Abstract
Muckle–Wells syndrome (MWS), a subclass of cryopyrin-associated periodic syndrome (CAPS), sometimes includes complications of bilateral progressive sensorineural hearing loss. A 48-year-old woman had been diagnosed with pediatric rheumatic arthritis at aged 6 years; however, systematic therapy with prednisolone and methotrexate showed limited efficacy for her general fatigue and arthritic pain, and it never improved the hearing level. She underwent a cochlear implant surgery for progressive profound bilateral hearing loss. After 7 years of cochlear implant surgery, she was diagnosed with MWS by genetic tests. Interleukin (IL)-1β monoclonal antibody therapy (canakinumab) improved general fatigue and arthritic pain but showed no effect on cochlear symptoms. Owing to successful cochlear implant surgery, she reacquired the hearing and communication function while being able to understand over 90% of monosyllables and words in the sound field of her daily life at 65 dB SPL for the next 13 years of her life. This suggests that peripheral cochlear damage induced by chronic inflammation contributes to the sensorineural hearing loss in cases with MWS, and that cochlear implantation can provide long-term hearing efficacy for patients with MWS with irreversible profound hearing loss.
Keywords: Muckle-Wells syndrome, cochlear implant, IL-1β monoclonal antibody
INTRODUCTION
Cryopyrin-associated periodic syndrome (CAPS) is very rare, with a prevalence of 1–2 in 1 million people in the US, and presents with persistent and periodic inflammation of some organs accompanied by the abnormal production of the inflammatory cytokine interleukin (IL)-1β [1]. CAPS consists of three types as classified by seriousness: familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS), and chronic infantile neurological cutaneous and articular syndrome/neonatal-onset multisystem inflammatory disease (CINCA/NOMID) [1, 2]. It is related to a heterozygous mutation in nucleotide-binding oligomerization domain-like receptor pyrin domain-containing-3 (NALP3) gene encoding cryopyrin.
Patients with MWS and CINCA/NOMID often complain of progressive bilateral hearing loss [3]. A few studies have suggested that IL-1β blockage therapy may improve the hearing loss in some cases with CAPS [4]. However, patients with CAPS who fail to respond to IL-1β blockage therapy (Canakinumab; ILARIS®,Novartis International AG, Basel, Switzerland) require a new alternative therapy. A few case reports have described the efficacy of a cochlear implant for cases with CAPS with bilateral profound hearing loss [5, 6]. However, the long-term efficacy of cochlear implant has not been evaluated because the pathogenesis of the hearing disturbance in CAPS remains unclear.
Here, to the best of our knowledge, we present the first published report of the long-term efficacy of a cochlear implant for a case with MWS with bilateral profound hearing loss in an Asian patient. The relevant literature on hearing loss due to MWS was reviewed regarding the present case.
CASE PRESENTATION
A 48-year-old woman consulted our department for bilateral profound hearing loss. She suddenly suffered from rotational dizziness when she was aged 33 years. Since similar vertigo attacks repeatedly occurred with bilateral hearing loss, she had been diagnosed with bilateral Meniere’s disease. However, her hearing level progressively worsened until eventually hearing aids were required, although these devices completely lost their efficacy by aged 40 years.
According to her medical history, she had been diagnosed with rheumatic arthritis at aged 6 years. She developed right facial paralysis and underwent surgical treatment for an ovarian cyst at aged 43 years. Systematic therapy with prednisolone and methotrexate for rheumatic arthritis showed limited efficacy for her general fatigue and arthritic pain.
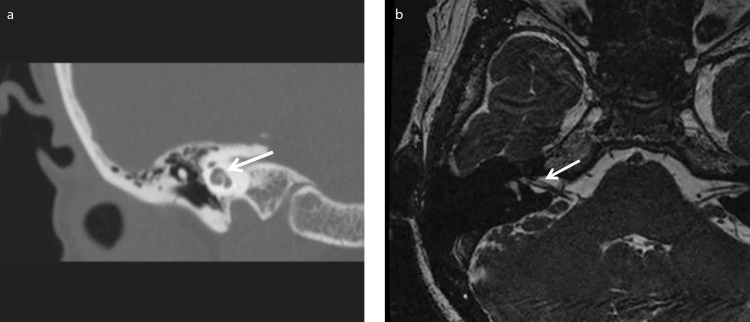
At her first visit to our department, an audiological examination revealed bilateral profound hearing loss (Figure 1). A caloric test by irrigation with cold water showed no response in the bilateral semicircular canals. No cervical vestibular evoked myogenic potential was noted on either side following stimulation with 135 dB SPL to assess the otolith function. Equilibrium tests revealed bilateral vestibular dysfunction. However, her ability to remain upright was normal according to stabilometry before the cochlear implant surgery, with both eyes open and closed, because of central compensation. Computed tomography imaging findings revealed no ossification or fibrosis in the cochlea (Figure 2a), and the cochlear nerves and perilymph in the cochlea were visible on T2-weighted magnetic resonance imaging (Figure 2b).
Figure 1.
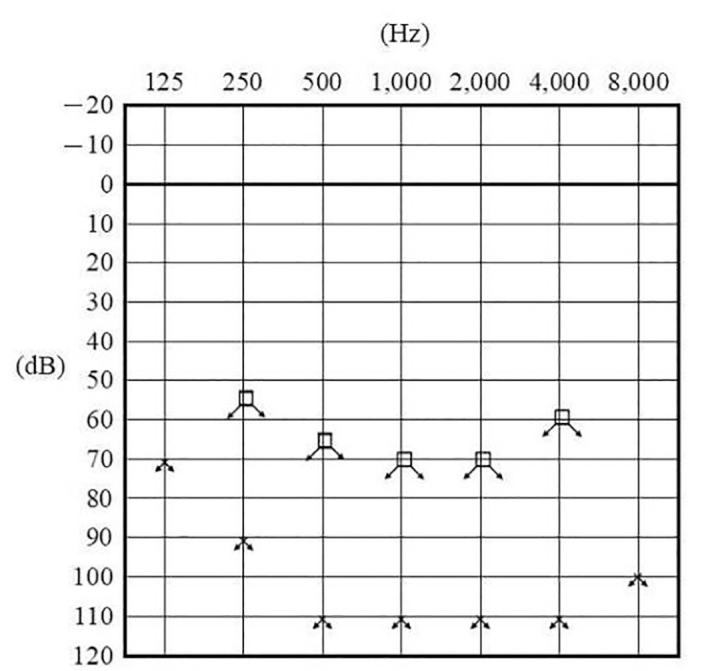
Pure tone audiogram of the patient at her first visit to our department.
Figure 2. a, b.
Computed tomography imaging (A) and magnetic resonance imaging T2 image (B) of our case before the cochlear implant surgery.
A cochlear implant (nucleus CI24RE Contour Advance; Cochlear Corporation, Sydney, Australia) electrode was completely inserted into the fenestration of the right cochlea under general anesthesia. She never complained of dizziness or tinnitus postoperatively. The behavioral threshold and comfortable levels on a 900 Hz advanced combination encoder map were acquired within a normal dynamic range with relatively low impedance.
After 3 months of surgery, she was able to grasp >90% of monosyllables and words from an open set in the sound field at 65 dB SPL. In addition, she reacquired excellent hearing function in the 13 years following the cochlear implant surgery and has been able to grasp >95% of words from an open set in the sound field at 65 dB SPL. She has obtained good hearing in conversations with family members and friends on a mobile telephone.
Seven years after she underwent the cochlear implantation, her son was diagnosed with CINCA/NOMID syndrome by compound heterozygous gene variations (E688K and G809S of NALP3), and he died at aged 19 years. In addition, the genetic tests confirmed that she had one mutation (E688K of NALP3), resulting in that she was diagnosed with MWS based on clinical symptoms (Table 1).
Table 1.
Characteristics of our case and her son with CAPS
| Onset age | Diagnosis | Genotyp (NLRP3) | Meningitis | Urticaria like rash | Arthritis | hearing loss | Renal amyloidosis | |
|---|---|---|---|---|---|---|---|---|
| Son | 11 months | CINCA/NOMID | E688K, G809S | + | + | + | − | − |
| Present case | unknown | MWS | E688K | − | + | + | + | + |
CAPS: cryopyrin-associated periodic syndrome; MWS: Muckle–Wells syndrome; CINCA/NOMID: chronic infantile neurological cutaneous and articular syndrome/neonatal-onset multisystem inflammatory disease; NALP3: nucleotide-binding oligomerization domain-like receptor pyrin domain-containing-3.
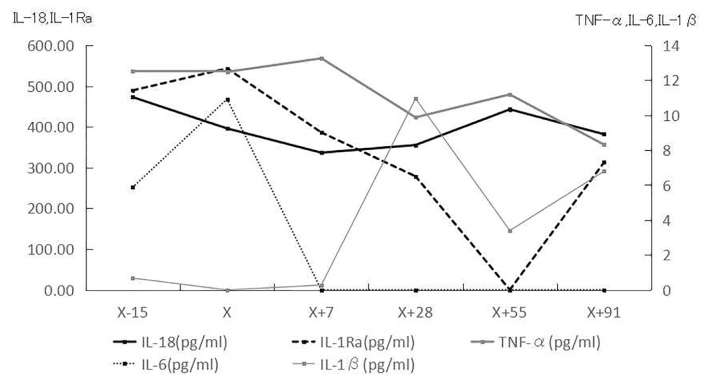
On additional examinations, the blood findings, including the hepatic and renal functions, were normal except for a high C-reactive protein level (5.34 mg/dL) and high leukocyte count (white blood corpuscles, 9150/μL). She showed significantly higher values of IL-18 (472.8 pg/mL), IL-6 (5.88 pg/mL), and IL-1 receptor antagonist (IL-1Ra) (489.6 pg/mL) than control subjects (average levels: 170 pg/mL, 0 pg/mL, and 213 pg/mL, respectively). The subcutaneous injection of human anti-human IL-1β monoclonal antibody (canakinumab 150 mg) significantly normalized the IL-6 and IL-1Ra values. In addition, the tumor necrosis factor-α (TNF-α) level gradually decreased with the administration of canakinumab. In contrast, IL-18 was decreased temporarily by canakinumab but returned to its original value after 8 weeks (Figure 3). Canakinumab also improved her subjective symptoms of general fatigue and arthritic pain. However, her hearing level did not change following the administration of canakinumab.
Figure 3.
Changes in IL-18/IL-1Ra/TNF-α/IL-6/IL-1β by the administration of the IL-1β monoclonal antibody (canakinumab 150 mg). X day: administration day (arrow).
DISCUSSION
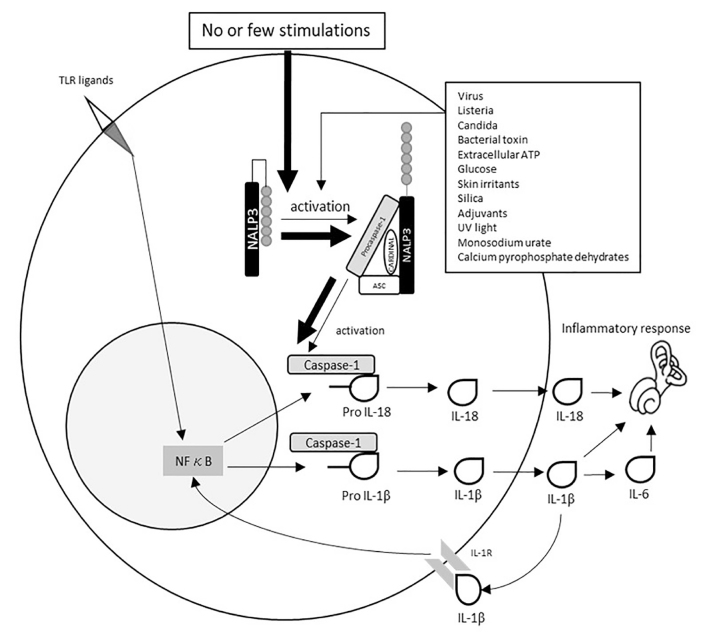
The protein cryopyrin in NALP3, which is strongly expressed in monocytes, has several ligands, including intracellular-invaded microorganism-derived antigens and harmful metabolites, consequently forms the NALP3 inflammasome, and then activates caspase-1 with other molecules. The mutation in the NALP3 gene in CAPS leads to the erratic formation of the inflammasome even without any ligands and increases the secretion of the proinflammatory cytokines IL-1β and IL-18, causing a series of inflammatory reactions [2, 7] (Figure 4).
Figure 4.
The flow of activation of NALP3 inflammasome and generation of active IL-1β and IL-18 in healthy subjects (thin arrows) and patients with CAPS (thick arrows). In cases with CAPS, the NALP3 inflammasome can be activated, resulting in increasing the release of IL-6 and IL-18 even under no or few stimulations. TLR, Toll-like receptor; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; NF-κB, nuclear factor kappa B.
The subclasses of the CAPS (FCAS, MWS, and CINCA/NOMID) are not separate independent syndromes but rather form a spectrum. Patients with CINCA/NOMID show the most severe symptoms and inflammatory changes in their bodies with permanent damage, and approximately 20% of children with CINCA/NOMID die before they reach adulthood. However, the prognosis has recently improved with the advent of IL-1β blockade therapy, and most cases with FCAS and MWS survive to adulthood. There are examples of two or more of these features overlapping. However, the subclasses of CAPS are still differentiated based on the severity of symptoms at the worst point of the disease [2, 7, 8] (Table 2).
Table 2.
CAPS is distinguished among three subclasses by the severity of symptoms in the worst time. The main differences are persistent rash, joint, neurological, and others
| Subclass | Symptoms of CAPS | |||
|---|---|---|---|---|
| Rash persistent | Joint | Neurological | Others | |
| FCAS | withen 24 hours | arthralgia | headache | fever |
| MWS | with a few days | arthralgia arthritis | headache ensorineural hearing loss | fever/amyloidosis |
| CINCA/NOMID | persistently | arthropathy | headache sensorineural hearing loss meningitis | fever/amyloidosis/growth disease |
CAPS: cryopyrin-associated periodic syndrome; FCAS: familial cold autoinflammatory syndrome; MWS: Muckle–Wells syndrome; CINCA/NOMID: chronic infantile neurological cutaneous and articular syndrome/neonatal-onset multisystem inflammatory disease.
Previous reports have shown that 89%–91% of cases with MWS have sensorineural hearing loss, and women had the higher risk of hearing loss [3, 9]. However, whether or not the vestibular functions of patients with MWS are preserved is unclear. Our patient experienced some episodic vertigo attacks, such as Meniere’s disease; however, she never complained of dizziness when she visited our hospital. It suggests that episodic vertigo attacks disappear with deteriorating vestibular function. In addition, signs of musculoskeletal complaints, skin rash, and fever were found in 87.5%, 83.3%, and 54.2% of patients with CAPS, respectively [10]. In cases with FCAS, these symptoms disappear within 24 h and a few days, respectively, whereas cases with CINCA/NOMID suffer from the symptoms persistently [11].
Although most cases with MWS and CINCA/NOMID never respond to steroid therapy, a recent study found that anti-IL-1β antibody (canakinumab) had marked efficacy, helping 72%–94% of the patients acquire complete remission [12, 13]. The early treatment with an anti-IL-1β antibody may improve the hearing level for patients with CAPS [7, 10]. However, no report has so far described the effects of this therapy in cases with profound hearing loss; therefore, a new alternative therapy is required.
The pathogenesis of progressive sensorineural hearing loss in CAPS remains unclear. The deposition of amyloidosis, which is found in some organs of patients with CAPS, has not been observed in the cochlea of autopsied cases with MWS. The chronic inflammatory changes induced by the overproduction of inflammatory cytokines (IL-6 and IL-18) may deteriorate the cochlear function. An animal study has shown that lipopolysaccharide intraperitoneal injections increase the expression of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, in the spiral ligaments and stria vascularis of the cochlea [14]. However, cases with CAPS often suffer from meningitis, which may induce the retro-labyrinth hearing loss [15]. Our patient showed no ossification or fibrosis of the cochlea, which is found in most cases with severe deafness due to meningitis.
Only two cases with CAPS with bilateral profound hearing deafness successfully treated with a cochlear implant have been reported in the English literature [5, 6], and the present patient also acquired an excellent hearing threshold on audiometry with a cochlear implant, which has remarkably improved her communication skill. Furthermore, our patient with MWS has reacquired an excellent hearing function in the 13 years since her cochlear implant surgery. These results suggest that the progressive sensorineural hearing loss in cases with MWS may be derived from peripheral cochlear damage, and a cochlear implant may be effective for such cases with bilateral profound hearing loss. Furthermore, our patient is still being treated with canakinumab, suggesting that IL-1β blockade therapy may preserve the efficacy of the cochlear implant.
Although some studies have suggested that anti-IL-1β monoclonal antibody might improve the prognosis of MWS symptoms, including hearing loss, in some cases, the therapeutic effect has not been evaluated in cases with profound hearing loss. Our case study indicates that the early intervention with a cochlear implant may provide long-term clinical efficacy in cases with MWS with IL-1β blockade therapy-resistant profound hearing loss.
CONCLUSION
It is necessary for otolaryngology doctors to consider this disease when encountering cases with steroid-resistant sensory hearing loss, although CAPS, including MWS, is a rare disease. The early treatment with an anti-IL-1β antibody can improve the subjective symptoms and hearing loss. However, cochlear implant surgery may be considered as an optional therapy for cases with MWS with profound hearing loss.
Acknowledgements
We are grateful to Dr. Takahide Teramoto for his cooperation.
Footnotes
This study was presented at the Gifu University, Otolaaryngology, October 22, 2019, Gifu, Japan.
Informed Consent: Written informed consent was obtained from the patient who participated in the present study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.O., M.A.; Design - B.O, M.A.; Supervision -M.A., B.K, Y.I.; Resource - M.A, H.O.; Materials - M.A., H.O., T.O., H.H.; Data Collection and/or Processing - M.A., H.O., T.O., H.H.; Analysis and/or Interpretation - B.O., M.A.; Literature Search - B.O., M.A.; Writing - B.O., M.A.; Critical Reviews - B.O., M.A., Y.I.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this this study has received no financial support.
REFERENCES
- 1.Kuemmerle-Deschner JB, Haug I. Canakinumab in patients with cryopyrin-associated periodic syndrome: an update for clinicians. Ther Adv Musculoskelet Dis. 2013;5:315–29. doi: 10.1177/1759720X13502629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubota T, Koike R. Biological and clinical aspects of Muckle-Wells syndrome. Nihon Rinsho Meneki Gakkai Kaishi. 2007;30:114–22. doi: 10.2177/jsci.30.114. [DOI] [PubMed] [Google Scholar]
- 3.Koitschev A, Gramlich K, Hansmann S, Benseler S, Plontke SK, Koitschev C, et al. Progressive familial hearing loss in Muckle-Wells syndrome. Acta Otolaryngologica. 2012;132:756–62. doi: 10.3109/00016489.2012.656321. [DOI] [PubMed] [Google Scholar]
- 4.Kuemmerle-Deschner JB, Wittkowski H, Tyrrell PN, Koetter I, Lohse P, Ummenhofer K, et al. Treatment of Muckle-Wells syndrome: analysis of two IL-1-blocking regimens. Arthritis Res Ther. 2013;15:R64. doi: 10.1186/ar4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall AC, Leong AC, Jiang D, Fitzgerald-O’Connor A. Sudden bilateral sensorineural hearing loss associated with urticarial vasculitis. J Laryngol Otol. 2013;127:708–11. doi: 10.1017/S0022215113001047. [DOI] [PubMed] [Google Scholar]
- 6.Bates JE, Bruce IA, Henderson L, Melling C, Green KM. Cochlear implantation in a child with CINCA syndrome who also has wide vestibular aqueducts. Cochlear Implants Int. 2012;13:173–6. doi: 10.1179/1754762811Y.0000000005. [DOI] [PubMed] [Google Scholar]
- 7.Kuemmerle-Deschner JB. CAPS--pathogenesis, presentation and treatment of an autoinflammatory disease. Semin Immunopathol. 2015;37:377–85. doi: 10.1007/s00281-015-0491-7. [DOI] [PubMed] [Google Scholar]
- 8.Ohnishi H, Teramoto T, Iwata H, Kato Z, Kimura T, Kubota K, et al. Characterization of NALP3 variants in Japanese cryopyrin-associated periodic syndrome patients. J Clin Immunol. 2012;32:221–9. doi: 10.1007/s10875-011-9629-0. [DOI] [PubMed] [Google Scholar]
- 9.Kuemmerle-Deschner JB, Koitschev A, Tyrrell PN, Plontke SK, Deschner N, Hansmann S, et al. Early detection of sensorineural hearing loss in Muckle-Wells-syndrome. Pediatr Rheumatol Online J. 2015;13:43. doi: 10.1186/s12969-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulders-Manders CM, Kanters TA, van Daele PLA, Hoppenreijs E, Legger GE, van Laar JAM, et al. Decreased quality of life and societal impact of cryopyrin-associated periodic syndrome treated with canakinumab: a questionnaire based cohort study. Orphanet J Rare Dis. 2018;20:13–59. doi: 10.1186/s13023-018-0799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giat E, Lidar M. Cryopyrin-associated periodic syndrome. Isr Med Assoc J. 2014;16:659–61. [PubMed] [Google Scholar]
- 12.Kuemmerle-Deschner JB, Hofer F, Endres T, Kortus-Goetze B, Blank N, Weissbarth-Riedel E, et al. Real-life effectiveness of canakinumab in cryopyrin-associated periodic syndrome. Rheumatology (Oxford) 2016;55:1340–1. doi: 10.1093/rheumatology/kew060. [DOI] [PubMed] [Google Scholar]
- 13.Imagawa T, Nishikomori R, Takada H, Takeshita S, Patel N, Kim D, et al. Safety and efficacy of canakinumab in Japanese patients with phenotypes of cryopyrin-associated periodic syndrome as established in the first open-label, phase-3 pivotal study (24-week results) Clin Exp Rheumatol. 2013;31:302–9. [PubMed] [Google Scholar]
- 14.Zhang J, Songlin C, Zhiqiang H, Jing C, Mingmin D, Xiaorui ShiLipopolysaccharide-Induced Middle Ear Inflammation Disrupts the cochlear Intra-Strial Fluid-Blood Barrier through Down-Regulation of Tight Junction Proteins. PLoS One. 2015;10:e0122572. doi: 10.1371/journal.pone.0122572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker T, Keddie S, Kidd D, Lane T, Maviki M, Hawkins PN, et al. Neurology of the cryopyrin-associated periodic fever syndrome. Eur J Neurol. 2016;23:1145–51. doi: 10.1111/ene.12965. [DOI] [PubMed] [Google Scholar]