Abstract
The intra-aortic balloon pump (IABP) neither benefits nor harms patients with acute myocardial infarction (AMI) with cardiogenic shock (CS) but may stabilize those with chronic heart failure who decompensate into CS. We sought to compare its hemodynamic effects in these 2 populations. We performed a retrospective analysis of the hemodynamic effects of IABP for AMI or acute decompensated heart failure (ADHF) patients with hemodynamic evidence of CS. The primary outcome was cardiac output (CO) change following insertion. In total, 205 patients were treated for CS resulting from AMI (73;35.6%) or ADHF (132;64.4%). At baseline, both cohorts had significant hemodynamic compromise with mean arterial pressure 75.6±12.3mmHg, CO 3.02±0.84L/min, and cardiac power index 0.26±0.06W/m2; these parameters were nearly identical between groups though ADHF-CS patients had a higher pre-IABP mean pulmonary artery (PA) pressure than AMI-CS patients. After IABP insertion, ADHF-CS patients had moderate CO augmentation while AMI-CS experienced almost no improvement (0.58±0.79L/min vs. 0.12±1.00L/min; p=0.0009). Intra-cardiac filling pressures were reduced by similar amounts in both cohorts. Systemic vascular resistance (SVR) was reduced among patients with ADHF-CS but not among those with AMI-CS. In conclusion, following IABP insertion, ADHF-CS patients experience roughly a 5-fold greater CO augmentation compared to AMI-CS patients. Pre-IABP PA pressure differences and differential SVR reduction may explain these results and shed light on recent evidence supporting IABP use in ADHF-CS and curbing it in AMI-CS.
Keywords: cardiogenic shock, acute myocardial infarction, heart failure, intra-aortic balloon pump, IABP
Introduction
Despite advances in medical and device-based therapies, the prognosis of cardiogenic shock (CS) remains poor.1 Temporary mechanical support devices (MCSDs) are often utilized in CS refractory to medical therapy to stabilize patients sufficiently, then wean from the device or bridge to heart replacement therapy (HRT) such as a durable left ventricular assist device (LVAD) or heart transplantation (HT). Commonly used percutaneous options include the intra-aortic balloon pump (IABP), percutaneous ventricular assist device (pVAD), and extracorporeal membrane oxygenation (ECMO).2 Of these, the IABP is used most commonly due to its widespread availability, ease of insertion, and low complication rate.3 Despite decades of IABP experience, its optimal use is poorly defined. It has been shown to be of neither benefit nor harm for CS following AMI. However, there is new interest in its use to stabilize patients with acutely decompensated heart failure (ADHF) with CS.4-5 We sought to compare the hemodynamic response to IABP insertion in patients with CS from either ADHF or AMI to determine whether this response might differ between different etiologies of CS.
Methods
We retrospectively reviewed medical records of all patients in the cardiac care unit who underwent IABP implantation at our institution from January 2011 to April 2016. We identified patients ≥ 18 years of age with AMI or ADHF and hemodynamic evidence of CS defined as pre-IABP cardiac index (CI) < 2.2 L/min/m2 and either systolic blood pressure (SBP) < 90 mmHg or need for vasoactive medications to achieve this blood pressure. We restricted our population to those with hemodynamic evidence of CS as the study goal was to understand the hemodynamic effects of the IABP. ADHF was defined as an acute presentation of a patient with left ventricular ejection fraction (LVEF) ≤ 40% for ≥ 6 months. AMI patients included those with ST-elevation myocardial infarction and non-ST elevation myocardial infarction. Patients were excluded from analysis if (1) IABP placement occurred after cardiac surgery; (2) support with another MCSD had occurred prior to IABP implantation (e.g. ECMO); or (3) pre-implant hemodynamics were not obtained. The study was approved by the Columbia University Institutional Review Board.
Demographic data for the ADHF-CS and AMI-CS cohorts were collected, including co-morbidities and echocardiographic parameters. For the AMI-CS cohort, angiographic data, culprit vessel, and type of revascularization (e.g. coronary artery bypass graft surgery [CABG] vs. percutaneous coronary intervention [PCI]) were also identified. Hemodynamic data included pulmonary artery (PA) catheter measurements, including cardiac output (CO) and CI by Fick method, cardiac power output/index (CPO/CPI), PA pulsatility index (PAPi). Change in CO, change in CI, and percent change in CO were calculated based on the pre- and post-IABP implantation hemodynamic differences.
The primary outcome was CO change as a marker of hemodynamic response to IABP implantation. Secondary outcomes included other hemodynamic parameters including CPO and CPI, in-hospital mortality, and the need for escalation to another MCSD. Continuous variables are presented as mean ± standard deviation or median with interquartile range and were compared using the Student’s t-test or Wilcoxon’s rank sum test, as appropriate. For outcomes of interest, continuous variables were compared using ANCOVA to control for important covariates. Categorical variables are presented as percentages and were compared using chi-square tests. All analyses were performed using STATA version 15.0 (StataCorp LP, College Station, TX).
Results
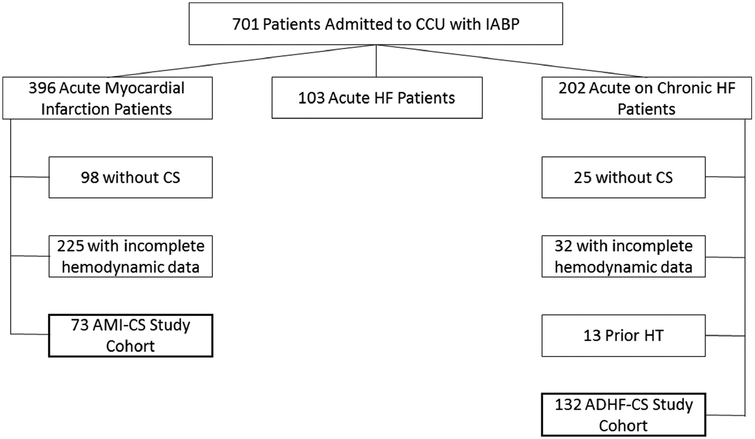
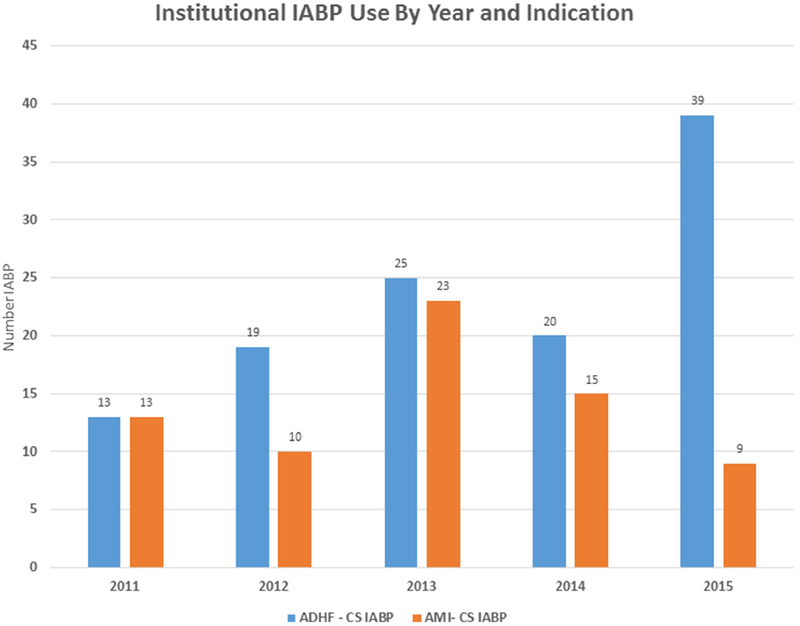
Figure 1 details IABP use at our institution during the study period. Overall, 701 patients underwent IABP implantation from January 2011 to April 2016. Of these, 396 patients presented with AMI and 202 presented with ADHF. Seventy-three AMI patients and 132 ADHF patients met our pre-defined inclusion criteria with hemodynamic evidence of pre-IABP CS. Of those with AMI, 323 patients were excluded for the following reasons: incomplete hemodynamic data (n=225) and pre-implant hemodynamics not consistent with CS (n=98). Of 202 ADHF patients, 70 were excluded for the following reasons: previous HT (n=13), lack of complete pre-implant hemodynamics (n=32), and hemodynamics not consistent with CS (n=25). Our annual institutional utilization of IABP for these two etiologies of CS is represented in Figure 2. IABP use in ADHF-CS rose steadily during the study period while it declined for AMI-CS.
Figure 1:
Institutional Use of IABP. The study cohort was derived from an analysis of all IABP use in the CCU from January, 2011 to April, 2016. Patients with AMI or ADHF and hemodynamic evidence of CS prior to IABP insertion were included. CCU, Cardiac intensive Care Unit; HF, heart failure, CS, cardiogenic shock; AMI, acute myocardial infarction; HT, heart transplant; ADHF, acute decompensated heart failure.
Figure 2:
Institutional IABP Use by Year and Indication. The use of IABP for AMI decreased during the study period while its use for ADHF increased. ADHF, acute decompensated heart failure; CS, cardiogenic shock; AMI, acute myocardial infarction.
The study cohort’s baseline demographics and echocardiographic characteristics are presented in Table 1. There was a significant difference in age, gender, body surface area (BSA), diabetes mellitus (DM) prevalence, left ventricular ejection fraction (LVEF), and left ventricular end-diastolic diameter (LVEDd) between the ADHF-CS and AMI-CS groups. ADHF-CS patients had a higher serum creatinine at baseline but lower serum lactate than AMI-CS patients.
Table 1.
Baseline Demographics for Patients with IABP Placed for Cardiogenic Shock
| All (N=205) | ADHF-CS (N=132) | AMI-CS (N=73) | p-value | |
|---|---|---|---|---|
| Age (years) | 65.0 ± 13.8 | 61.2 ± 13.0 | 71.8 ± 12.7 | <0.0001 |
| Men | 148 (72.2%) | 111 (84.1%) | 37 (50.7%) | <0.001 |
| Body Surface Area (m2) | 1.90 ± 0.24 | 1.93 ± 0.25 | 1.85 ± 0.22 | 0.02 |
| Diabetes Mellitus | 82 (40.0%) | 44 (33.3%) | 38 (52.1%) | 0.009 |
| Left Ventricular | ||||
| Ejection Fraction (percent) | 22.2 ± 11.7 | 18.0 ± 8.9 | 30.2 ± 12.2 | <0.0001 |
| Left Ventricular End | ||||
| Diastolic Diameter (cm) | 6.2 ± 1.5 | 7.0 ± 1.1 | 4.8 ± 0.9 | <0.0001 |
| Serum Creatinine (mg/dL) | 1.83 ± 1.09 | 1.97 ± 1.06 | 1.59 ± 1.11 | 0.02 |
| Serum Lactate (mg/dL) | 3.65 ± 3.58 | 2.54 ± 2.50 | 4.92 ± 4.21 | 0.003 |
ADHF-CS, acute decompensated heart failure – cardiogenic shock; AMI-CS, acute myocardial infarction – cardiogenic shock.
Procedural characteristics for patients who underwent IABP implantation for AMI-CS are presented in Table 2. The left anterior descending (LAD) or left main (LM) coronary arteiy was the culprit vessel in 46.6% of the cases. The burden of coronary arteiy disease was high with an average of 2.2 ± 0.8 coronary vessels narrowed (defined as >50% stenosis of a major epicardial vessel). Twenty-two (30.1%) AMI patients had IABP implanted after revascularization as opposed to prior to it. For 7 patients, revascularization could not be performed (either unsuccessful, deferred, or the patient expired prior to revascularization).
Table 2.
Procedural Characteristics for Acute Myocardial Infarction Patients
| Variable | AMI-CS |
|---|---|
| Access | |
| Femoral | 73 (100%) |
| Axillary | 0 |
| Acute Coronary Syndrome Type | |
| ST Elevation Myocardial Infarction | 40 (54.8%) |
| Non ST Elevation Myocardial Infarction | 33 (45.2%) |
| Timing of IABP | |
| Pre-percutaneous coronary intervention | 51 (69.9%) |
| Post-percutaneous coronary intervention | 22 (30.1%) |
| Culprit Coronary Artery | |
| Right | 9 (12.3%) |
| Left Circumflex | 5 (6.9%) |
| Left Main or Left Anterior Descending | 34 (46.6%) |
| Multiple Coronary Arteries | 25 (34.3%) |
| Number Coronary Arteries Narrowed | 2.2 ± 0.8 |
| Revascularization | |
| Percutaneous Coronary Intervention | 59 (80.8%) |
| Coronary Artery Bypass Graft Surgery | 7 (9.6%) |
| Revascularization not performed | 7 (9.6%) |
AMI, acute myocardial infarction; CS, cardiogenic shock.
Baseline hemodynamic characteristics are displayed in Table 3. The overall cohort manifested significant hemodynamic compromise with a mean arterial blood pressure (MAP) of 75.6 ± 12.3mmHg on 1.6 ± 1.0 vasopressors/inotropes with a CI of 1.58 ± 0.39L/min/m2 and a CPI of 0.26 ± 0.07W/m2. The MAP was nearly identical between those with AMI and ADHF though the latter had a lower pulse pressure. The CO and CPI were also nearly identical for the 2 cohorts. The baseline stroke volume (SV) was 33.9 ± 11.9ml in ADHF-CS patients and 35.4 ± 12.4ml in AMI-CS patients (p=0.40). Systemic vascular resistance (SVR) was similar between the 2 cohorts (1680.2 ± 541.4 dyn·s·cm−5 for ADHF-CS patients vs. 1745.1 ± 821.8 dyn·s·cm−5 for AMI-CS patients, p = 0.52). However, pulmonary artery pressures and PAPi were higher in the ADHF cohort.
Table 3.
Baseline Hemodynamic Data for Patients with IABP Placed for Cardiogenic Shock
| All (N=205) | ADHF-CS (N=132) | AMI-CS (N=73) | p-value | |
|---|---|---|---|---|
| Systolic Blood Pressure (mmHg) | 101.8 ± 18.2 | 98.4 ± 14.6 | 108.0 ± 22.2 | 0.0003 |
| Diastolic Blood Pressure (mmHg) | 62.6 ± 12.7 | 64.2 ± 10.9 | 59.7 ± 15.2 | 0.02 |
| Mean Arterial Pressure (mmHg) | 75.6 ± 12.3 | 75.6 ± 10.6 | 75.7 ± 14.8 | 0.97 |
| Cardiac Output (L/min) | 3.02 ± 0.84 | 3.01 ± 0.78 | 3.02 ± 0.93 | 0.91 |
| Cardiac Index (L/min/m2) | 1.58 ± 0.39 | 1.56 ± 0.36 | 1.62 ± 0.44 | 0.33 |
| Cardiac Power Output (W) | 0.50 ± 0.16 | 0.50 ± 0.14 | 0.51 ± 0.18 | 0.87 |
| Cardiac Power Index (W/m2) | 0.26 ± 0.06 | 0.26 ± 0.06 | 0.27 ± 0.09 | 0.32 |
| Central Venous Pressure (mmHg) | 14.3 ± 6.7 | 14.6 ± 7.2 | 13.6 ± 5.6 | 0.32 |
| Pulmonary Artery systolic pressure (mmHg) | 52.4 ± 14.5 | 56.6 ± 14.0 | 44.9 ± 12.3 | <0.0001 |
| Pulmonary Artery diastolic pressure (mmHg) | 26.6 ± 8.1 | 28.2 ± 8.1 | 23.7 ± 7.3 | 0.0001 |
| Mean Pulmonary Artery pressure (mmHg) | 35.3 ± 9.6 | 37.9 ± 9.3 | 30.7 ± 8.2 | <0.0001 |
| Pulmonary Artery Pulsatility Index | 2.59 ± 2.91 | 2.91 ± 3.35 | 2.00 ± 1.69 | 0.04 |
| Systemic Vascular Resistance (dyn·s·cm−5) | 1702.3 ± 649.0 | 1680.2 ± 541.4 | 1745.1 ± 821.8 | 0.52 |
| Vasoactive agents (number) | 1.6 ± 1.0 | 1.7 ± 1.0 | 1.4 ± 0.8 | 0.048 |
ADHF, acute decompensated heart failure; CS, cardiogenic shock; AMI, acute myocardial infarction.
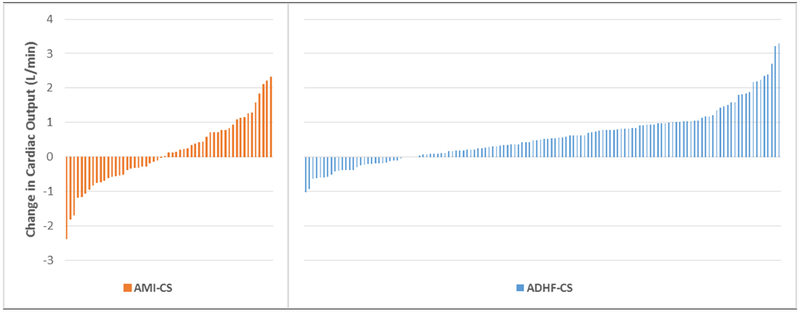
Figure 3 displays the CO change with IABP for each patient and the overall hemodynamic changes observed with IABP insertion are displayed in Table 4. The median duration of IABP support between pre- and post-insertion measurements was 5.0 hours (IQR:3.5 – 9.0). The mean number of vasoactive infusions administered during post-insertion measurements was 1.5 ± 1.1; 27 (14.2%) patients had an increase in the number of inotropic or vasopressor medications between the pre- and post-implant measurements while 115 (60.5%) had no change and 48 (25.3%) had a reduction in these medications. No patients received vasodilator therapy during the peri-implantation period.
Figure 3:
Cardiac Output Change Following IABP insertion. The cardiac output change is displayed for each patient with AMI or ADHF. AMI, acute myocardial infarction; CS, cardiogenic shock; ADHF, acute decompensated heart failure.
Table 4.
Hemodynamic Changes Observed with IABP Insertion
| All (N=205) | ADHF-CS (N=132) | AMI-CS (N=73) | p-value | |
|---|---|---|---|---|
| Mean Arterial Pressure (mmHg) | −0.5 ± 15.0 | −1.4 ± 13.9 | 1.3 ± 17.1 | 0.26 |
| Change in Cardiac Output (L/min) | 0.44 ± 0.88 | 0.58 ± 0.79 | 0.12 ± 1.00 | 0.0009 |
| Change in Cardiac Index (L/min/m2) | 0.24 ± 0.46 | 0.30 ± 0.42 | 0.08 ± 0.51 | 0.003 |
| Cardiac Output Percent change (%) | 20.0 ± 36.1 | 23.9 ± 35.2 | 10.1 ± 36.6 | 0.02 |
| Cardiac Power Output (W) | 0.07 ± 0.18 | 0.09 ± 0.17 | 0.03 ± 0.20 | 0.06 |
| Cardiac Power Index (W/m2) | 0.04 ± 0.10 | 0.05 ± 0.0.9 | 0.02 ± 0.11 | 0.09 |
| Central Venous Pressure (mmHg) | −2.1 ± 5.6 | −2.0 ± 5.2 | −2.5 ± 6.6 | 0.59 |
| Mean Pulmonary Artery pressure (mmHg) | −5.2 ± 7.5 | −5.3 ± 7.4 | −5.0 ± 7.6 | 0.78 |
| Systemic Vascular Resistance (dyn·s·cm−5) | −173.3 ± 625.5 | −253.1 ± 493.0 | 21.3 ± 843.0 | 0.01 |
| Vasoactive agents (number) | −0.1 ± 0.9 | −0.1 ± 0.8 | −0.2 ± 1.0 | 0.30 |
ADHF, acute decompensated heart failure; CS, cardiogenic shock; AMI, acute myocardial infarction.
The mean CO change for the entire cohort was 0.44 ± 0.88L/min. However, there was a significant difference in the mean augmentation between the ADHF-CS and AMI-CS cohorts with the former experiencing almost a 5-fold greater CO augmentation compared to the latter (0.58 ± 0.79 L/min vs. 0.12 ± 1.00 L/min, p=0.0009). This amounted to only a 10% CO increase for AMI-CS patients while those with ADHF-CS experienced an increase by almost a quarter of their baseline CO. Among those in the ADHF cohort, there was a trend towards a greater CO increase with IABP insertion in patients with a non-ischemic dilated cardiomyopathy as opposed to an ischemic dilated cardiomyopathy (0.68 ± 0.72 L/min vs. 0.40 ± 0.89 L/min, p = 0.052). We examined univariable predictors of CO change with IABP insertion and included these in an ANCOVA model to control for potential baseline differences in the cohorts. After controlling for age, gender, BSA, DM prevalence, vasopressor/inotropic medications, baseline CO, mean PA pressure (mPAP), LVEDd, and SV, the underlying etiology of CS (i.e. AMI vs. ADHF) remained a significant predictor of IABP hemodynamic response (p = 0.03).
In addition to CO augmentation, we examined PA pressure reduction with IABP insertion and found similar mPAP reduction in the cohorts with IABP therapy. There was negligible change in MAP overall and no difference in MAP change between the cohorts. Lastly, the systemic vascular resistance (SVR) difference between the pre- and post-implant hemodynamic evaluation was −253.1 ± 493.0 dyn·s·cm−5 among ADHF-CS patients and 21.3 ± 843.0 dyn·s·cm−5 for AMI-CS patients (p=0.01).
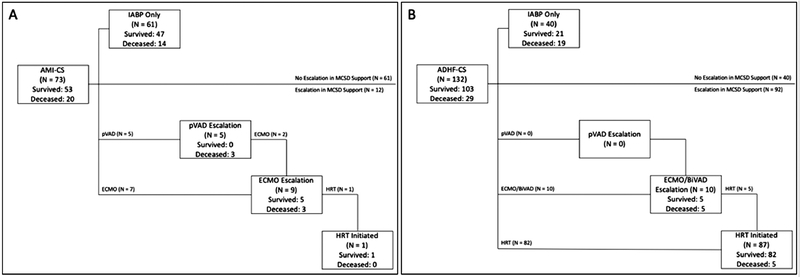
Figure 4 displays the clinical outcomes of study patients. The median duration of IABP support was 3.0 days (IQR: 2.0 – 5.0). In the AMI-CS cohort, 53 (72.6%) patients survived to discharge including 1 who required durable LVAD implantation. Of 73 patients with AMI-CS, 47 (64.4%) patients survived to discharge with only IABP for mechanical circulatory support while 14 (19.2%) died without escalation to another MSCD. Twelve (16.4%) patients had another MCSD implanted after IABP. Of these, 5 received a pVAD and 7 received VA-ECMO and of those initially receiving pVAD, 2 patients also had VA-ECMO implanted due to persistent CS. Of those requiring escalation to either of these devices, 6 (50.0%) died. Lastly, the median time on IABP support was 2.0 days (IQR: 2.0 – 4.0).
Figure 4.
Patient Outcomes Following IABP Insertion for Cardiogenic Shock. Patient outcomes are displayed following A) AMI with CS and B) ADHF with CS according to the MCSDs used. AMI, acute myocardial infarction; CS, cardiogenic shock; MCSD, mechanical circulatory support device; pVAD, percutaneous ventricular assist device; ECMO, extracorporeal membrane oxygenation; HRT, heart replacement therapy BiVAD, biventricular assist device (short-term, surgically implanted).
In contrast to those with AMI-CS, the vast majority of ADHF-CS patients underwent HRT during the index admission. While a similar number of patients (10, 7.6%) required escalation to another more potent short-term circulatory support device, 87 patients ultimately underwent HRT initiation (11 HT and 76 durable LVAD). Overall, 103 (78.0%) ADHF-CS survived to discharge with or without HRT. The median time on IABP support for those with ADHF-CS was 4.0 days (IQR: 2.0 – 6.0).
Discussion
In this study, we performed a comprehensive examination of the hemodynamic response to IABP implantation in patients presenting with AMI-CS and ADHF-CS. Our principal finding is that AMI-CS patients had minimal CO augmentation in response to IABP insertion, while those with ADHF-CS had moderate CO augmentation which was roughly a 5-fold increase compared to that seen with AMI-CS. To our knowledge, this is the first comparison of the hemodynamic response to aortic counterpulsation between 2 commonly supported patient phenotypes.
IABPs have been implanted for CS for >40 years. The earliest studies contained a heterogeneous population and demonstrated improvements in CO of roughly 0.5L/min in CS patients.6-7 We observed a similar CO change in our cohort but noted a large discrepancy between patients with different CS etiologies. Recent evidence has highlighted the importance of CS etiology;8-9 the CardShock registry demonstrated a worse prognosis in AMI-CS compared to other CS etiologies. Thus, it should not be surprising that the response to IABP may differ by etiology as well. While the hemodynamics are similar in each cohort, the differential IABP response suggests that these phenotypes are quite different.
Our findings are consistent with prior studies demonstrating negligible CO augmentation with IABP in AMI-CS.10-12 Together with these data, ours suggests that in AMI-CS, IABP does little to improve hemodynamics. In this way, our study sheds light on the previous studies that demonstrated lack of clinical efficacy of IABP in AMI-CS. Notably the IABP-SHOCK II trial demonstrated no reduction in 30-day mortality with IABP for AMI-CS.13 Hemodynamic measurements were not required for trial inclusion but our findings may explain why no benefit was observed. This finding is especially noteworthy in light of data highlighting the importance of CO and its derivatives in predicting outcomes in CS.14-15
Despite the lack of benefit for AMI-CS patients, there has been growing interest in IABP use for ADHF-CS patients. This has been fueled by the observation that many such patients can be stabilized by IABP with favorable clinical outcomes, particularly when bridged to HRT.4-5 While the mean CO augmentation in ADHF-CS patients was slightly >0.5L/min, this may be sufficient improvement to stabilize a chronic heart failure patient with a relatively low CO at baseline. We have previously demonstrated that ADHF-CS patients were stabilized with IABP at low complication rates and characterized robust hemodynamic response in this cohort.4 Sintek et al. showed a >50% stabilization rate with IABP in patients with chronic heart failure bridged to LVAD.5 Furthermore, new counterpulsation devices implanted in the subclavian artery have led to successful (and even long-term) bridging to HRT, recovery, and improvement in biventricular function.16-19
While our data suggest that different CS etiologies may have significantly different responses to IABP, the reason for this is not obvious. One explanation historically proposed is the need for intrinsic pulsatility for effective IABP support. However, the SV was almost identical between the 2 cohorts despite a difference in arterial pulse pressure. SVR was also comparable between the 2 populations, though ADHF patients experienced a reduction in SVR while AMI patients did not. The differential in response may be related to differential PA pressures and SVR reduction. Not surprisingly, those with ADHF-CS had significantly higher mPAP than those with AMI-CS. By reducing afterload, the IABP may allow for more forward flow in the setting of higher filling pressures. This would fit with our prior observation that the strongest predictor of IABP “super-response” (i.e. robust CO augmentation) was an elevated mPAP.4 Another possible explanation is that those with greater right ventricular (RV) contractility, manifested by a higher PAPi which we observed in the ADHF-CS cohort at baseline, are primed to have greater response to IABP which does not directly support the RV. Thus, PAPi might be a good marker for patients with an expected favorable IABP hemodynamic response, as we have previously demonstrated.4
The outcomes of our AMI-CS patient cohort reflect that of a population with severe hemodynamic compromise including a CPI comparable to that observed in the SHOCK trial and registry and a significantly elevated serum lactate.14 Importantly, a subset of patients underwent escalation to another MCSD and had a worse prognosis (as expected) than the overall AMI-CS cohort. While ADHF-CS patients appear to derive greater hemodynamic support from the IABP than those with AMI-CS, the IABP alone is unlikely to stabilize ADHF-CS patients with more advanced forms of CS. The most hemodynamically unstable ADHF-CS patients typically require more powerful MCSDs.20 However, the utilization of IABP for CS at our institution has shifted in response to the growing literature supporting use of IABP for ADHF-CS and the data supporting lack of benefit for AMI-CS patients. Our data provide hemodynamic evidence supporting these changes which mirror national trends with a decline in IABP use for AMI-CS and a rise in use of other MCSDs for the same indication.21
Our study has several significant limitations. First, our data represent a singlecenter experience and are retrospective in nature. Importantly, there were patients treated with IABP during the study lacking pre-implantation hemodynamic data who were excluded from analysis. Though this resulted in a selected patient population, our goal was to understand hemodynamic effects of this device in CS and therefore we restricted our inclusion criteria to those with hemodynamic evidence of this state. Decisions regarding escalation to other MCSDs reflect physician practice and were not protocolized for a portion of the study period. Detailed information regarding which vasopressors and inotropes were used for the entire cohort were not available to further understand the differential effects of the device on the SVR. Lastly, the timing of pre- and post-implantation hemodynamics were not uniform, nor were vasoactive medications held constant in all cases, limiting our ability to attribute all hemodynamic changes to the IABP. However, the majority of patients had either no change or a reduction in the number of vasoactive medications administered between the hemodynamic timepoints.
In conclusion, patients presenting with AMI-CS have minimal hemodynamic improvement with IABP insertion while those with ADHF-CS have a more robust improvement with this intervention. The reasons for this discrepancy remain unclear but may relate to differences in pre-implant PA pressures and differential SVR reduction in these CS phenotypes. These findings highlight the fact that although CS may be present in each, there are significant differences in treatment response among different CS etiologies. Furthermore, they shed light on the lack of benefit with IABP for AMI and the mounting evidence that IABP may stabilize many ADHF patients who deteriorate into CS.
Acknowledgments
Disclosures:
Dr. Garan is supported by National Institutes of Health Grant Nos. KL2TR001874 and UL1TR001873, has received honoraria from Abiomed, and is currently an unpaid advisor for Abiomed. Dr. Kirtane reports institutional grant support from Abbott Vascular, Medtronic, Boston Scientific, Abiomed, CSI, Siemens, Philips, and ReCor Medical. None of the listed entities has had any involvement with the development of this manuscript. All other authors have no relationships relevant to the contents of this paper to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade-long trends (2001-2011) in the Incidence and Hospital Death Rates Associated with the In-Hospital Development of Cardiogenic Shock after Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes 2016;9:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014; 35:156–167. [DOI] [PubMed] [Google Scholar]
- 3.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014;64:407–415. [DOI] [PubMed] [Google Scholar]
- 4.Fried JA, Nair A, Takeda K, Clerkin K, Topkara VK, Masoumi A, Yuzefpolskaya M, Takayama H, Naka Y, Burkhoff D, Kirtane A, Karmpaliotis D, Moses J, Colombo PC, Garan AR. Clinical and Hemodynamic Effects of Intra-Aortic Balloon Pump Therapy in Chronic Heart Failure Patients with Cardiogenic Shock. J Heart Lung Transplant 2018;37:1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sintek MA, Gdowski M, Lindman BR, Nassif M, Lavine KJ, Novak E, Bach RG, Silvestry SC, Mann DL, Joseph SM. Intra-aortic balloon counterpulsation in patients with chronic heart failure and cardiogenic shock: Clinical Response and Predictors of Stabilization. J Card Fail 2015;21:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheidt S, Wilner G, Mueller H, Summers D, Lesch M, Wolff G, Krakauer J, Rubenfire M, Fleming P, Noon G, Oldham N, Killip T, Kantrowitz A. Intra-aortic balloon counterpulsation in cardiogenic shock. Report of a co-operative clinical trial. N Engl J Med 1973;288:979–984. [DOI] [PubMed] [Google Scholar]
- 7.Dunkman WB, Leinbach RC, Buckley MJ, Mundth ED, Kantrowitz AR, Austen WG, Sanders CA. Clinical and hemodynamic results of intraaortic balloon pumping and surgery for cardiogenic shock. Circulation 1972;46:465–477. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246–2256. [DOI] [PubMed] [Google Scholar]
- 9.Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva-Cardoso J, Carubelli V, Di Somma S, Tolppanen H, Zeymer U, Thiele H, Nieminen MS, Mebazaa A; CardShock Study Investigators; GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 2015;17:501–509. [DOI] [PubMed] [Google Scholar]
- 10.Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, Schuler G. Randomized comparison of intra-aortic balloon support versus a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26:1276–1283. [DOI] [PubMed] [Google Scholar]
- 11.Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra - aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584–1588. [DOI] [PubMed] [Google Scholar]
- 12.Prondzinsky R, Unverzagt S, Russ M, Lemm H, Swyter M, Wegener N, Buerke U, Raaz U, Ebelt H, Schlitt A, Heinroth K, Haerting J, Werdan K, Buerke M. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP shock trial. Shock 2012;37:378–384. [DOI] [PubMed] [Google Scholar]
- 13.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K; IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012:367:1287–1296. [DOI] [PubMed] [Google Scholar]
- 14.Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, Lejemtel TH, Cotter G; SHOCK Investigators. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004;44:340–348. [DOI] [PubMed] [Google Scholar]
- 15.Garan AR, Eckhardt C, Takeda K, Topkara VK, Clerkin K, Fried J, Masoumi A, Demmer RT, Trinh P, Yuzefpolskaya M, Naka Y, Burkhoff D, Kirtane A, Colombo PC, Takayama H. Predictors of survival and ability to wean from short-term mechanical circulatory support device following acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2018;7:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka A, Tuladhar SM, Onsager D, Asfaw Z, Ota T, Juricek C, Lahart M, Lonchyna VA, Kim G, Fedson S, Sayer G, Uriel N, Jeevanandam V. The subclavian intraaortic balloon pump: A compelling bridge device for advanced heart failure. Ann Thorac Surg 2015;100:2151–2157. [DOI] [PubMed] [Google Scholar]
- 17.Jeevanandam V, Song T, Onsager D, Ota T, LaBuhn CJ, Lammy T, Sayer G, Kim G, Patel-Raman S, Uriel N. The first-in-human experience with a minimally invasive, ambulatory, counterpulsation heart assist system for advanced congestive heart failure. J Heart Lung Transplant 2018;37:1–6. [DOI] [PubMed] [Google Scholar]
- 18.Imamura T, Juricek C, Song T, Ota T, Onsager D, Sarswat N, Kim G, Raikhelkar J, Kalantari S, Sayer G, Burkhoff D, Jeevanandam V, Uriel N. Improvement in biventricular cardiac function following ambulatory counterpulsation. J Card Fail 2019;25:20–26. [DOI] [PubMed] [Google Scholar]
- 19.Estep JD, Cordero-Reyes AM, Bhimaraj A, Trachtenberg B, Khalil N, Loebe M, Bruckner B, Orrego CM, Bismuth J, Kleiman NS, Torre-Amione G. Percutaneous placement of an intraaortic balloon pump in the left axillary/subclavian position provides safe, ambulatory longterm support as bridge to heart transplantation. JACC Heart Fail 2013;1:382–388. [DOI] [PubMed] [Google Scholar]
- 20.Garan AR, Malick W, Habal M, Topkara VK, Fried J, Masoumi A, Hasan AK, Karmpaliotis D, Kirtane A, Yuzefpolskaya M, Farr M, Naka Y, Burkhoff D, Colombo PC, Kurlansky P, Takayama H, Takeda K. Predictors of Survival for Patients with Acute Decompensated Heart Failure Requiring Extra-Corporeal Membrane Oxygenation Therapy. ASAIO J 2018. doi: 10.1097/MAT.0000000000000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah M, Patnaik S, Patel B, Ram P, Garg L, Agarwal M, Agrawal S, Arora S, Patel N, Wald J, Jorde UP. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol 2018;107:287–303. [DOI] [PubMed] [Google Scholar]