mean) | 6.5±1.7 | 5.9±1.9 | 0.0325 |
The Rao-Scott χ2 test or t-test was performed to determine differences between groups with/without depressive symptoms. Missing values/non-responses were excluded from analysis. Individuals with PHQ-9 scores >10 were considered to have depressive symptoms. Levels of depressive symptoms were divided into five quartiles: none (0–4), mild (5–9), moderate (10–14), moderately severe (15–19) and severe (20–27) according to the PHQ-9 score.
BMI, body mass index; PHQ-9, Patient Health Questionnaire-9.
Associations between depressive symptoms and chronic knee pain
Multiple logistic regression models were used to analyse the effects of depressive symptoms on chronic knee pain. Univariate analyses revealed a strong association between depressive symptoms and chronic knee pain (model 1, OR=3.553, 95% CI 2.558 to 4.935, p<0.0001). After adjusting for sex and age, a clear association between depressive symptoms and chronic knee pain was present (model 2, OR=2.722, 95% CI 1.844 to 4.017, p<0.0001). After adjusting for sex, age, smoking status, alcohol use, education level, household income, physical activity, sleep duration and comorbidities, there was still a clear association between depressive symptoms and chronic knee pain (model 3, OR=2.333, 95% CI 1.605 to 3.391, p<0.0001). The OR of depressive symptom levels also indicated an association between the severity of depressive symptoms and chronic knee pain. When compared with the absence of depressive symptoms, the probability of having chronic knee pain increased with the severity of depressive symptoms: mild (OR=3.715, 95% CI 2.687 to 5.138, p<0.0001), moderate (OR=4.525, 95% CI 2.964 to 6.909, p<0.0001), moderately severe (OR=4.124, 95% CI 2.256 to 7.539, p=0.0002) and severe depressive symptoms (model 1, OR=6.93, 95% CI 2.519 to 19.068, p for trend <0.0001). In the fully adjusted logistic regression model (model 3), the association between severe depressive symptom and chronic knee pain was strongly significant. When compared with the absence of depressive symptoms, the probability of having chronic knee pain increased with depressive symptom severity showing mild depressive symptoms (OR=2.944, 95% CI 2.112 to 4.103, p<0.0001) and severe depressive symptoms (OR=4.552, model 3, 95% CI 1.489 to 13.92, p for trend <0.0001; table 3). Driven by the Cochran-Armitage trend test and complex samples logistic regression, a linear relationship was found between chronic knee pain and depressive symptoms (online supplementary table 1, figures 1 and 2).
Table 3.
Association between severity of depressive symptoms and chronic knee pain
| Model 1 | Model 2 | Model 3 | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Diagnosis of depressive symptoms | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 3.553 (2.558 to 4.935) | <0.0001 | 2.722 (1.844 to 4.017) | <0.0001 | 2.333 (1.605 to 3.391) | <0.0001 |
| Levels of depressive symptom | ||||||
| None (0–4) | 1 | 1 | 1 | |||
| Mild (5–9) | 3.715 (2.687 to 5.138) | <0.0001 | 3.266 (2.35 to 4.541) | <0.0001 | 2.944 (2.112 to 4.103) | <0.0001 |
| Moderate (10–14) | 4.525 (2.964 to 6.909) | <0.0001 | 3.619 (2.233 to 5.865) | <0.0001 | 3.211 (1.977 to 5.217) | <0.0001 |
| Moderately severe (15–19) | 4.124 (2.256 to 7.539) | <0.0001 | 2.805 (1.553 to 5.066) | 0.0007 | 2.43 (1.355 to 4.359) | 0.0031 |
| Severe (20–27) | 6.93 (2.519 to 19.068) | 0.0002 | 5.109 (1.606 to 16.257) | 0.006 | 4.552 (1.489 to 13.92) | 0.0082 |
| P value for trend | <0.0001 | <0.0001 | <0.0001 | |||
Logistic regression analysis with complex sampling design was performed by adjusting for covariates. Individuals with PHQ-9 scores >10 were considered to have depressive symptoms. Levels of depressive symptom were divided into five quartiles: none (0–4), mild (5–9), moderate (10–14), moderately severe (15–19) and severe (20–27) according to the PHQ-9 score. Model 1 was unadjusted ORs. Model 2 was adjusted by age and sex. Model 3 was fully adjusted by age, sex and other environmental factors, such as smoking, alcohol consumption, educational level, household income, physical activity, duration of sleep and comorbidities.
PHQ-9, Patient Health Questionnaire-9.
bmjopen-2019-032451supp001.pdf (17.6KB, pdf)
bmjopen-2019-032451supp002.pdf (42.2KB, pdf)
bmjopen-2019-032451supp003.pdf (40.3KB, pdf)
Association between depressive symptom state and chronic knee pain intensity
The comparison between severity of depressive symptoms and intensity of chronic knee pain, measured by NRS, showed a positive linear association. When the chronic knee pain NRS score ranged from 0 to 4, the prevalence of moderate depressive symptom was 3.4%, and the prevalence of severe depressive symptoms was 0.6%. In the presence of severe chronic knee pain (NRS=8–10), the prevalence of moderate and severe depressive symptoms was high (10.1% and 5.6%, respectively) (p<0.001; table 4, figure 2).
Table 4.
Association between severity of chronic knee pain NRS and severity of depressive symptoms
| Chronic knee pain NRS | Depressive symptoms | |||||
| None (0–4) | Mild (5–9) | Moderate (10–14) | Moderately severe (15–19) | Severe (20–27) | P value | |
| NRS (0–4) (%) | 1872 (82.4) | 272 (12) | 77 (3.4) | 38 (1.7) | 13 (0.6) | <0.0001 |
| NRS (5–7) (%) | 136 (58.1) | 63 (26.9) | 25 (10.7) | 8 (3.4) | 2 (0.9) | |
| NRS (8–10) (%) | 65 (46.8) | 37 (26.6) | 14 (10.1) | 15 (10.8) | 8 (5.8) | |
The Rao-Scott χ2 test was performed to compare severity of depressive symptoms according to chronic knee pain NRS. Individuals without knee pain were regarded as NRS 0.
NRS, Numerical Rating Scale.
Figure 2.
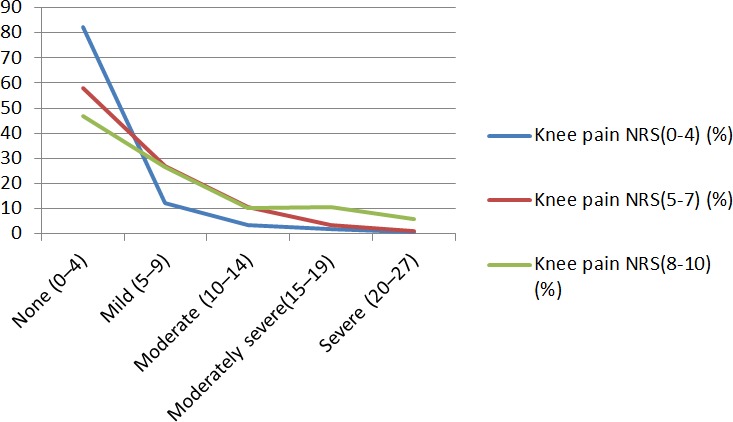
Severity of depressive symptoms according to chronic knee pain NRS. Individuals without knee pain were regarded as NRS 0. Severity of depressive symptoms according to PHQ-9 score: none (0–4), mild (5–9), moderate (10–14), moderately severe (15–19) and severe (20–27). Individuals without knee pain were regarded as NRS 0. NRS, Numerical Rating Scale.
Discussion
This study analysed the association between the severity of depressive symptoms and chronic knee pain by examining a sample of 7550 Koreans representing the general population from the reliable nationwide survey data. All the participants selected for this study were aged 50 years or older and shared information about their chronic knee pain, for which NRS was used to measure the pain intensity.
The association between depressive symptoms and pain is well established by previous studies.13–15 Moreover, a study indicated that persistent depressive symptoms was significantly associated with worsening of osteoarthritis (OA) knee pain.25 Another previous study also demonstrated that physical pain symptoms were significantly associated with the mean score of depressive symptoms severity.17 Furthermore, a longitudinal analysis revealed that pain severity was a strong predictor of subsequent depressive symptom severity, and conversely, depressive symptom severity was a strong predictor of subsequent pain severity.18 Further to this, our report is a cross-sectional study which explores the association between severity of depressive symptoms and intensity of chronic knee pain.
In our study, the positive association became stronger in the group with severe depressive symptoms, when compared with that of those having the symptoms of mild to moderate depressive symptoms. The strong linear correlation between depressive symptoms and chronic knee pain was still present after adjusting for variables, such as socioeconomic indicators and comorbidity. In the PHQ-9 survey, a significantly strong association was observed in the severely depressed group, identified with PHQ-9 score of 20 or above. Such a highly significant association persisted even after adjusting for variables, such as sex, age, socioeconomic indicators, smoking status and alcohol use. Although a previous study using KNHANES revealed a relationship between self-reported depressive symptoms and Kellgren-Lawrence knee OA grade, our study is the first to demonstrate the association between depressive symptoms and chronic knee pain using PHQ-9 and NRS in the Korean population.15
In our study, a positive linear relationship existed between intensity of chronic knee pain and severity of depressive symptoms as shown in table 4. We divided severity of depressive symptoms as not depressed (0–4 scores), mild (5–9 scores), moderate (10–14), moderately severe (15–19) and severe (20–27), and divided severity of chronic knee pain as light (0–4), moderate (5–7) and intense (8–10). In the participants with no chronic knee pain or light pain (NRS=0–4), the prevalence of severe depressive symptoms was 0.6. However, it increased to 5.8% in the participants with more intense chronic knee pain (NRS=8–10). To our knowledge, our study is the first to divide and compare both aspects of severity of depressive symptoms and intensity of chronic knee pain. As a result, we found a strong association between severity of depressive symptoms and intensity of chronic knee pain. It is a cross-sectional proof of a previous longitudinal study that suggested severe pain and severe depressive symptoms can be a predictor of each other.18
Although there is no clear evidence that depressive symptoms can cause chronic knee pain, many potential hypotheses have been suggested. Depressive symptom can interfere with the mechanism of inflammatory cytokines and the regulation of autonomic nervous system. Moreover, it can also destabilise the hypothalamic–pituitary–adrenal axis.26–28 Depressive symptom-induced pathological conditions act as the catalyst for chronic pain syndrome.29 30 Furthermore, neurotransmitters of norepinephrine and serotonin are associated with the pathological mechanism of depressive symptom. These neurotransmitters also play an important role in pain inhibitory pathways.31–33 The association between depressive symptoms and chronic knee pain can also be explained by reduced physical activity, which may be associated with depressive symptoms, consequently decreasing muscle strength and joint stability, whereby negative outcomes, such as OA, can be induced.34 35 Taken together, these findings suggest the effects of depressive symptoms on the pathogenic mechanism of chronic pain.
Strength and limitations
This study has the following strengths. First, this study was based on highly reliable data from the national survey using the Korean population-based questionnaire. Second, to our knowledge, this is the first study to describe the correlation between the severity of depressive symptoms and the intensity of chronic knee pain.
However, this study had certain limitations. First, this study was a cross-sectional study. Therefore, it assessed depressive symptoms and chronic knee pain using ORs without the analysis of the cause–effect relationship between the variables. Second, because the questionnaire regarding chronic knee pain was only asked to a population aged over 50 years, the younger population with chronic knee pain was excluded in the analysis. Third, self-reporting questionnaires were used to assess the NRS and PHQ-9 scores for chronic knee pain and depressive symptoms, respectively. Therefore, response biases cannot be ruled out.
Conclusions
This study identified a strong association between the presence and severity of depressive symptoms and the presence and intensity of chronic knee pain. Furthermore, the association became stronger with higher levels of depressive symptoms, demonstrating greater NRS scores for chronic knee pain. This study suggests depressive symptoms as an independent risk factor when screening for chronic knee pain.
Supplementary Material
Acknowledgments
We thank Park for providing data necessary for our analysis.
Footnotes
Contributors: Conceptualisation: S-BH, S-HL. Data curation: S-HL, I-HH, E-JK. Formal analysis: S-HL, I-HH. Writing-original draft: S-BH. Writing review and editing: I-HH, E-JK.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required
Ethics approval: The VI-2 version of KNHANES was approved by the KCDC Institutional Review Board (IRB approval no: 2013-12EXP-03-5C). Informed consent was obtained from all participants when the surveys were conducted. The approval of IRB was not required because the study did not deal with any sensitive information, but rather accessed only publicly available data from the KNHANES (JASENG IRB File No: 2018-11-017).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository.
References
- 1. Jääskeläinen E, Juola T, Korpela H, et al. Epidemiology of psychotic depression - systematic review and meta-analysis. Psychol Med 2018;48:905–18. 10.1017/S0033291717002501 [DOI] [PubMed] [Google Scholar]
- 2. Gu L, Xie J, Long J, et al. Epidemiology of major depressive disorder in mainland China: a systematic review. PLoS One 2013;8:e65356 10.1371/journal.pone.0065356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nigatu YT, Liu Y, Wang J. External validation of the international risk prediction algorithm for major depressive episode in the US general population: the PredictD-US study. BMC Psychiatry 2016;16:256 10.1186/s12888-016-0971-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patten SB, Williams JVA, Lavorato DH, et al. Depressive episode characteristics and subsequent recurrence risk. J Affect Disord 2012;140:277–84. 10.1016/j.jad.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 5. Lotfaliany M, Bowe SJ, Kowal P, et al. Depression and chronic diseases: co-occurrence and communality of risk factors. J Affect Disord 2018;241:461–8. 10.1016/j.jad.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 6. Lee S-H, Son C, Yeo S, et al. Cross-sectional analysis of self-reported sedentary behaviors and chronic knee pain among South Korean adults over 50 years of age in KNHANES 2013-2015. BMC Public Health 2019;19:1375 10.1186/s12889-019-7653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cecchi F, Mannoni A, Molino-Lova R, et al. Epidemiology of hip and knee pain in a community based sample of Italian persons aged 65 and older. Osteoarthritis Cartilage 2008;16:1039–46. 10.1016/j.joca.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dawson J, Linsell L, Zondervan K, et al. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology 2004;43:497–504. 10.1093/rheumatology/keh086 [DOI] [PubMed] [Google Scholar]
- 9. Takahashi A, Kitamura K, Watanabe Y, et al. Epidemiological profiles of chronic low back and knee pain in middle-aged and elderly Japanese from the Murakami cohort. J Pain Res 2018;11:3161–9. 10.2147/JPR.S184746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nazeri M, Ghahrechahi H-R, Pourzare A, et al. Role of anxiety and depression in association with migraine and myofascial pain temporomandibular disorder. Indian J Dent Res 2018;29:583–7. 10.4103/0970-9290.244932 [DOI] [PubMed] [Google Scholar]
- 11. Liu F, Fang T, Zhou F, et al. Association of Depression/Anxiety symptoms with neck pain: a systematic review and meta-analysis of literature in China. Pain Res Manag 2018;2018:1–9. 10.1155/2018/3259431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Maharbi S, Abolkhair AB, Al Ghamdi H, et al. Prevalence of depression and its association with sociodemographic factors in patients with chronic pain: a cross-sectional study in a tertiary care hospital in Saudi Arabia. Saudi J Anaesth 2018;12:419–25. 10.4103/sja.SJA_771_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phyomaung PP, Dubowitz J, Cicuttini FM, et al. Are depression, anxiety and poor mental health risk factors for knee pain? A systematic review. BMC Musculoskelet Disord 2014;15:10 10.1186/1471-2474-15-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sugai K, Takeda-Imai F, Michikawa T, et al. Association between knee pain, impaired function, and development of depressive symptoms. J Am Geriatr Soc 2018;66:570–6. 10.1111/jgs.15259 [DOI] [PubMed] [Google Scholar]
- 15. Han H-S, Lee J-Y, Kang S-B, et al. The relationship between the presence of depressive symptoms and the severity of self-reported knee pain in the middle aged and elderly. Knee Surg Sports Traumatol Arthrosc 2016;24:1634–42. 10.1007/s00167-015-3628-2 [DOI] [PubMed] [Google Scholar]
- 16. Rathbun AM, Schuler MS, Stuart EA, et al. Depression subtypes in persons with or at risk for symptomatic knee osteoarthritis. Arthritis Care Res 2019. 10.1002/acr.23898. [Epub ahead of print: 05 Apr 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oon-Arom A, Likhitsathian S, Maneeton B, et al. Subjective depressive symptoms associated with pain in patients with major depressive disorder: findings from the study on the aspect of Asian depression. Perspect Psychiatr Care 2019. 10.1111/ppc.12403. [Epub ahead of print: 30 May 2019]. [DOI] [PubMed] [Google Scholar]
- 18. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain 2011;12:964–73. 10.1016/j.jpain.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi Y, Mayer TG, Williams MJ, et al. What is the best screening test for depression in chronic spinal pain patients? Spine J 2014;14:1175–82. 10.1016/j.spinee.2013.10.037 [DOI] [PubMed] [Google Scholar]
- 20. Feingold D, Brill S, Goor-Aryeh I, et al. The association between severity of depression and prescription opioid misuse among chronic pain patients with and without anxiety: a cross-sectional study. J Affect Disord 2018;235:293–302. 10.1016/j.jad.2018.04.058 [DOI] [PubMed] [Google Scholar]
- 21. Gilbody S, Richards D, Brealey S, et al. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med 2007;22:1596–602. 10.1007/s11606-007-0333-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Man-van Ginkel JM, Gooskens F, Schepers VPM, et al. Screening for poststroke depression using the Patient Health Questionnaire. Nurs Res 2012;61:333–41. 10.1097/NNR.0b013e31825d9e9e [DOI] [PubMed] [Google Scholar]
- 23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park S-M, Kim H-J, Jang S, et al. Depression is closely associated with chronic low back pain in patients over 50 years of age: a cross-sectional study using the sixth Korea National health and nutrition examination survey (KNHANES VI-2). Spine 2018;43:1281–8. 10.1097/BRS.0000000000002595 [DOI] [PubMed] [Google Scholar]
- 25. Rathbun AM, Stuart EA, Shardell M, et al. Dynamic effects of depressive symptoms on osteoarthritis knee pain. Arthritis Care Res 2018;70:80–8. 10.1002/acr.23239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayley S, Poulter MO, Merali Z, et al. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience 2005;135:659–78. 10.1016/j.neuroscience.2005.03.051 [DOI] [PubMed] [Google Scholar]
- 27. Narasimhan M, Campbell N. A tale of two comorbidities: understanding the neurobiology of depression and pain. Indian J Psychiatry 2010;52:127–30. 10.4103/0019-5545.64586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pace TWW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun 2007;21:9–19. 10.1016/j.bbi.2006.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ross RL, Jones KD, Bennett RM, et al. Preliminary evidence of increased pain and elevated cytokines in fibromyalgia patients with defective growth hormone response to exercise. Open Immunol J 2010;3:9–18. 10.2174/1874226201003010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D'Andrea G, Leon A. Pathogenesis of migraine: from neurotransmitters to neuromodulators and beyond. Neurol Sci 2010;31:S1–7. 10.1007/s10072-010-0267-8 [DOI] [PubMed] [Google Scholar]
- 31. Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci 2009;14:5291–338. 10.2741/3598 [DOI] [PubMed] [Google Scholar]
- 32. Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci 2006;101:107–17. 10.1254/jphs.CRJ06008X [DOI] [PubMed] [Google Scholar]
- 33. Millan MJ. Descending control of pain. Prog Neurobiol 2002;66:355–474. 10.1016/S0301-0082(02)00009-6 [DOI] [PubMed] [Google Scholar]
- 34. Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000;85:317–32. 10.1016/S0304-3959(99)00242-0 [DOI] [PubMed] [Google Scholar]
- 35. Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am 1999;25:283–98. 10.1016/S0889-857X(05)70068-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-032451supp001.pdf (17.6KB, pdf)
bmjopen-2019-032451supp002.pdf (42.2KB, pdf)
bmjopen-2019-032451supp003.pdf (40.3KB, pdf)