Abstract
Tumour progression involves interactions among various cancer cell clones, including the cancer stem cell subpopulation and exogenous cellular components, termed cancer stromal cells. The latter include a plethora of tumour infiltrating immunocompetent cells, among which are also immuno-modulatory mesenchymal stem cells, which by vigorous migration to growing tumours and susequent transdifferentiation into various types of tumour-residing stromal cells, may either inhibit or support tumour progression. In the light of the scarce therapeutic options existing for the most malignant brain tumour glioblastoma, mesenchymal stem cells may represent a promising novel tool for cell therapy, e.g. drug delivery vectors. Here, we review the increasing number of reports on mutual interactions between mesenchymal stem cells and glioblastoma cells in their microenvironment. We particularly point out two novel aspects: the different responses of cancer cells to their microenvironmental cues, and to the signalling by kinin receptors that complement the immuno-modulating cytokine-signalling networks. Inflammatory glioblastoma microenvironment is characterised by increasing expression of kinin receptors during progressive glioma malignancy, thus making kinin signalling and kinins themselves rather important in this context. In general, their role in tumour microenvironment has not been explored so far. In addition, kinins also regulate blood brain barrier-related drug transfer as well as brain tumour angiogenesis. These studies support the on-going research on kinin antagonists as candidates in the development of anti-invasive agents for adjuvant glioblastoma therapy.
Keywords: Co-culture, Glioma, Kinin receptors, Mesenchymal stem cells, Microenvironment, Tumour heterogeneity
Brain Tumours - Glioblastoma
Brain tumours originate from various types of cells, of which astroglial tumours being the most frequent. The World Health Organisation (WHO) distinguishes four grades of glioma, of which glioblastoma (GBM) is the most aggressive, invasive, and lethal among all types of brain tumours. Unfortunately, it is also the most common among glial tumours with 5–7 cases per 100.000 individuals yearly, and represents 50% of all gliomas [1, 2]. It is characterised by histological features such as necrosis, vascular proliferation and pleomorphism. Contrary to most tumour types, irradiation and chemotherapy has proven to be ineffective to impair long term GBM progression, demonstrating its remarkable therapeutic resistance [3–5]. Radiotherapy is combined with chemotherapy, for which the alkylating agent temozolomide has been used in most cases. There is a constant search for novel adjuvant therapeutics, including kinase inhibitors, anti-angiogenic agents and recently also immunotherapy to increase average survival of GBM patients.
Several reasons for GBM resistance to therapy has been recognised, such as diffuse infiltration of cancer cells into neighbouring brain parenchyma that are hard to target by surgery [6–8]. Furthermore, the presence of heterogenic GBM cell populations is nowadays one of the hallmarks of these tumours. The Cancer Genome Atlas (TCGA) defined four different GBM subtypes, proneural (PN), neural (N), mesenchymal (MES) and classical (CL) by genomic characteristics [9]. These GBM subtypes differ in survival rate, being shorter with the MES subtype. The four subtypes can be clustered into two major subpopulations: PN subtype, characterised by high expression of the platelet-derived growth factor receptor (PDGFR), OLIG2, TCF3 and IDH1 mutation, and the MES subtype, highly expressing MET, CD44 and CHI3LI/YLK40 [10]. Patel [11] was the first to establish that GBM subtype-specific markers are variably expressed across individual cells, even within one tumour, and demonstrated that higher intra-tumour heterogeneity resulted in worse prognosis of GBM patients [12]. However, GBM patients of the PN subtype are unlikely to benefit from aggressive therapies, which on the other hand, are beneficial for patients of the MES and CL subtypes. Rapid genetic evolution [12] and a frequently observed shift of PN GBM towards MES-like GBM phenotypes during the the course of therapy [5, 13] are unfavourable. Wang [5] and others showed that two-thirds of primary GBM cases switched transcriptional subtypes at recurrence. It has been pointed out that, among these, the mesenchymal subtype was the most stable primary GBM subtype. Clinical reports demonstrated worse prognosis of patients with tumours with a higher expression of MES-related genes [11]. It has been also experimentally confirmed that PN GBM can reoccur as a more aggressive MES GBM.
Transcriptome diversity is underscored by a broad spectrum of recurrent oncogenic driver mutations, such as amplification of the epidermal growth factor receptor (EGFR), EGFR variant III mutation (EGFRvIII) and isocitrate dehydrogenase 1 R132C (IDH1 R132C) mutation [14, 15]. IDH1 mutations are peculiar, as these are present in the secondary PN GBM subtype, and also in all low-grade gliomas, where they convey better prognosis of patient survival. Mutated IDH1 protein acquires the ability to convert alpha-ketogluterate (α-KG) to R (−)-2-hydroxyglutarate (2-HG) [15]. This is supported by the findings that 2-HG levels are elevated in gliomas containing an IDH1 mutation and led to the hypothesis that mutant IDH is an oncogene and 2-HG is an “oncometabolite” [16].
Common somatic mutations and copy number deletion/amplification in GBM genome were already grouped into three frequently amplified pathways: the p53 signalling pathway, the retinoblastoma RB signalling pathway, and the receptor tyrosine kinase (RTK) signalling pathway [17]. p53 pathway was particularly affected by genomic alteration via CDKN2A deletion, and MDM2 and MDM4 amplification, as well as by mutations and deletions of TP53. 88% of GBM samples harboured at least one genetic mutation in the RTK pathways, besides EGFR amplification also mutation or deletion in NF1 and PTEN. Alterations in the RB signalling pathway included deletion of CDKN2 A/B, amplification of CDK4, CDK6, and CCND2, and deletion or mutation of RB1. More complexity to GBM heterogeneity has lately been added to the genomic heterogeneity [5, 18]. TCGA pilot project sequenced 601 target genes, and identified frequently mutated genes, including TP53 with frequency of 42%, PTEN (33%), Neurofibromin −1 (NF1) (21%), EGFR (18%), RB1 (11%), PIK3R1 (10%), and 7% of PIK3CA mutations. The whole-exome sequencing of 291 GBM patients revealed similar results.
Clinical application of these findings comprise drugs directly targeting these frequent genomic alterations, although the efforts have not yet been very effective. Superimposed to these are epigenetic changes, comprising CP island methylation, histone acetylation and miRNA transcriptional regulation. However, the most clinically relevant so far has been promoter region methylation of O6-methylguanine DNA methyltransferase (MGMT), as most relevant biomarker for the response DNA-alkylating activity of temozolomide (TMZ) [19], proving in many clinical studies that MGMT methylation status is predictive of TMZ response. Radiation concomitant to TMZ administration, the so called Stupp protocol for GBM treatment, has increased the survival rates of patients with methylated MGMT.
Tumour Heterogeneity
Although molecular subtypes-based high-dimensional profiling in GBM [5, 18] improved our understanding of the disease progression, this does not correlate with a significant increase in patient overall survival. Obstacles to successful therapy also include tumour non-autonomous heterogeneity due to its microenvironment. Infiltrating stromal cells and their secretomes, extracellular matrix plasticity and angiogenesis, all have an impact on cancer cells’ invasion, and tumour recurrence regardless of the GBM subtype. To this end, Wang et al. [5] leveraged RNA sequencing profiles of single glioma cells and glioma stem cells as well as bulk tissues to study the tumour intrinsic- and tumour microenvironment-independent transcriptional heterogeneity of GBM tumours. They identified the so called “bona fide” glioma genes that are uniquely expressed in glioma cells, but not in tumour associated host cells. Based on these findings, they (re)identifed three tumour-intrinsic transcriptional subtypes, corresponding to Verhaak’s PN, CL and MES subtypes, but not the N-subtype. This suggests that the signature defining the neuronal (N) subtype belonged to the normal neuron lineage cells within these tumours. This leads to questioning whether MES GBM subtypes may not be the results of interactions with infiltrating mesenchymal components of tumour stroma, such as mesenchymal stem cells (MSCs) and cancer-associated fibroblasts (CAFs). Indeed, the work by Hossain [20] showed that the majority of GBM-associated MSCs isolated from fresh tumours were non-tumourigenic stromal cells of bone marrow MSC phenotype that had been recruited to the tumour. However, approximately 10% of GBM-associated MSCs may originate directly from GBM stem cell (GSCs) transdifferentiation or display genetic patterns intermediate between these two types. Moreover, Oliveira [21] recently described in vitro cell fusion between MSCs and GBM cells, resulting in hybrid cells, similar to those, observed as minor fraction by Hossain [20]. In contrast, Appaix [22] proposed that MSC transdifferentiation gives rise to GSCs and not vice versa.
The clinically observed heterogeneity is also reflected in the isolated, established cell lines that are widely used in the in vitro experiments [23]. Comprehensive characterization of the genetic background and comparison of the differentially regulated genes in cell lines U87, T98G, LN229, U343 and U373 with the genes from GBM TCGA had a ~73% match, suggesting that the transcriptomic make-up of these cell lines closely resembles that of GBM tissues. The cell lines all harbour promoter activating mutations in the hTERT genes and up-regulation of hTERT transcript levels, but none of them presented the IDH1 mutation. The reason is the fact that IDH1-mutated cells, except the cells with particular IDH mutatuon IDH1-R314C, cannot be cultured in vitro [24]. The most frequent alterations were observed in TP53, PTEN, TCHH and MLL3 genes, and mutations in EGFR, NF1 and PDGFRA were found in some of the cell lines. However, the differences on the phenotypes of these cells were only addressed in our work [25, 26]. Moreover, we were first to address the mutual interaction of U87 and U373 cells upon indirect co-culturing [27] and found that U373 cell-conditioned medium increased U87 genomic stability, whilst decreasing proliferation rates and increasing invasion, due to a plethora of produced cytokines identified in the co-culture media. This cross-talk altered the expression of 264 genes associated with proliferation, inflammation, migration and adhesion in U87 cells, as well as 221 genes associated with apoptosis, the cell cycle, cell differentiation and migration in U373 cells. Indirect and direct co-culture of U87 and U373 cells showed mutual-opposite effects on temozolomide resistance, which was higher in U373 cells. Furthermore, we explored genetic backgrounds of the two cell lines with respect to TCGA subtype classification [9]. We applied a modified Behnan [28] algorithm, based on a set of 12 genes, discriminating between PN, CL and MES subtypes [26]. We have shown that U373 harbours more MES GBM genes than U87 cells [26, 27].
Glioblastoma Stem Cells
GBM stem cells (GSCs) and their progenies [29] represent major obstacles in therapy, associated with the most resistance-related phenotypes. GSCs define the functional progression of various GBM cell populations within the GBM, expressing a panel of stemness markers. Many of the transcription factors or structural proteins essential for normal stem and progenitor cell (NSPC) function also mark GSCs, including SOX2, NANOG, OLIG2, MYC, MUSASHI1, BMI1, nestin and inhibitor of differentiation protein 1 (ID1) [30]. A multitude of potential cell surface markers have additionally been suggested to facilitate GSC isolation by flow cytometry, including CD133, CD15 and SSEA-1 (stage-specific embryonic antigen 1), integrin α6, CD44, L1CAM and A2B5. In addition, these types of cell surface markers mediate interactions between cells and the microenvironment, but dissociation of cells from their surroundings gradually degrades their informational content, pointing to the so-called plasticity of CSCs found in GSCs isolates. A reflection of the variable expression of defined GSC biomarkers, such as CD133/prominin1, CD15, CD9, Nestin, OCT4, SOX2, LICAM and OCT4, was confirmed by many authors and summarized by Teng and co-workers [31]. They showed that hypoxic environment and the presence of serum in vitro may already be sufficient for changing the GSC phenotype. In vitro, when grown in serum-free neurobasal medium culture, the GSCs phenotype change after several passages (our unpublished data on GSC cell lines). GSCs isolated from MES GBM tumours tend to lose their MES properties and present a PN signature, even at early passages [32]. This loss of MES identity has been attributed to the change of tumour microenvironment that likely promotes and maintains MES properties, and vice versa: in the presence of serum-free neurobasal medium and hypoxic conditions the MES PN transition occurs. Animals co-implanted with PN GSCs and MES GSCs developing tumours composed of two cell subtypes were worse in survival rates when compared with those formed by MES GSCs or by PN GSCs alone. Analysis of tumour composition revealed that despite the initial dominance of PN GSCs, by day 9 most of the tumour was composed of MES GSCs and by the terminal phase almost the entire tumour was composed of MES GSCs [33]. Additionally, Liu and co-workers show that GBM cells from peripheric blood of patients acquire a GSC-like phenotype, evidenced by stemness, resistant to genotoxic drugs, and capacity to home to a primary tumor site to proliferate, contributing to relapse of GBM. This GSC-like phenotype, when in the blood, and proliferative rate, when in primary tumor site, reveal the microenvironment influence on GBM cell fate [34].
GSCs, while capable of self-renewal, retain their ability to activate cellular programmes that lead to the generation of rapidly dividing, committed, and differentiated bulk GBM cell populations. GSCs thus carry tumour initiation potential and can play indirect or even direct roles in tumour invasion, repopulation, therapeutic resistance and relapse. Radiotherapy was shown to promote stemness genotype and phenotype, leading to the tumour-resistant MES subtype [3, 35, 36]. In recurrent tumours, epigenetically-induced MES-related expression of CD44, cMET, YKL40 and/or high expression of NFκB was observed due to PN GSC transdifferentiation into MES GSC, which correlates with increased tumour resistance, leading to lower patient survival rates. Radiation-resistant GSCs revealed gene signatures enriched for coding epithelial–to-mesenchymal (EMT) pathways and leading to increased recurrent invasive patterns [37]. On the other hand, EMT is involved in the generation of refractory cancer cells with an accumulation of specific stem cell markers. As reviewed by Iwadate [38], there are two different pathways involved in EMT induction in glioma: (a) TGF-β initiated, possibly derived from MSC in the tumour microenvironment and (b) reactive oxidative species (ROS)-initiated, activating extracellular signalling-regulated by check kinases (CHK) 1/2. Each pathway leads to nuclear localization and activation of transcription factor SNAIL or TWIST expression. Qazi [39] emphasized that due to the observed GBM subtype variability, not all malignant tumours respond similarly to radiotherapy.
Thus, Novel therapies may target GSC in addition to standard treatments that eradicate the bulk of the tumour, while normal neural stem cells (NPSC) should be left unharmed. We were the first to describe the stemness-maintaining gene tetraspanin CD9 in GSC in primary biopsies, GBM spheroids, as well as in GBM cell lines, but not in their normal counterparts, which is an excellent target choice [40]. CD9 silencing in three CD133+ GSC cells lines reduced cell proliferation, survival, invasion, and self-renewal capacity, and altered expression of other stem-cell markers, such as CD133, Nestin and SOX2, indicating that it may be the “master” GSC gene. On the basis of our discovery, Shi [41] recently revealed that disrupting CD9 markedly reduced its interaction with the membrane protein gp130, which sustains signalling pathways via STAT3, and demonstrated that disruption of CD9 signalling promoted differentiation of GSC in animal models. Although sharing many markers with neural stem cells, GSCs have significantly different evolutionary and physiological role, leading to host self-destruction, whereas normal stem cells generally lead to homeostasis and higher levels of tissues organisation, promoting host survival [31]. This concept led Teng and co-workers [31] to propose that cancer stem cells should be renamed into tumour cell survival cells.
Glioblastoma Stem Cell Niches
In CSCs, both internal (intrinsic) and external (extrinsic) forces play a key role in cell regulation and therapy resistance [30]. The external factors include the cues from protective tumour tissue niches, similar to NPSC niches in the subventricular zone of the brain [30, 42] and the haematopoietic stem cells niches [43]. In the tumour microenvironment, it is common for GSC niches to form in hypoxic areas [44]. However, it has been also demonstrated that GSCs reside around a fraction of arterioles in human GBM samples [44, 45]. Arterioles are transport vessels that do not take part in oxygenation, so they retain hypoxic conditions and nourish the stemness of GSCs. Therefore, the hypoxic peri-arteriolar GSC niche is a logical explanation for this seemingly contradictory necessity of both hypoxic conditions and the presence of endothelial cells.
Moreover, the tunica adventitia of arteriolar walls is inhabited by MSCs, smooth muscle cells (SMCs) and other niche components [22]. These stromal cells residing around the peri-arteriolar endothelium release several chemokines, such as stromal derived factor-1α (SDF-1α), whereas GSC express C-X-C receptors 4/7, thus residing in close proximity of arteriolar endothelial cells. The strategy to exploit the environmental cues to break this axis would activate low proliferating GSCs and release them from the niche. GSCs may then differentiate into rapidly dividing progenitors, more vulnerable to radiation or transdifferentiate PN to MES GBM phenotype that present an aggressive invasive behaviour [45–48].
Homotypic and Heterotypic Cell Communication by Extracellular Vesicles
In recent years, it has become increasingly clear that cells use several mechanisms to facilitate intercellular cross-talk, such as exchange of cytokines and other molecules, extracellular vesicles (EVs) [49], and closer contact via gap junctions, nanotubules formation and fusion. GBM tumour cells release microvesicles (exosomes) that contain mRNA, miRNA and angiogenic proteins [50]. The tumour-specific receptor EGFRVIII was detected in serum microvesicles of a third of GBM patients [51]. These microvesicles are also enriched in angiogenic proteins and elicit tubule formation by endothelial cells [52]. Spinelli [53] have recently shown that PN GSC and MES GSC lines produce different sets of EVs, that can vary in size, marker distributions and proteomes.
Interestingly, EV-borne protein cargos transferred between proneural and mesenchymal GSC increased pro-tumourigenic behaviours in vitro and in vivo [33]. Clinically, analysis of GBM patient data from the Cancer Genome Atlas database revealed that proneural tumours with increased expression of genes, encoding for mesenchymal EV profile, or mesenchymal tumours with increased expression of genes of proneural EV profile were both associated with worse outcomes, suggesting influences by the proportion of tumour cells of varying subtypes in tumours, as we have demonstrated in vitro [27, 33]. These datasets indicated that heterogeneity within tumours, which may be propagated throughout the tumour via EV communication, was associated with decreased survival. This is in agreement with the scenario proposed by Patel [11] where the clinical outcome of a GBM subclass is influenced by the proportion of tumour cells of alternate subtypes and emphasized the clinical importance of intratumoural heterogeneity.
Moreover, EV proteome patterns analyses also identified key proteins, exchanged between dormant GSCs and their microenvironment, and thus may reveal biomarkers, predicting GBM/GSC therapeutic resistance. Also, serum induced differentiation of these GSC subsets resulted in considerable differences in cellular phenotype, increased EV emission and content. Notably, differentiation of GSCs of MES subtype is accompanied by production of EVs with better ability to stimulate growth of brain endothelial cells than that that of EVs from undifferentiated MES GSCs. These results point to a role of GSC heterogeneity and differentiation potential in EV-mediated communication of GBM cells with their vascular system [53]. GBM and GSCs also communicate with infiltating MSCs, as dicussed below.
Mesenchymal Stem Cells
Adult mesenchymal stem cells (MSCs), also called “tissue stem cells”, can be found in many tissues and are necessary for its regeneration. As reviewed by Stuckey & Shah [54], MSCs, along with other types of normal tissue stem cells, such as hematopoietic (HSCs) and neural stem cells (NSCs), are considered as novel cell therapy tools, and/or vehicles for drug delivery, in cancer. In particular, MSCs are increasingly used in cell therapies because of their availability, multi-potency, and immunomodulatory activity, and lack of tumourigenic behaviour [55]. As it turns out, the lack of knowledge regarding their mutual interaction with cancer and even more so with cancer stem cells hindered their application. We first have to understand MSC role in complex tumour microenvironment, specifically their indirect and direct cross-talk with cancer stem cells, particularly within their niches, as it occurs in vivo.
MSCs are multipotent stromal cells, characterised by adherent growth in vitro, expression of CD105, CD73 and CD90, and the lack of expression of CD45, CD34, CD14, CD31, CD106 markers (the latter two being endothelial precursor markers). MSCs possess the ability to differentiate into adipocytes, osteoblasts, and chondrocytes. These cells are present in a variety of tissues, but are most abundant in bone marrow, adipose tissue, placenta and umbilical cord [56]. Most importantly, MSCs origin may have crucial effects on the microenvironment of the tumour, as has been demonstrated in a variety of in vitro studies [54]. Various modulatory factors/chemokines that regulate inflammation, cell death, angiogenesis, fibrosis and tissue regeneration are produced and secreted by MSCs [57, 58]. Therefore, it is crucial to standardize methods used in different studies in order to better understand whether MSCs provide valid and safe therapeutic approaches for tackling cancer [56].
Mesenchymal Stem Cells in Glioblastoma
Another intrinsic characteristic of MSCs, worth researching, is the ability to home from bone marrow, adipose tissue or other tissues to glioma tumors [59]. MSCs affect GBM and GSCs directly and/or modulate other stromal components, such as immune cells. A good example is the MSC-induced T cell anergy by downregulation of CD80 and CD86 expression on antigen presenting cells [60]. MSCs prevented differentiation of monocytes into dendritic cells and by releasing inhibitory factors, such as PGE2 and transforming growth factor (TGF)-β, modulating natural killer cell activity, which is aimed to destroy cancer stem cells. TGF-β has been recently reported to be the most relevant MSC-mediated cytokine, exerting paracrine and direct effects on GBM proliferation and invasion [61]. This cytokine plays a role in direct MSC-GBM cell interactions as the key EMT-inducing cytokine. It affects U87 cells in two ways: (a) by switching from proliferation to invasion and (b) by exocrine function [62]. Recently, these authors pointed out similar interaction mechanisms between U87 and haematopoietic stem cells, such as adhesive intercellular contacts, interdigitating of cytoplasm and cells fusion [63]. TGF β may not be unique for MSC heterotypic interactions. Direct MSC effects relate to their anti-inflammatory action by reducing the production of tumour necrosis factor (TNF)-α and interleukin (IL)-12 and by increasing the synthesis of IL-10 by macrophages [22]. MSCs are stimulated by local factors, such as hypoxia, cytokine milieu and the toll-like receptor ligands (TLRL). Several studies describe two classes of polarized MSCs [64]. Depending on the expression of TRL, MSCs are primed by TRL4 into pro-inflammatory phenotypes, thus making MSCs capable of potentiating tumour aggressiveness. Other studies, however, claimed that MSCs can be safely used as drug delivery vectors [64]. Lastly, after their engraftment to the tumour, MSCs can differentiate into tumour-associated fibroblasts, endothelial-, pericytes [59, 65] and macrophage-like cells [64], possibly even to GSCs as hypothesized by Appaix [22]. In conclusion, MSCs in the tumour microenvironment interact with tumour cells and other stromal cells via a number of signalling molecules, forming a complex cellular cross-talk.
Indirect and Direct Mesenchymal Stem Cell and Glioblastoma Cell Cross-Talk
Indirect Interactions
GBM microenvironment is comprised of various populations of non-cancerous cells, so-called stromal cells, which have the ability to infiltrate into the brain tumour tissue. Similar to their chemotactic response to the sites of injury, MSCs also exert, possibly by the same cues, tropism to brain tumours. Noteworthy, MSCs have the ability to cross the blood-brain barrier in particular in a loose intratumour vasculature in angiogenic GBM, where they have immunosuppressive effects [59]. Further, as GBM/GSC cells diffusely spread into the neighbouring parenchyma and interact with MSCs, immune cells, fibroblasts, endothelial cells, and astrocytes. Also, several recent reports describe the association of MSCs with GBM in the microenvironment of the tumour and MSCs affecting tumour progression [61, 66–68]. MSCs are attracted to the GBM microenvironment through secretion of several soluble factors, cytokines and growth factors, such as TGF-β, CXCL12/SDF-1α, CCL5, IL-8 and CCL2/MCP-1 [60]. Motaln and Lah [69] have identified vigorous cytokine responses upon indirect MSC/GBM cross-talk, where paracrine effects were reflected by a panel of chemokines with upregulated expression, such as cKit, cMET, CXCL12/SDF-1α, CCL5, CCL2/MCP1, IL-6,IL-8 and LIF. The receptors of these cytokines are expressed by immune cells as well as by GBM cells. However, MSCs also expressed higher levels of the chemokine receptors, such as CXCR1 (IL-8 R), CXCR2 (IL-8 R) CCR2 (CCL2 R) and CXCR4 (CXCL12/SDF-1α R). To inhibit intratumour capillary formation, MSCs secrete anti-angiogenic chemokines down-regulating the PDGF/PDGFR axis, which plays a key role in periycte stabilization of new vessels. In our studies on the effects of MSCs on cell growth, proliferation and invasion, we used bioinformatics to correlate chemokine array data to cDNA microarray data of GBM U87 cells and MSC gene expression, showing that MSCs impaired growth, induced senescence, decreased invasion of U87 GBM cells and enhanced expression of adhesion proteins. We found CCL2/MCP-1 to be the most significantly regulated chemokine in U87/MSC paracrine signalling in addition to the above mentioned chemokines that may have affected the observed gene expression alterations, associated with proliferation (Pmepa-1, NF-κb, IL-6, IL-1b), invasion (ephb2, Sod2, Pcdh18, Col7A1, Gja1, Mmp1/2), and senescence (Kiaa1199, serpinb2). We confirmed the functional role of CCL2/MCP-1 and, therefore, have proposed a novel mechanism of CCL2-induced antimigratory effects on GBM cells, distinct from its immunomodulatory roles. Our data was corroborated by several recent studies on high CCL2 levels in the secretome of umbilical cord-MSC co-cultures with U251 and SNB19 GBM cells, where MSCs increased tumour cell growth and migration [56]. Moreover, CCL2 and CXCL12 were recently demonstrated to mediate the migration of MSC towards CD133+ GSC cells. In another study, where we exposed GSCs to conditioned media of bone marrow- and umbilical cord-derived MSCs, reduced GSC sphere formation and induced GSC senescence and differentiation were observed, thereby increasing GSC sensitivity to chemotherapeutic temozolomide [70]. This was in contrast to MSC effects on U251 and SNB19 cells. Hossain [20] also demonstrated that MSC-conditioned medium increased patient-derived GSCs self-renewal and the expression of stem cell genes, via IL-6/STAT3 pathways. In a similar fashion, MSC-derived exosomes, isolated from glioma-associated MSCs, increased proliferation of GSCs in vitro through transfer of RNAmir-1587 [71]. Bajetto [67] has recently demonstrated that direct interaction between umbilical cord-derived MSCs and GSCs in 3D spheroids diminished GSC proliferation as wells as reciprocal trophism between these cells.
As mentioned above, indirect modulation of immune microenvironment of GBM would indirectly affect GBM/GSC cells. Similar to dendritic cells, MSCs also overexepressed CD54 (ICAM-1) and CD106 (VCAM-1) adhesion molecule and attached to vascular cells when exposed to CCL15, CCL19, CXCL12, CXCL13 that are secreted by endothelial and immune cells. These cytokines may play a crucial role in MSC intra-tumour distribution, where the adhesion to vessel ining is required for MSC integration into the GSC artrioral niche. By secreting cytokines and modulating their signalling, MSC may also regulate the extent of intra-tumoural immune cell trafficking. The outcome of these interactions is still not clear, but may be the reason of the dual role that MSCs have on glioma progression, both in in vitro and in vivo studies [26, 70, 72–74]. On the other hand, GBM cells also induced changes in MSC phenotype upon their indirect interactions: GBM cells increased MSC proliferation as well as their invasion upon exposure to GBM-conditioned medium [25, 65].
Direct Interactions
MSCs and GBM cells direct confrontation in vitro better resemble their interactions in tumour microenvironment in vivo. As the discrepancies among the studies, using different GBM cell lines and tissues, and knowing that these are heterogeneous, we aimed to address this question by studying the differential effects of the same MSC line on U373 GBM cells, expressing more MES GBM genes and TGF-β responsive elements than in U87 cells, a less mesenchymal GBM subtype [26, 27, 75]. MSCs increased invasion of U373 cells, accompanied by the increased expression and activity of proteolytic enzymes, such as cathepsin B, calpain 1 and matrix metalloproteases (MMPs), and urokinase-type plasminogen activator (uPA), whereas invasion capability of the U87 GBM cell line was even decreased and expression of proteases was down-regulated or not altered.
Extracellular Vesicles (EVs)
Another communication pathway of intratumour interactions is EVs exchange. Figueroa et al. [71] showed that GBM-associated MSCs released exosomes, which augmented proliferation and clonogenicity of GSCs in orthotopic xenografts. This event lead to a significantly greater tumour burden and decreased host survival compared to untreated GSCs. Analysis of the EVs content identified miR-1587 as a mediator of the exosomal effects on GSCs, in part via down-regulation of the tumour suppressive nuclear receptor co-repressor NCO1, enhancing GBM aggressiveness. Secretome profiling of MSC-GBM (U87 cell) co-cultures identified 126 differentially expressed proteins. Ten of these were exclusively detected under direct cell-cell contact conditions, and most of these proteins were involved in cell motility and tissue development [61].
Heterotypic Interaction Cell Interactions in Animal Models
Several in vivo studies have shown that MSCs, systematically or locally delivered, are capable of migrating into glioma and tracking single glioma cells in the brain tissue [66, 68, 72, 73]. MSCs are able to cross the blood-brain barrier of the rats and migrate to tumour site [68]. There are reports suggesting that MSCs increased GBM cell tumourigenicity after their co-injection in mice [61, 66]. MSCs increased GSC proliferation and caused GBM dissemination, invasiveness and vascular proliferation after injection of MSCs into the caudeal vein of rats with established GBM tumours [68]. In addition, co-injection of GSCs and MSCs, derived from GBM tissue or bone marrow, respectively enhanced GSC growth and mesenchymal features in nude mice [20]. Glioma-associated MSCs release EV, which increased GSC proliferation and clonogenicity and, therefore, increased tumour burden and decreased the survival rate of nude mice [71].
On the other hand, some studies have demonstrated that intracranial injection of umbilical cord blood MSCs to the pre-established intracranial glioma prevented GBM progression [73]. MSC-treated tumours in mice showed decreased expression of apoptosis inhibitory protein (XIAP) and lower pro-migratory proteins Akt, pAkt and FAK. Simultaneously, increased levels of tumour suppressor gene PTEN, involved in the migration and invasion of cancer cells were observed [76, 77]. Furthermore, intracranial administration of umbilical cord blood MSCs to GBM tumours inhibited tumour angiogenesis in mice through downregulation of FAK, VEGF and Akt [77]. The zebrafish model is becoming an emerging tool for cancer progression studies, having several advantages, as described by Vittori et al. [78]. Xenotransplantation of human cancer cells into zebrafish embryos has another benefit as these animals have not yet developed functional immune system and thus immunosuppression is not needed. We have studied the effects of MSCs on GBM cell proliferation and invasion in the orthotopic xenotransplantation of zebrafish embryos [26]. After co-injection of MSCs and GBM cells into the zebrafish embryo brain, MSCs affected differentially the invasion of two phenotypically-different GBM cells (Fig. 1). U373 GBM cell line is less invasive in spheroid monocultures in vitro and in zebrafish brain than U87 cell line. Co-injecting GBM cells with MSCs lead to an increase of U373 GBM cell invasion and decreased U87 GBM cell invasion. Increased invasion was associated with upregulaton of cathepsin B, metalloproteases and plasminogen activation system. However, MSCs decreased the proliferation of both GBM cells. Taken together, the outcome of MSC-glioma cell interactions on glioma growth and invasion depends on the source of MSC population, i.e. bone marrow, adipose tissue, umbilical cord blood and tumour tissue, and the phenotype of glioma cells, used for studies. The timing and mode of MSC administration to animal models may affect the interactions between MSCs and glioma in vitro deepening on the mode of interactions [26, 73, 79]. Further investigations are needed to deepen our understanding of the mechanisms of MSC behaviour in tumour microenvironment and their effects on glioma pathobiology before to successful MSC-based anti-cancer therapy will be developed.
Fig. 1.
Zebrafish embryo as an animal model to study MSC-GBM cell interactions. The interactions between MSCs and U373 GBM cell line increase the invasion of U373 cells in zebrafish embryo brain (a) as described by Breznik et al. [26]. Image taken at higher magnification shows MSC-GBM mixed tumours with invasive U373 cells. MSCs follow U373 cells into the zebrafish brain parenchyma (b). Scale bars: 250 μm (A); 50 μm (B). Image courtesy of Miloš Vittori
Kinin System in Cancer
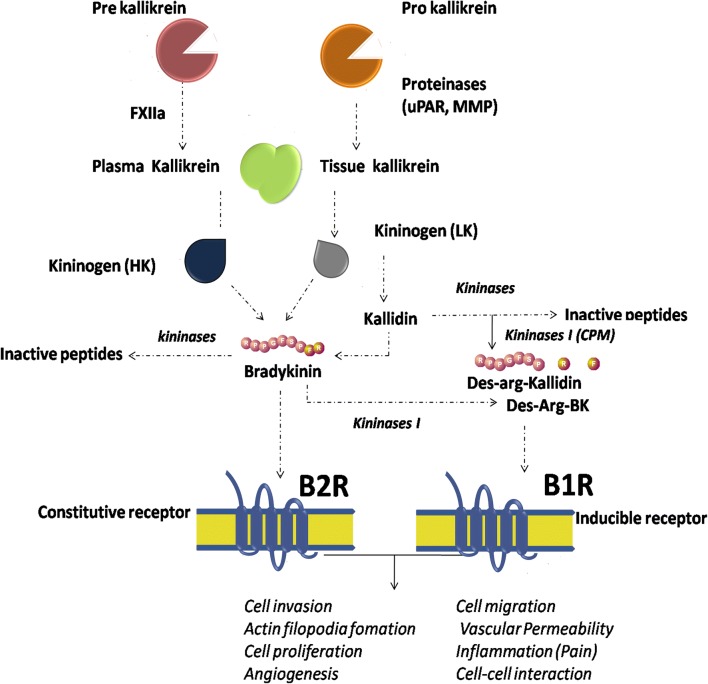
Bradykinin (BK) was first described and isolated from plasma globulins in 1949 based on its effects in reducing cardiac contractions in the presence of Bothrops jararaca snake venom [80]. BK is a low molecular weight nonapeptide of about 1.1 kDA size. In plasma and tissues, BK is rapidly proteolytically degraded by kininases and metalloproteinases (MMPs) with a half-life of 15 s, resulting in very low blood levels (0.2–7.1pM) under physiological conditions [81]. The kallikrein-kinin system activation starts with the kallikrein-mediated cleavage of kininogen to bradykinin (BK) and/or kallidin (KD) peptides. Once released, BK and KD can be proteolytically processed to its bioactive derivatives des-Arg9-bradykinin (DBK) and des-Arg9-kallidin (DKD) by M type- or N type-carboxypeptidases, through cleavage of the terminal arginine [82]. BK and KD are the main ligand of the kinin-B2 receptor (B2R), whereas DBK and DKD bind to the kinin-B1 receptor (B1R) [83] (Fig. 2). The B2R is constitutively and widely expressed throughout the central and peripheral nervous system, mediating most of the physiological effects of kinins [84], whereas B1R is not expressed to a great extent under normal conditions, but displays an essential role when rapidly upregulated following chronic inflammatory [85], infection, traumatic stimuli [86] and in cancer [87].
Fig. 2.
Schematic diagram of the kallikrein-kinin system (KKS) metabolic cascade. Plasma- and kallikrein-related peptidases are secreted as zymogens and become activated by a cascade of pro-peptide proteolytic cleavages. Kallikreins cleave serum and tissue high molecular-weight (HK) and low molecular-weight (LK) kininogens to generate bradykinin (BK) and kallidin (Lys-BK) which, in turn, are cleaved either by kininase (originating inactive peptides), or arginine aminopeptidyl carboxy-peptidases (M and N), originating their des-Arg metabolites. Kinins are rapidly inactivated, mainly by angiotensin converting enzyme (ACE, kininase II). Des-Arg-Bradykinin (DBK) and Bradykinin bind to BR1 and BR2 receptors, respectively, thus inducing signalling pathways involved in the regulation of several (patho)physiological processes, such as blood pressure variations, inflammation and cancer
Kinins are important inflammatory mediators that are also involved in other pathophysiological processes. The B1R plays a pro-tumour role via cross-talk with the B2R [88] and its activation induces cell migration [89], while B1R-specific antagonists diminish proliferation in breast cancer cells [90]. BK has been shown to regulate proliferation and promote migration of neural stem cells and neurogenic differentiation [91]. Further, it has been suggested that the binding of kinins to their respective receptors correlated with cancer cell proliferation and invasion, which was indeed observed in breast carcinoma and glioma [92–94]. However, the dynamics of brain cancer microenvironment remains poorly explored, more specifically the abnormaly permissive behaviour of the blood-brain barrier (BBB) in malignant brain tumours (often referred to as blood-tumour barrier (BTB)), which leads to various bioactive derivatives infiltrating the tumour mass [95, 96].
The Role of Kinins in Glioblastoma
GBM, like many other solid tumours, compresses zones of inflammation and necrosis that are, in fact, needed for sustained tumour growth and angiogenesis [97]. This process is partly dependent on the GBM-derived cytokines IL-1β and TNF-α, that may trigger expression and activity of B1R, thus affecting the brain tumour microcirculatory system [98]. B1R was found to actively modulate blood-brain-barrier permeability both in normal and cancerous brains [99]. We have demonstrated that MSC-GBM interaction changed the expression of BK receptors 1 and 2 in U87 cell lines [100]. We also found that certain cell-cell interactions between GBM cells and MSCs, such as vesicle transfer and nanotube formation, decreased after B1R activity was blocked [21]. In contrast, when B1R intrinsic expression is high, such as in GBM U87 cells vs U373 cells, significantly more heterotypic interactions were observed. As we demonstrated previously, [26], these two GBM cell lines also responsed differently to the presence of MSCs in vitro and in zebrafish models. In 3D co-culture with MSCs, U87 cell line increased CD29 expression, led to high compaction of the spheroid and substantial cell fusion with MSCs. In accordance with our previous work [26], this was followed by diminished U87 cell migration and invasion out of the mixed spheroids. However, after stimulation with BK and DBK, the migration was, again, highly increased [21].
Cell Migration and Invasion
Migration is a process by which cells interact with their microenvironment via different adhesion molecules, presented by other cells and the extracellular matrix, in which the latter is critical in dictating the type of cell movements [101]. Mesenchymal movement is typical in GBM cells invasion [102], where binding of adhesion proteins to their receptors generates signals that regulate the migration, releasing invasion- associated proteases [7, 103, 104]. Ifuku et al. [105] suggested that BK promoted microglial migration by activation of B1R. On the other hand, B1R and B2R activation promoted the oscillation of intracellular free calcium concentration ([Ca2+]i) in glioma cells and induced amoeboid movement of glioma cells [106]. This is analogous to modulating neuron differentiation and neuronal migration of growth cone by [Ca2+]i oscillations [91]. Our results also revealed an important role of B1R in GBM U87 cell migration [100], showing GBM cells expression of functional B1R and B2R genes and proteins that can be further activated under the influence of indirect and direct cellular cross-talk with MSCs. We have suggested that kinin receptor-mediated downstream signalling, which in direct co-cultures may affect expression of MMP9 key enzymes in pericellular proteolytic cascades, favours GBM invasion. Further work has shown that in co-culture of glioma and MSCs pharmacological blockade of B1R dramatically diminished cell migration of 3D spheroids [21]. Migrating cells form protrusions, such as invadopodia and other extensions including ruffles or spikes, all containing filamentous actin, and lead to dynamic interactions with ECM substrates [107]. The regulation in myosin-actin contractions is also under the calcium oscillations control [108] which, as mentioned above, are regulated by kinin receptors and have been proven as crucial for glioma invasion [109, 110]. BK also induced expression of smooth muscle actin in human MSCs [111]. In indirect co-cultures of GBM and MSCs, long exposure to BK led to [Ca2+]i oscillations [100], and low BK levels caused a prolonged persistence of intracellular calcium [112]. These alterations in [Ca2+]i resulted in the activation of ion channels crucial for volume and morphology changes of glioma cells during migration through narrow spaces [106]. [Ca2+]i oscillations reprogram Cl− and K+ channel activities that allow for the reduction of glioma cell volume down to 33% of its original size, and expelling free cytoplasmic water via Cl− efflux [113]. It has been suggested that BK is not only a key ligand binding to BR2 but, also, that it enhances migration of human and rat glioma cells via binding to B1R in vitro [89]. Pillat et al. [100] confirmed a BK-promoted feedback control on the expression of kinin, both in monocultures and co-cultures: treatment with BK resulted in down-regulation of B1 and B2 receptors in MSC, with simultaneous upregulation of these receptors in U87 cells, suggesting a function of BK in information flow between these cells, being important for tumour progression and invasion. Noteworthy, in addition to BK activation of B2R, the cross-talk with B1R activation is seen as an indirect response or compensation for the signalling responses between them. This points out the importance of both kinin receptor in a cross-talk and potential use of their selective inhibitors in GB therapy.
Decreased cell adhesion and increased mobility results from epithelial-to-mesenchymal-like transition (EMT), necessary for tumour cell dissemination [38]. We have observed higher levels of mesenchymal genes expression in U87 cells after coculturing with MSCs, indicating a kind of epigenetic transdifferentiation of U87 cells resulting in their increased invasion [21]. Interestingly, treatment with BK and DBK resulted in even more invasive U87 cells in 3D spheroids. Invasiveness decreased after treatment with B2R antagonist. Similarly, MSCs and breast cancer cell hybrid formations also acquired an EMT phenotype that lead to enhanced metastatic cancer cells abilities [114].
Cell Fusion and Entosis
Entosis is a form of cell cannibalism that is prevalent in human cancer, typically triggered by a loss of matrix adhesion [115]. Recently, Durgan [116] reported on an alternative mechanism for entosis in human epithelial cells, driven by mitosis, that is regulated by G protein Cdc42, which controls mitotic morphology. Cdc42 plays a crucial role in cytokinesis, cell polarization, mitosis, and induces the formation of filopodia and retraction fibreswith associated focal complexes [117]. Cdc42 depletion enhances mitotic de-adhesion and rounding. These biophysical changes, which depend on RhoA activation and are phenocopied by Rap1 inhibition, permit subsequent entosis. Interestingly, BK treatment - but not that with PDGF, insulin, PMA, LPA, or bombesin- of cells resulted in morphological and cytoskeletal effects, similar to those seen upon Cdc42 microinjection [118]. Treatment with a B1R antagonist promoted cell de-polarization and potentially cell cannibalism (Fig. 3).
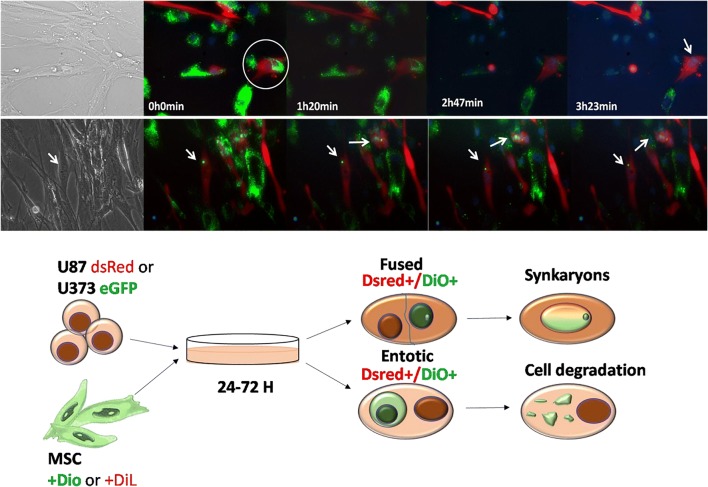
Fig. 3.
Heterotypic cell fusion and cell cannibalism. Time-lapse frame imaging of BM-MSC DiO/U87 dsRED co-culture showing entosis, cell fusion. (a) Demonstration of the entosis-like process on BM-MSC DiO (green) and U87dsRed cells (red), marked with a circle, followed by a cell fusion event, after 3 h, which is marked with an arrow (nuclear stained in blue). (b) Time-lapse depicting the nuclear fusion process. Fused nuclei exhibit a combined green staining marked by arrows. Scale bar: 50 μm. (c) Illustration of GBM cell-cell interaction with BM-MSC after co-culture, showing fused cells and the cellular degradation process. Original data published in Oliveira et al. [21]
Cell fusion occurs when cell membranes merge and cytoplasm merges to form multinucleated cells [119, 120]. Cancer cells fuse with normal cells, MSCs and other cancer cells. Homotypic cancer cell fusion contributes to the formation of polyploid giant cancer cells (PGCCs) that have been shown to be highly tumourigenic and chemoresistant [121]. Polyploidy is an intermediate karyotype that often occurs via fusion of healthy diploid cells and neoplastic aneuploidy cells [122]. It has been suggested that 0.01% of tumour cells are tumour hybrid cells with higher resistance towards chemotherapeutics [123, 124]. Chronic inflammation potentiates cell fusion in the brain, muscle, liver and heart, suggesting that inflammatory mediators affect this process [125]. It has been previously reported that bone marrow-derived cells fuse with interstinal stem or progenitor cells after inflammation-induced epithelial injury and that these events markedly increased intestinal tumour [126, 127]. Cell fusion-associated characteristics are aneuploidy and the expression of mesenchymal phenotype markers on tumour cells [128].
Cell fusion phenomena have an impact on cancer therapy, thus, the genesis of drug-resistant cancer hybrid cells is an important issue to be explored [129]. Inflammatory conditions per se or /and pro-inflammatory cytokines foster cell fusion [124]. The chemokines and cytokines prompt tumour-associated macrophages (TAMs) and MSCs to migrate into tumour microenvironments [130]. TAMs consist of M1 phenotype cells, which kill pathogens and M2 phenotype cells, which induce angiogenesis and tissue remodelling, and intermediate TAMs phenotypes. They have been suggested as major inflammatory components in tumour microenvironment that trigger expression and activity of B1R and B2R. In glioma, this process partly depends on glioma-derived IL-6 [20] and chemokines CXCR7 and CXCR4 [131]. Interestingly, B1Rs are absent in cortical areas, albeit present in endothelial cells surrounding the tumour area, possibly regulating angiogenesis [87] or cancer stem cell niches [43]. We have recently shown that treatment of GBM U87/MSC co-cultures with B1R antagonist R715 partially inhibited cell fusion [21]. Moreover, Figueroa et al. [132] have reported that endothelial cells stimulated with BK, and neutrophils primed with TNF-α (and vice versa), resulted in enhanced adhesion between both cells. This suggests that the B1R is a potential modulator, which promotes adhesion between leukocytes and endothelial cells. Following blocking B1R by antagonists, a partial compensation of cell fusion by B2R activity occurs. Therefore, a better understanding of the interplay between both kinin receptors in cell fusion modulation is needed (Fig. 4).
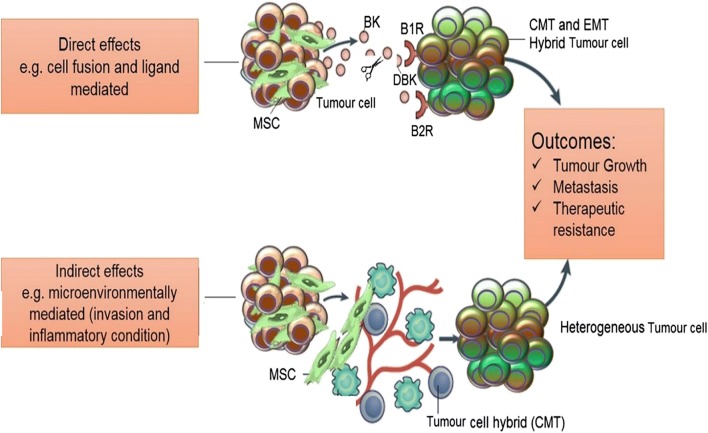
Fig. 4.
Summary of the proposed role of bradykinins in MSC-GBM cross-talk. Depiction of the heterotypic cell-cell interaction between GBM cells and MSCs in complex tumour microenvironment. This interaction is regulated by bradykinin and Des-Arg–bradykinin binding to their respective receptors in direct (above) and indirect (bellow) binding conditions. Direct binding occurs when tumour cells secret BK which, in turn, is converted to its metabolite. Indirect binding is mediated by the tumour microenvironment- cytokines and tumour cell hybrids influence MSC behaviour and lead to the formation of the cancer mesenchymal transition cells that make up the heterogenous tumour
Bradykinin-Targeting in Cancer as Adjuvant Therapy
The understanding of the roles of kinins in cancer therapy has significantly improved in recent years. The duality of B1R and B2R activation, in controlling permeability of the blood brain barrier and in enhancing tumour progression, makes them excellent candidates for therapeutic targets. An Investigational New Drug (IND) application has been approved by the Food and Drug Administration (FDA) for the clinical evaluation of B9870 in lung cancer [133]. B9870 is a potent B1R and B2R antagonist with excellent in vivo antitumour activity. However, a randomized phase 2 trial in patients with glioma receiving carboplatin with or without RMP-7 (a B2R antagonist), did not improve carboplatin efficiency [134]. In the malignant F98 glioma rat model, dual B1R and B2R activation provided enhanced blood–brain barrier permeability after carboplatin drug delivery [99]. The advancement of kinin receptor antagonists into the clinics in some tumours [92] highlights the importance of continued research also in the highly invasive GBM, where the role of kinins in aggressive tumour progression needs to be better identified in respect to GBM microenvironment.
Icatibant, a B2R inhibitor, was tested in rat glioma models and has been shown to be effective in reducing glioma cell invasion of cerebral parenchyma, ultimately resulting in a smaller tumour mass [135] . Other BK antagonists that play an important role in cancer are: B-9870, B-10054 and M-516 (listed in the Table 1) [92, 134]. B9870 is the most studied BK antagonist dimer as anti-cancer agent. This dimer is selectively cytotoxic for many types of cancer cells and acts at very low concentrations, not damaging normal cells [133]. Interestingly, this group has shown that B1R knockout of B1R (KOB1R) mice have revealed an uncontrolled tumour growth in SSR240612 (antagonist of B1R)-treated mice, which was blunted by B2R blockade with HOE-140, suggesting a crosstalk between B1R and B2R in tumour growth [94]. Combined treatment with B1R and B2R antagonists normalized the upregulation of tumour B1R and decreased both tumour size and mitotic index, and has been shown in double knockout receptors (KOB1B2R). In F98 rat glioma transient BTB disruption was promoted by activation of B1R, causing COX metabolites concentration increase, and thereby transient BTB disruption. On the other hand, B1R and B2R agonists may be used as selective BTB modulators of increased local delivery of various therapeutic agents to (peri)tumoural sites [99].
Table 1.
Kinin receptors in glioblastoma and other types of cells: Potential functionality in cancer progression
| Target | Cell type | Kinin receptor expression | Associated function | References |
|---|---|---|---|---|
| B1 receptor | C6 Mouse glioma | B1R expression and activity | Cell migration by COX and AP-1 activation | [89] |
| B1 receptor | Human microglia | B1R upregulation in response to activation | Cell motility and chemotaxis of BK-induced microglial migration is mediated by B1R but not by B2R | [105] |
| B2 receptor | D54 human GBM | Receptor expression not verified | Glioma invasion is stimulated by DBK to amoeboid type of migration | [136] |
| B1 receptor | U87 human GBM | B1R expression stimulated by bradykinin | Increase in 3D cell invasion after co-culture with MSCs | [21, 100] |
| B1 and B2 receptor | U138 and U251 human GBM | Endogenous expression of both B1R and B2R | Both B1R and B2R are involved in glioma cell proliferation | [94] |
| B1 and B2 Receptors | PC3 prostate cancer | Endogenous expression of both B1R and B2R | B1R and B2R activation is required for the proliferation of androgen-independent prostate cancer cells | [137] |
| B1R- carboxipeptidase M (CPM) heterodimers | HEK human embryonic kidney | B1R activity and CPM expression | CPM and B1 receptor mediates BK or KD stimulation of B1R signalling, independent of the enzymatic activity of CPM | [138] |
| B1R-CPM heterodimers | HEK human embryonic kidney | B1R and B2R activity and CPM expression | CPM expression is required to generate a B1R-dependent [Ca2+]i increase by kallidin or bradykinin | [139] |
| B2 receptor | Adipose–derived human MSCs (ADSCs) | B1R and B2R endogenous expression | BK induced α-Smooth Muscle Actin expression in hADSCs by TGF-β1-Smad2 signalling pathway | [111] |
| B2 receptor | 3 T3 fibroblasts | B2R endogenous expression and activity | Bradykinin promotes formation of peripheral actin microspikes and filopodia in fibroblasts | [140] |
In conclusion, it is imperative to study the role of kinin receptors as well as the B1R-B2R cross-talk compensation mechanism in the regulation of cell-cell adhesion, which could induce cell fusion and proliferation. The complex interaction between stromal cells and tumour cells in the tumour microenvironment increases the tumour non-autonomous heterogeneity and delays or alters response to therapy. Thus, understanding homotypic and heterotypic cell fusion, cell migration and tumour permeability that is regulated by kinin receptors in tumour microenvironment can be useful to design treatment protocols. Particularly ones related to potential MSC therapy application with engineered MSCs. These are suggested to be genetically modified to carry the “killing weapons,” inducing programmed cell death [54]. The summary of most relevant findings in this matter are presented in Table 1.
Acknowledgements
We acknowledge the support by an ARRS Programme P1-0245 awarded to TTL in Slovenia and the National Counsel of Technological and Scientific Development granted to HU with a Visiting Professorship (CNPq Linha 2 – Bolsa Pesquisador Visitante Especial, Edital No 61/2011) awarded to TTL in Brazil. HU further acknowledges grant support from the São Paulo Research Foundation (FAPESP proj. No. 2012/50880-4). BB was a PhD Fellow of the Jožef Stefan International Postgraduate School, Ljubljana, Slovenia. MNO was a double PhD fellow at the Biochemistry Department of the University of São Paulo-Brazil and Fellow of the Jožef Stefan International Postgraduate School, Ljubljana, Slovenia, with a fellowship from National Counsel of Technological and Scientific Development (CNPq). RLP is a graduate student at the Biochemistry Department of the University of São Paulo, with a fellowship for his PhD thesis granted by the Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil. We thank Dr. Miloš Vittori for images of glioblastoma tumours in zebrafish embryos.
Author Contributions
BB and MNO share first authorship, TTL and HU share correspondence.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Mona N. Oliveira and Barbara Breznik equally share the first authorship.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Philips A, Henshaw DL, Lamburn G, O’Carroll MJ. Brain tumours: rise in glioblastoma multiforme incidence in England 1995-2015 suggests an adverse environmental or lifestyle factor. J Environ Public Health. 2018;2018:7910754–7910710. doi: 10.1155/2018/7910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Wood MD, Reis GF, Reuss DE, Phillips JJ. Protein analysis of glioblastoma primary and posttreatment pairs suggests a mesenchymal shift at recurrence. J Neuropathol Exp Neurol. 2016;75:925–935. doi: 10.1093/jnen/nlw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q, Hu X, Muller F, et al. Tumor evolution of glioma intrinsic gene expression subtype associates with immunological changes in the microenvironment. Cancer Cell. 2017;32:42–56. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lah Tamara T, Durán Alonso María Beatriz, Van Noorden Cornelis JF. Antiprotease therapy in cancer: hot or not? Expert Opinion on Biological Therapy. 2006;6(3):257–279. doi: 10.1517/14712598.6.3.257. [DOI] [PubMed] [Google Scholar]
- 7.Gole B, Huszthy PC, Popović M, Jeruc J, Ardebili YS, Bjerkvig R, Lah TT. The regulation of cysteine cathepsins and cystatins in human gliomas. Int J Cancer. 2012;131:1779–1789. doi: 10.1002/ijc.27453. [DOI] [PubMed] [Google Scholar]
- 8.Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15:455–465. doi: 10.1038/nrn3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, Anjum S, Wang J, Manyam G, Zoppoli P, Ling S, Rao AA, Grifford M, Cherniack AD, Zhang H, Poisson L, Carlotti CG, Jr, Tirapelli DPC, Rao A, Mikkelsen T, Lau CC, Yung WKA, Rabadan R, Huse J, Brat DJ, Lehman NL, Barnholtz-Sloan JS, Zheng S, Hess K, Rao G, Meyerson M, Beroukhim R, Cooper L, Akbani R, Wrensch M, Haussler D, Aldape KD, Laird PW, Gutmann DH, Noushmehr H, Iavarone A, Verhaak RGW, Anjum S, Arachchi H, Auman JT, Balasundaram M, Balu S, Barnett G, Baylin S, Bell S, Benz C, Bir N, Black KL, Bodenheimer T, Boice L, Bootwalla MS, Bowen J, Bristow CA, Butterfield YSN, Chen QR, Chin L, Cho J, Chuah E, Chudamani S, Coetzee SG, Cohen ML, Colman H, Couce M, D’Angelo F, Davidsen T, Davis A, Demchok JA, Devine K, Ding L, Duell R, Elder JB, Eschbacher JM, Fehrenbach A, Ferguson M, Frazer S, Fuller G, Fulop J, Gabriel SB, Garofano L, Gastier-Foster JM, Gehlenborg N, Gerken M, Getz G, Giannini C, Gibson WJ, Hadjipanayis A, Hayes DN, Heiman DI, Hermes B, Hilty J, Hoadley KA, Hoyle AP, Huang M, Jefferys SR, Jones CD, Jones SJM, Ju Z, Kastl A, Kendler A, Kim J, Kucherlapati R, Lai PH, Lawrence MS, Lee S, Leraas KM, Lichtenberg TM, Lin P, Liu Y, Liu J, Ljubimova JY, Lu Y, Ma Y, Maglinte DT, Mahadeshwar HS, Marra MA, McGraw M, McPherson C, Meng S, Mieczkowski PA, Miller CR, Mills GB, Moore RA, Mose LE, Mungall AJ, Naresh R, Naska T, Neder L, Noble MS, Noss A, O’Neill BP, Ostrom QT, Palmer C, Pantazi A, Parfenov M, Park PJ, Parker JS, Perou CM, Pierson CR, Pihl T, Protopopov A, Radenbaugh A, Ramirez NC, Rathmell WK, Ren X, Roach J, Robertson AG, Saksena G, Schein JE, Schumacher SE, Seidman J, Senecal K, Seth S, Shen H, Shi Y, Shih J, Shimmel K, Sicotte H, Sifri S, Silva T, Simons JV, Singh R, Skelly T, Sloan AE, Sofia HJ, Soloway MG, Song X, Sougnez C, Souza C, Staugaitis SM, Sun H, Sun C, Tan D, Tang J, Tang Y, Thorne L, Trevisan FA, Triche T, van den Berg DJ, Veluvolu U, Voet D, Wan Y, Wang Z, Warnick R, Weinstein JN, Weisenberger DJ, Wilkerson MD, Williams F, Wise L, Wolinsky Y, Wu J, Xu AW, Yang L, Yang L, Zack TI, Zenklusen JC, Zhang J, Zhang W, Zhang J, Zmuda E. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(80):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sottoriva A, Spiteri I, Piccirillo SGM, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavare S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Felsberg J, Hentschel B, Kaulich K, Gramatzki D, Zacher A, Malzkorn B, Kamp M, Sabel M, Simon M, Westphal M, Schackert G, Tonn JC, Pietsch T, von Deimling A, Loeffler M, Reifenberger G, Weller M. Epidermal growth factor receptor variant III (EGFRvIII) positivity in EGFR-amplified glioblastomas: prognostic role and comparison between primary and recurrent tumors. Clin Cancer Res. 2017;23:6846–6855. doi: 10.1158/1078-0432.CCR-17-0890. [DOI] [PubMed] [Google Scholar]
- 15.Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JWM, Boots-Sprenger SHE, Wesseling P, Hulsebos TJM, Troost D, van Tilborg AA, Leenstra S, Vandertop WP, Bardelli A, van Noorden CJF, Bleeker FE. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro-Oncology. 2014;16:1263–1273. doi: 10.1093/neuonc/nou005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molenaar RJ, Maciejewski JP, Wilmink JW, van Noorden CJF. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene. 2018;37:1949–1960. doi: 10.1038/s41388-017-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HY, Hong IS. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci. 2017;108:1939–1946. doi: 10.1111/cas.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegi ME, Diserens A-C, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JEC, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from Temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 20.Hossain A, Gumin J, Gao F, Figueroa J, Shinojima N, Takezaki T, Priebe W, Villarreal D, Kang SG, Joyce C, Sulman E, Wang Q, Marini FC, Andreeff M, Colman H, Lang FF. Mesenchymal stem cells isolated from human gliomas increase proliferation and maintain Stemness of glioma stem cells through the IL-6/gp130/STAT3 pathway. Stem Cells. 2015;33:2400–2415. doi: 10.1002/stem.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira MN, Pillat MM, Motaln H, Ulrich H, Lah TT. Kinin-B1 receptor stimulation promotes invasion and is involved in cell-cell interaction of co-cultured glioblastoma and mesenchymal stem cells. Sci Rep. 2018;8:1299. doi: 10.1038/s41598-018-19359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appaix F, Nissou M-F, van der Sanden B, et al. Brain mesenchymal stem cells: the other stem cells of the brain? World J Stem Cells. 2014;6:134–143. doi: 10.4252/wjsc.v6.i2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patil V, Pal J, Somasundaram K (2015) Elucidating the cancer-specific genetic alteration spectrum of glioblastoma derived cell lines from whole exome and RNA sequencing. Oncotarget 6:43452–71. Doi: 10.18632/oncotarget.6171 [DOI] [PMC free article] [PubMed]
- 24.van Lith SA, Navis AC, Lenting K, et al. Identification of a novel inactivating mutation in Isocitrate dehydrogenase 1 (IDH1-R314C) in a high grade astrocytoma. Sci Rep. 2016;6:30486. doi: 10.1038/srep30486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motaln H, Gruden K, Hren M, Schichor C, Primon M, Rotter A, Lah TT. Human mesenchymal stem cells exploit the immune response mediating chemokines to impact the phenotype of glioblastoma. Cell Transplant. 2012;21:1529–1545. doi: 10.3727/096368912X640547. [DOI] [PubMed] [Google Scholar]
- 26.Breznik B, Motaln H, Vittori M, et al (2017) Mesenchymal stem cells differentially affect the invasion of distinct glioblastoma cell lines. Oncotarget 8:25482–25499. Doi: 10.18632/oncotarget.16041 [DOI] [PMC free article] [PubMed]
- 27.Motaln H, Koren A, Gruden K, et al. Heterogeneous glioblastoma cell cross-talk promotes phenotype alterations and enhanced drug resistance. Oncotarget. 2015;6:40998–41017. doi: 10.18632/oncotarget.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behnan J, Stangeland B, Hosainey SAM, Joel M, Olsen TK, Micci F, Glover JC, Isakson P, Brinchmann JE. Differential propagation of stroma and cancer stem cells dictates tumorigenesis and multipotency. Oncogene. 2017;36:570–584. doi: 10.1038/onc.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 30.Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CLL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng YD, Wang L, Kabatas S, Ulrich H, Zafonte RD. Cancer stem cells or tumor survival cells? Stem Cells Dev. 2018;27:1466–1478. doi: 10.1089/scd.2018.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teng J, Carla da Hora C, Kantar RS, Nakano I, Wakimoto H, Batchelor TT, Chiocca EA, Badr CE, Tannous BA. Dissecting inherent intratumor heterogeneity in patient-derived glioblastoma culture models. Neuro-Oncology. 2017;19:820–832. doi: 10.1093/neuonc/now253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricklefs F, Mineo M, Rooj AK, Nakano I, Charest A, Weissleder R, Breakefield XO, Chiocca EA, Godlewski J, Bronisz A. Extracellular vesicles from high-grade glioma exchange diverse pro-oncogenic signals that maintain Intratumoral heterogeneity. Cancer Res. 2016;76:2876–2881. doi: 10.1158/0008-5472.CAN-15-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T, Xu H, Huang M, Ma W, Saxena D, Lustig RA, Alonso-Basanta M, Zhang Z, O Rourke DM, Zhang L, Gong Y, Kao GD, Dorsey JF, Fan Y. Circulating glioma cells exhibit stem cell-like properties. Cancer Res. 2018;78:6632–6642. doi: 10.1158/0008-5472.CAN-18-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhat KPL, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, Conroy S, Long L, Lelic N, Wang S, Gumin J, Raj D, Kodama Y, Raghunathan A, Olar A, Joshi K, Pelloski CE, Heimberger A, Kim SH, Cahill DP, Rao G, den Dunnen WFA, Boddeke HWGM, Phillips HS, Nakano I, Lang FF, Colman H, Sulman EP, Aldape K. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohr M, Zänker KS, Dittmar T. Cancer (stem) cell differentiation: an inherent or acquired property? Med Hypotheses. 2015;85:1012–1018. doi: 10.1016/j.mehy.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Wood MD, Reis GF, Reuss DE, Phillips JJ. Protein analysis of glioblastoma primary and posttreatment pairs suggests a mesenchymal shift at recurrence. J Neuropathol Exp Neurol. 2016;75:925–935. doi: 10.1093/jnen/nlw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwadate Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol Lett. 2016;11:1615–1620. doi: 10.3892/ol.2016.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qazi MA, Vora P, Venugopal C, Sidhu SS, Moffat J, Swanton C, Singh SK. Intratumoral heterogeneity: pathways to treatment resistance and relapse in human glioblastoma. Ann Oncol. 2017;28:1448–1456. doi: 10.1093/annonc/mdx169. [DOI] [PubMed] [Google Scholar]
- 40.Podergajs N, Motaln H, Rajčević U, et al. Transmembrane protein CD9 is glioblastoma biomarker, relevant for maintenance of glioblastoma stem cells. Oncotarget. 2016;7:593–609. doi: 10.18632/oncotarget.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y, Zhou W, Cheng L, Chen C, Huang Z, Fang X, Wu Q, He Z, Xu S, Lathia JD, Ping Y, Rich JN, Bian XW, Bao S. Tetraspanin CD9 stabilizes gp130 by preventing its ubiquitin-dependent lysosomal degradation to promote STAT3 activation in glioma stem cells. Cell Death Differ. 2017;24:167–180. doi: 10.1038/cdd.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tavazoie M, Van der Veken L, Silva-Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hira VVV, Verbovšek U, Breznik B, Srdič M, Novinec M, Kakar H, Wormer J, der Swaan BV, Lenarčič B, Juliano L, Mehta S, van Noorden CJF, Lah TT. Cathepsin K cleavage of SDF-1α inhibits its chemotactic activity towards glioblastoma stem-like cells. Biochim Biophys Acta - Mol Cell Res. 2017;1864:594–603. doi: 10.1016/j.bbamcr.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Aderetti DA, Hira VVV, Molenaar RJ, van Noorden CJF. The hypoxic peri-arteriolar glioma stem cell niche, an integrated concept of five types of niches in human glioblastoma. Biochim Biophys Acta - Rev Cancer. 2018;1869:346–354. doi: 10.1016/j.bbcan.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Hira VVV, Ploegmakers KJ, Grevers F, Verbovšek U, Silvestre-Roig C, Aronica E, Tigchelaar W, Turnšek TL, Molenaar RJ, van Noorden CJF. CD133 + and nestin + glioma stem-like cells reside around CD31 + arterioles in niches that express SDF-1α, CXCR4, Osteopontin and Cathepsin K. J Histochem Cytochem. 2015;63:481–493. doi: 10.1369/0022155415581689. [DOI] [PubMed] [Google Scholar]
- 46.Verbovšek U, Van Noorden CJF, Lah TT. Complexity of cancer protease biology: Cathepsin K expression and function in cancer progression. Semin Cancer Biol. 2015;35:71–84. doi: 10.1016/j.semcancer.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Edalat L, Stegen B, Klumpp L, et al (2016) BK K+ channel blockade inhibits radiation-induced migration/brain infiltration of glioblastoma cells. Oncotarget 7:14259–78. Doi: 10.18632/oncotarget.7423 [DOI] [PMC free article] [PubMed]
- 48.Steinbichler TB, Dudás J, Skvortsov S, Ganswindt U, Riechelmann H, Skvortsova II. Therapy resistance mediated by cancer stem cells. Semin Cancer Biol. 2018;53:156–167. doi: 10.1016/j.semcancer.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Zomer A, Van Rheenen J. Implications of extracellular vesicle transfer on cellular heterogeneity in cancer: what are the potential clinical ramifications? Cancer Res. 2016;76:2071–2075. doi: 10.1158/0008-5472.CAN-15-2804. [DOI] [PubMed] [Google Scholar]
- 50.Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14:14240–14269. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skog J, Wurdinger T, Van Rijn S, et al. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers Johan. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800.Glioblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howcroft TK, Zhang HG, Dhodapkar M, Mohla S. Vesicle transfer and cell fusion: emerging concepts of cell-cell communication in the tumor microenvironment. Cancer Biol Ther. 2011;12:159–164. doi: 10.4161/cbt.12.3.17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spinelli C, Montermini L, Meehan B, Brisson AR, Tan S, Choi D, Nakano I, Rak J. Molecular subtypes and differentiation programmes of glioma stem cells as determinants of extracellular vesicle profiles and endothelial cell-stimulating activities. J Extracell vesicles. 2018;7:1490144. doi: 10.1080/20013078.2018.1490144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stuckey DW, Shah K. Stem cell-based therapies for cancer treatment: separating hope from hype. Nat Rev Cancer. 2014;14:683–691. doi: 10.1038/nrc3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torsvik A, Røsland GV, Svendsen A, et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track - letter. Cancer Res. 2010;70:6393–6396. doi: 10.1158/0008-5472.CAN-10-1305. [DOI] [PubMed] [Google Scholar]
- 56.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917–394920. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee HK, Kim HS, Kim JS, Kim YG, Park KH, Lee JH, Kim KH, Chang IY, Bae SC, Kim Y, Hong JT, Kehrl JH, Han SB. CCL2 deficient mesenchymal stem cells fail to establish long-lasting contact with T cells and no longer ameliorate lupus symptoms. Sci Rep. 2017;7:41258. doi: 10.1038/srep41258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi S, Zhang M, Guo R, Miao Y, Li B. Bone marrow–derived mesenchymal stem cell–mediated dual-gene therapy for glioblastoma. Hum Gene Ther. 2019;30:106–117. doi: 10.1089/hum.2018.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V. Concise review: Cancer cells, Cancer stem cells, and mesenchymal stem cells: influence in Cancer development. Stem Cells Transl Med. 2017;6:2115–2125. doi: 10.1002/sctm.17-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodini CO, da Silva PBG, Assoni AF, et al. Mesenchymal stem cells enhance tumorigenic properties of human glioblastoma through independent cell-cell communication mechanisms. Oncotarget. 2018;9:24766–24777. doi: 10.18632/oncotarget.25346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bryukhovetskiy I, Shevchenko V. Molecular mechanisms of the effect of TGF-β1 on U87 human glioblastoma cells. Oncol Lett. 2016;12:1581–1590. doi: 10.3892/ol.2016.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milkina E, Ponomarenko A, Korneyko M, Lyakhova I, Zayats Y, Zaitsev S, Mischenko P, Eliseikina M, Khotimchenko Y, Shevchenko V, Sharma H, Bryukhovetskiy I. Interaction of hematopoietic CD34+ CD45+ stem cells and cancer cells stimulated by TGF-β1 in a model of glioblastoma in vitro. Oncol Rep. 2018;40:2595–2607. doi: 10.3892/or.2018.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta - Rev Cancer. 2013;1836:321–335. doi: 10.1016/j.bbcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Yi D, Xiang W, Zhang Q, Cen Y, Su Q, Zhang F, Lu Y, Zhao H, Fu P. Human glioblastoma-derived mesenchymal stem cell to Pericytes transition and Angiogenic capacity in glioblastoma microenvironment. Cell Physiol Biochem. 2018;46:279–290. doi: 10.1159/000488429. [DOI] [PubMed] [Google Scholar]
- 66.Behnan J, Isakson P, Joel M, Cilio C, Langmoen IA, Vik-Mo EO, Badn W. Recruited brain tumor-derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells. 2014;32:1110–1123. doi: 10.1002/stem.1614. [DOI] [PubMed] [Google Scholar]
- 67.Bajetto A, Pattarozzi A, Corsaro A, Barbieri F, Daga A, Bosio A, Gatti M, Pisaturo V, Sirito R, Florio T. Different effects of human umbilical cord mesenchymal stem cells on glioblastoma stem cells by direct cell interaction or via released soluble factors. Front Cell Neurosci. 2017;11:312. doi: 10.3389/fncel.2017.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pavon LF, Sibov TT, de Souza AV, da Cruz EF, Malheiros SMF, Cabral FR, de Souza JG, Boufleur P, de Oliveira DM, de Toledo SRC, Marti LC, Malheiros JM, Paiva FF, Tannús A, de Oliveira SM, Chudzinski-Tavassi AM, de Paiva Neto MA, Cavalheiro S. Tropism of mesenchymal stem cell toward CD133+ stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res Ther. 2018;9:310. doi: 10.1186/s13287-018-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Motaln H, Turnsek TL. Cytokines play a key role in communication between mesenchymal stem cells and brain cancer cells. Protein Pept Lett. 2015;22:322–331. doi: 10.2174/0929866522666150131123808. [DOI] [PubMed] [Google Scholar]
- 70.Kološa K, Motaln H, Herold-Mende C, Koršič M, Lah TT. Paracrine effects of mesenchymal stem cells induce senescence and differentiation of glioblastoma stem-like cells. Cell Transplant. 2015;24:631–644. doi: 10.3727/096368915X687787. [DOI] [PubMed] [Google Scholar]
- 71.Figueroa J, Phillips LM, Shahar T, Hossain A, Gumin J, Kim H, Bean AJ, Calin GA, Fueyo J, Walters ET, Kalluri R, Verhaak RG, Lang FF. Exosomes from glioma-associated mesenchymal stem cells increase the Tumorigenicity of glioma stem-like cells via transfer of miR-1587. Cancer Res. 2017;77:5808–5819. doi: 10.1158/0008-5472.CAN-16-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 73.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., III Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Del Fattore A, Luciano R, Saracino R, et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther. 2015;15:495–504. doi: 10.1517/14712598.2015.997706. [DOI] [PubMed] [Google Scholar]
- 75.Schichor C, Albrecht V, Korte B, Buchner A, Riesenberg R, Mysliwietz J, Paron I, Motaln H, Turnšek TL, Jürchott K, Selbig J, Tonn JC. Mesenchymal stem cells and glioma cells form a structural as well as a functional syncytium in vitro. Exp Neurol. 2012;234:208–219. doi: 10.1016/j.expneurol.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 76.Dasari VR, Kaur K, Velpula KK, Gujrati M, Fassett D, Klopfenstein JD, Dinh DH, Rao JS. Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PLoS One. 2010;5:e10350. doi: 10.1371/journal.pone.0010350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Dasari VR, Velpula KK, Kaur K, Fassett D, Klopfenstein JD, Dinh DH, Gujrati M, Rao JS. Cord blood stem cell-mediated induction of apoptosis in glioma downregulates X-linked inhibitor of apoptosis protein (XIAP) PLoS One. 2010;5:e11813. doi: 10.1371/journal.pone.0011813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Vittori M, Motaln H, Turnšek TL. The study of glioma by xenotransplantation in zebrafish early life stages. J Histochem Cytochem. 2015;63:749–761. doi: 10.1369/0022155415595670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomes ED, Vieira de Castro J, Costa BM, Salgado AJ. The impact of mesenchymal stem cells and their secretome as a treatment for gliomas. Biochimie. 2018;155:59–66. doi: 10.1016/j.biochi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 80.e Silva M. Rocha, Beraldo Wilson T., Rosenfeld G. BRADYKININ, A HYPOTENSIVE AND SMOOTH MUSCLE STIMULATING FACTOR RELEASED FROM PLASMA GLOBULIN BY SNAKE VENOMS AND BY TRYPSIN. American Journal of Physiology-Legacy Content. 1949;156(2):261–273. doi: 10.1152/ajplegacy.1949.156.2.261. [DOI] [PubMed] [Google Scholar]
- 81.Negraes PD, Trujillo CA, Pillat MM, Teng YD, Ulrich H. Roles of Kinins in the nervous system. Cell Transplant. 2015;24:613–623. doi: 10.3727/096368915X687778. [DOI] [PubMed] [Google Scholar]
- 82.Björkqvist J, Jämsä A, Renné T. Plasma kallikrein: the bradykinin-producing enzyme. Thromb Haemost. 2013;110:399–407. doi: 10.1160/TH13-03-0258. [DOI] [PubMed] [Google Scholar]
- 83.Leeb-Lundberg LMF, Kang DS, Lamb ME, Fathy DB. The human B1 bradykinin receptor exhibits high ligand-independent, constitutive activity: roles of residues in the fourth intracellular and third transmembrane domains. J Biol Chem. 2001;276:8785–8792. doi: 10.1074/jbc.M007396200. [DOI] [PubMed] [Google Scholar]
- 84.Teng YD. Kinin-B1 and B2 receptors and neural differentiation of pluripotent or multipotent stem cells. Cytom Part A. 2015;87:979–981. doi: 10.1002/cyto.a.22688. [DOI] [PubMed] [Google Scholar]
- 85.Ehrenfeld P, Matus CE, Pavicic F, Toledo C, Nualart F, Gonzalez CB, Burgos RA, Bhoola KD, Figueroa CD. Kinin B 1 receptor activation turns on exocytosis of matrix metalloprotease-9 and myeloperoxidase in human neutrophils: involvement of mitogen-activated protein kinase family. J Leukoc Biol. 2009;86:1179–1189. doi: 10.1189/jlb.0109012. [DOI] [PubMed] [Google Scholar]
- 86.Marceau F. Kinin B1 receptors: a review. Immunopharmacology. 1995;30:1–26. doi: 10.1016/0162-3109(95)00011-H. [DOI] [PubMed] [Google Scholar]
- 87.Raidoo DM, Sawant S, Mahabeer R, Bhoola KD. Kinin receptors are expressed in human astrocytic tumour cells. Immunopharmacology. 1999;43:255–263. doi: 10.1016/S0162-3109(99)00097-1. [DOI] [PubMed] [Google Scholar]
- 88.Barki-Harrington L, Bookout AL, Wang G, et al. Requirement for direct cross-talk between B1 and B2 kinin receptors for the proliferation of androgen-insensitive prostate cancer PC3 cells. Biochem J. 2003;371:581–587. doi: 10.1042/BJ20021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu DY, Leung YM, Huang SM, Wong KL. Bradykinin-induced cell migration and COX-2 production mediated by the bradykinin B1 receptor in glioma cells. J Cell Biochem. 2010;110:141–150. doi: 10.1002/jcb.22520. [DOI] [PubMed] [Google Scholar]
- 90.Molina L, Matus CE, Astroza A, Pavicic F, Tapia E, Toledo C, Perez JA, Nualart F, Gonzalez CB, Burgos RA, Figueroa CD, Ehrenfeld P, Poblete MT. Stimulation of the bradykinin B1 receptor induces the proliferation of estrogen-sensitive breast cancer cells and activates the ERK1/2 signaling pathway. Breast Cancer Res Treat. 2009;118:499–510. doi: 10.1007/s10549-009-0314-4. [DOI] [PubMed] [Google Scholar]
- 91.Trujillo CA, Negraes PD, Schwindt TT, Lameu C, Carromeu C, Muotri AR, Pesquero JB, Cerqueira DM, Pillat MM, de Souza HDN, Turaça LT, Abreu JG, Ulrich H. Kinin-B2 receptor activity determines the differentiation fate of neural stem cells. J Biol Chem. 2012;287:44046–44061. doi: 10.1074/jbc.M112.407197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.da Costa PLN, Sirois P, Tannock IF, Chammas R. The role of kinin receptors in cancer and therapeutic opportunities. Cancer Lett. 2014;345:27–38. doi: 10.1016/j.canlet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 93.Kashuba E, Bailey J, Allsup D, Cawkwell L. The kinin-kallikrein system: physiological roles, pathophysiology and its relationship to cancer biomarkers. Biomarkers. 2013;18:279–296. doi: 10.3109/1354750X.2013.787544. [DOI] [PubMed] [Google Scholar]
- 94.Nicoletti NF, Sénécal J, da Silva VD, Roxo MR, Ferreira NP, de Morais RLT, Pesquero JB, Campos MM, Couture R, Morrone FB. Primary role for Kinin B1 and B2 receptors in glioma proliferation. Mol Neurobiol. 2017;54:7869–7882. doi: 10.1007/s12035-016-0265-9. [DOI] [PubMed] [Google Scholar]
- 95.Uchida M, Chen Z, Liu Y, Black KL. Overexpression of bradykinin type 2 receptors on glioma cells enhances bradykinin-mediated blood-brain tumor barrier permeability increase. Neurol Res. 2002;24:739–746. doi: 10.1179/016164102101200753. [DOI] [PubMed] [Google Scholar]
- 96.Ronaldson PT, Davis TP. Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery. Ther Deliv. 2011;2:1015–1041. doi: 10.4155/tde.11.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ishihara K, Hayash I, Yamashina S, Majima M. A potential role of bradykinin in angiogenesis and growth of S-180 mouse tumors. Jpn J Pharmacol. 2001;87:318–326. doi: 10.1254/jjp.87.318. [DOI] [PubMed] [Google Scholar]
- 98.Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol. 2012;181:1126–1141. doi: 10.1016/j.ajpath.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Côté J, Bovenzi V, Savard M, Dubuc C, Fortier A, Neugebauer W, Tremblay L, Müller-Esterl W, Tsanaclis AM, Lepage M, Fortin D, Gobeil F. Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model. PLoS One. 2012;7:e37485. doi: 10.1371/journal.pone.0037485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pillat MM, Oliveira MN, Motaln H, Breznik B, Glaser T, Lah TT, Ulrich H. Glioblastoma-mesenchymal stem cell communication modulates expression patterns of kinin receptors: possible involvement of bradykinin in information flow. Cytometry A. 2016;89:365–375. doi: 10.1002/cyto.a.22800. [DOI] [PubMed] [Google Scholar]
- 101.Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21:736–744. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 102.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 103.Levicar N, Strojnik T, Kos J, et al. Lysosomal enzymes, cathepsins in brain tumour invasion. J Neuro-Oncol. 2002;58:21–32. doi: 10.1023/A:1015892911420. [DOI] [PubMed] [Google Scholar]
- 104.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 105.Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, Merrino VF, Kita S, Iwamoto T, Komuro I, Wang B, Cheung G, Ishikawa E, Ooboshi H, Bader M, Wada K, Kettenmann H, Noda M. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci. 2007;27:13065–13073. doi: 10.1523/JNEUROSCI.3467-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seifert S, Sontheimer H. Bradykinin enhances invasion of malignant glioma into the brain parenchyma by inducing cells to undergo amoeboid migration. J Physiol. 2014;592:5109–5127. doi: 10.1113/jphysiol.2014.274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 108.Clapham DE. Calcium Signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 109.Montana V, Sontheimer H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J Neurosci. 2011;31:4858–4867. doi: 10.1523/jneurosci.3825-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diksin M, Smith SJ, Rahman R. The molecular and phenotypic basis of the glioma invasive perivascular niche. Int J Mol Sci. 2017;18:2342. doi: 10.3390/ijms18112342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim YM, Jeon ES, Kim MR, Lee JS, Kim JH. Bradykinin-induced expression of α-smooth muscle actin in human mesenchymal stem cells. Cell Signal. 2008;20:1882–1889. doi: 10.1016/j.cellsig.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 112.Bao WY, Peng C, Hui LY. Low dose of bradykinin selectively increases intracellular calcium in glioma cells. J Neurol Sci. 2007;258:44–51. doi: 10.1016/j.jns.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 113.Thompson EG, Sontheimer H. A role for ion channels in perivascular glioma invasion. Eur Biophys J. 2016;45:635–648. doi: 10.1007/s00249-016-1154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Melzer C, Yang Y, Hass R. Interaction of MSC with tumor cells. Cell Commun Signal. 2016;14:20. doi: 10.1186/s12964-016-0143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 116.Durgan J, Tseng Y-Y, Hamann JC, Domart MC, Collinson L, Hall A, Overholtzer M, Florey O (2017) Mitosis can drive cell cannibalism through entosis. Elife 6. 10.7554/eLife.27134 [DOI] [PMC free article] [PubMed]
- 117.Maiuri P, Rupprecht JF, Wieser S, Ruprecht V, Bénichou O, Carpi N, Coppey M, de Beco S, Gov N, Heisenberg CP, Lage Crespo C, Lautenschlaeger F, le Berre M, Lennon-Dumenil AM, Raab M, Thiam HR, Piel M, Sixt M, Voituriez R. Actin flows mediate a universal coupling between cell speed and cell persistence. Cell. 2015;161:374–386. doi: 10.1016/j.cell.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 118.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/MCB.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mercapide J, Rappa G, Lorico A. The intrinsic fusogenicity of glioma cells as a factor of transformation and progression in the tumor microenvironment. Int J Cancer. 2012;131:334–343. doi: 10.1002/ijc.26361. [DOI] [PubMed] [Google Scholar]
- 120.Wang R, Sun X, Wang CY, Hu P, Chu CY, Liu S, Zhau HE, Chung LWK. Spontaneous Cancer-stromal cell fusion as a mechanism of prostate Cancer androgen-independent progression. PLoS One. 2012;7:e42653. doi: 10.1371/journal.pone.0042653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bastida-Ruiz D, Van Hoesen K, Cohen M. The dark side of cell fusion. Int J Mol Sci. 2016;17:638. doi: 10.3390/ijms17050638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang D, Wang Y, Zhang S. Asymmetric cell division in Polyploid Giant Cancer cells and low eukaryotic cells. Biomed Res Int. 2014;2014:1–8. doi: 10.1155/2014/432652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dittmar T, Schwitalla S, Seidel J, Haverkampf S, Reith G, Meyer-Staeckling S, Brandt BH, Niggemann B, Zänker KS. Characterization of hybrid cells derived from spontaneous fusion events between breast epithelial cells exhibiting stem-like characteristics and breast cancer cells. Clin Exp Metastasis. 2011;28:75–90. doi: 10.1007/s10585-010-9359-3. [DOI] [PubMed] [Google Scholar]
- 124.Dittmar T, Zänker KS. Tissue regeneration in the chronically inflamed tumor environment: implications for cell fusion driven tumor progression and therapy resistant tumor hybrid cells. Int J Mol Sci. 2015;16:30362–30381. doi: 10.3390/ijms161226240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singec I, Snyder EY. Inflammation as a matchmaker: revisiting cell fusion. Nat Cell Biol. 2008;10:503–505. doi: 10.1038/ncb0508-503. [DOI] [PubMed] [Google Scholar]
- 126.Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, Grompe M, Fleming WH, Wong MH. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci. 2006;103:6321–6325. doi: 10.1073/pnas.0508593103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ogle BM, Cascalho M, Platt JL. Biological implications of cell fusion. Nat Rev Mol Cell Biol. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 128.Pfannkuche Kurt., editor. Cell Fusion. New York, NY: Springer New York; 2015. [Google Scholar]
- 129.Yan B, Wang J, Liu L. Chemotherapy promotes tumour cell hybridization in vivo. Tumor Biol. 2016;37:5025–5030. doi: 10.1007/s13277-015-4337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim SM, Jeong CH, Woo JS, Ryu CH, Lee JH, Jeun SS (2015) In vivo near-infrared imaging for the tracking of systemically delivered mesenchymal stem cells: tropism for brain tumors and biodistribution. In vivo near-infrared imaging for the tracking of systemically delivered mesenchymal stem cells: tropism for brain tumors and biodistribution Int J Nanomedicine:13. 10.2147/IJN.S97073 [DOI] [PMC free article] [PubMed]
- 131.Würth R, Bajetto A, Harrison JK, et al. CXCL12 modulation of CXCR4 and CXCR7 activity in human glioblastoma stem-like cells and regulation of the tumor microenvironment. Front Cell Neurosci. 2014;8:1–19. doi: 10.3389/fncel.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Figueroa CD, Matus CE, Pavicic F, Sarmiento J, Hidalgo MA, Burgos RA, Gonzalez CB, Bhoola KD, Ehrenfeld P. Kinin B 1 receptor regulates interactions between neutrophils and endothelial cells by modulating the levels of mac-1, LFA-1 and intercellular adhesion molecule-1. Innate Immun. 2015;21:289–304. doi: 10.1177/1753425914529169. [DOI] [PubMed] [Google Scholar]
- 133.Stewart JM, Gera L, Chan DC, Bunn Jr PA, York EJ, Simkeviciene V, Helfrich B. Bradykinin-related compounds as new drugs for cancer and inflammation. Can J Physiol Pharmacol. 2002;80:275–280. doi: 10.1139/y02-030. [DOI] [PubMed] [Google Scholar]
- 134.Whalley ET, Figueroa CD, Gera L, Bhoola KD. Discovery and therapeutic potential of kinin receptor antagonists. Expert Opin Drug Discov. 2012;1:1–20. doi: 10.1517/17460441.2012.729038. [DOI] [PubMed] [Google Scholar]
- 135.Montana V, Sontheimer H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J Neurosci. 2011;31:4858–4867. doi: 10.1523/JNEUROSCI.3825-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seifert S, Sontheimer H. Bradykinin enhances invasion of malignant glioma into the brain parenchyma by inducing cells to undergo amoeboid migration. J Physiol. 2014;592:5109–5127. doi: 10.1113/jphysiol.2014.274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barki-Harrington L, Bookout AL, Wang G, et al. Requirement for direct cross-talk between B1 and B2 kinin receptors for the proliferation of androgen-insensitive prostate cancer PC3 cells. Biochem J. 2003;371:581–587. doi: 10.1042/bj20021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the Kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J Biol Chem. 2011;286:18547–18561. doi: 10.1074/jbc.M110.214940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang X, Tan F, Zhang Y, Skidgel RA. Carboxypeptidase M and Kinin B1 receptors interact to facilitate efficient B1 signaling from B2 agonists. J Biol Chem. 2008;283:7994–8004. doi: 10.1074/jbc.M709837200. [DOI] [PubMed] [Google Scholar]
- 140.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/MCB.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]