Abstract
In foregut-fermenting mammals (e.g., colobine monkeys, artiodactyl ruminants) the enzymes pancreatic ribonuclease (RNASE1) and lysozyme C (LYZ), originally involved in immune defense, have evolved new digestive functions. Howler monkeys are folivorous non-colobine primates that lack the multi-chambered stomachs of colobines and instead digest leaves using fermentation in the caeco-colic region. We present data on the RNASE1 and LYZ genes of four species of howler monkey (Alouatta spp.). We find that howler monkey LYZ is conserved and does not share the substitutions found in colobine and cow sequences, whereas RNASE1 was duplicated in the common ancestor of A. palliata, A. seniculus, A. sara, and A. pigra. While the parent gene (RNASE1) is conserved, the daughter gene (RNASE1B) has multiple amino acid substitutions that are parallel to those found in RNASE1B genes of colobines. The duplicated RNase in Alouatta has biochemical changes similar to those in colobines, suggesting a novel, possibly digestive function. These findings suggest that pancreatic ribonuclease has, in parallel, evolved a new role for digesting the products of microbial fermentation in both foregut- and hindgut-fermenting folivorous primates. This may be a vital digestive enzyme adaptation allowing howler monkeys to survive on leaves during periods of low fruit availability.
Subject terms: Biological anthropology, Evolutionary genetics, Molecular ecology, Evolutionary ecology
Introduction
Howler monkeys (Alouatta spp.) are some of the few New World monkeys with a diet rich in leaves. While the amount of leaves consumed varies greatly between sites and populations1, as well as across seasons2, on average young and mature leaves make up half or more of the howler monkey diet3–7. Howler monkeys manage to persist on such a diet without the sacculated, foregut-fermenting stomachs found in folivorous Old World monkeys, the colobines. However, howler monkeys have a suite of other adaptations for a folivorous diet.
Adaptations for a leaf-rich diet include behavioral strategies, such as minimizing energy expenditure2,8,9 and feeding preferentially on young leaves6,10,11 or leaves with a higher protein to fiber ratio5, which are less tough and may be easier to digest12. Howler monkeys are unique among platyrrhines in having routine trichromacy, a trait that has been proposed as an adaptation for detecting such young leaves13,14. Gut and digestive adaptations include very long gut transit times of approx. 20 hours15,16 and an enlarged caecum and colon17.
These gut adaptations are important for folivorous mammals because the cell walls of plants are made of structural carbohydrates, cellulose and hemicellulose, that cannot be digested by the enzymes produced by vertebrates18. Instead, leaf-eating mammals rely on microbial fermentation to break down plant material in the forestomach (foregut fermenters, like colobines) or in the large intestine (“hindgut” or caeco-colic fermenters)18–20. The enlarged caecum and colon in howlers are important sites for microbial fermentation and are also enlarged in other folivorous or herbivorous caeco-colic fermenters, such as horses and elephants21. During microbial fermentation, the plant material is broken down by symbiotic bacteria found in the host’s gut. This process releases volatile fatty acids, which are easily absorbed22 but the bacteria themselves are also digested by the host and are an important source of nitrogen22,23. Both ruminant artiodactyls and colobine monkeys have convergent digestive enzyme adaptations for the digestion of fermenting gut bacteria, but we do not know whether howler monkeys share any of these adaptations. Endogenous digestive enzymes are a crucial component of an animal’s digestive system and include important dietary adaptations24, such as the enzymes lysozyme and pancreatic ribonuclease.
RNase and Lysozyme Evolution in Primates and Ruminants
The enzyme lysozyme is found in many vertebrates and invertebrates where it has an immunological function25. In cows and colobines this enzyme exhibits parallel amino acid changes that allow lysozyme to function at a much lower pH, an adaptation for bacteriolytic activity in the acidic stomach fluid26,27. Similarly, the pancreatic ribonuclease enzyme (RNase1), which has an original function in pathogen defense28, has acquired a new digestive function in both colobines and ruminant artiodactyls23,29–31. In this case, the RNASE1 gene underwent one or more duplications, and the duplicated gene(s) (RNASE1B and RNASE1C, or alternatively RNASE1β and RNASE1γ) evolved a new function in digesting the nucleic acids of fermenting microbes found in the digestive system. These duplications and subsequent functional changes evolved independently in artiodactyl ruminants and colobines and, indeed, via different amino acid substitutions30. They may have also evolved separately in African and Asian colobines31,32, although this has been questioned by some studies33,34 and the evolutionary history of RNASE1 duplications in colobines has not yet been completely resolved.
Significantly, the duplicated RNases of both ruminants and colobines have a set of different, taxon-specific amino acid changes leading to convergent changes of the isoelectric point (pI), optimal pH, and charge of the resulting protein. The digestive RNases (RNase1B and RNase1C in colobines, pancreatic RNase1 in bovines) exhibit a lower pI, a lower optimal pH, and a decreased charge at pH 7.030,31 compared to the ancestral RNase1. Previous research has shown that the charge of pancreatic ribonuclease affects the enzyme’s ability to degrade double-stranded RNA35. A high pI and positive charge is indicative of a ribonuclease that is involved in defense against pathogens, while a decrease in pI and charge suggest a novel function for the enzyme36,37, as shown in the case of cows and colobines30.
RNase and Lysozyme Evolution in Other Mammals
Duplications of the RNASE1 gene have also been found in other mammal groups. In rodents, both rats (Rattus spp.) and guinea pigs (Cavia spp.) independently evolved two duplicated genes, RNASE1B and RNASE1C, from the ancestral RNASE1 gene37–39. Whether these duplicated genes remain involved in immune function or have acquired novel, possibly digestive, functions has not been determined. Lang and colleagues (2017) suggest that the high pI (9.66) of rat RNase1B and its expression in the spleen point to retention of the original immunological function, while rat RNase1C (pI = 7.71) may have acquired a novel function39. Similar to cows and colobines, the duplicated ribonuclease in guinea pigs has a lower pI and decreased charge and is less effective at degrading double-stranded RNA than the ancestral enzyme, suggesting a novel and possibly digestive function35,40. Like howler monkeys, guinea pigs are herbivorous animals with caeco-colic rather than foregut fermentation. This suggests that ribonuclease could have a function in the digestion of microbial fermentation products, regardless of the presence of a foregut.
RNASE1 also underwent multiple duplications independently in two families of bats, the Vespertilionidae and Molossidae, but the duplicate proteins have high isoelectric points suggesting an immunological rather than dietary function41. Seven RNASE1 genes were found in Myotis lucifugus, an insectivorous Vesper bat37. The authors propose that this may be an immunological adaptation, as the communal roosting behavior of these bats potentially increases their exposure to pathogens and RNase1 may improve their resilience to them37. In the superfamily Musteloidea, a group that includes red pandas, weasels, raccoons, and skunks, RNASE1 was duplicated independently in four families but the functional significance of these duplicates is not yet clear36. As summarized here, RNASE1 genes have an interesting history of duplications and functional diversification in mammals, including for novel dietary functions in herbivorous foregut and caeco-colic fermenters.
Present Study
The RNASE1 gene(s) of folivorous foregut-fermenting primates (colobines) and many non-folivorous primates are now well characterized31–33, but it is not clear whether folivorous caeco-colic fermenting primates, such as howler monkeys, share the digestive enzyme adaptations of colobines. In this study, we therefore investigated both the RNASE1 and LYZ genes in four howler monkey species – the mantled howler (Alouatta palliata), the Venezuelan red howler (A. seniculus), Guatemalan black howler (A. pigra), and the Bolivian red howler (A. sara) – a group of leaf-eating primates with caeco-colic fermentation. We used a 10X Chromium draft genome assembly for Alouatta palliata and conducted amplicon sequencing of the RNASE1 gene for all four howler monkey species. We hypothesize that howler monkey RNase1 and lysozyme exhibit similar changes in charge and isoelectric point as the proteins in colobines, as adaptations for a folivorous diet. To find the predicted shared amino acid changes, we assembled a comparative dataset of RNASE1 and LYZ gene sequences across primates and translated and aligned the coding sequences. To better understand the evolutionary history and selective pressures acting on RNASE1, we tested for positive/purifying selection and reconstructed the inferred ancestral gene sequences. Finally, the pI and charge at pH 7.0 were calculated for both extant and inferred ancestral sequences, in order to identify the predicted shared biochemical properties of the proteins in different species.
Results
Genome mining and sequencing
Our Alouatta palliata draft genome assembly is 2.51 Gb with a contig N50 of 58.2 kb, a scaffold N50 of 3.47 Mb, and an effective read depth of 38X (Burrell et al., in prep; Janiak et al., in prep). From these reads, we were able to identify sequences in the Alouatta palliata draft genome putatively orthologous to the query LYZ and RNASE1 gene sequences.
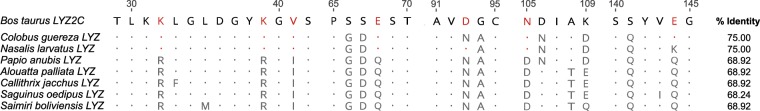
The LYZ nucleotide sequence of A. palliata was 96.42% and 96.20% identical to that of Saimiri boliviensis and Callithrix jacchus, respectively. The LYZ amino acid sequence was also very similar to those of other platyrrhines, being 91.22–91.89% identical. A protein alignment of the lysozyme C sequences from A. palliata, S. boliviensis, C. jacchus, Saguinus oedipus, Papio anubis, Colobus guereza, Nasalis larvatus, and the cow (Bos taurus) is shown in Fig. 1. While colobines and cows have a number of parallel amino acid changes, these substitutions are not found in howler monkey lysozyme C (Fig. 1). Pairwise distances of lysozyme C amino acid sequences are shown in Supplemental Table S3. As found in previous studies26, the two colobines (C. guereza and N. larvatus) have overall greater sequence similarity with the cow (111/148 amino acids, 75%) than another closely related catarrhine, Papio anubis, has with the cow (102/148 amino acids, 68.92%). The howler monkey, on the other hand, does not share the parallel changes found in the lysozyme sequences of colobines and cows and its sequence identity with these groups is comparable to those of other platyrrhines (Fig. 1, Suppl. Table S3). Like the other platyrrhines, the howler monkey shares 124–125/148 amino acids (83.78–84.46%) with the LYZ sequence of colobines and 102/148 amino acids (68.92%) with the LYZ sequence in cows.
Figure 1.
Primate LYZ protein sequences aligned to cow (Bos taurus) reference sequence. Parallel amino acid changes between cow and colobines are highlighted. Percent identity to the cow reference sequence is indicated on the right.
The RNASE1 BLASTN search of the howler monkey Supernova 10X genome pseudohap assembly initially returned no hits that matched the Callithrix jacchus RNASE1 query. Because RNASE1 is conserved in all other primates, it is implausible that this gene was lost in Alouatta. We, therefore, repeated our BLASTN search on the raw Supernova assembly output which contains every edge in the assembly and does not flatten bubbles42. The BLASTN search of the raw Supernova assembly output produced a total of twelve significant alignments ranging in similarity from 92–98%. However, none of the hits covered the entire query sequence and parts of the query produced multiple non-identical hits.
Amplicon sequencing
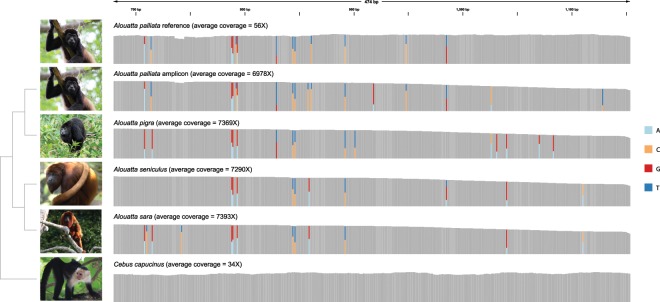
Sequencing of the RNASE1 amplicons on an Illumina MiSeq produced an average of 1,812,320 reads per sample (SD = 100,204 reads), of which an average of 65.86% mapped to the reference (range = 44.07–83.42%) (Suppl. Fig. S7). Average coverage of the RNASE1 coding region ranged from 6978–7393X (Fig. 2) for reads generated from amplicon sequencing. The average coverage of the RNASE1 coding region for the reads generated for the A. palliata genome assembly was 56X, while reads from whole genome sequencing of Cebus capucinus imitator had an average coverage of 34X (Fig. 2).
Figure 2.
Coverage of reads (duplicates removed) mapped against RNASE1 coding region. Variable sites are colored by proportion of reads with each base at that site. Cebus capucinus imitator is a non-folivorous platyrrhine that was included as a control. Reads mapping to RNASE1 in C. capucinus do not indicate the presence of variable sites. Images by Simon Pierre Barrette (Alouatta palliata), Dave Johnson (A. pigra), Alessandro Catenazzi (A. seniculus), Raul Ignacio (A. sara), and Steven G. Johnson (Cebus capucinus), all via Wikimedia Commons.
Assembly of RNASE1
Read mapping, followed by variant calling revealed 10–19 variable sites above a PHRED-scaled quality threshold of 20 in the coding region of RNASE1 (Suppl. Table S4). Six of these variants were shared between all four species. Haplotype calling and assembly of the RNASE1 coding region yielded three distinct haplotypes for each of the Alouatta species and a single haplotype for Cebus capucinus imitator (Fig. 2). The two most similar haplotypes were 98.28–99.57% identical, while the two most divergent haplotypes were 94.18–97.20% identical in each species (Suppl. Table S5).To exclude the possibility that these sequences were of another, closely related gene, we conducted BLAST searches with all haplotype sequences as queries against publicly-available platyrrhine genomes (n = 4). All searches only returned hits to RNASE1 and no other platyrrhine genomes investigated here showed evidence of a second RNASE1-like gene. Therefore, it is most likely that the additional RNASE1-like sequences found in the howler monkey genome represent a duplication of the ancestral RNASE1 gene. The observed read depths and haplotype frequencies (Suppl. Table S4) suggest that Alouatta species have two RNASE1 genes and that the third haplotype represents small polymorphisms within one or both of these genes. We refer to these duplicated sequences as RNASE1B.
Sequence analyses
Primate RNASE1 genes generally appear to be conserved and lack premature stop codons in all species included here (n = 29). The overall amino acid sequence divergence across RNASE1 in these species was low (mean pairwise identity = 88.63%, SD = 4.78%). When including the duplicated RNASE1 genes found in colobines and Alouatta, overall divergence only increased slightly (mean pairwise identity = 87.20%, SD = 4.86%).
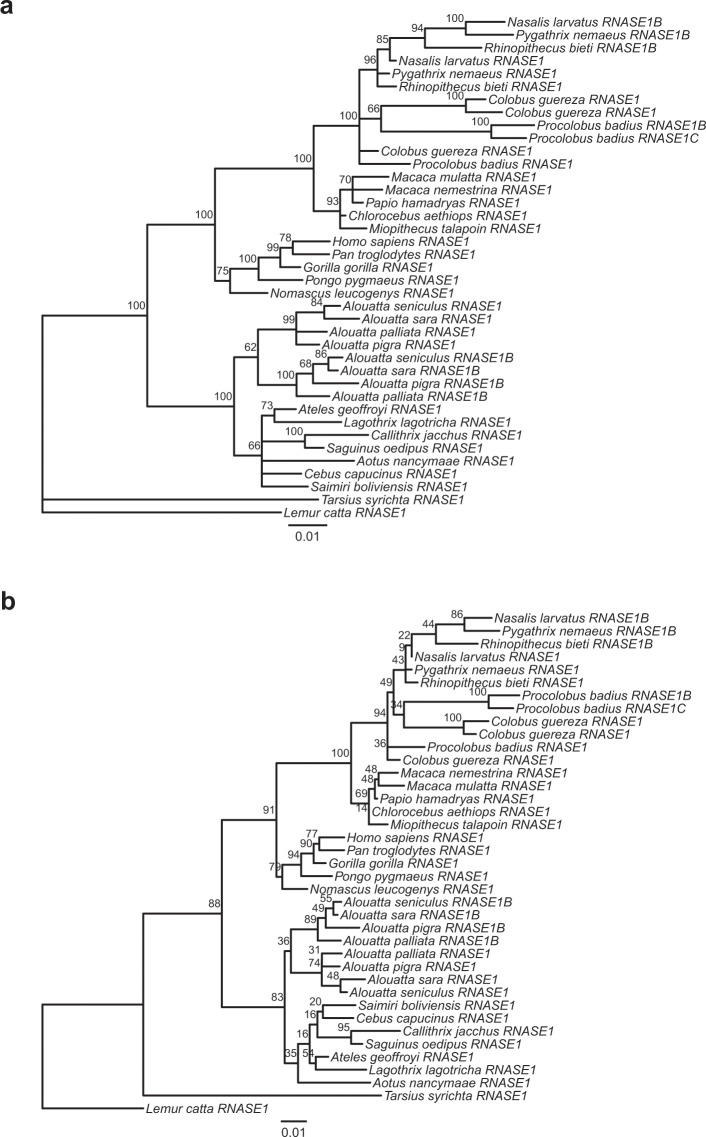
Trees built from the coding region of RNASE1 failed to accurately resolve the phylogenetic relationships of all primate species we examined (Fig. 3). Different programs (MrBayes, PHYML) and approaches (neighbor-joining, maximum likelihood) gave different topologies and did not resolve the history of RNASE1 duplications in colobines with confidence. This is likely due to the short size of (471 bp) and overall conservation of RNASE1. However, all approaches supported a scenario in which RNASE1 was duplicated once in the common ancestor of Alouatta species. Sequence information for non-coding regions of RNASE1 was not available for all species in our sample, so it was not possible to use a longer sequence to construct a phylogenetic tree.
Figure 3.
Phylogenies of primates based on coding sequences (474 bp) of RNASE1 and duplications. Trees were built using a (a) Bayesian approach with MrBayes60 and a b) maximum-likelihood method with PHYML61. Branch labels indicate (a) the posterior probability in percent and (b) bootstrap support in percent (1000 replicates). Scale bars indicate rate of substitutions per site.
CODEML provided evidence that there was an increase in the underlying mutation rate of the ancestral RNASE1B branch (H1: ΔLRT = 5.77, p = 0.016; Table 1) and that there has been positive selection for functional divergence in RNASE1B following the duplication event (H2: ΔLRT = 6.47, p = 0.011; Table 1). This is further supported by the dN/dS ratios in model H2, which were above one for the duplicated branches (ω = 1.229) but below one on the background branches (ω = 0.287). Note that the value for omega for the foreground branches in model H1 (ω = 999.0) is due to dS = 0, which makes it unreliable (Table 1). However, the LRT is unaffected by this, so we are basing our conclusions for model H1 only on the comparisons with the null model. The branch-site model did not support the hypothesis that any sites along the howler monkey RNASE1B genes are under positive selection, as the alternative model did not have a significantly better fit than the null model (ΔLRT = 3.41, p = 0.065; Table 1).
Table 1.
Results of CODEML analyses for primate RNASE1 and Alouatta duplicated sequences (n = 33).
| Analysis | Model | ω (dN/dS) | lnL | LRT | p | Test |
|---|---|---|---|---|---|---|
| Branch | H0 | 0.313 | −2159.77 | Null model, one ω for all branches | ||
| H1 | Background = 0.301 | −2156.89 | 5.77 | 0.016 | Higher ω on branch leading to duplicated genes? | |
| Foreground = 999.0 | ||||||
| H2 | Background = 0.287 | −2156.54 | 6.47 | 0.011 | Higher ω on duplicated branches? | |
| Foreground = 1.229 | ||||||
| Branch-site | Null | −1948.78 | Are there positively selected sites along RNASE1B? | |||
| Alternative | −1947.08 | 3.41 | 0.065 |
Protein properties and ancestral sequence reconstruction
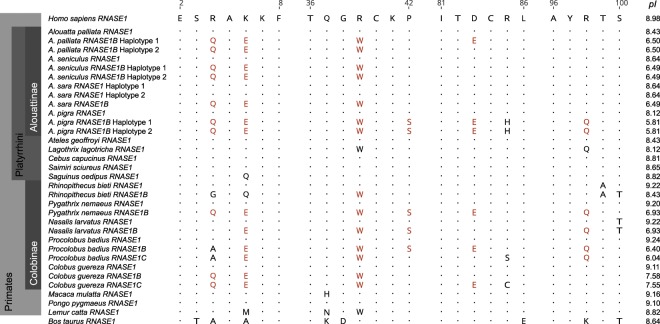
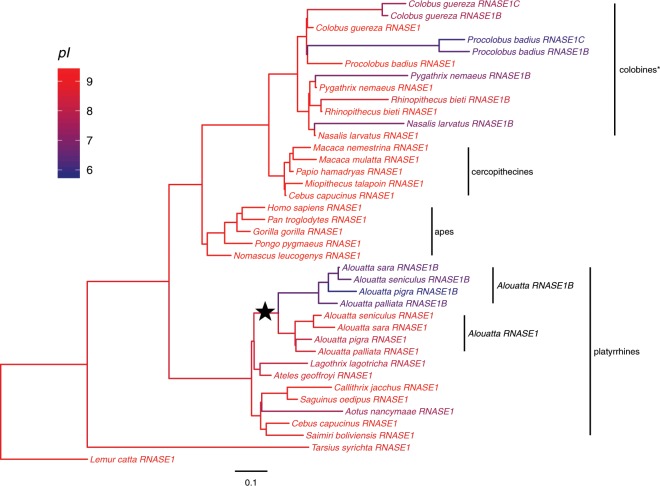
Aligning all RNase1 protein sequences shows several parallel amino acid changes between the duplicated howler monkey and colobine genes, RNASE1B and RNASE1C (Fig. 4), but not with the bovine pancreatic RNASE1 (Suppl. Fig. S8). These include functionally important changes that have been experimentally shown to decrease the enzyme’s activity against double-stranded RNA in colobine monkeys30,31,43. The duplicated sequences in all Alouatta species have a change from arginine to glutamine at site 4, a change from lysine to glutamic acid at site 6, and a change from arginine to tryptophan at site 39. A. palliata and A. pigra share an additional change from aspartic acid to glutamic acid at site 83 with the duplicated colobine proteins. Arginine and lysine are positively charged amino acids, while glutamic acid is negatively charged, so these changes contribute to a change in the charge of the resulting protein. Alouatta pigra further shares changes from proline to serine at site 42 and from arginine to glutamine at site 98. All numbering is in relation to the start of the mature peptide sequence and consistent with site numbering used in Zhang (2003)30. The isoelectric point (pI) of RNase1 and the duplicated RNase1 proteins are shown in Fig. 5. Compared to the high pI (9.11–9.24) and higher charge of parent RNase1 proteins, the duplicated proteins in colobines have a lower pI (6.04–8.43) (Fig. 5) and a reduced charge at pH 7.0 (Fig. 4). Likewise, the parent howler monkey RNase1 proteins have a higher pI (8.12–8.64) and charge (2.9–4.9) than the duplicated howler proteins (pI = 5.81–6.50, charge = −2.9 to −0.1) (Figs. 4,5).
Figure 4.
Primate RNASE1, RNASE1B, and RNASE1C sequences aligned to human (Homo sapiens) reference sequence. Parallel amino acid changes between duplicated genes (RNASE1B and RNASE1C) in Alouatta palliata and colobines are highlighted in red. Numbering is in relation to the start of the mature peptide sequence and consistent with numbering used in previous studies30,31. Isoelectric points (pI) shown on the right. PI calculated with ExPASy Compute pI tool.
Figure 5.
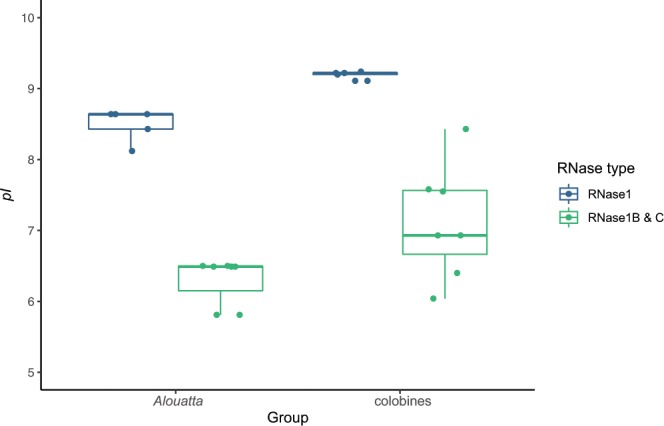
Computed isoelectric points (pI) of RNase1 and the duplicated proteins RNase1B and RNase1C in colobines and Alouatta spp.
Reconstruction of the ancestral RNASE1 sequences showed that isoelectric points of RNase1 proteins are consistently high across the primate phylogeny, with the exception of the duplicated proteins in colobines and Alouatta (Fig. 6). Another exception is the RNase1 protein of the owl monkey (Aotus nancymaae). While we found no evidence of a duplicated gene, the Aotus RNASE1 sequence has three amino acid changes that are parallel to changes found in the colobine RNASE1B sequences (K1G, K6E and R39W). Consistent with these changes, the pI of owl monkey RNase1 is lower (7.56) than any other non-duplicated RNase1 in primates, but still higher than howler monkey RNase1B (Fig. 6, Supplemental Table S6). The RNASE1 sequence of the brown woolly monkey (Lagothrix lagotricha) shares two of the amino acid changes found in RNASE1B (R39W and R98Q) but the protein does not exhibit a strong decrease in pI (Fig. 4, Supplemental Table S6) and there is no evidence of a gene duplication43. Other non-folivorous New World primates have conserved RNase1 sequences that do not share any or at most one of these amino acid substitutions (Fig. 4, Suppl. Fig. S8).
Figure 6.
Evolutionary relationships and isoelectric point (pI) of the proteins encoded by RNASE1, RNASE1B, and RNASE1C in primates. Branches are colored based on computed pI of extant primate protein sequences and reconstructed ancestral protein sequences. The timing of the RNASE1 duplication in Alouatta is indicated by the black star. *The colobine clade as shown here likely does not reflect the true evolutionary history of RNASE1 and gene duplications. Because the evolutionary history of the RNASE1 genes in colobines has not been fully resolved, the colobine genes are grouped by species here to illustrate the differences in pI between the parent and daughter proteins.
Discussion
In many mammal groups RNASE1 genes have a history of duplications and functional diversification. This study identified a previously unknown RNASE1 duplication event in the common ancestor of extant howler monkey (Alouatta) species and found amino acid substitutions in the duplicated gene that are parallel to those in duplicated RNASE1 genes in colobines and consistent with a new function in the digestive system. The howler monkey LYZ gene, however, was found to be conserved and not to have evolved changes consistent with a new role as a digestive enzyme.
While all other platyrrhine species studied so far only have one RNASE1 gene43, two different RNASE1-like sequences were identified in Alouatta palliata, A. seniculus, A. sara, and A. pigra. One sequence (RNASE1) retained a high pI and positive charge, while the other sequence (RNASE1B) had several amino acid changes (Fig. 4) that resulted in a reduction of the proteins’ pI and charge (Figs. 4,5). Such changes have also been found in RNASE1 duplications in Asian and African colobine monkeys and artiodactyl ruminants30–33. Colobine RNase1B and RNase1C have up to nine amino acid changes that reduce the enzymes’ effectiveness against double stranded RNA43 and howler monkey RNase1B proteins share three (R4Q, K6E, R39W), four (R4Q, K6E, R39W, D83E), or six (R4Q, K6E, R39W, P42S, D83E, R98Q) of these substitutions, depending on the species (Fig. 4). It is therefore likely that the duplicated howler monkey proteins are not as effective against double-stranded RNA as the ancestral protein. Combined with the lowered pI and reduced charge, this supports the idea that this RNASE1 duplication in howler monkeys has diverged in function from the original, immunological role of the ancestral protein23,35–37,43. Zhang (2006) had previously calculated the probability of three or more parallel amino acid substitutions arising in two lineages by chance to be between 0.0001 and 0.002631. The more likely explanation is that the parallel substitutions in howler monkey and colobine RNASE1B/C arose due to shared selective pressures. Results from the CODEML branch models further support the hypothesis that there was positive selection for functional divergence following the duplication event (Table 1). However, we want to caution that omega values are often elevated following gene duplication44,45, potentially due to loss of function and relaxed selection. Thus, relaxed selection following gene duplication rather than positive selection for functional divergence may be an alternate explanation for the results of the CODEML analyses.
In colobines and ruminant artiodactyls the duplicated RNase1 proteins are thought to be adaptations for the digestion of bacteria that ferment leaves in the foregut of these animals, an important source of nitrogen29,33. While howler monkeys do not have sacculated forestomachs like colobines and ruminants, they do rely on microbial fermentation, just in the caeco-colic region, rather than forestomach, to break down the foliage they consume19. The duplicated RNase1B may thus fill a role similar to the duplicated proteins in colobines and artiodactyl ruminants, by efficiently digesting bacterial RNA in the caeco-colic region, which has a similar pH (6.8) to that reported for the small intestine in colobines (6.0–7.0)19,22,43. In a study of fermentative digestion in Alouatta palliata the authors found that up to 31% of the monkeys’ daily required energy may come from the digestion of fermentation end products19. Although caeco-colic fermentation of leaves has been shown to be slightly less efficient at digesting fiber than foregut fermentation21,46, the overall nutritional gains may be comparable if the digestion of fermentation end products is considered. During times of fruit scarcity when the howler monkey diet consists entirely of leaves, the ability to efficiently digest such products may therefore be crucial to their survival19 and the RNase1B enzyme may be a key factor ensuring digestive efficiency.
Unlike cows and colobines, howler monkeys did not have parallel amino acid changes in the LYZ gene (Fig. 1). Lysozyme is an enzyme found in many vertebrates and invertebrates and is thought to play a role in immune function25. In cows and colobines, however, lysozyme exhibits parallel amino acid changes that allow the enzyme to function at a much lower pH, possibly as an adaptation for bacteriolytic activity in the acidic stomach fluid26,27. In artiodactyl ruminants the LYZ gene underwent multiple duplications and some of the daughter genes acquired a novel digestive function47. Interestingly, in the colobines there is only a single LYZ gene that was adapted for a digestive function. In non-colobine primates, including howler monkeys, LYZ is conserved (Fig. 1)48. It may be that the utility of lysozyme as a digestive enzyme is tied to foregut-fermentation, while ribonuclease can be adaptive for microbial fermentation both in the foregut, as well as in the “hindgut.” Some support for this is provided by a study of lysozyme in the only avian species with foregut-fermentation, the hoatzin (Opisthocomus hoazin), a leaf-eating bird from South America49. Despite arising from a different lysozyme gene family, a lysozyme expressed in the hoatzin stomach has biochemical properties and amino acid substitutions that are parallel to those found in artiodactyl ruminants and colobines50. Another possible example of ribonuclease adaptation for a digestive purpose comes from the ancient DNA of the extinct subfossil lemur Megaladapis (Perry and colleagues, in prep). Based on studies of dental microwear and dental topography Megaladapis was likely folivorous51,52 and its RNASE1 gene shares several amino acid substitutions with the duplicated RNASE1B and RNASE1C genes of colobines and howler monkeys (Perry and colleagues, in prep). Since no extant lemurs have a colobine-like digestive system18, it is most likely that Megaladapis relied on caeco-colic fermentation to digest leaves, like howler monkeys.
A limitation of the current study is that data are lacking on where in the howler monkey body RNASE1 and RNASE1B are expressed. Finding that RNASE1B is expressed in the howler monkey caecum and/or colon would provide strong evidence that this protein has been repurposed as a digestive enzyme. Future expression studies should therefore be a priority. The role of RNASE1 in owl monkeys (Aotus spp.) likewise deserves additional study. While there is no evidence of a gene duplication, A. nancymaae RNASE1 shares three amino acid substitutions with colobine RNASE1B/C and consequently has a lower pI than RNase1 in other species (Fig. 6, Suppl. Table S6). Zhang (2006) has noted that evolution of a digestive role for pancreatic RNase is likely impossible without gene duplication, given the need to retain an RNase with efficient double-stranded RNA degradation activity31. Kinetic assays of owl monkey RNase1 are, therefore, a priority for future research. Finally, some populations of the New World primate genus Brachyteles53 and several lemur species54 are also quite folivorous, making them targets for future studies.
Conclusions
The RNASE1 gene family has a history of duplications and functional divergence in many mammals, including the colobine primates30–33,36,37,39,41. Here we present evidence that RNASE1 has also been duplicated in the common ancestor of a group of folivorous non-colobine primates, the howler monkeys (Alouatta spp.), and that the duplicated gene (RNASE1B) has biochemical properties and amino acid substitutions that are parallel to those found in foregut-fermenting primates. This protein may therefore be used for an analogous function in howler monkeys, digesting the products of microbial fermentation in the caeco-colic region, a potentially substantial source of energy and nitrogen19. Along with behavioral and morphological adaptations, this duplicated protein may be a crucial digestive enzyme adaptation allowing howler monkeys to survive on a folivorous diet during times of fruit scarcity.
Methods
Genome mining and sequencing
To assemble a comparative dataset of pancreatic ribonuclease (RNASE1) and lysozyme (LYZ) gene, we mined primate genomes and gene sequences available on GenBank, as well as an unpublished draft genome assembly of the mantled howler monkey (Alouatta palliata) that we generated using Chromium Genome library preparation (10X Genomics) at New York University’s Langone School of Medicine Genome Technology Center for another project (Burrell et al., in prep; Janiak et al., in prep). Genomic DNA was already extracted and came from the collection of the Molecular Anthropology Lab at New York University. We size-selected high-molecular weight DNA via a Blue Pippin (Sage Science) for fragments > 50 kb long prior to library prep. The Chromium Genome library was then run on two lanes of an Illumina HiSeq. 2500 with v. 4.0 chemistry. Reads were assembled with the 10X Genomics Supernova pipeline42.
The RNASE1 sequence from the common marmoset (Callithrix jacchus) reference genome and the LYZ sequence from the black-capped squirrel monkey (Saimiri boliviensis) reference genome were used as queries to run BLASTN searches (default search parameters) on the Supernova 10X genome pseudohap and raw assemblies of Alouatta palliata. Primate RNASE1 sequences generated in previous studies31–33,43 were downloaded from the National Center for Biotechnology (NCBI, accession numbers in Supplemental Table S1). For a better understanding of the history of RNASE1 in primates, especially in platyrrhines, the published reference genomes of Aotus nancymaae, Cebus capucinus imitator, Microcebus murinus, and Tarsius syrichta were searched for RNASE1 sequences using BLASTN with the same query and parameters as above. Primate sequences of LYZ generated in a previous study48 were retrieved from GenBank. The LYZ gene sequence found in the Bos taurus reference genome differed from the sequence reported in Stewart et al. (1987)26. The sequence for Bos taurus LYZC2 was used, because it was most similar and almost identical to the bovine lysozyme sequence reported in Stewart et al. (1987). A full list of sequences used in this study and their accession numbers are presented in Supplemental Table S1.
Amplicon sequencing
In addition to genome sequencing of A. palliata, we also conducted amplicon sequencing of the RNASE1 gene region in four howler monkey species (Alouatta palliata, A. seniculus, A. pigra, A. sara). Briefly, we amplified a 1180 bp region surrounding RNASE1 using conserved PCR primers (Suppl. Table S2) and KAPA HiFi HotStart ReadyMix (thermocycler settings in Suppl. Table S2), gel purified the amplicons (PureLink Quick Gel Extraction Kit, Invitrogen), before shearing them with a Covaris S2 focused-ultrasonicator (settings used: intensity = 2, duty factor = 10%, cycles/burst = 200, time = 45 sec, cycles = 2). The sheared samples were prepped with an NEBNext Ultra II DNA Library Prep Kit (New England Biolabs) and sequenced together on an Illumina MiSeq using a Micro Kit with v2 chemistry. Extracted DNA samples used in this study were from the collection of the Molecular Anthropology Lab at New York University.
Assembly of RNASE1
Assembly with the 10X Genomics Supernova pipeline was unable resolve the RNASE1 region in Alouatta palliata. We, therefore, attempted to complete a targeted re-assembly of the region of interest from the A. palliata Illumina short reads. In order to use reads generated with the 10X Genomics system with standard mapping and assembly tools, it is necessary to remove the 10X linked barcodes from the reads. We removed barcodes with the script process_10xReads.py (https://github.com/ucdavis-bioinformatics/proc10xG). The 10X Genomics short reads (with barcodes removed), the short reads resulting from amplicon sequencing (after demultiplexing and adapter trimming), and short reads from Cebus capucinus imitator were mapped to the human RNASE1 region with bwa mem55 (https://github.com/lh3/bwa), followed by sorting and indexing with samtools56. After the initial mapping to the human RNASE1 reference, we identified the consensus sequence of each set of mapped reads and repeated the mapping step with this consensus sequence as a reference.
After mapping, sorting, indexing, and removing duplicates, we called variants and then haplotypes (using the previously called variants as a guide) with freebayes v1.2.0–17-ga78ffc057. We then reviewed and confirmed the short haplotypes identified by freebayes in the Integrative Genomics Viewer (IGV)58 and manually assembled the full-length RNASE1 haplotypes for each species.
Sequence analyses
Coding regions of all sequences for RNASE1 (464–474 bp) and LYZ (447 bp) were translated using Geneious 9.1.8. Coding regions and translated amino acid sequences were aligned using MAFFT v759 and Geneious 9.1.8 (alignments are available as supplementary materials). The following analyses were only conducted with data for RNASE1, because no evidence of duplication or convergence between Alouatta and colobines was found for LYZ.
Phylogenetic trees were constructed from both nucleotide and protein alignments of RNASE1 with MrBayes60 and PHYML61 programs using the Hasegawa-Kishino-Yano (HKY) substitution model with a discrete Gamma distribution (+G). The nucleotide substitution model was chosen based on the Akaike Information Criterion (AIC) statistics calculated by the jModelTest program (http://jmodeltest.org/)62. In PHYML, 1000 bootstrap replicates were completed. Because these relatively short sequences did not resolve the primate phylogeny accurately, trees based on the best available primate phylogeny63 were used in CODEML analyses and ancestral sequence reconstructions.
We used branch and branch-site models in the program CODEML which is part of the PAML package64 to test for positive selection acting on the duplicated RNASE1 genes in howler monkeys. Branch-specific models (H0, H1, and H2) were used to determine if there is an increase in the underlying mutation rate of the ancestral RNASE1B branch (H1) and if there is evidence of positive selection for functional divergence in RNASE1B following the duplication event (H2). Model fit was evaluated using likelihood ratio tests (LRT). Variation in the values of ω (nonsynonymous/synonymous substitutions, dn/ds) across sites along the RNASE1B genes was evaluated with branch-site models. For these, the duplicated Alouatta RNASE1 genes (RNASE1B) and the ancestral branch leading to them were designated as foreground branches, while all parent RNASE1 genes were designated as background branches. In this model, ω is allowed to vary both between sites and across branches to determine whether any sites are under positive selection in the foreground branches64. This alternative model is compared to the null model in which ω is fixed at 1 and model fit is evaluated with a LRT.
Protein properties
To compare the biochemical properties of proteins across species, the isoelectric points (pI) of lysozyme, extant RNAse1 and inferred ancestral RNAse1 proteins were calculated with the Compute pI Tool on the ExPASy webserver (http://web.expasy.org/compute_pi/). Charge at pH 7 was calculated with ProteinCalculator v3.4 (http://protcalc.sourceforge.net/).
Ancestral sequence reconstruction
To better understand the evolutionary history of RNASE1 in primates, ancestral sequences were reconstructed (n = 38) using FastML (http://fastml.tau.ac.il/)65 with a dataset of 39 sequences (shown in Fig. 6). The following running parameters were used: sequence type = codons, model of substitution = yang, use gamma distribution = yes and probability cutoff to prefer ancestral indel over character = 0.5. Reconstructed ancestral sequences were used to calculate shifts in pI across primate evolution and to determine whether the amino acid changes observed in the duplicated howler monkey sequences may have occurred in a common ancestor with other Atelines.
Acknowledgements
Support for this study was provided by the Center for Human Evolutionary Studies at Rutgers University, the National Science Foundation (BCS-1640515 to ASB and TRD, DDRIG BCS-1650864 to MCJ). Genome sequencing was conducted at the NYU Genome Technology Center (supported by Cancer Center Support Grant P30CA016087 from the Laura and Isaac Perlmutter Cancer Center). MCJ is supported by a postdoctoral fellowship from the Alberta Children’s Hospital Research Institute. JDO is supported by the Beatriu de Pinós postdoctoral programme of the Government of Catalonia’s Secretariat for Universities and Research of the Ministry of Economy and Knowledge. We are grateful for the computational resources provided by the University of Calgary’s Helix cluster, WestGrid, and ComputeCanada. We thank Colin MacFarland, Salman Reza, and Gwen Duytschaever for their help with amplicon sequencing at UCVM. We thank Marcella Nidiffer, Liliana Cortes-Ortiz, and Nicole Torosin for their help, and Robert Scott, Erin Vogel, and Ryne Palombit for comments on earlier versions of the manuscript.
Supporting Information
Author contributions
M.C.J. designed the study, performed laboratory work for amplicon sequencing, performed computational analyses, and wrote the manuscript. A.S.B. and T.R.D. designed and performed lab work for A. palliata genome sequencing and contributed to the preparation of the manuscript. J.D.O. performed computational analyses and contributed to the preparation of the manuscript. All authors gave final approval for publication.
Data availability
Sequence reads generated for this study are accessible via the SRA (BioProject accession number PRJNA593273) and assembled sequences have been uploaded as supplemental alignments. Accession numbers for all sequences mined from GenBank are included in Supplementary Table S1. Scripts used for data analyses are available on GitHub (github.com/MareikeJaniak/Alouatta-RNASE1).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56941-7.
References
- 1.Garber, P. A., Righini, N. & Kowalewski, M. M. Evidence of alternative dietary syndromes and nutritional goals In the genus Alouatta in Howler Monkeys (eds. Garber, P. A., Cortés-Ortiz, L., Urbani, B. & Youlatos, D.) 85–109 (Springer New York, 2015).
- 2.Milton K. Physiological ecology of howlers (Alouatta): energetic and digestive considerations and comparison with the Colobinae. Int. J. Primatol. 1998;19:513–548. doi: 10.1023/A:1020364523213. [DOI] [Google Scholar]
- 3.Milton Katharine. Food Choice and Digestive Strategies of Two Sympatric Primate Species. The American Naturalist. 1981;117(4):496–505. doi: 10.1086/283730. [DOI] [Google Scholar]
- 4.Chapman C. Flexibility in diets of three species of Costa Rican primates. Folia Primatol. 1987;49:90–105. doi: 10.1159/000156311. [DOI] [Google Scholar]
- 5.Glander, K. E. Feeding patterns in mantled howling monkeys. In Foraging Behavior: Ecological, Ethological, and Psychological Approaches (eds. Kamil, A. C. & Sargent, T. D.) 231–257 (Garland Press, 1981).
- 6.Stoner KE. Habitat selection and seasonal patterns of activity and foraging of mantled howling monkeys (Alouatta palliata) in northeastern Costa Rica. Int. J. Primatol. 1996;17:1–30. doi: 10.1007/BF02696156. [DOI] [Google Scholar]
- 7.Williams-Guillén, K. The Behavioral Ecology of Mantled Howling Monkeys (Alouatta palliata) Living in a Nicaraguan Shade Coffee Plantation. (PhD Dissertation, New York University, 2003).
- 8.Strier KB. Atelinae adaptations: behavioral strategies and ecological constraints. Am. J. Phys. Anthropol. 1992;88:515–524. doi: 10.1002/ajpa.1330880407. [DOI] [PubMed] [Google Scholar]
- 9.Da Cunha RGT, Byrne RW. Roars of black howler monkeys (Alouatta caraya): evidence for a function in inter-group spacing. Behaviour. 2006;143:1169–1199. doi: 10.1163/156853906778691568. [DOI] [Google Scholar]
- 10.Milton Katherine. Factors Influencing Leaf Choice by Howler Monkeys: A Test of Some Hypotheses of Food Selection by Generalist Herbivores. The American Naturalist. 1979;114(3):362–378. doi: 10.1086/283485. [DOI] [Google Scholar]
- 11.Amato KR, Garber PA. Nutrition and foraging strategies of the black howler monkey (Alouatta pigra) in Palenque National Park, Mexico. Am. J. Primatol. 2014;76:774–787. doi: 10.1002/ajp.22268. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda I, et al. Factors affecting leaf selection by foregut-fermenting proboscis monkeys: new insight from in vitro digestibility and toughness of leaves. Sci. Rep. 2017;7:42774. doi: 10.1038/srep42774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominy NJ, Lucas PW. Ecological importance of trichromatic vision to primates. Nature. 2001;410:363–366. doi: 10.1038/35066567. [DOI] [PubMed] [Google Scholar]
- 14.Lucas PW, et al. Evolution and function of routine trichromatic vision in primates. Evolution. 2003;57:2636–2643. doi: 10.1111/j.0014-3820.2003.tb01506.x. [DOI] [PubMed] [Google Scholar]
- 15.Milton K, Van Soest PJ, Robertson JB. Digestive efficiencies of wild howler monkeys. Physiol. Zool. 1980;53:402–409. doi: 10.1086/physzool.53.4.30157878. [DOI] [Google Scholar]
- 16.Espinosa-Gómez F, Gómez-Rosales S, Wallis IR, Canales-Espinosa D, Hernández-Salazar L. Digestive strategies and food choice in mantled howler monkeys Alouatta palliata mexicana: bases of their dietary flexibility. J. Comp. Physiol. B. 2013;183:1089–1100. doi: 10.1007/s00360-013-0769-9. [DOI] [PubMed] [Google Scholar]
- 17.Chivers DJ, Hladik CM. Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J. Morphol. 1980;166:337–386. doi: 10.1002/jmor.1051660306. [DOI] [PubMed] [Google Scholar]
- 18.Lambert JE. Primate digestion: interactions among anatomy, physiology, and feeding ecology. Evolutionary Anthropology: Issues, News, and Reviews. 1998;7:8–20. doi: 10.1002/(SICI)1520-6505(1998)7:1<8::AID-EVAN3>3.0.CO;2-C. [DOI] [Google Scholar]
- 19.Milton K, McBee RH. Rates of fermentative digestion in the howler monkey, Alouatta palliata (primates: ceboidea) Comp. Biochem. Physiol. A Comp. Physiol. 1983;74:29–31. doi: 10.1016/0300-9629(83)90706-5. [DOI] [PubMed] [Google Scholar]
- 20.Chivers, D. J. & Langer, P. The Digestive System in Mammals: Food Form and Function. (Cambridge University Press, 1994).
- 21.Alexander R. The relative merits of foregut and hindgut fermentation. J. Zool. 1993;231:391–401. doi: 10.1111/j.1469-7998.1993.tb01927.x. [DOI] [Google Scholar]
- 22.Kay, R. N. B. & Davies, A. G. Digestive physiology In Colobine Monkeys: Their Ecology, Behaviour and Evolution (eds. Davies, A. G. & Oates, J. F.) 229–249 (Cambridge University Press, 1994).
- 23.Barnard EA. Biological function of pancreatic ribonuclease. Nature. 1969;221:340–344. doi: 10.1038/221340a0. [DOI] [PubMed] [Google Scholar]
- 24.Janiak MC. Digestive enzymes of human and nonhuman primates. Evol. Anthropol. 2016;25:253–266. doi: 10.1002/evan.21498. [DOI] [PubMed] [Google Scholar]
- 25.Jollès P, Jollès J. What’s new in lysozyme research? Mol. Cell. Biochem. 1984;63:165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- 26.Stewart CB, Schilling JW, Wilson AC. Adaptive evolution in the stomach lysozymes of foregut fermenters. Nature. 1987;330:401–404. doi: 10.1038/330401a0. [DOI] [PubMed] [Google Scholar]
- 27.Swanson KW, Irwin DM, Wilson AC. Stomach lysozyme gene of the langur monkey: tests for convergence and positive selection. J. Mol. Evol. 1991;33:418–425. doi: 10.1007/BF02103133. [DOI] [PubMed] [Google Scholar]
- 28.Cho S, Beintema JJ, Zhang J. The ribonuclease A superfamily of mammals and birds: identifying new members and tracing evolutionary histories. Genomics. 2005;85:208–220. doi: 10.1016/j.ygeno.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Beintema JJ. The primary structure of langur (Presbytis entellus) pancreatic ribonuclease: adaptive features in digestive enzymes in mammals. Mol. Biol. Evol. 1990;7:470–477. doi: 10.1093/oxfordjournals.molbev.a040619. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J. Parallel functional changes in the digestive RNases of ruminants and colobines by divergent amino acid substitutions. Mol. Biol. Evol. 2003;20:1310–1317. doi: 10.1093/molbev/msg143. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J. Parallel adaptive origins of digestive RNases in Asian and African leaf monkeys. Nat. Genet. 2006;38:819–823. doi: 10.1038/ng1812. [DOI] [PubMed] [Google Scholar]
- 32.Yu L, et al. Adaptive evolution of digestive RNASE1 genes in leaf-eating monkeys revisited: new insights from ten additional colobines. Mol. Biol. Evol. 2010;27:121–131. doi: 10.1093/molbev/msp216. [DOI] [PubMed] [Google Scholar]
- 33.Schienman JE, Holt RA, Auerbach MR, Stewart C-B. Duplication and divergence of 2 distinct pancreatic ribonuclease genes in leaf-eating African and Asian colobine monkeys. Mol. Biol. Evol. 2006;23:1465–1479. doi: 10.1093/molbev/msl025. [DOI] [PubMed] [Google Scholar]
- 34.Xu L, Su Z, Gu Z, Gu X. Evolution of RNases in leaf monkeys: being parallel gene duplications or parallel gene conversions is a problem of molecular phylogeny. Mol. Phylogenet. Evol. 2009;50:397–400. doi: 10.1016/j.ympev.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Libonati M, Furia A, Beintema JJ. Basic charges on mammalian ribonuclease molecules and the ability to attack double‐stranded RNA. Eur. J. Biochem. 1976;69:445–451. doi: 10.1111/j.1432-1033.1976.tb10929.x. [DOI] [Google Scholar]
- 36.Liu J, et al. Evolutionary and functional novelty of pancreatic ribonuclease: a study of Musteloidea (order Carnivora) Sci. Rep. 2014;4:5070. doi: 10.1038/srep05070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goo SM, Cho S. The expansion and functional diversification of the mammalian ribonuclease a superfamily epitomizes the efficiency of multigene families at generating biological novelty. Genome Biol. Evol. 2013;5:2124–2140. doi: 10.1093/gbe/evt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubois J-YF, et al. Pancreatic-type ribonuclease 1 gene duplications in rat species. J. Mol. Evol. 2002;55:522–533. doi: 10.1007/s00239-002-2347-8. [DOI] [PubMed] [Google Scholar]
- 39.Lang D-T, Wang X-P, Wang L, Yu L. Molecular evolution of pancreatic ribonuclease gene (RNase1) in Rodentia. J. Genet. Genomics. 2017;44:219–222. doi: 10.1016/j.jgg.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Dubois J-YF, Ursing BM, Kolkman JA, Beintema JJ. Molecular evolution of mammalian ribonucleases 1. Mol. Phylogenet. Evol. 2003;27:453–463. doi: 10.1016/S1055-7903(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, et al. Multiple bursts of pancreatic ribonuclease gene duplication in insect-eating bats. Gene. 2013;526:112–117. doi: 10.1016/j.gene.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 42.Weisenfeld NI, Kumar V, Shah P, Church DM, Jaffe DB. Direct determination of diploid genome sequences. Genome Res. 2017;27:757–767. doi: 10.1101/gr.214874.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Zhang Y-P, Rosenberg HF. Adaptive evolution of a duplicated pancreatic ribonuclease gene in a leaf-eating monkey. Nat. Genet. 2002;30:411–415. doi: 10.1038/ng852. [DOI] [PubMed] [Google Scholar]
- 44.Hahn MW. Distinguishing among evolutionary models for the maintenance of gene duplicates. J. Hered. 2009;100:605–617. doi: 10.1093/jhered/esp047. [DOI] [PubMed] [Google Scholar]
- 45.Han, M. V. & Hahn, M. W. Identifying parent-daughter relationships among duplicated genes. Pac. Symp. Biocomput. 114–125 (2009). [PubMed]
- 46.Edwards MS, Ullrey DE. Effect of dietary fiber concentration on apparent digestibility and digesta passage in non-human primates. II. Hindgut-and foregut-fermenting folivores. Zoo Biol. 1999;18:537–549. doi: 10.1002/(SICI)1098-2361(1999)18:6<537::AID-ZOO8>3.0.CO;2-F. [DOI] [Google Scholar]
- 47.Irwin DM. Evolution of the bovine lysozyme gene family: changes in gene expression and reversion of function. J. Mol. Evol. 1995;41:299–312. doi: 10.1007/BF01215177. [DOI] [PubMed] [Google Scholar]
- 48.Messier W, Stewart CB. Episodic adaptive evolution of primate lysozymes. Nature. 1997;385:151–154. doi: 10.1038/385151a0. [DOI] [PubMed] [Google Scholar]
- 49.Grajal A, Strahl SD, Parra R, Gloria Dominguez M, Neher A. Foregut fermentation in the hoatzin, a neotropical leaf-eating bird. Science. 1989;245:1236–1238. doi: 10.1126/science.245.4923.1236. [DOI] [PubMed] [Google Scholar]
- 50.Kornegay JR, Schilling JW, Wilson AC. Molecular adaptation of a leaf-eating bird: stomach lysozyme of the hoatzin. Mol. Biol. Evol. 1994;11:921–928. doi: 10.1093/oxfordjournals.molbev.a040173. [DOI] [PubMed] [Google Scholar]
- 51.Scott JR, et al. Dental microwear texture analysis of two families of subfossil lemurs from Madagascar. J. Hum. Evol. 2009;56:405–416. doi: 10.1016/j.jhevol.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Godfrey LR, Winchester JM, King SJ, Boyer DM, Jernvall J. Dental topography indicates ecological contraction of lemur communities. Am. J. Phys. Anthropol. 2012;148:215–227. doi: 10.1002/ajpa.21615. [DOI] [PubMed] [Google Scholar]
- 53.Di Fiore, A., Link, A. & Campbell, C. J. The Atelines In Primates in Perspective (eds. Campbell, C. J., Fuentes, A., MacKinnon, K. C., Bearder, S. K. & Stumpf, R. M.) (Oxford University Press, 2011).
- 54.Gould, L., Sauther, M. L. & Cameron, A. Lemuriformes In Primates in Perspective (eds. Campbell, C. J., Fuentes, A., MacKinnon, K. C., Bearder, S. K. & Stumpf, R. M.) (Oxford University Press, 2011).
- 55.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at https://arxiv.org/abs/1303.3997v2 (2013).
- 56.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garrison, E. & Marth, G. Haplotype-based variant detection from short-read sequencing. Preprint at https://arxiv.org/abs/1207.3907 (2012).
- 58.Robinson JT, Thorvaldsdóttir H, Wenger AM, Zehir A, Mesirov JP. Variant review with the Integrative Genomics Viewer. Cancer Res. 2017;77:e31–e34. doi: 10.1158/0008-5472.CAN-17-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 61.Guindon S, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 62.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9:772–772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perelman P, et al. A molecular phylogeny of living primates. PLoS Genet. 2011;7:e1001342–17. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 65.Ashkenazy H., Penn O., Doron-Faigenboim A., Cohen O., Cannarozzi G., Zomer O., Pupko T. FastML: a web server for probabilistic reconstruction of ancestral sequences. Nucleic Acids Research. 2012;40(W1):W580–W584. doi: 10.1093/nar/gks498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence reads generated for this study are accessible via the SRA (BioProject accession number PRJNA593273) and assembled sequences have been uploaded as supplemental alignments. Accession numbers for all sequences mined from GenBank are included in Supplementary Table S1. Scripts used for data analyses are available on GitHub (github.com/MareikeJaniak/Alouatta-RNASE1).