Abstract
Muscle sympathetic neural activity (MSNA) influences the mechanical properties (i.e., vascular smooth muscle tone and stiffness) of peripheral arteries, but it remains controversial whether MSNA contributes to stiffness of central arteries such as the aorta and carotids. We examined whether elevated MSNA (age-related) would be independently associated with greater stiffness of central [carotid-femoral pulse wave velocity (PWV)] and peripheral (carotid-brachial PWV) arteries, in addition to lower carotid compliance coefficient (CC), in healthy men and women (n=88, age:19-73 years, 52%men). Also, we examined whether acute elevations in MSNA without increases in mean arterial pressure (MAP) using graded levels of lower body negative pressure (LBNP) would augment central and peripheral artery stiffness in young (YG, n=15, 60%men) and middle-age/older adults (MA/O, n=14, 43%men). Resting MSNA burst frequency (bursts·min−1) was significantly correlated with carotid-femoral PWV (R=0.44, P<0.001), carotid-brachial PWV (R=0.32, P=0.003), and carotid CC (R=0.28, P=0.01) after controlling for sex, MAP, heart rate, and waist-to-hip ratio (central obesity), but these correlations were abolished after further controlling for age (all P>0.05). In YG and MA/O adults, MSNA was elevated during LBNP (P<0.001) and produced significant increases in carotid-femoral PWV (YG:Δ+1.3±0.3 vs. MA/O:Δ+1.0±0.3 m·s−1, P=0.53) and carotid-brachial PWV (YG: Δ+0.7±0.3 vs. MA/O: Δ+0.7±0.5 m·s−1, P=0.92), whereas carotid CC during LBNP was significantly reduced in YG but not MA/O (YG:Δ-0.04 ±0.01 vs. MA/O:Δ0.001±0.008 mm2·mmHg−1, P<0.01). Collectively, these data demonstrate the influence of MSNA on central artery stiffness and its potential contribution to age-related increases in stiffness of both peripheral and central arteries.
Keywords: Arterial stiffness, MSNA, pulse wave velocity, aging
Summary
Our findings demonstrate the influence of MSNA on central artery stiffness and its potential contribution to age-related increases in stiffness of both peripheral and central arteries.
Introduction
Stiffening of the large central arteries (i.e., aorta and carotid arteries) increases with advancing age and is associated with an elevated risk of developing cardiovascular disease (CVD)1–5 Several common structural features of arterial stiffness include loss of elastin proteins and increased collagen deposition and cross-linking in the extra-cellular matrix of the vascular wall.6 The contribution of mechanical features such as vascular smooth muscle tone has recently garnered more attention.7 However, the underlying mechanisms that promote increased arterial stiffness with older age are still incompletely understood.
Muscle sympathetic nerve activity (MSNA) increases markedly with advancing age.8–12 Elevated MSNA with aging in both men and women impairs vascular endothelium-dependent vasodilation of large peripheral arteries such as the brachial artery.13, 14 In response to acute and sustained elevations in MSNA, studies have consistently found corresponding increases in functional measures of stiffness in peripheral arteries (e.g., brachial, radial, and femoral compliance) attributable to adrenergic-mediated increases in smooth muscle tone.15–20 However, the influence of age-related increases in MSNA on the mechanical properties of the large central arteries, which also possess smooth muscle tone,21 is unclear. In a small group of men (n=25), carotid-femoral PWV was significantly correlated with MSNA independent of age.22 Yet, in a comparison between young (YG) and middle-age/older (MA/O) adults, the age-related reduction in carotid compliance (inverse of stiffness) was abolished when statistically correcting for resting MSNA,23 suggesting that the relation between MSNA and large elastic artery stiffness is age-dependent. Given these inconclusive findings from studies with limited size groups of only men and different measures of arterial stiffness (i.e., carotid-femoral PWV vs. carotid compliance), larger comprehensive studies using microneurography in YG and MA/O men and women varying in age are needed to directly assess the relation between age-related increases in MSNA and central and peripheral artery stiffness as well as carotid compliance.
Results from studies examining central artery stiffness in response to acute changes in MSNA in MA/O adults have also been inconclusive.24–26 For example, Sugawara et al. (2007) reported no significant effect of systemic α-adrenergic receptor blockade on carotid β-stiffness index in a small sample of mostly MA/O women (n=7).27 In contrast, an increase in carotid artery compliance (decrease in artery wall stiffness) was observed in the same group of subjects following systemic α-adrenergic receptor blockade.28 Notably, mean arterial pressure (MAP) was concomitantly reduced ~15 mmHg during α-adrenergic receptor blockade and likely contributed to inconsistent results. Alterations in MAP (i.e., distending pressure), which typically occurs with acute changes in vasoconstrictor tone, markedly confounds the interpretation of changes in arterial stiffness because the mechanical properties of the artery are dependent on the distending pressure.29 Similarly, a reduction in sympathetic outflow via ganglionic blockade significantly reduced carotid-femoral PWV with a concomitant reduction in MAP of ~28 mmHg in MA/O women.26 The limitation of changes in MAP when modulating MSNA has been overcome in some studies by examining carotid-femoral PWV in response to application of mild lower body negative pressure (LBNP), which elicits reflex increases in MSNA and vasoconstriction without increases in MAP.30–32 However, these studies have been performed primarily in young adults, and therefore it remains unclear whether carotid-femoral PWV is modulated by acute changes in MSNA independent of MAP in MA/O adults.
With this background in mind, we sought to examine the extent to which MSNA influences the mechanical properties of the large central arteries and contributes to increased stiffness in YG and MA/O adults. We tested two distinct hypotheses. First, we hypothesized that higher MSNA would be associated with greater carotid-femoral PWV and lower carotid compliance independent of age in a cross-sectional analysis of adults varying in age (19–73 years) without history of cardiovascular disease. Second, to directly examine the contribution of increased MSNA to the mechanical properties of the central arteries in MA/O adults, we tested the hypothesis that acute elevations in MSNA in the absence of increased MAP using graded levels of mild LBNP would result in increases in carotid-femoral PWV and reductions in carotid compliance similarly in YG and MA/O adults.
Methods
All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the Institutional Review Board at the University of Iowa (IRB ID# 201701762). Each subject received a verbal and written explanation of the study objectives, measurement techniques, and risks and benefits associated with the investigation prior to providing written informed consent on the initial visit. The data that support the findings of this study can be made available by the corresponding author upon reasonable request.
Subjects:
A total of 88 participants (age range: 19-73 yr) who were nonsmokers and free of CVD, metabolic, or neurological disease were recruited through the University of Iowa. Study 2 included 15 YG (age: 19-45 years) and 14 MA/O participants (age: ≥45 years) (Table 2). No pregnant women were studied as confirmed by a urine pregnancy test the morning of the initial visit and the morning of the study visit. The phase of the menstrual cycle was not controlled because previous data demonstrate no temporal changes in carotid-femoral PWV across the phases of the menstrual cycle in healthy women.33, 34
Table 2.
Subject characteristics (Study 2)
| Variable | YG (n=15) | MA/O (n=14) | P-value |
|---|---|---|---|
| Age, years | 26 ± 2 | 57 ± 2 | <0.001 |
| Women/Men (%) | 40/60 | 57/43 | 0.687 |
| BMI, kg·m2 | 24 ± 1 | 23 ± 1 | 0.333 |
| Waist/hip ratio | 0.79 ± 0.02 | 0.82 ± 0.03 | 0.304 |
| Triglycerides, mg·dL−1 | 65 ± 8 | 67 ± 5 | 0.122 |
| LDL, mg·dL−1 | 93 ± 6 | 89 ± 6 | 0.698 |
| HDL, mg·dL−1 | 53 ± 4 | 68 ± 5 | 0.029 |
| Total cholesterol, mg·dL−1 | 169 ± 11 | 171 ± 5 | 0.909 |
| Ambulatory BP | |||
| 24-hr systolic BP, mmHg | 123 ± 2 | 116 ± 2 | 0.042 |
| 24-hr diastolic BP, mmHg | 72 ± 2 | 72 ± 2 | 0.781 |
| 24-hr mean BP, mmHg | 89 ± 2 | 86 ± 2 | 0.210 |
| Arterial stiffness | |||
| Carotid-femoral PWV, m·s−1 | 5.8 ± 0.2 | 7.8 ± 0.5 | 0.001 |
| Carotid CC, mm2·mmHg−1 | 0.14 ± 0.01 | 0.10 ± 0.01 | 0.005 |
| Carotid-brachial PWV, m·s−1 | 6.1 ± 0.2 | 7.8 ± 0.5 | 0.002 |
| History | |||
| HTN or borderline HTN, n (%) | 1 (7) | 0 (0) | -- |
| Family HTN, n (%) | 7 (47) | 5 (43) | 0.938 |
| Smoking, n (%) | 2 (20) | 3 (21) | 0.894 |
| Cardiovascular medications | |||
| Statin, n (%) | 0 (0) | 0 (0) | -- |
| Diuretic, n (%) | 0 (0) | 0 (0) | -- |
| ACE inhibitor, n (%) | 0 (0) | 0 (0) | -- |
Values are means ± SE. YG, young adults; MA/O, middle-age/older adults BMI, body mass index; LDL and HDL, low/high density lipoprotein; BP, blood pressure; PWV, pulse wave velocity; CC, compliance coefficient; HTN, hypertension.
Experimental Measurements
Cardiovascular variables:
Heart rate (HR) was determined from lead II of a three-lead ECG, and auscultatory BP at the brachial artery was recorded in triplicate using the Noninvasive Hemodynamics (NIHem) workstation (Cardiovascular Engineering, Inc., Norwood, MA).2
Central and peripheral artery stiffness:
Aortic stiffness was quantified by carotid-femoral PWV using the NIHem workstation, and with a Millar tonometer (SPT-301) (Millar, Inc. Houston, TX) during the LBNP protocol (i.e., baseline, −15mmHg, −30mmHg). Because the brachial artery is a large-size conduit vessel known to be influenced by MSNA,13, 15 carotid-brachial PWV was assessed as an index of peripheral muscular artery stiffness.1, 2
Carotid compliance:
Common carotid artery compliance coefficient (CC) was assessed using high-resolution B-mode ultrasound imaging with a 12-MHz linear transducer (Logiq 7; GE Healthcare) and analyzed using Vascular Analysis Tools Analyzer 5.5 (Medical Imaging Applications, Coralville, IA).
Sympathetic nerve activity:
Multiunit postganglionic MSNA was recorded from the peroneal nerve using standard microneurographic techniques as described in previous studies 35–38 and in the Online Data Supplement. Plasma norepinephrine concentration was assessed using high performance liquid chromatography.
Experimental protocol:
On the first visit to the laboratory, subjects received verbal explanation of the study and provided written informed consent. Subjects completed a health history survey and were instrumented with a 24-hr BP monitor. On the experimental day (within 2 weeks of the initial visit), participants were instructed to refrain from medication use and fast overnight prior to arriving at the laboratory between 0700 and 0900 h. Subjects were also instructed to abstain from caffeinated beverages the morning of the study and strenuous physical activity and alcohol for at least 24 h before experimental sessions. All experiments were performed in a dimly-lit room at an ambient temperature of 22-24°C.
Study 1: Resting MSNA and arterial stiffness
Upon arrival, a venous catheter was inserted into the subject’s antecubital or hand vein for blood sampling of norepinephrine and a metabolic panel after 20 min of resting supine. Among the 88 subjects, a venous catheter was unable to be placed to collect circulating norepinephrine in 14 subjects, and a butterfly needle was instead used to collect blood for a metabolic panel. Resting assessments of PWV and carotid CC were completed, followed by instrumentation for MSNA. Once the MSNA signal was acquired, data were collected for at least a 10-min baseline period.
Study 2: Acute elevations in MSNA and arterial stiffness
Subjects (n=29) underwent LBNP to achieve progressive elevations in MSNA without increases in MAP.39, 40 The subject’s lower body was enclosed to the level of the iliac crest in a negative pressure chamber adapted for acquisition of MSNA and with a custom-built glove port at the level of the pelvic region that allowed acquisition of the femoral artery pulse waveform with tonometry (Millar). Pressure inside the chamber was measured continuously. Acquisition of the MSNA signal was followed by a 10-min baseline period and measurements of PWV and CC. One MA/O subject elected to not undergo MSNA recording, and a quality MSNA signal was unable to be attained in the LBNP chamber in 2 YG and 3 MA/O subjects. Negative pressure in the chamber was then applied gradually (~5 mmHg per 15s) to minimize potential movement of the MSNA electrode. After 5 min of steady-state LBNP, assessments of PWV and carotid CC were performed, and a venous blood sample was taken for norepinephrine concentrations. LBNP at −15 mmHg and −30 mmHg were separated by 15 min of rest and were not randomized because potential for movement of the MSNA electrode increases with progressive decreases in chamber pressure. In this regard, the MSNA electrode moved and no signal was obtained during LBNP −15mmHg in 2 YG subjects and during LBNP −30mmHg in 1 YG and 1 MA/O subject.
Statistical Analysis
Partial correlation was used to test the strength of association between MSNA and arterial stiffness while controlling for relevant confounding factors [age, MAP, HR, and waist-to-hip ratio (central obesity)] that have been shown to influence carotid-femoral PWV in previous studies.41, 42 Sex, which was categorized as nominal binary variable (W=0, M=1), was also controlled given known age- and sex-related differences in MSNA.9 Multiple linear regression was used to examine whether MSNA was an independent determinant of arterial stiffness beyond MSNA, MAP, HR, sex, and waist-to-hip ratio (Model 1) and age, MSNA, MAP, HR, sex, and waist-to-hip ratio (Model 2). Analysis of covariance (ANCOVA) was used to determine differences between YG and MA/O in arterial stiffness after controlling for MSNA in Study 2. Chi-squared test was used to examine group differences in categorical variables. Bivariate correlations were examined using Pearson correlation coefficient. Two-way repeated measures analysis of variance (2-way RM ANOVA) was performed to test the difference between means of arterial stiffness, MSNA, and variables across levels of LBNP in YG verses MA/O. For 2-way RM ANOVA, the estimate of an absent data value was calculated as the marginal sums of squares (type III, adjusted sums of squares) using a general linear model approach. The Holm-Sidak method was used for all pairwise multiple comparison testing. Statistical significance was set at P < 0.05.
Results
Study 1: Resting MSNA and arterial stiffness (larger cohort, n=88)
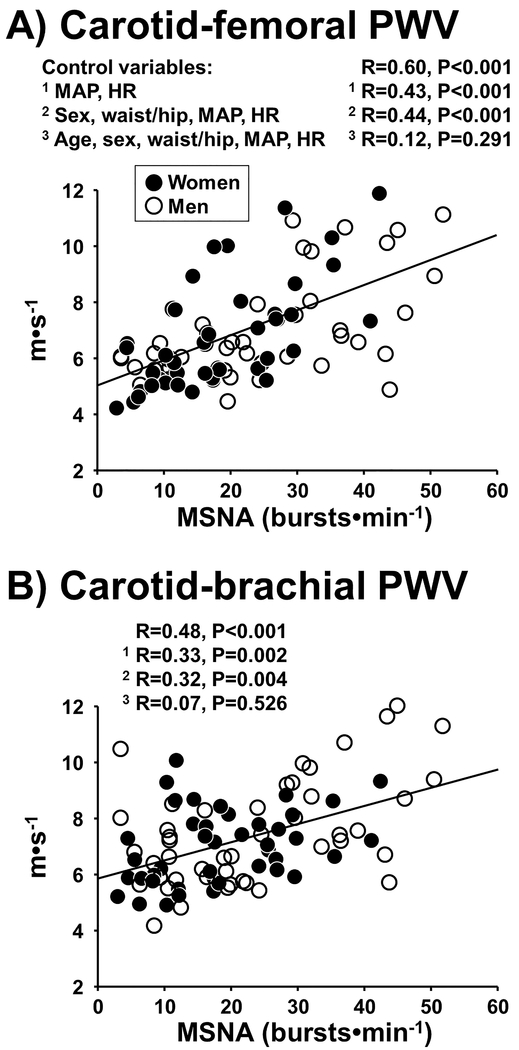
Subject characteristics, including MSNA, arterial stiffness, and cardiovascular variables are shown in Table 1. Resting MSNA burst frequency (bursts•min−1) was significantly correlated with central artery stiffness (carotid-femoral PWV: R=0.60, P<0.001) (Figure 1A) and peripheral artery stiffness (carotid-brachial PWV: R=0.48, P<0.001) (Figure 1B). Similar results were observed when considering MSNA burst incidence (bursts•100hb−1). In comparison, circulating norepinephrine concentration (295 ± 24 pg·ml−1, n=74) was only moderately correlated with carotid-femoral PWV (R=0.24, P=0.041) and not related to carotid-brachial PWV (R=0.12, P=0.292). After controlling for confounding factors (sex, MAP, HR, and waist-to-hip ratio), MSNA burst frequency remained significantly correlated with carotid-femoral PWV (R=0.44, P<0.001) and carotid-brachial PWV (R=0.32, P=0.004) (Figure 1A,B). However, when further adjusting for age, no relation was observed between MSNA burst frequency and carotid-femoral PWV (R=0.12, P=0.291), or between MSNA and carotid-brachial PWV (R=0.07, P=0.526).
Table 1.
Subject characteristics (Study 1)
| Variable | Value (n=88) |
|---|---|
| Age, yrs | 37 ± 2 |
| Age range, yrs | 19-73 |
| Women/Men (%) | 48/52 |
| BMI, kg·m2 | 28 ± 1 |
| Waist/hip ratio | 0.84 ± 0.01 |
| Triglycerides, mg·dL−1 | 90 ± 9 |
| LDL, mg·dL−1 | 100 ± 5 |
| HDL, mg·dL−1 | 54 ± 3 |
| Total cholesterol, mg·dL−1 | 174 ± 6 |
| Cardiovascular variables | |
| Heart rate, beats·min−1 | 61 ± 2 |
| Systolic BP, mmHg | 119 ± 3 |
| Diastolic BP, mmHg | 71 ± 1 |
| Mean BP, mmHg | 87 ± 2 |
| 24-hr systolic BP, mmHg | 126 ± 2 |
| 24-hr diastolic BP, mmHg | 75 ± 1 |
| 24-hr mean BP, mmHg | 92 ± 2 |
| MSNA | |
| Burst frequency, bursts·min−1 | 18 ± 2 |
| Burst incidence, bursts·100hb−1 | 31 ± 3 |
| Arterial stiffness | |
| Carotid-femoral PWV, m·s−1 | 6.7 ± 0.3 |
| Carotid CC, mm2·mmHg−1 | 0.11 ± 0.01 |
| Carotid-brachial PWV, m·s−1 | 7.0 ± 0.2 |
| History | |
| HTN or borderline HTN, n (%) | 18 (20) |
| Family HTN, n (%) | 48 (55) |
| Smoking, n (%) | 17 (19) |
| Cardiovascular medications | |
| Statin, n (%) | 2 (2) |
| Diuretic, n (%) | 2 (2) |
| ACE inhibitor, n (%) | 2 (2) |
Values are means ± SE. BMI, body mass index; HDL, high density lipoprotein; BP, blood pressure; MSNA, muscle sympathetic nerve activity; HTN, hypertension.
Figure 1.
Regression analysis between resting muscle sympathetic nerve activity (MSNA) burst frequency and central artery stiffness (carotid-femoral pulse wave velocity, PWV) (Panel A) and peripheral artery stiffness (carotid-brachial PWV) (Panel B) in 88 young and middle-age/older men and women. Partial correlation was used to control for mean arterial pressure (MAP) and heart rate (HR) in Model 1; sex, waist/hip ratio (central obesity), MAP, and HR in Model 2; age, sex, waist/hip ratio, MAP, and HR in Model 3.
Results from multiple linear regression analysis [independent variables: MSNA, MAP, HR, sex, and waist-to-hip ratio (Model 1)] showed that MSNA (P<0.001) and MAP (P<0.001) were significant determinants of carotid-femoral PWV (model R2=0.55), and that MSNA (P=0.004) and MAP (P=0.003) were significant determinants of carotid-brachial PWV (model R2=0.33). In Model 2, which added age as an independent variable, there was a greater proportion of the variance explained (increase in R2) for carotid-femoral PWV (model R2=0.74) and carotid-brachial PWV (model R2=0.45). However, MSNA was not a significant determinant in Model 2, suggesting that MSNA as a determinant of arterial stiffness was subordinate to age.
Resting MSNA burst frequency was correlated with carotid CC (R=−0.35, P=0.001) (Figure S1). Circulating norepinephrine concentration was not significantly correlated with carotid CC (R=−0.20, P=0.082). After controlling for confounding factors (sex, MAP, HR, and waist-to-hip ratio), MSNA burst frequency remained significantly correlated with carotid CC (R=−0.28, P=0.012). However, when further adjusting for age, no relation was observed between MSNA and carotid CC (R=−0.11, P=0.321). Results from multiple linear regression analysis (Model 1) showed that MSNA (P=0.012) and sex (P=0.003) were significant determinants of carotid CC (model R2=0.25). In Model 2, which added age as an independent variable (P=0.013), MSNA was not a significant determinant of carotid CC (model R2=0.30).
There were no sex differences in the relation between MSNA and central artery stiffness. Resting MSNA burst frequency was significantly correlated with carotid-femoral PWV and carotid CC in both men and women (Table S1). When controlling for MAP, HR, and waist-to-hip ratio in both groups, MSNA burst frequency remained significantly correlated with carotid-femoral PWV (Men: R=0.52, P<0.001; Women: R=0.41, P=0.011) but not carotid CC (Men: R=−0.29, P=0.057; Women: R=−0.29, P=0.082). Importantly, the association between MSNA burst frequency and carotid-femoral PWV was abolished in both men and women after also controlling for age (both P>0.05), consistent with the entire cohort. The relation between MSNA burst frequency and peripheral artery stiffness (carotid-brachial PWV) was more prominent in men compared with women because the association remained statistically significant after controlling for MAP, HR, and waist-to-hip ratio in men (R=0.47, P=0.001) but not women (R=0.15, P=0.376).
Study 2. Increased arterial stiffness during acute elevations in MSNA
Characteristics for YG (n=15) and MA/O (n=14) subjects are shown in Table 2. As expected, MSNA, carotid-femoral PWV, and carotid-brachial PWV were significantly greater and carotid CC significantly lower in MA/O compared with YG (all P<0.05). However, there was no significant difference between YG and MA/O adults in central artery stiffness after controlling for MSNA (carotid-femoral PWV: P=0.530; carotid CC: P=0.076, ANCOVA).
MSNA and cardiovascular responses to LBNP:
MSNA burst frequency, burst incidence, and total activity were significantly elevated during LBNP −15mmHg and LBNP −30mmHg compared with baseline (Table 3). Although MSNA was greater in MA/O compared with YG at baseline, the increase in MSNA during LBNP was similar between groups (P>0.05 for all). However, the rise in circulating norepinephrine concentrations during LBNP was significantly greater in MA/O compared with YG subjects (group • time point interaction: P=0.003). By design, there were no significant increases in MAP during LBNP (P=0.722) (Table 3), and therefore no increases in distending pressure. LBNP elicited baroreflex-mediated increases in HR, and the increase in HR was significantly greater in YG (+9 beats•min−1) compared with MA/O (+4 beats•min−1) at LBNP −30mmHg (P=0.004).
Table 3.
MSNA and cardiovascular variables at baseline and during lower body negative pressure (LBNP)
| Stimulus | 2-way RM ANOVA P-value | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Group | Baseline | LBNP −15 | LBNP −30 | Group | Time point | Interaction |
| MSNA, bursts·min−1 | YG (n=12) | 10 ± 1 | 15 ± 2 | 22 ± 3 | 0.004 | <0.001 | 0.237 |
| MA/O (n=10) | 25 ± 4 | 30 ± 4 | 33 ± 5 | ||||
| MSNA, bursts·100hb−1 | YG (n=12) | 17 ± 2 | 24 ± 3 | 32 ± 5 | <0.001 | <0.001 | 0.280 |
| MA/O (n=10) | 45 ± 7 | 53 ± 7 | 57 ± 8 | ||||
| MSNA, AU·min−1 | YG (n=12) | 490 ± 66 | 780 ± 101 | 1317 ± 293 | 0.021 | <0.001 | 0.655 |
| MA/O (n=10) | 1180 ± 221 | 1611 ± 233 | 1871 ± 408 | ||||
| Norepinephrine, pg·mL−1 | YG (n=12) | 211 ± 34 | 221 ± 20 | 306 ± 37 | <0.001 | <0.001 | 0.003 |
| MA/O (n=13) | 298 ± 34 | 552 ± 47*† | 598 ± 68*† | ||||
| Systolic BP, mmHg | YG (n=15) | 113 ± 2 | 112 ± 3 | 112 ± 3 | 0.520 | <0.001 | 0.135 |
| MA/O (n=14) | 117 ± 3 | 117 ± 4 | 112 ± 4 | ||||
| Diastolic BP, mmHg | YG (n=15) | 68 ± 1 | 68 ± 1 | 71 ± 1* | 0.165 | 0.272 | 0.049 |
| MA/O (n=14) | 73 ± 2 | 73 ± 3 | 72 ± 3 | ||||
| Mean BP, mmHg | YG (n=15) | 83 ± 1 | 83 ± 2 | 84 ± 2 | 0.248 | 0.722 | 0.038 |
| MA/O (n=14) | 88 ± 2 | 88 ± 3 | 86 ± 3 | ||||
| Heart rate, beats·min−1 | YG (n=15) | 59 ± 2 | 61 ± 2 | 68 ± 3# | 0.028 | <0.001 | 0.021 |
| MA/O (n=14) | 54 ± 2 | 55 ± 3 | 58 ± 3#† | ||||
Values are means ± SE. YG, young adults; MA/O, middle-age/older adults; MSNA, muscle sympathetic nerve activity; AU, arbitrary units; BP, arterial blood pressure;
P<0.05 vs. baseline,
P<0.05 vs. baseline and LBNP −15mmHg;
P<0.05 vs. YG; Holm-Sidak post hoc did not detect significant differences between YG and MA/O in mean BP during LBNP despite 2-way RM ANOVA significant interaction.
Central and peripheral artery stiffness responses to LBNP:
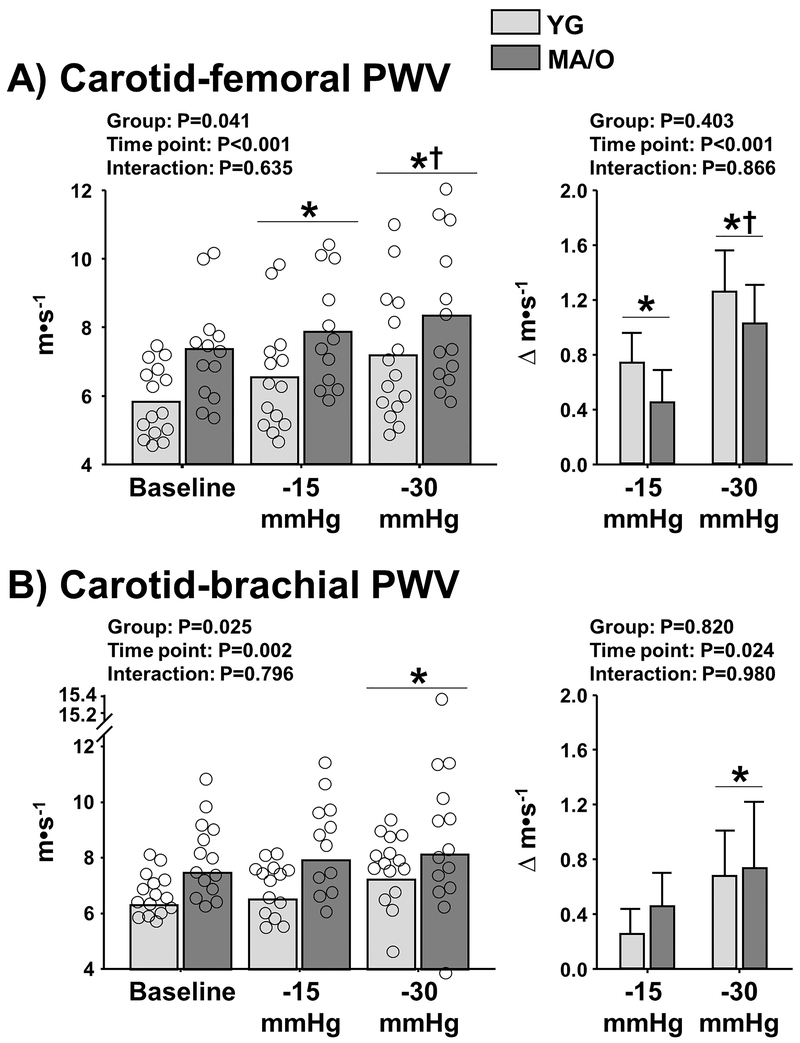
Central artery stiffness (carotid-femoral PWV) was significantly increased during LBNP compared with baseline in YG and MA/O (time point: P<0.001) (Figure 2A, left panel). The magnitude of increase (Δ) in carotid-femoral PWV during LBNP (P<0.001) was similar between YG (−15mmHg: Δ+0.8 ± 0.2; −30mmHg: Δ+1.3 ± 0.3 m·s−1) and MA/O (−15mmHg: Δ+0.5 ± 0.2; −30mmHg: Δ+1.0 ± 0.3 m·s−1) (P=0.403) (Figure 2A, right panel). Carotid-femoral PWV was significantly correlated with MSNA burst frequency at baseline and during LBNP (Baseline: R=0.77, P<0.001; LBNP −15mmHg: R=0.62, P=0.005; LBNP −30mmHg: R=0.52, P=0.023). Interestingly, after controlling for age, a correlational analysis between the average Δ MSNA and Δ carotid-femoral PWV during LBNP revealed a stronger association among women (R=0.80, P=0.009) compared with men (R=0.20, P=0.578), despite no significant difference between women and men in Δ MSNA (P=0.292) and Δ carotid-femoral PWV (P=0.129). Carotid-femoral PWV was not correlated with circulating norepinephrine concentration (Baseline: R=0.07, P=0.744; LBNP −15mmHg: R=0.37, P=0.098; LBNP −30mmHg: R=0.12, P=0.625), consistent with Study 1.
Figure 2.
Central artery stiffness [carotid-femoral pulse wave velocity (PWV), Panel A] and peripheral artery stiffness (carotid-brachial PWV, Panel B) at baseline and during acute elevations in muscle sympathetic nerve activity using progressive levels of lower body negative pressure (−15 mmHg and −30 mmHg) in 15 young (YG) and 14 middle-age/older (MA/O) adults. Right panels depict changes (deltas, Δ) in carotid-femoral PWV and carotid-brachial PWV during LBNP in YG and MA/O. * P<0.05 vs. baseline; † P<0.05 vs. −15 mmHg.
Peripheral artery stiffness (carotid-brachial PWV) was not significantly elevated at LBNP −15mmHg compared with baseline but was significantly elevated at LBNP −30mmHg (time point: P=0.002) (Figure 2B, left panel). The magnitude of increase in carotid-brachial PWV during LBNP −30mmHg (P=0.024) was similar between YG and MA/O (YG: Δ+0.7±0.3 vs. MA/O: Δ+0.7±0.5 m·s−1, P=0.82) (Figure 2B, right panel).
Carotid compliance responses to LBNP:
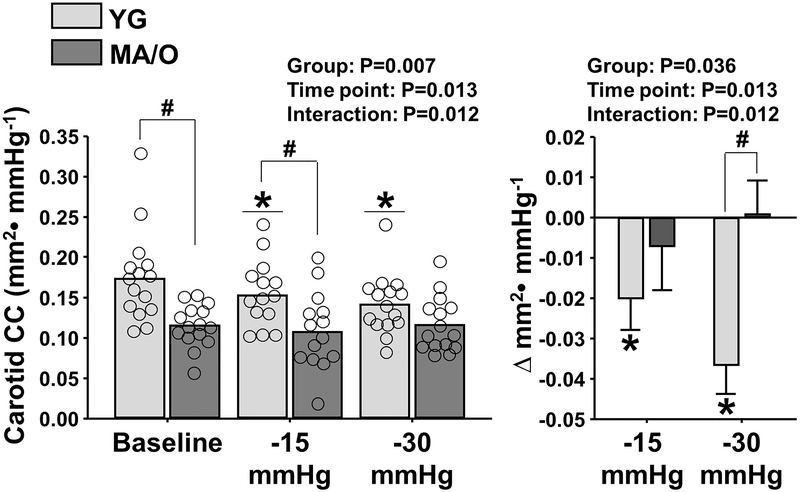
Compared with baseline, carotid CC was significantly reduced during LBNP (time point: P=0.013) (Figure 3, left panel). The reduction in carotid CC during LBNP was observed in YG (−15mmHg: Δ−0.02 ± 0.01; −30mmHg: Δ−0.04 ± 0.01 mm2·mmHg−1), but not MA/O (−15mmHg: Δ−0.007 ± 0.011; −30mmHg: Δ0.001 ± 0.008 mm2·mmHg−1) (interaction: P=0.012) (Figure 3, right panel), despite comparable increases in MSNA. Carotid CC was significantly correlated with MSNA burst frequency only during LBNP −30mmHg (Baseline: R=−0.40, P=0.067; LBNP −15mmHg: R=−0.42, P=0.063; LBNP −30mmHg: R=−0.55, P=0.012), and was not related to circulating norepinephrine concentrations at baseline or during LBNP (P>0.05) (data not shown).
Figure 3.
Common carotid artery compliance coefficient (CC) at baseline and during acute elevations in muscle sympathetic nerve activity using progressive levels of lower body negative pressure (LBNP) in 15 young (YG) and 14 middle-age/older (MA/O) adults. Right panel depicts changes (deltas, Δ) in carotid CC during LBNP in YG and MA/O. * P<0.05 vs. baseline; † P<0.05 vs. −15 mmHg; # P<0.05 vs. YG.
Discussion
The present study reveals a significant contribution of acute and age-related increases in MSNA to increased stiffness of both peripheral and central arteries. Three important and novel findings of this study are as follows. First, in the larger group of YG and MA/O men and women, the robust relation between MSNA and central artery stiffness (carotid-femoral PWV) and the more moderate relation between MSNA and carotid CC remained significant after controlling for sex, MAP, HR, and waist-to-hip ratio, but these associations were abolished when also controlling for age. Second, acute elevations in MSNA during LBNP produced age-independent increases in carotid-femoral PWV, whereas reductions in carotid CC during LBNP were age-dependent because reductions occurred in YG but not MA/O adults. Third, increases in carotid-femoral PWV during LBNP appeared greater than those observed in carotid-brachial PWV (peripheral artery stiffness), demonstrating increases in both central and peripheral artery stiffness during sympathetic activation in YG and MA/O adults. Together, these findings demonstrate the influence of MSNA on central artery stiffness and its potential contribution to age-related increases in stiffness of both peripheral and central arteries.
Resting MSNA and central artery stiffness
The aim of the present study was to examine the extent to which SNA influences the mechanical properties of the central arteries and contributes to increased stiffness in YG and MA/O adults. We found a robust relation between MSNA and carotid-femoral PWV independent of MAP in a larger group of YG and MA/O men and women (n=88), but this association was abolished when controlling for age. Similarly, when examining only men (n=46), higher MSNA was significantly correlated with greater carotid-femoral PWV after controlling for MAP, HR, and waist-to-hip ratio, but not after additional adjustment for age. Recently, Tanaka et al. (2017) reported that the age-related difference in central artery stiffness assessed by carotid compliance between young and older men was abolished after statistically controlling for MSNA using ANCOVA, suggesting that the relation between MSNA and central artery stiffness is age-dependent. Similar results were found when performing this analysis between YG and MA/O subjects in the present study (Study 2). These data support our main findings that MSNA was associated with carotid-femoral PWV and carotid CC, but these associations were dependent on age. Therefore, the association between MSNA and central artery stiffness appears to change with age, which is likely promoted by the age-related increase in resting MSNA. It is possible that higher MSNA with advancing age increases vascular smooth muscle tone that is reflected by greater functional measures of arterial stiffness. It is also tempting to speculate that age might alter the association between MSNA and central artery stiffness when structural changes in the vascular wall also become present such as higher collagen/elastin ratio and vascular smooth muscle hypertrophy. Additional studies are needed to examine whether chronically elevated (age-related) SNA-adrenergic stimulation interacts with the vascular wall to influence the structural changes that occur with advancing age.
Acute increases in MSNA and arterial stiffness
Stiffness of peripheral arteries can be modulated by sympathetic innervation and resultant changes in smooth muscle tone.15–20 However, examinations of SNA-adrenergic influence on stiffness of the large elastic central arteries have provided mixed results because some studies have relied on more indirect measures of central artery stiffness and MSNA 25, 43 that can be highly variable and interpretation has been controversial.3, 44, 45 Also, study methodologies that allow assessment of central artery stiffness in response to acute changes in MSNA in the absence of major changes in MAP (i.e., distending pressure) have been limited to only a few studies. Sonesson at al. (1997) did not detect significant changes in mechanical properties of the abdominal aorta in MA/O adults when limiting increases in distending pressure during sympatho-excitation with LBNP,46 which is in contrast to findings of the present study. Although the reasons for these discrepant findings are unclear, it should be noted that Sonesson at al. (1997) examined local distensibility of the abdominal aorta, whereas regional stiffness of the aorta was assessed in the present study using carotid-femoral PWV. In the present study, acute increases in MSNA and carotid-femoral PWV during LBNP were observed and similar in magnitude in YG and MA/O subjects. In accordance, the magnitude of increase in peripheral artery stiffness during LBNP assessed by carotid-brachial PWV was similar between YG and MA/O subjects, although the overall increase in carotid-brachial PWV was relatively less compared with carotid-femoral PWV. The relative difference between changes in carotid-brachial PWV vs. carotid-femoral PWV during sympathetic activation may be related to less α-adrenergic receptor density or sensitivity in the brachial region compared with the femoral region.47, 48 Nonetheless, our results support and extend previous findings demonstrating MSNA-mediated increases in carotid-femoral PWV in young, healthy subjects independent of distending pressure.30, 31 To our knowledge, the present study is the first to demonstrate that MSNA-mediated increases in carotid-femoral PWV also occur in MA/O adults independent of distending pressure. These findings also provide evidence that acute increases in MSNA influence functional measures of both central and peripheral artery stiffness where central artery stiffness is as responsive (or more) as peripheral stiffness, and that these acute changes in stiffness occur in both YG and MA/O.
Sympathetic innervation
MA/O subjects demonstrated a significantly greater increase in circulating norepinephrine concentration during LBNP compared with YG. Thus, a greater increase in carotid-femoral PWV during LBNP would be expected among MA/O if circulating norepinephrine rather than direct sympathetic innervation was a primary mediator of vascular smooth muscle tone in the central arteries. However, increases in carotid-femoral PWV during LBNP were similar in YG and MA/O and parallel with the elevations in MSNA. Indeed, carotid-femoral PWV during LBNP was significantly correlated with MSNA but not with circulating norepinephrine concentration. These findings lend support to the idea that increases in carotid-femoral PWV were more likely MSNA-driven rather than an increased vasoconstrictor tone via adrenergic receptor binding by circulating norepinephrine. Although few studies have focused on sympathetic innervation of the large central arteries, evidence from animal models show that large central arteries such as the thoracic aorta possess smooth muscle tissue and express α1-adrenergic receptors,21, 49, 50 although evidence also shows that sympathetic innervation of the ascending aorta may be more sparse.51 MSNA innervating vascular smooth muscle in different skeletal muscle beds is strikingly similar 52, 53 and is positively correlated with sympathetic activity at the level of the heart.54 Thus, it remains plausible that MSNA reflects sympathetic innervation of vascular smooth muscle in the central arteries. An alternative vasoconstrictor that increases in the circulation during LBNP is arginine-vasopressin (AVP). However, an increase in circulating concentration of AVP requires a more intense LBNP stimulus (~ −50 mmHg or higher vs. only −30 mmHg in the present study) that is often accompanied by hypotension and syncopal symptoms.55–57 Therefore, AVP likely did not contribute to changes in central artery stiffness during LBNP in the present study. Taken together, the findings suggest that increases in central and peripheral artery stiffness during LBNP are primarily a result of increased sympathetic nerve activity and less attributable to increased circulating norepinephrine or AVP concentration.
Carotid compliance vs. carotid-femoral PWV
In contrast to the observed increase in carotid-femoral PWV during LBNP in YG and MA/O adults, carotid compliance was reduced during LBNP in YG, but not MA/O, despite similar increases in MSNA. These results are in contrast to previous findings demonstrating changes in carotid compliance in MA/O adults when given systemic α-adrenergic receptor blockade to attenuate sympathetic influence on the vasculature.28 However, MAP was also reduced ~ 15 mmHg during α-adrenergic receptor blockade, which decreases arterial distending pressure and confounds the interpretation of the change in carotid compliance. In the present study, no change in carotid compliance was observed among MA/O during limited changes in MAP. The difference in findings can be explained, at least in part, by the pressure-volume (or diameter) relation by which arterial compliance is expressed. Estimation of arterial compliance depends on local arterial pressure, and changes in arterial pressure can lead to measurement discrepancies because compliance is not the same at different points on the pressure-volume curve.
Potential limitations
SNA to central arteries cannot be directly assessed, at least in humans. It is therefore unclear whether SNA to central arteries is proportional to MSNA in the peroneal nerve. Although MSNA is similar to vascular beds in different skeletal muscle groups,52, 53 this obvious limitation allows only an inference of SNA to the central arteries.
PWV is typically greater in medium-size peripheral arteries such as the radial artery compared with central arteries such as the aorta, and there is a strong association between MSNA and mechanical properties of medium-size peripheral arteries.16, 18, 19 Thus, the relation between MSNA and PWV may have been more pronounced in other peripheral arteries compared with carotid-brachial PWV used in the present study. However, it is established that mechanical properties of large-size peripheral muscular arteries such as the brachial artery are influenced by MSNA.13, 15 While literature has characterized the aorta and brachial arteries as central and peripheral muscular arteries, respectively, 1, 2 our findings support the idea that PWV increases in response to elevated MSNA in both central elastic and peripheral muscular arteries.
LBNP elicited baroreflex-mediated increases in HR that were significantly greater in YG compared with MA/O subjects. Since the increase in HR and reduction in carotid compliance during LBNP was significantly greater among YG compared with MA/O, this raises concern that the increase in HR during LBNP contributed to the observed reductions in carotid compliance. Studies in humans and animals have demonstrated that increases in HR are accompanied by reductions in arterial compliance.58, 59 Some studies,59, 60 but not all,61 have reported a contribution of HR to changes in carotid-femoral PWV. However, several lines of reasoning support that increases in carotid-femoral PWV during LBNP were not attributed to elevations in HR. First, YG had a significantly greater HR response to LBNP compared with MA/O (Table 3), yet the increase in carotid-femoral PWV was not significantly different between groups (Figure 2). Second, significant increases in carotid-femoral PWV during LBNP in the absence of changes in HR have previously been reported.31 Third, controlled studies in humans showed that elevations in HR via atrial pacing did not significantly increase carotid-femoral PWV when there was no corresponding change in MAP.59 Given that observations in the present study were characterized by significant increases in HR without corresponding changes in MAP, the contribution of HR to increased carotid-femoral PWV was likely minimal.
Perspectives
Elevated SNA with age is associated with peripheral conduit artery stiffness and remodeling.15–20 We report that SNA contributes to increased stiffness of the central arteries, and the association between SNA and central artery stiffness was as prominent, or more, as the association with peripheral artery stiffness. This is important because, unlike peripheral artery stiffness, increased central artery (aorta) stiffness is associated with major CVD outcomes.2, 62 Our findings also challenge the classical concept of arterial aging focused on structural features (e.g., loss of elastin, increased collagen) to also include an influence of SNA that modulates smooth muscle tone of the central arteries and promotes an increase in functional measures of arterial stiffness.
Conclusion
In summary, findings from the present study demonstrate an age-dependent association between resting SNA and central and peripheral artery stiffness, whereas acute elevations in SNA produce age-independent increases in central artery stiffness that are at least as pronounced as increases observed in peripheral artery stiffness. Findings from the present study reconcile mixed findings from previous investigations that have been confounded by alterations in mean arterial pressure (i.e., distending pressure) which typically occurs with acute changes in vasoconstrictor tone. SNA and central artery stiffness both increase with advancing age and are associated with an increased risk of developing CVD. Future intervention studies targeting reductions in SNA may provide important insight into the clinical feasibility of limiting age-related increases in central artery stiffness.
Supplementary Material
What is New?
We report a significant association between sympathetic nerve activity and central artery stiffness independent of arterial distending pressure, and that acute increases in sympathetic nerve activity influence functional measures of both central and peripheral artery stiffness in young and middle-aged/older men and women.
What is Relevant?
The contribution of sympathetic nerve activity to central artery stiffness is important because, unlike peripheral artery stiffness, increased central artery (aorta) stiffness is associated with major cardiovascular disease outcomes.
Acknowledgements
We acknowledge Allene L. Gremaud and the University of Iowa Institute for Clinical and Translational Science Clinical Research Unit staff for assistance during studies.
Sources of funding
This work was supported in part by an Iowa Cardiovascular Interdisciplinary Research Fellowship (T32HL007121) (S.W. Holwerda), American Heart Association Grants 17POST33440101 (S.W. Holwerda) and 13SDG143400012 (G.L. Pierce) and National Institutes of Health Grants P01 HL-014388-48 (F.M. Abboud, G.L. Pierce) and CTSA UL1TR002537 (University of Iowa).
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J Appl Physiol (1985). 2008;105:1652–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, European Network for Non-invasive Investigation of Large A. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605 [DOI] [PubMed] [Google Scholar]
- 4.van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, Kavousi M, Mattace-Raso F, Franco OH, Boutouyrie P, Stehouwer CDA. Carotid stiffness is associated with incident stroke: A systematic review and individual participant data meta-analysis. J Am Coll Cardiol. 2015;66:2116–2125 [DOI] [PubMed] [Google Scholar]
- 5.Abboud FM, Huston JH. The effects of aging and degenerative vascular disease on the measurement of arterial rigidity in man. J Clin Invest. 1961;40:933–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacolley P, Regnault V, Avolio AP. Smooth muscle cell and arterial aging: Basic and clinical aspects. Cardiovasc Res. 2018;114:513–528 [DOI] [PubMed] [Google Scholar]
- 8.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol. 2008;93:715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525 [DOI] [PubMed] [Google Scholar]
- 10.Ziegler MG, Lake CR, Kopin IJ. Plasma noradrenaline increases with age. Nature. 1976;261:333–335 [DOI] [PubMed] [Google Scholar]
- 11.Hajduczok G, Chapleau MW, Abboud FM. Increase in sympathetic activity with age. Ii. Role of impairment of cardiopulmonary baroreflexes. Am J Physiol. 1991;260:H1121–1127 [DOI] [PubMed] [Google Scholar]
- 12.Hajduczok G, Chapleau MW, Johnson SL, Abboud FM. Increase in sympathetic activity with age. I. Role of impairment of arterial baroreflexes. Am J Physiol. 1991;260:H1113–1120 [DOI] [PubMed] [Google Scholar]
- 13.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–688 [DOI] [PubMed] [Google Scholar]
- 14.Kaplon RE, Walker AE, Seals DR. Plasma norepinephrine is an independent predictor of vascular endothelial function with aging in healthy women. J Appl Physiol (1985). 2011;111:1416–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosch M, Barenbrock M, Kisters K, Rahn KH, Hausberg M. Relationship between muscle sympathetic nerve activity and large artery mechanical vessel wall properties in renal transplant patients. J Hypertens. 2002;20:501–508 [DOI] [PubMed] [Google Scholar]
- 16.Failla M, Grappiolo A, Emanuelli G, Vitale G, Fraschini N, Bigoni M, Grieco N, Denti M, Giannattasio C, Mancia G. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. J Hypertens. 1999;17:1117–1123 [DOI] [PubMed] [Google Scholar]
- 17.Mangoni AA, Mircoli L, Giannattasio C, Mancia G, Ferrari AU. Effect of sympathectomy on mechanical properties of common carotid and femoral arteries. Hypertension. 1997;30:1085–1088 [DOI] [PubMed] [Google Scholar]
- 18.Grassi G, Giannattasio C, Failla M, Pesenti A, Peretti G, Marinoni E, Fraschini N, Vailati S, Mancia G. Sympathetic modulation of radial artery compliance in congestive heart failure. Hypertension. 1995;26:348–354 [DOI] [PubMed] [Google Scholar]
- 19.Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. Am J Physiol. 1994;267:H1368–1376 [DOI] [PubMed] [Google Scholar]
- 20.Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278:H1205–1210 [DOI] [PubMed] [Google Scholar]
- 21.Pagani M, Mirsky I, Baig H, Manders WT, Kerkhof P, Vatner SF. Effects of age on aortic pressure-diameter and elastic stiffness-stress relationships in unanesthetized sheep. Circ Res. 1979;44:420–429 [DOI] [PubMed] [Google Scholar]
- 22.Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Boutouyrie P, Somers VK, Narkiewicz K. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens. 2010;28:979–984 [DOI] [PubMed] [Google Scholar]
- 23.Tanaka H, Dinenno FA, Seals DR. Reductions in central arterial compliance with age are related to sympathetic vasoconstrictor nerve activity in healthy men. Hypertens Res. 2017 [DOI] [PubMed] [Google Scholar]
- 24.Lydakis C, Momen A, Blaha C, Herr M, Leuenberger UA, Sinoway LI. Changes of elastic properties of central arteries during acute static exercise and lower body negative pressure. Eur J Appl Physiol. 2008;102:633–641 [DOI] [PubMed] [Google Scholar]
- 25.Maki-Petaja KM, Barrett SM, Evans SV, Cheriyan J, McEniery CM, Wilkinson IB. The role of the autonomic nervous system in the regulation of aortic stiffness. Hypertension. 2016;68:1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey RE, Barnes JN, Hart EC, Nicholson WT, Joyner MJ, Casey DP. Influence of sympathetic nerve activity on aortic hemodynamics and pulse wave velocity in women. Am J Physiol Heart Circ Physiol. 2017;312:H340–H346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Yokoi T, Otsuki T, Shimojo N, Miyauchi T, Maeda S, Tanaka H. Effect of systemic nitric oxide synthase inhibition on arterial stiffness in humans. Hypertens Res. 2007;30:411–415 [DOI] [PubMed] [Google Scholar]
- 28.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, Miyauchi T, Yokoi T, Maeda S, Tanaka H. Reduction in alpha-adrenergic receptor-mediated vascular tone contributes to improved arterial compliance with endurance training. Int J Cardiol. 2009;135:346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chirinos JA. Arterial stiffness: Basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5:243–255 [DOI] [PubMed] [Google Scholar]
- 30.Phillips AA, Bredin SS, Cote AT, Drury CT, Warburton DE. Aortic distensibility is reduced during intense lower body negative pressure and is related to low frequency power of systolic blood pressure. Eur J Appl Physiol. 2013;113:785–792 [DOI] [PubMed] [Google Scholar]
- 31.Nardone M, Incognito AV, Millar PJ. Evidence for pressure-independent sympathetic modulation of central pulse wave velocity. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoller RP, Mark AL, Abboud FM, Schmid PG, Heistad DD. The role of low pressure baroreceptors in reflex vasoconstrictor responses in man. J Clin Invest. 1972;51:2967–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: Fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood). 2010;235:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:5389–5395 [DOI] [PubMed] [Google Scholar]
- 35.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957 [DOI] [PubMed] [Google Scholar]
- 36.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;302:H2419–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holwerda SW, Vianna LC, Restaino RM, Chaudhary K, Young CN, Fadel PJ. Arterial baroreflex control of sympathetic nerve activity and heart rate in patients with type 2 diabetes. Am J Physiol Heart Circ Physiol. 2016;311:H1170–H1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holwerda SW, Luehrs RE, Gremaud AL, Wooldridge NA, Stroud AK, Fiedorowicz JG, Abboud FM, Pierce GL. Relative burst amplitude of muscle sympathetic nerve activity is an indicator of altered sympathetic outflow in chronic anxiety. J Neurophysiol. 2018;120:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Victor RG, Leimbach WN Jr., Effects of lower body negative pressure on sympathetic discharge to leg muscles in humans. J Appl Physiol (1985). 1987;63:2558–2562 [DOI] [PubMed] [Google Scholar]
- 40.Joyner MJ, Shepherd JT, Seals DR. Sustained increases in sympathetic outflow during prolonged lower body negative pressure in humans. J Appl Physiol (1985). 1990;68:1004–1009 [DOI] [PubMed] [Google Scholar]
- 41.Johansen NB, Vistisen D, Brunner EJ, Tabak AG, Shipley MJ, Wilkinson IB, McEniery CM, Roden M, Herder C, Kivimaki M, Witte DR. Determinants of aortic stiffness: 16-year follow-up of the whitehall ii study. PLoS One. 2012;7:e37165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207 [DOI] [PubMed] [Google Scholar]
- 43.Casey DP, Curry TB, Joyner MJ, Charkoudian N, Hart EC. Relationship between muscle sympathetic nerve activity and aortic wave reflection characteristics in young men and women. Hypertension. 2011;57:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995;25:1276–1286 [DOI] [PubMed] [Google Scholar]
- 45.Saul JP, Rea RF, Eckberg DL, Berger RD, Cohen RJ. Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. Am J Physiol. 1990;258:H713–721 [DOI] [PubMed] [Google Scholar]
- 46.Sonesson B, Vernersson E, Hansen F, Lanne T. Influence of sympathetic stimulation on the mechanical properties of the aorta in humans. Acta Physiol Scand. 1997;159:139–145 [DOI] [PubMed] [Google Scholar]
- 47.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC 2nd, Wray DW, Davis MJ, Fadel PJ. The role of alpha-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. The Journal of physiology. 2013;591:3637–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: A gravitational effect? J Appl Physiol (1985). 2002;92:2105–2113 [DOI] [PubMed] [Google Scholar]
- 49.Okazaki M, Hu ZW, Fujinaga M, Hoffman BB. Alpha 1 adrenergic receptor-induced c-fos gene expression in rat aorta and cultured vascular smooth muscle cells. J Clin Invest. 1994;94:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macia RA, Matthews WD, Lafferty J, DeMarinis RM. Assessment of alpha-adrenergic receptor subtypes in isolated rat aortic segments. Naunyn Schmiedebergs Arch Pharmacol. 1984;325:306–309 [DOI] [PubMed] [Google Scholar]
- 51.Kienecker EW, Knoche H. Sympathetic innervation of the pulmonary artery, ascending aorta, and coronar glomera of the rabbit. A fluorescence microscopic study. Cell Tissue Res. 1978;188:329–333 [DOI] [PubMed] [Google Scholar]
- 52.Hansen J, Thomas GD, Jacobsen TN, Victor RG. Muscle metaboreflex triggers parallel sympathetic activation in exercising and resting human skeletal muscle. Am J Physiol. 1994;266:H2508–2514 [DOI] [PubMed] [Google Scholar]
- 53.Ray CA, Mark AL. Sympathetic nerve activity to nonactive muscle of the exercising and nonexercising limb. Med Sci Sports Exerc. 1995;27:183–187 [PubMed] [Google Scholar]
- 54.Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. The Journal of physiology. 1992;453:45–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldsmith SR, Francis GS, Cowley AW, Cohn JN. Response of vasopressin and norepinephrine to lower body negative pressure in humans. Am J Physiol. 1982;243:H970–973 [DOI] [PubMed] [Google Scholar]
- 56.Norsk P, Ellegaard P, Videbaek R, Stadeager C, Jessen F, Johansen LB, Kristensen MS, Kamegai M, Warberg J, Christensen NJ. Arterial pulse pressure and vasopressin release in humans during lower body negative pressure. Am J Physiol. 1993;264:R1024–1030 [DOI] [PubMed] [Google Scholar]
- 57.Baylis PH, Stockley RA, Heath DA. Influence of lower body negative pressure upon arginine vasopressin release. Clin Endocrinol (Oxf). 1978;9:89–95 [DOI] [PubMed] [Google Scholar]
- 58.Mangoni AA, Mircoli L, Giannattasio C, Ferrari AU, Mancia G. Heart rate-dependence of arterial distensibility in vivo. J Hypertens. 1996;14:897–901 [DOI] [PubMed] [Google Scholar]
- 59.Liang YL, Gatzka CD, Du XJ, Cameron JD, Kingwell BA, Dart AM. Effects of heart rate on arterial compliance in men. Clin Exp Pharmacol Physiol. 1999;26:342–346 [DOI] [PubMed] [Google Scholar]
- 60.Tan I, Butlin M, Liu YY, Ng K, Avolio AP. Heart rate dependence of aortic pulse wave velocity at different arterial pressures in rats. Hypertension. 2012;60:528–533 [DOI] [PubMed] [Google Scholar]
- 61.Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, Levy T, Cockcroft JR. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15:24–30 [DOI] [PubMed] [Google Scholar]
- 62.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.