Abstract
Jugular foramen (JF) metastasis is rare and often presents as JF syndrome. A 73-year-old male complained of left-sided mastoid pain that irradiated to the neck since the past 3 months. Onset of facial nerve (FN) palsy and persistence of the symptomatology despite corticosteroid therapy demanded radiologic evaluation. Computed tomography and magnetic resonance imaging showed a wide osteolytic lesion of the left JF with involvement of the third segment of the FN. The patient underwent transmastoid incisional biopsy. Histopathological examination showed an adenocarcinoma that was suggested to be of respiratory origin. A primary pulmonary lesion and metastasis to other sits were detected. The patient died 1 month after the initiation of the chemotherapy. Persistent mastoid pain and progressive FN palsy must be considered indicative of JF malignant lesions. Despite early diagnosis, secondary lesions of the JF are characterized by a poor prognosis; however, accurate diagnosis may avoid unnecessary aggressive surgery.
Keywords: Jugular foramen, metastasis, paragangliomas, facial nerve palsy, lung cancer, misdiagnosis
INTRODUCTION
The jugular foramen (JF) might be affected in various tumors, mostly paragangliomas, meningiomas, schwannomas of the lower cranial nerves, and chondrosarcomas. Metastasis in this anatomical region is rare, as previously reported [1, 2]. Although lung cancer is the second most common malignant lesion and the most common cause of cancer-related deaths, only three cases of lung cancer metastasis to JF have been reported in the medical literature to date [3–5].
CASE PRESENTATION
A 73-year-old man complained of left-sided mastoid pain that radiated to the neck and shoulder and caused mild left hearing loss, ipsilateral ear fullness, mild instability, and episodes of vertigo since the past 3 months. At another hospital, he was diagnosed with secretory otitis media; however, he suggested that the treatment produced no improvement in his symptoms. One month later, the patient developed facial nerve (FN) palsy (grade VI, House-Brackmann scale) and was treated with corticosteroids. Three months later, he was referred to our university hospital owing to persistence of the previous complaints. Physical examination revealed left-sided FN weakness (grade III, House–Brackmann scale); otomicroscopy was bilaterally normal, and otoneurological evaluation showed no signs of IX, X, XI, and XII nerve palsies and Horner’s syndrome. Pure-tone audiometry revealed moderate-to-severe left-sided and mild right-sided sensorineural hearing loss. Computed tomography (CT) showed a left-sided, wide, irregular osteolytic lesion centered on JF and extending from occipital condyle through the mastoid tip, with involvement of the third segment of FN (Figure 1). Magnetic resonance imaging (MRI) confirmed the presence of an expansive, invasive mass sized 30×21×30 mm that was centered on the left JF and was hypointense on T1- and T2-weighted images (Figure 2a) with intense and heterogeneous enhancement after gadolinium administration (Figure 2b). The pattern of expansion was irregularly centrifugal. The lesion was moderately hyperintense on diffusion weighted imaging (DWI) (Figure 3), with a calculated apparent diffusion coefficient (ADC) value of 899×10−3 mm2/s (versus 701×10−3 mm2/s of white matter), suggesting the mass was somewhat highly cellular. No signs of sigmoid sinus infiltration and jugular bulb were present. The radiological features, presence of the mastoid pain, and sudden occurrence of facial palsy did not match the diagnosis of common JF lesions. The patient underwent transmastoid incisional biopsy. Pathological results revealed a mucinous adenocarcinoma that was suggested to be of respiratory origin (Figure 4). Immunohistochemical studies confirmed lung cancer metastasis; in the following findings were observed: positive stain for cytokeratin 7 and 19; polyclonality of carcinoembryonic antigen (CEA) and negative stain for anaplastic lymphoma kinase, programmed cell death-ligand 1 (PD-L1), and epidermal growth factor receptor. Positron emission tomography/CT (PET-CT) showed hypermetabolic lesions in the left petrous bone as well as in the intervertebral joint of C2–C3, traverse process of C3, right first rib, and the eighth dorsal vertebral body. In addition, left pleural thickening with contextual effusion with mild hyper-accumulation of the radio-tracer was observed in the costophrenic recess. Chest CT confirmed severe left pleural effusion with wide and irregular pleural thickening; the left lung was atelectatic with a dishomogeneous contrast-enhancement 3×2.5 cm in the interazigos-esophagus recess. The patient was referred to the oncologic department, where he was treated with chemotherapy and chest drainage. However, he died 1 month after treatment initiation. Written informed consent was obtained from the patient prior to study initiation for the publication of this case report.
Figure 1.
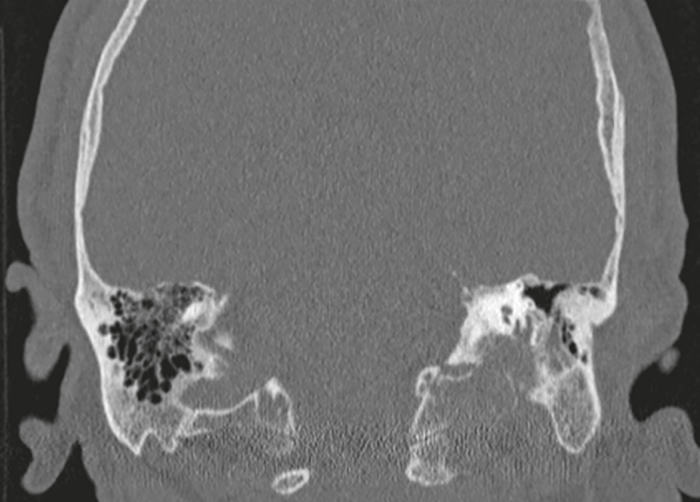
Coronal T-bone CT of the left ear showed destructive bone changes on the JF margins by the expansive mass.
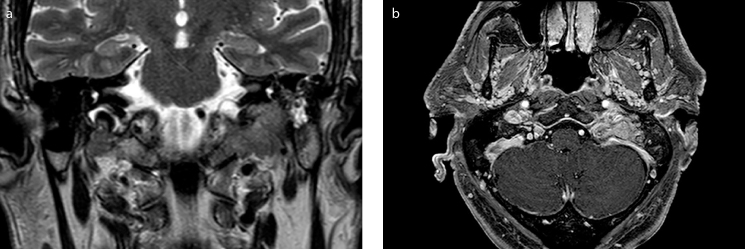
Figure 2. a, b.
(a) Coronal T2-weighted scan. (b) post-contrast axial T1-weighted scan. The lesion showed an irregular centrifugal spread pattern, was hypointense on T2 and T1-weighted images, and showed intense and heterogeneous contrast-enhancement after gadolinium administration.
Figure 3.
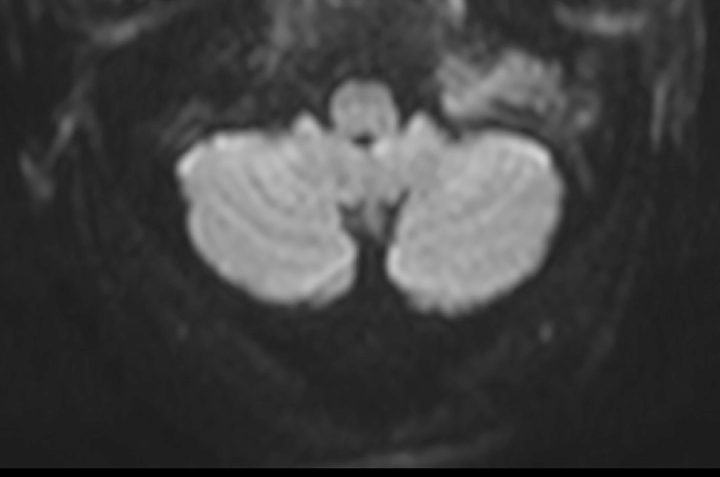
DWI-MRI images. The mass had a moderate-to-low diffusivity on DWI images and an ADC value of 899 × 10−3 mm2/s.
Figure 4.
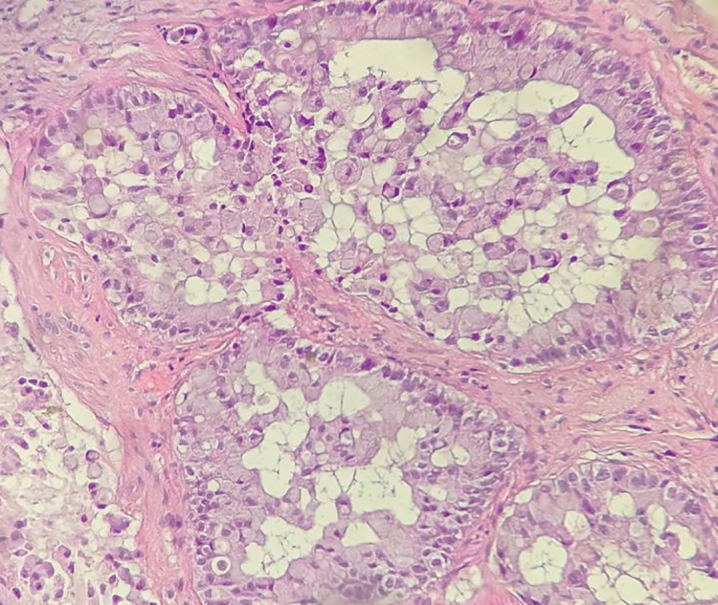
Photomicrograph shows an mucinousadenocarcinoma pattern (hematoxylin and eosin staining; ×400).
DISCUSSION
The jugular foramen lesions are most commonly paragangliomas, meningiomas, schwannomas of lower cranial nerves, or chondrosarcomas. Metastatic localization in JF region is rare. Reviews on secondary malignant tumors of the temporal bone reported that the incidence of JF metastasis is 3.5%–36% [1, 2]. Interestingly, only 3 patients with lung cancer metastasized to JF have been reported in medical literature; 2 of those were initially misdiagnosed with JF benign tumors [3, 4], whereas the last one was initially considered a medullary brainstem stroke secondary to carotid artery stenosis [5]. Metastasis from renal cell carcinoma, prostate cancer, and colorectal cancer misdiagnosed as tympano–jugular paragangliomas has been also reported [6–9]. Clinically, persistent pain should be suspected as malignancy. Rapid onset of peripheral nerves deficit (VII, VII, IX, X, XI, XII cranial nerves) also represents a frequent, even if not specific, sign of secondary lesions. On CT, aggressive and progressive bony erosions represent more specific radiologic features of secondary malignant disease [10]. On MRI, metastasis appears as focal lesions of low-intensity signals on precontrast T1-weighted images. Thomas et al. [8] suggested a rule of DWI for the differential diagnosis of the secondary malignant lesions to the JF benign lesions; the ADC value for renal cancer metastasis of JF reported in their study significantly differed from that reported in ours. Skeletal scintigraphy and CT-PET might help confirm the suspect of metastases in cases without previous diagnosis of malignancy; it may also help grade the disease and detect other metastatic locations [10]. Biopsy, histopathological evaluations, and complete disease staging are necessary to perform proper treatment and to estimate the prognosis.
CONCLUSION
Although JF metastasis is rare, it must be considered in diagnostic workup, particularly when persistent pain and rapid cranial nerve deficit onset are present. Even if the prognosis is poor, transmastoid biopsy may reduce the delay in achieving accurate diagnosis and may to avoid unnecessary aggressive surgery [3–9].
Footnotes
Informed Consent: Written informed consent was obtained from the patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.F.; Design - G.C., M.F.; Supervision – M.F.; Resources - G.C, M.F., F.B.; Materials - G.C., M.F., F.B.; Data Collection and/or Processing - G.C., M.F., F.B.; Analysis and/or Interpretation - G.C., M.F., F.B.; Literature Search - G.C.; Writing Manuscript - G.C., M.F., F.B.; Critical Review - G.C., F.B., M.F..
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Laigle-Donadey F, Taillibert S, Martin-Duverneuil N, Hildebrand J, Delattre JY. Skull-base metastases. J Neurooncol. 2005;75:63–9. doi: 10.1007/s11060-004-8099-0. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg HS, Deck MDF, Vikram B, Chu FCH, Posner JB. Metastasis to the base of the skull: clinical findings in 43 patients. Neurology. 1981;31:530–7. doi: 10.1212/WNL.31.5.530. [DOI] [PubMed] [Google Scholar]
- 3.Chao CK, Sheen TS, Lien HC, Hsu MM. Metastatic carcinoma to the jugular foramen. Otolaryngol Head Neck Surg. 2000;122:922–3. doi: 10.1067/mhn.2000.104522. [DOI] [PubMed] [Google Scholar]
- 4.Hayward D, Morgan C, Emami B, Biller J, Prabhu VC. Jugular foramen syndrome as initial presentation of metastatic lung cancer. J Neurol Surg Rep. 2012;73:14–8. doi: 10.1055/s-0032-1301406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal A, Baisakhiya N, Kakani A, Bhake A, Nagrale M, Reddy S. Metastatic lung cancer presenting with jugular foramen syndrome in a case of von Recklinghausens disease. J Cancer Res Ther. 2010;6:391–3. doi: 10.4103/0973-1482.73344. [DOI] [PubMed] [Google Scholar]
- 6.Taxy JB. Renal adenocarcinoma presenting as a solitary metastasis: contribution of electron microscopy to diagnosis. Cancer. 1981;48:2056–62. doi: 10.1002/1097-0142(19811101)48:9<2056::aid-cncr2820480923>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Boileau MA, Grotta JC, Borit A, Van der Linden C, Nath A, Ostrow P, et al. Metastatic renal cell carcinoma simulating glomus jugulare tumor. J Surg Oncol. 1987;35:201–3. doi: 10.1002/jso.2930350313. [DOI] [PubMed] [Google Scholar]
- 8.Thomas AJ, Wiggins RH, Gurgel RK. Metastatic Renal Cell Carcinoma Masquerading as Jugular Foramen Paraganglioma: A Role for Novel Magnetic Resonance Imaging. Ann Otol Rhinol Laryngol. 2017;126:606–10. doi: 10.1177/0003489417714487. [DOI] [PubMed] [Google Scholar]
- 9.Hellier WP, Crockard HA, Cheesman AD. Metastatic carcinoma of the temporal bone presenting as glomus jugulare and glomus tympanicum tumours: a description of two cases. J Laryngol Otol. 1997;111:963–6. doi: 10.1017/S0022215100139088. [DOI] [PubMed] [Google Scholar]
- 10.Löwenheim H, Andrei Koerbel A, Ebner FH, Kumagami H, Tatagiba UEM. Differentiating imaging findings in primary and secondary tumors of the jugular foramen. Neurosurg Rev. 2006;29:1–11. doi: 10.1007/s10143-005-0420-7. [DOI] [PubMed] [Google Scholar]