Abstract
Pyrroloiminoquinone alkaloids represent a structurally intriguing class of natural products that display an array of useful biological properties. Here, we present a versatile and scalable platform for the synthesis of this diverse family – and in particular the antitumor discorhabdins – built upon sequential selective C–H functionalization of tryptamine. The utility of this strategy is showcased through short formal syntheses of damirones A–C, makaluvamines D and I, and discorhadbin E. Additionally, we describe efforts to develop the first catalytic asymmetric entry to the discorhabdin subclass.
Graphical Abstract:

Alkaloids have long captured the imagination of synthetic chemists and medical practitioners alike due to the challenge their intricate structures present and the wide array of useful biological properties often encoded therein.1 Among this large collection of natural products, the pyrroloiminoquinone alkaloids represent a unique subset of structural complexity. These compounds are typically isolated from marine sources and encompass many diverse classes, such as the discorhabdins, makaluvamines, and damirones (Figure 1), displaying antitumor, antimalarial, antiviral, antifeedant and antibacterial properties.2 Within this larger group of alkaloids, arguably the most complex and interesting biologically are the discorhabdins, a family of over 50 members isolated from various species of marine sponge.3 The discorhabdins display noteworthy anticancer activities, with nanomolar cytotoxicity (IC50 often <50 nM) being observed in vitro against a range of cancer cell lines; however, in vivo studies have proven less promising, either due to compound instability or nonspecific cytotoxicity.3,4,5 For this reason, a flexible de novo synthetic entry to the family would be desirable for detailed SAR studies,5 ideally allowing their selectivity profile to be fine-tuned while also providing access to related pyrroloiminoquinone targets.
Figure 1.
Representative pyrroloiminoquinone alkaloids.
Structurally, the discorhabdins contain a unique polycyclic framework, comprising a tricyclic pyrroloiminoquinone ring system fused to a spirodienone fragment via a quaternary stereogenic spirocenter, as exemplified in discorhabdin E6 (1, Figure 1). More complex members feature additional ring systems (e.g., 4, 5) and the class as a whole contains an impressive array of heteroatoms, with nitrogen, sulfur, bromine and oxygen substituents all commonly found within the same molecule. Of note is the particularly challenging arrangement of N-atoms in the pyrroloiminoquinone portion of the molecules, which results in a highly basic doubly vinylogous guanidinine (i.e. 7); in fact, such substructures have typically required more than 10 steps to construct.3
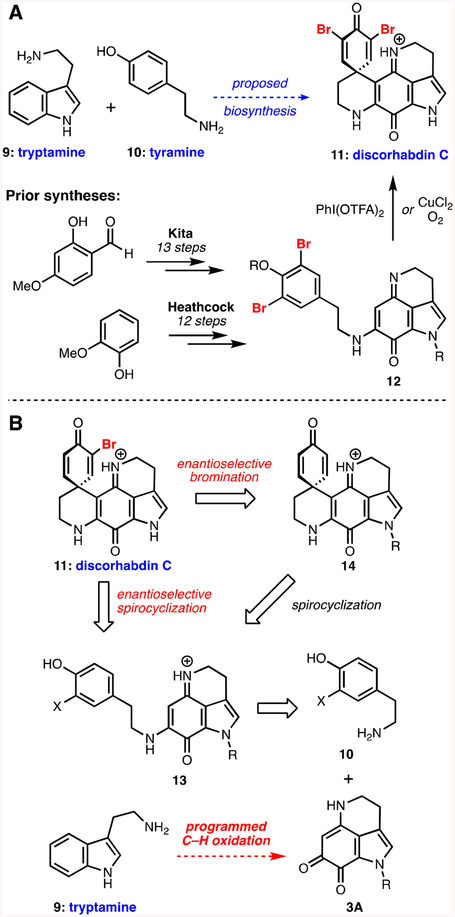
Biosynthetically, these natural products are postulated to arise from the combination of two amino acid-derived fragments: tryptamine (9) and tyramine (10), which form makaluvamine-type structures (e.g., 2, Figure 1) that are in turn spirocyclized to the discorhabins (e.g., discorhabdin C 11, Scheme 1A).3 Indeed, while there have been many creative approaches to these molecules,7 the majority of successful synthetic efforts have followed this biomimetic blueprint. For example, notable work from the Kita and Heathcock groups involved synthesizing makaluvamine-type structures 12 from simple aromatic building blocks which are then oxidatively spirocyclized under hypervalent iodine(III) or aerobic copper-mediated conditions en route to discorhabdins C (11),8,9,10 E (1),10 and A (4)11 (Scheme 1A).
Scheme 1.
(A) Proposed biosynthetic origins, notable prior art, (B) our approach to the discorhabdins.
In planning our own bioinspired approach to these important targets, we noted two key areas in these prior studies where significant improvement might be possible: first, while highly effective in their sequential transformation of oxidized tryptamine fragments to makaluvamine-type intermediates to spirocyclic compounds, these routes invested the majority of their synthetic effort in preparation of such tryptamine frameworks, typically via lengthy de novo sequences from simple aromatics (Scheme 1A).8–11 Second, no enantioselective approach to the family has been described to date,12 meaning that a catalytic asymmetric entry to the class could prove especially enabling. Given our lab’s interest in both catalytic asymmetric halofunctionalization transformations13 and novel synthetic strategies enabled by C–H functionalization,14 we formulated a plan towards one of the prototypical chiral members of the discorhabdin family, discorhabdin E (1). As outlined in Scheme 1B, we hoped to set the chirality of its lone stereocenter through the development of either an asymmetric spirocyclization of a brominated makaluvamine-type precursor 13 (X = Br) or via a brominative desymmetrization of achiral spirodienone 14, also available from a similar precursor (13, X = H). We postulated that 13 could be formed through a condensation reaction between an appropriate tyramine partner 10A and an orthoquinone tricycle 3A, encompassing the framework of the natural product damirone C15 (3). In contrast to the relatively lengthy prior syntheses of tricycles of type 3A,16 we sought to streamline our preparation of this key fragment by beginning with the readily available, unsubstituted tryptamine scaffold, and simply installing the necessary carbon–heteroatom bonds through selective C–H oxidations. Importantly, 3A could also serve as a versatile intermediate for accessing other classes of pyrroloiminoquinone alkaloids (see Figure 1). Herein, we report the execution of this plan, resulting in convenient, scalable access to such an intermediate, along with our efforts to develop the first catalytic asymmetric entry to the discorhabdins as a prelude to optimizing their antitumor properties.
Our route began with the quantitative N-Boc-protection of tryptamine (9), followed by the application of a modified one-pot C–H diborylation/monodeborylation procedure under Ir- and Pd-catalysis, developed by Movassaghi and co-workers (Scheme 2).17 This process proceeds via diborylated intermediate 15 and achieves the net installation of a C-7 Bpin substituent, which could be easily transformed to the corresponding phenol through treatment with alkaline H2O2 to deliver 17 in 56% overall yield from tryptamine. This sequence proved highly scalable and was reliably conducted on decagram quantities of 17 with little variation in yield. With a C-7 phenol in place, selective oxidation to the corresponding orthoquinone 18A with IBX proceeded effectively,18 with this being the first demonstration of this process in an indole setting to the best of our knowledge. Although precedent exists for cyclization of tryptamine orthoquinones similar to 18A under basic aerobic conditions,16b–d,f our efforts to cyclize the corresponding amine salts (available from acidic N-Boc deprotection), routinely resulted in extensive decomposition, with no damirone C (3) being isolated. We found, however, that protection of the indole nitrogen of 18A with a tosyl group (62% over two steps from phenol 17) gave a material (18B) that could be cleanly converted to tricycle 19 in 48% yield by treatment with TFA, evaporation of the volatiles, and exposure to Et N in MeOH under air.19 This protocol can reliably be conducted on gram scale, and to date we have prepared over 2.5 g of 19. Indeed, the synthesis of 19 in 6 steps and 15% overall yield from tryptamine represents the shortest preparation of this material to date (15 steps previously),16a and also constitutes the formal synthesis of several pyrroloiminoquinone alkaloids including damirones A–C and makaluvamines D and I.16 Furthermore, we found that 19 could be condensed with tyramine fragments 10 and 10A under mild conditions to give makaluvamine-type compounds 2A and 20 in 71 and 29% yield, respectively (Scheme 3). In these reactions, careful control of reaction time and purification was important for maximizing material throughput; even so, achieving high conversions in the brominated series proved unexpectedly challenging. We observed that longer reaction times led to competitive degradation of both 20 and its condensation product. One such identified pathway was transfer of the toluenesulfonyl group from the indole nitrogen to the primary amine.20
Scheme 2.
Synthesis of Ts-damirone C via sequential C–H functionaiization of tryptamine.
Scheme 3.
Preparation of makaluvamine-type materials and attempted transform-ations to enantioenriched spirocycle.
While the preparation of 20 constituted a racemic formal synthesis of discorhabdin E (1),10 we aimed to provide an asymmetric entry to the family. It should be noted, however, that the properties of compounds post condensation rendered the development of such a process challenging, with the basic and heteroatom-rich scaffolds limiting the choice of strategies or, in the case of polar salt forms of 20 (and 21), solvents. While our initial efforts focusing on an asymmetric spirocyclization of 2A and 20 were largely unfruitful, explorations of a brominative desymmetrization approach on spirodienone 21 proved more rewarding. This material was reliably prepared in 74% yield by treatment of 2A under conditions of Kita et al. employing PIFA and Montmorillonite K-10 clay in 2,2,2-trifluoroethanol.11 Although the aerobic Cu-based conditions10 of Heathcock and Aubart provided high yields of 21 on small scale (<50 mg of 2A), in our hands their method proved much less effective on scale-up. Initial attempts at effecting the desired bromination of 21 showed that this could be readily accomplished in a racemic sense using n-Bu4NBr3 (66% yield of rac-1A). In contrast, achieving an analogous enantioselective transformation proved challenging. For example, while explorations in a model system showed organocatalytic methods to be viable, the complications inherent to the structure of 21 (either as its TFA salt or free base) led to no productive reactivity. Efforts to condense chiral auxiliaries such as (S)-1-amino-2-methoxymethylpyrrolidine (SAMP) onto the ketone were unfortunately unsuccessful. Similarly, attempted bromination of chiral salt forms of 21 (formed from 21 and an equivalent amount of chiral phosphoric acid) delivered the desired product in poor yield and with no enantioselectivity.
Given these failures, we then proceeded to explore Baylis–Hillman-type brominations with an appropriate combination of nucleophile and brominating reagent. Inspired by a recent report by Feng and co-workers on the asymmetric haloazidation of acyclic enones,21 we found in initial trials that the combination of TMS azide and NBS as nucleophile and bromonium source, respectively, in the presence of a catalytic amount of Sc(OTf)3 delivered rac-1A directly (34%, 43% 21 recovered) without isolation of the intermediate bromoazide. We then proceeded to test various combinations of Lewis acidic metals and chiral ligands (see SI for details) in this process. Among the many systems screened, we ultimately found that treatment of 21 with TMS azide and NBS in the presence of a combination of Sc(OTf)3 and chiral N,N’-dioxide ligand 2222 (30 mol% of each) in CH2Cl2 at −30 °C with 4Å MS provided the desired bromoenone 1A in 23% yield and 33% ee. The use of other Br+ sources (e.g. NBA, DBDMH, TBCHD, BsNMeBr; for a complete list, see Supporting Information), nucleophiles (TsNH2, p-NsNH2)23 and various solvents did not improve the enantioinduction (see SI for full details). While the selectivity achieved to date is admittedly moderate, it is important to note that this nevertheless represents the first catalytic asymmetric inroad towards the discorhabdin family, hinting at the challenge posed by their unique scaffolds.
In summary, we have described a short and scalable entry to the pyrroloiminoquinone alkaloids via a key tricyclic intermediate, available through a series of selective C–H functionalization reactions on the parent tryptamine framework. Through this synthetic platform we have achieved concise formal syntheses of damirones A–C, makaluvamines D and I, and discorhadbin E. Finally, we have disclosed our preliminary efforts toward a catalytic asymmetric solution to the discorhabdin alkaloids, providing the key spirocycle of discorhadbin E with moderate enantioselectivity. It is our hope that the tools and strategies presented herein will prove useful in future synthetic endeavors toward this broad class of bioactive alkaloids.
Supplementary Material
Acknowledgments:
This work was supported by Stanford University and the National Institutes of Health (R01 GM114061). We are grateful to Dr. S. Lynch (Stanford University) for assistance with NMR spectroscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.(a) Nicolaou KC; Sorensen EJ Classics in Total Synthesis: Targets, Strategies, Methods; VCH: Weinheim, 1996; [Google Scholar]; (b) Schmeller T; Wink M Utilization of Alkaloids in Modern Medicine In Alkaloids; Roberts MF; Wink M, Eds; Springer: Boston, MA, 1998; [Google Scholar]; (c) Nicolaou KC; Snyder SA; Classics in Total Synthesis II: More Targets, Strategies, Methods; Wiley-VCH: Weinheim, 2003; [Google Scholar]; (d) Modern Alkaloids: Structure, Isolation, Synthesis, and Biology; Fattorusso E; Taglialatela-Scafati O, Eds; Wiley-VCH: Weinheim, 2008. [Google Scholar]
- 2.(a) For reviews, see:Antunes EM; Copp BR; Davies-Coleman MT; Samaai T Pyrroloiminoquinone and related metabolites from marine sponges. Nat. Prod. Rep 2005, 22, 62–72; [DOI] [PubMed] [Google Scholar]; (b) Hu J-F; Fan H; Xiong J; Wu S-B Discorhabdins and Pyrroloiminoquinone-Related Alkaloids. Chem. Rev 2011, 111, 5465–5491. [DOI] [PubMed] [Google Scholar]
- 3.(a) For reviews, see:Harayama Y; Kita Y Pyrroloiminoquinone Alkaloids: Discorhabdins and Makaluvamines Current Organic Chemistry, 2005, 9, 1567–1588; [Google Scholar]; (b) Wada Y; Fujioka H; Kita Y Synthesis of the Marine Pyrroloiminoquinone Alkaloids, Discorhabdins. Mar. Drugs 2010, 8, 1394–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Perry NB; Blunt JW; Munro MHG Cytotoxic Pigments from New Zealand Sponges of the Genus Latrunculia: Discorhabdins A, B, and C. Tetrahedron 1988, 44, 1727–1734. [Google Scholar]; (b) Perry NB; Blunt JW; Munro MHG; Discorhabdin D, an Antitumor Alkaloid from the Sponges Latrunculia brevis and Prianos sp. J. Org. Chem 1988, 53, 4127–4128. [Google Scholar]; (c) Hamann MT, et al. Anti-infective Discorhabdins from a Deep-Water Alaskan Sponge of the Genus Latrunculia. J. Nat. Prod 2010, 73, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kita Y; et al. The Synthetic and Biological Studies of Discorhabdins and Related Compounds. Org. Biomol. Chem 2011, 9, 4959–4976. [DOI] [PubMed] [Google Scholar]
- 6.For its isolation, see: Copp BR; Fulton KF; Perry NB; Blunt JW; Munro MHG Natural and Synthetic Derivatives of Discorhabdin C, a Cytotoxic Pigment from the New Zealand Sponge Latrunculia cf. bocagei. J. Org. Chem 1994, 59, 8233–8238. [Google Scholar]
- 7.(a) For formal syntheses of discorhabdin C (11), see:White JD; Yager KM; Yakura T Synthetic Studies of the Pyrroloquinoline Nucleus of the Makaluvamine Alkaloids. Synthesis of the Topoisomerase II Inhibitor Makaluvamine D. J. Am. Chem. Soc 1994, 116, 1831–1838; [Google Scholar]; (b) Sadanandan EV; Pillai SK; Lakshmikantham MV; Billimoria AD; Culpepper JS; Cava MP Efficient Syntheses of the Marine Alkaloids Makaluvamine D and Discorhabdin C: The 4,6,7-Trimethoxyindole Approach. J. Org. Chem 1995, 60, 1800–1805; [Google Scholar]; (c) Roberts D; Joule JA Synthesis of Pyrrolo[4,3,2-de]quinolines from 6,7-Dimethoxy-4-methylquinoline. Formal Total Syntheses of Damirones A and B, Batzelline C, Isobatzelline C, Discorhabdin C, and Makaluvamines A–D. J. Org. Chem 1997, 62, 568–577; [DOI] [PubMed] [Google Scholar]; (d) Zhao R; Lown JW A Concise Synthesis of the Pyrroloquinoline Nucleus of the Makaluvamine Alkaloids. Synth. Commun, 1997, 27, 2103–2110. For studies towards the discorhabdins, see: [Google Scholar]; (e) Kublak GG; Confalone PN The Preparation of the Aza-spirobicyclic System of Discorhabdin C via an Intramolecular Phenolate Alkylation. Tetrahedron Lett. 1990, 31, 3845–3848; [Google Scholar]; (f) Confalone PN The Use of Heterocyclic Chemistry in the Synthesis of Natural and Unnatural Products. J. Heterocycl. Chem 1990, 27, 31–46; [Google Scholar]; (g) Knölker H-J; Hartmann K Transition Metal-Diene Complexes in Organic Synthesis; Part 8. Iron-Mediated Approach to the Discorhabdin and Prianosin Alkaloids. Synlett 1991, 6, 428–430; [Google Scholar]; (h) Makosza M; Stalewski J; Maslennikova OS Synthesis of 7,8-Dimethoxy-2-oxo-1,3,4,5-tetrahydropyrrolo[4,3,2-de]quinoline: A Key Intermediate en Route to Makaluvamines, Discorhabdin C and Other Marine Alkaloids of this Group via Vicarious Nucleophilic Substitions of Hydrogen. Synthesis 1997, 10, 1131–1133. [Google Scholar]
- 8.Kita Y; Tohma H; Inagaki M; Hatanaka K; Yakura T Total Synthesis of Discorhabdin C: A General Aza Spiro Dienone Formation from O-Silylated Phenol Derivatives using a Hypervalent Iodine Reagent. J. Am. Chem. Soc 1992, 114, 2175–2180. [Google Scholar]
- 9.For the Yamamura synthesis of 11 using anodic oxidation, see: Tao XL; Cheng J-F; Nishiyama S; Yamamura S Synthetic Studies on Tetrahydropyrroloquinoline-containing Natural Products: Syntheses of Discorhabdin C, Batzelline C and Isobatzelline C. Tetrahedron 1994, 50, 2017–2028. [Google Scholar]
- 10.Aubart KM; Heathcock CH A Biomimetic Approach to Discorhabdin Alkaloids: Total Syntheses of Discorhabdins C and E and Dethiadiscorhabdin D. J. Org. Chem 1999, 64, 16–22. [DOI] [PubMed] [Google Scholar]
- 11.(a) Tohma H; Harayama Y; Hashizume M; Iwata M; Egi M; Kita Y Synthetic Studies on the Sulfur-Cross-Linked Core of Antitumor Marine Alkaloid, Discorhabdins: Total Synthesis of Discorhabdin A. Angew. Chem. Int. Ed 2002, 41, 348–350; [DOI] [PubMed] [Google Scholar]; (b) Tohma H; Harayama Y; Hashizume M; Iwata M; Kiyono Y; Egi M; Kita Y The First Total Synthesis of Discorhabdin A. J. Am. Chem. Soc 2003, 125, 11235–11240. [DOI] [PubMed] [Google Scholar]
- 12.The only asymmetric synthesis of a family member to date is Kita’s synthesis of discorhabdin A (ref. 10), which relied on a diastereoselective spirocyclization (optimal dr = 4.8:1) controlled by the chirality of a tyrosine-derived precursor.
- 13.(a) Hu DX; Shibuya GM; Burns NZ Catalytic Enantioselective Dibromination of Allylic Alcohols. J. Am. Chem. Soc 2013, 135, 12960–12963; [DOI] [PubMed] [Google Scholar]; (b) Hu DX; Seidl FJ; Bucher C; Burns NZ Catalytic Chemo-, Regio-, and Enantioselective Bromochlorination of Allylic Alcohols. J. Am. Chem. Soc 2015, 137, 3795–3798; [DOI] [PubMed] [Google Scholar]; (c) Landry ML; Burns NZ Catalytic Enantioselective Dihalogenation in Total Synthesis. Acc. Chem. Res 2018, 51, 1260–1271; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Seidl FJ; Min C; Lopez JA; Burns NZ Catalytic Regio- and Enantioselective Haloazidation of Allylic Alcohols. J. Am. Chem. Soc 2018, 2018, 140, 15646–15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer JAM; Cohen CM; Shuken SR; Wagner AM; Smith MW; Moss FR; Smith MD; Vahala R; Gonzalez-Martinez A; Boxer SG; Burns NZ Chemical Synthesis and Self-assembly of a Ladderane Phospholipid. J. Am. Chem. Soc 2016, 138, 15845–15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.For its isolation, see: Schmidt EW; Harper MK; Faulkner DJ Makaluvamines H–M and Damirone C from the Pohnpeian Sponge Zyzzya fuliginosa. J. Nat. Prod, 1995, 58, 1861–1867. [DOI] [PubMed] [Google Scholar]
- 16.(a) Inoue K; Ishikawa Y; Nishiyama S Synthesis of Tetrahydropyrroloiminoquinone Alkaloids Based on Electrochemically Generated Hypervalent Iodine Oxidative Cyclization. Org. Lett 2010, 12, 436–439. For the synthesis of similar scaffolds with additional substituents, see: [DOI] [PubMed] [Google Scholar]; (b) Baumann C; Brockelmann M; Fugmann B; Steffan B; Steglich W; Sheldrick WS Haematopodin, an Unusual Pyrroloquinoline Derivative Isolated from the Fungus Mycena haematopus, Agaricales. Angew Chem. Int. Ed 1993, 32, 1087–1089; [Google Scholar]; (c) Sadanandan EV; Cava MP Total Syntheses of Damirone A and Damirone B. Tetrahedron Lett. 1993, 34, 2405–2408; [Google Scholar]; (d) Wang H; Al-Said NH; Lown JW Convenient Syntheses of Pyrroloiminoquinone and its Lexitropsin-linked Derivative. Tetrahedron Lett. 1994, 35, 4085–4086; [Google Scholar]; (e) Roberts D; Venemalm L; Alvarez M; Joule JA Synthesis of Damirones A and B from a Quinoline. Tetrahedron Lett. 1994, 35, 7857–7860; [Google Scholar]; (f) Backenkohler J; Reck B; Plaumann M; Spiteller; P. Total Synthesis of Mycenarubin A, Sanguinolentaquinone and Mycenaflavin B and their Cytotoxic Activities. Eur. J. Org. Chem 2018, 2806–2816. [Google Scholar]
- 17.Loach RP; Fenton OS; Amaike K; Siegel DS; Ozkal E; Movassaghi M C7-Derivatization of C3-Alkylindoles Including Tryptophans and Tryptamines. J. Org. Chem 2014, 79, 11254–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magdziak D; Rodriguez AA; Van De Water RW Pettus TRR Regioselective Oxidation of Phenols to o-Quinones with o-Iodoxybenzoic Acid (IBX). Org. Lett 2002, 4, 285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Some loss of material was observed when compound 17 was subjected to silica gel chromatography, with crude yields of 17 (pure by 1H NMR) after work-up at 70–75%. However, we observed that chromatogaphed 17 performed better in the subsequent condensation reactions.
- 20.It is possible in this case that the more acidic brominated phenol results in a greater amount of a zwitterionic form of bromotyramine 10A, rendering it less nucleophilic. Efforts to employ basic additives or the O-TBS variant of 10A led to little improvement, however.
- 21.Zhou P; Lin L; Chen L; Zhong X; Liu X; Feng X Iron-Catalyzed Asymmetric Haloazidation of α,β-Unsaturated Ketones: Construction of Organic Azides with Two Vicinal Stereocenters. J. Am. Chem. Soc 2017, 139, 13414–13419. [DOI] [PubMed] [Google Scholar]
- 22.Liu X; Lin L; Feng X Chiral N,N’-Dioxides: New Ligands and Organocatalysts for Catalytic Asymmetric Reactions. Acc. Chem. Res 2011, 44, 574–587. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y; Liu X; Hui Y; Jiang J; Wang W; Chen W; Lin L; Feng X Catalytic Asymmetric Bromoamination of Chalcones: Highly Efficient Synthesis of Chiral α-Bromo-β-Amino Ketone Derivatives. Angew. Chem. Int. Ed 2010, 49, 6160–6164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.