Introduction
Clinical pharmacologists seek to advance optimal drug therapy in humans. The field has expanded beyond its roots in clinical medicine/drug action and pharmacokinetics/pharmacodynamics to encompass a wide range of more specialized foci, including basic/translational pharmacological sciences, -omics technologies, pharmacometrics, and real-world outcomes data, to name a few. Given the discipline’s breadth, clinical pharmacology training programs must provide foundational knowledge, complemented by specialized knowledge and soft skills, to supply the work force and advance the field.
Evolution of clinical pharmacology training
Clinical pharmacology training programs have changed markedly in recent decades, with substantial advances in our understanding of the processes that govern drug absorption, distribution, metabolism, and elimination.1 These programs are based in schools of medicine and pharmacy, and carry many names, including “experimental therapeutics”, “pharmacotherapy”, “translational science” as well as traditional “clinical pharmacology”. Today’s clinical pharmacologists are trained in more diverse and collaborative environments by both clinicians and scientists. Training for clinicians and non-clinicians may include either a PhD or post-doctoral fellowship program. For clinicians (i.e., MDs and PharmDs), fellowship training often serves as the primary avenue for research training.
Diversity in the backgrounds of entrants to clinical pharmacology -which includes physicians, pharmacists and non-clinician graduate students or post-doctoral trainees -and the range of titles under which clinical pharmacology training programs are housed, is viewed by some as a challenge or problem. In contrast, we view this as the strength of clinical pharmacology and its training programs because the variety of backgrounds and perspectives among today’s clinical pharmacologists adds to the richness of the discipline. Further, we view it as a strength that the discipline is not constrained by labels or backgrounds but is focused on optimizing drug therapy for humans – through evaluation of existing therapeutic agents and development of new medicines.2 Given the range of educational backgrounds and the rapidly evolving needs at the bench and bedside, we believe clinical pharmacology training programs must offer enough flexibility in design to enable trainees to acquire foundational knowledge and develop research techniques that complement their preexisting skill set(s). Subsequent specialization can take many paths (Figure 1). Herein, we describe what we believe represents the foundational and specialized knowledge, and provide examples of the opportunities and challenges in the decade ahead.
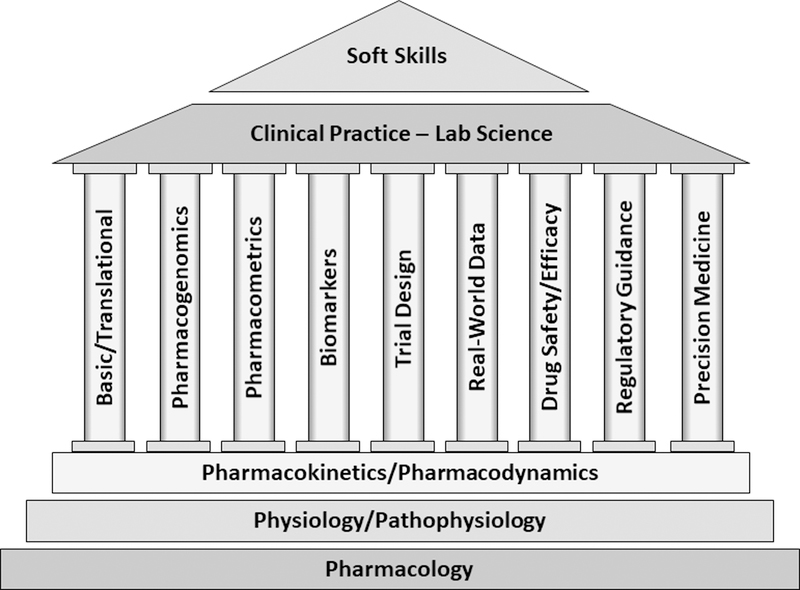
Figure 1. Clinical Pharmacology Education.
Clinical pharmacology training programs build on foundational knowledge, and provide opportunities for development of specialized skills (the discipline’s pillars), augmented by experiences in clinical practice and lab science. Soft skills training, depicted as an overarching skill, is key to success in today’s workforce.
Foundational knowledge in clinical pharmacology
Robust training programs must provide learners with foundational knowledge in general principles of clinical pharmacology, encompassing basic pharmacology (e.g., receptors, enzymes, mechanisms-of-action), pharmacokinetics (e.g., absorption, bioavailability, clearance, and variability therein), and pharmacodynamics (e.g., concentration-effect relationships), along with use of genetic and non-genetic biomarkers to inform clinical decision-making.
While not all clinical pharmacologists will have the same understanding of disease pathophysiology as physicians or pharmacists, they must have a deep knowledge of the disease(s) they are studying, including pathophysiology and approaches to drug therapy. A basic understanding of clinical trial design, biostatistics, as well as the ethical conduct of human subjects’ research are also critical elements of basic training in the discipline.
Historically, this knowledge, and the associated technical and experimental skills required to study the above elements, represented the majority of research and training in clinical pharmacology. For some clinical pharmacologists, this continues to represent the backbone of their work. With this foundational knowledge in hand, clinical pharmacology trainees are poised to take divergent paths that lead to development of specialized skills and more focused expertise to help address current and future challenges in the field (e.g., aging population, rising drug costs, increased regulatory requirements).
Specialized knowledge and skill sets in clinical pharmacology
The specialized knowledge and skills of clinical pharmacologists are those that will not be possessed by all, but that collectively are important for the discipline. Generally, these skill sets can be broken into basic research, translational and clinical research, and computational research, typically with human data.
Basic research often uses molecular biology tools to discover knowledge about proteins or biological networks important to drug pharmacokinetics, drug action or toxicity, as well as genetic and non-genetic variability therein. Specialized skills for individuals focused in this domain are similar to those in other basic biomedical disciplines, though typically their work will not involve use of animals.
Examples of translational and clinical research include traditional pharmacokinetic, drug-drug or food-drug interaction studies, clinical trials in special populations, and more recently, genetic and non-genetic biomarker studies to improve patient care. The specialized skill sets for those focused in translational and clinical research include expert knowledge in clinical trial design and execution, as well as bioanalysis and data analysis. The latter often requires knowledge beyond basic biostatistics, and may include complex analyses of big datasets, such as genomic, transcriptomic, and/or metabolomic data. Some working in this area will also develop expertise in clinical drug development, and the regulatory requirements for drug approval and labeling.
Specialized computational skills are needed to integrate multiple data sources (e.g., in vitro, preclinical, individual patient, population) for enhanced decision-making (e.g., dose selection, sampling strategy, optimal trial design), utilizing mathematical and statistical modeling and simulation. In the specialized field of pharmacometrics, practitioners must demonstrate proficiency with mathematical and statistical tools and methodologies, and possess a thorough understanding of how specific approaches are used to inform preclinical and clinical drug development. These pharmacometric approaches are often complemented by big data approaches in order to evaluate how much of the variability observed in clinical trials can be translated into real-world settings and/or to evaluate the mechanistic plausibility of signals (typically, toxicity) in real-world patients.
Challenges – current and future
The diversity of backgrounds of trainees entering clinical pharmacology programs presents challenges in terms of providing both comprehensive, foundational didactics and opportunities for specialized skill development. Medically-trained clinicians may have limited background in some foundational principles of clinical pharmacology, particularly pharmacokinetics. Most MDs and PharmDs are likely to have limited experience in basic research, statistics and mathematical modeling. Conversely, non-clinicians, particularly statisticians or engineers are likely to know little about medications or the diseases they are used to treat, and may lack solid knowledge of the biological sciences. A “one size fits all” educational approach is, consequently, unlikely to provide all trainees with the tools and skill sets needed to become a highly functional member of an interdisciplinary clinical pharmacology team. Therefore, programs must be flexible in design and tailored to the individual trainee’s background, needs and training goals while ensuring that core clinical pharmacology content is mastered. Given the diversity in trainees’ educational backgrounds, the time needed to acquire these foundational skills in formal training programs will differ. Some programs offer only postdoctoral fellowship training, minimally 2–3 years in length, while others offer PhD degrees, which typically require 4+ years of training. Informal, “on the job” training to continue skill and expertise development is an essential to maturation of the highly competent clinical pharmacologist.
Financial support for these time-and labor-intensive training programs continues to be a major challenge, despite the growing demand for highly qualified clinical pharmacologists in the industrial, regulatory and academic sectors. While the National Institutes of Health has played an important role in supporting clinical pharmacology training, it funds only a small fraction of those training in clinical pharmacology. Fellowship programs sponsored by the pharmaceutical industry or FDA also provide venues for high-quality training, though the majority remain in the academic setting. In order for academic institutions to meet the societal demands for individuals with knowledge and skills in clinical pharmacology, academic-private industry partnerships will become increasingly important. Expanded and sustained collaborations between these sectors in preparing a well-trained cadre of clinical pharmacologists for the workforce will help insure the strength of the discipline and its workforce into the future.
A caution for clinical pharmacology training programs is that focusing on highly specialized aspects of clinical pharmacology may come at the expense of developing a solid base of foundational knowledge – this must be avoided. The lack of foundational knowledge may result in the inability of the clinical pharmacologist to: a) properly develop experimental approaches, b) understand what their data can or should reveal, and c) accurately interpret the data and place it in the proper context. This presents a threat to the discipline and highlights the critical nature of the foundational knowledge in clinical pharmacology.
As the discipline evolves with new paths requiring specialized technical skills, it is also critical that educators simultaneously maintain focus on developing non-technical workplace skills. An increasing focus on the importance of “soft skills”, including proficient verbal and written communication, interpersonal, time management, problem solving, leadership and management skills, as well as emotional intelligence, represents a challenge in clinical pharmacology education. Trainees need to develop these skills alongside their technical skills and knowledge in order to become successful in the workforce. While many of these skills were developed “on the job” in earlier eras, today’s employers demand graduates that have demonstrable soft skills.
Opportunities for clinical pharmacology education
Opportunities in clinical pharmacology abound for the well-prepared clinical pharmacologist, and particularly for clinician-scientists for whom the foundation in diseases and drug therapy has already been laid. Understanding and predicting factors (e.g., genetics, drug interactions, environment, diet) that may influence drug dosing, efficacy and/or adverse effects is central to the discipline. The large volume of data that will be available to mine and use to inform clinical pharmacology decisions presents tremendous opportunities.
Clinical pharmacology will increasingly involve the use of big data, including large, structured datasets, and real-world (unstructured) data3. Clinical pharmacologists must be properly trained to analyze structured and unstructured data, the latter of which is expected to be an emergent area for clinical pharmacology in coming years. New approaches will be needed to manage this information, and analyze, interpret, and translate it into useful formats to guide decisions in drug development and therapeutics. Advanced analytical approaches (e.g., machine learning, artificial intelligence) will need to be evaluated for their value above currently used analytical methods.
There are also many opportunities for clinical pharmacologists as the therapeutic armamentarium extends from traditional small molecules to biologics, gene therapy and stem cell replacement therapies, along with novel dosage forms. Many new opportunities exist to identify and establish novel biomarkers and develop innovative clinical trial designs. Enhanced access to data and informatics approaches is also expected to increase the opportunities to use patient-specific data to inform precision medicine approaches that will then improve clinical care and outcomes at the individual level.
The future is bright for clinical pharmacologists, who are expected to have an expanding, critical role in drug development and regulatory decision-making. Even greater opportunities exist in optimizing medication therapy for individual patients, as the technology and data available for precision medicine approaches emerge and become commonly used in the coming decade. Clearly, those who can integrate knowledge of diseases and drug therapy with a sophisticated set of tools that have the potential to improve patient care and clinical outcomes will be in high demand. Given the discipline’s breadth and rapid advances in the field, clinical pharmacology training programs must be sufficiently dynamic and innovative to provide necessary foundational knowledge, and support development of both specialized skills and soft skills, to properly prepare tomorrow’s workforce.
Acknowledgments
Funding: Support of training grants from the National Institutes of Health, the National Institute of General Medical Sciences (award numbers T32GM086330 to the University of North Carolina Chapel Hill and Duke University, and T32GM007546 to University of California at San Francisco) and the National Human Genome Research Institute (award number T32 HG008958 to University of Florida) are gratefully acknowledged.
Footnotes
Conflict of Interest: The authors declared no competing interests for this work.
References
- 1.Eichelbaum M. Progress in clinical pharmacology over the last 20 years. Br J Clin Pharmacol. 55(5), 458–459 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson AJ, Huang S-M, Lertora JJL, Markey SP Principles of Clinical Pharmacology, 3rd edn. (Elsevier, London, 2012). [Google Scholar]
- 3.FDA Science and Research Special Topics: Real-World Evidence. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence. Accessed June 20, 2019.