Abstract
In most tumors, cancer cells show the ability to dynamically transit from a non-cancer stem-like cell to a cancer stem-like cell (CSC) state and vice versa. This cell plasticity has been associated with the epithelial-to-mesenchymal transition program (EMT) and can be regulated by tumor cell-intrinsic mechanisms and complex interactions with various tumor microenvironment (TME) components. These interactions favor the generation of a specific “CSC niche” that helps maintain the main properties, phenotypic plasticity and metastatic potential of this subset of tumor cells. For this reason, TME has been recognized as an important promoter of tumor progression and therapy resistance. Tumors have evolved a network of immunosuppressive mechanisms that limits the cytotoxic T cell response to cancer cells. Some key players in this network are tumor-associated macrophages, myeloid-derived suppressor cells and regulatory T cells, which not only favor a pro-tumoral and immunosuppressive environment that supports tumor growth and immune evasion, but also negatively influences immunotherapy. Here, we review the relevance of cytokines and growth factors provided by immunosuppressive immune cells in regulating cancer-cell plasticity. We also discuss how cancer cells remodel their own niche to promote proliferation, stemness and EMT, and escape immune surveillance. A better understanding of CSC-TME crosstalk signaling will enable the development of effective targeted or immune therapies that block tumor growth and metastasis.
Keywords: Immunosuppressive immune cells, Cancer-cell plasticity, Cancer stem cells, EMT, Metastasis, Therapy response
Introduction
In recent years, various studies have been directed towards understanding cancer-cell plasticity, which is defined as the ability to switch between cancer stem-like cell (CSC) and non-CSC states [1], along with transdifferentiation capability [2, 3], and how these processes affect tumor growth and progression. In some tumors, the dynamic acquisition of CSC features has been associated with the induction of the epithelial-to-mesenchymal transition (EMT) program, which promotes the motility and invasion of the epithelial-shaped cells and induces a mesenchymal-like phenotype that favors metastasis [4–6]. Although most studies have focused on tumor cell-intrinsic mechanisms, such as genetic and epigenetic alterations, attention has recently turned towards understanding how tumor cell-extrinsic factors provided by the tumor microenvironment (TME) influence cancer progression and response to therapy [6–8].
The TME is composed of fibroblasts, mesenchymal stem cells, endothelial cells and pericytes, extracellular matrix (ECM) proteins, immune cells, including macrophages, myeloid-derived suppressor cells (MDSCs), natural killer (NK) cells, dendritic cells (DCs) and B and T cells, among others, and a variety of factors secreted by these cells [9]. It is well known that the communication between cells and their microenvironment is important for tissue homeostasis, the immune cells being responsible for host defense, tissue remodeling and the elimination of dying cells. Similarly, acquisition of mutations and the altered expression of some proteins in cancer cells activate a cytotoxic immune response that eradicates these malignant cells. However, there is growing evidence that TME can change in response to cancer cell-derived signals to favor tumor growth and progression [7, 10]. In this scenario, tumor stromal cell-derived signaling contributes to sustain cancer cell proliferation, survival and invasion, as well as metastasis and angiogenesis [11]. Given that TME may display anti- or pro-tumoral properties, it has been suggested that the re-education of stroma cells towards anti-tumor activity may be an effective therapeutic strategy [12]. These bidirectional interactions favor the generation of a specific “CSC niche” that helps conserve the main properties of this subset of tumor cells [13] and preserves their phenotypic plasticity. As CSCs drive long-term tumor growth, are responsible for relapse after therapy [14], and are considered the most likely candidates for metastasis generation [15], a thorough understanding of CSC-TME crosstalk is fundamental for innovative therapeutic strategies development.
Tumors have evolved mechanisms to attenuate the effectiveness of T cells, whereby these cells become exhausted and dysfunctional, and therefore ineffective in attacking cancer cells [16, 17]. Tumors achieve this by creating an immunosuppressive network enriched in soluble mediators and specific immune cell populations. Components of TME, such as tumor-associated macrophages (TAMs), MDSCs and regulatory T (Treg) cells, which are highly effective in inhibiting T cell activation and proliferation, generate a tumor-promoting local environment that negatively influences immunotherapy [18, 19]. In addition, defects in antigen presentation, such as losing the expression of major histocompatibility complex (MHC) proteins in tumor cells, prevents them being recognized by cytotoxic T cells, precluding the elimination of these malignant cells [20]. Tumor cells and some immunosuppressive cells hijack the so-called ‘immune checkpoint’ pathways, which are important for maintaining self-tolerance and protecting tissues from damage. The best characterized immune-checkpoint receptors are cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), which bind to CD80/CD86 and PD-L1/PD-L2 ligands, respectively, to initiate checkpoint signaling and cytotoxic T cell inhibition. Tumor cells take advantage of these regulatory mechanisms and induce the expression of these ligands to prevent anti-tumor responses [21].
This review concerns itself with the relevance of cytokines and growth factors provided by immunosuppressive immune cells in regulating cancer-cell plasticity and tumor progression, and with how cancer cells remodel their own niche to promote their proliferation, stemness and EMT, and to escape immune surveillance. We also discuss recent therapeutic strategies that target the tumor-associated stroma to prevent disease progression.
Immune Cells Contributing to the Tumor-Immunosuppressive Network
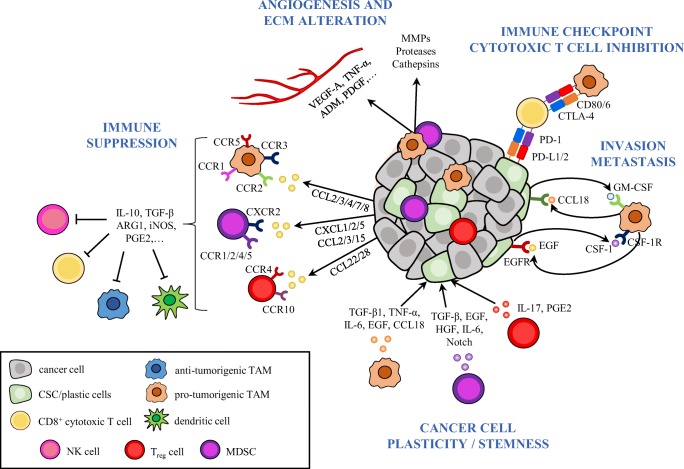
Several populations of tumor-immunosuppressive cells have been described in recent years. Here, we focus on the best characterized immune cells with an immunosuppressive function, notably TAMs, MDSCs and Treg cells (Fig. 1).
Fig. 1.
The tumor-immunosuppressive network limits cytotoxic immune-cell responses to cancer cells and promotes cancer-cell plasticity. This schema illustrates some of the mechanisms evolved by tumors to block cytotoxic immune cell responses and to promote tumor progression and metastasis. Immune cells, such as TAMs, MDSCs, and Treg cells are recruited into the tumor and become educated by cancer (stem) cell-derived cytokines and chemokines to acquire pro-tumorigenic functions. These cancer and tumor-infiltrating immune cells disrupt immune surveillance through multiple mechanisms, including inhibition of antigen presentation by DCs, T cell proliferation, M1 macrophage polarization and NK-cell cytotoxicity. In addition, cancer cells and immunosuppressive TAMs inhibit cytotoxic T cell function by activating immune checkpoint pathways. Immunosuppressive cells enhance tumor progression by directly promoting EMT and CSC properties in cancer cells through the secretion of cytokines such as TGF-β, IL-6, EGF, among others, and support invasion and metastasis by secreting a variety of pro-tumorigenic cytokines and growth factors. Paracrine signal loops between CSCs and TAMs have been described, which enhance cancer-cell plasticity, immunosuppression and tumor progression. Hence, CSC-derived CSF-1 and GM-CSF promote TAM activation and, in turn, macrophage-derived EGF or CCL18 induce EMT, CSC state, and tumor cell invasion. Finally, immunosuppressive TAMs and MDSCs produce pro-angiogenic factors such as VEGF, TNF-α, ADM, PDGF, among others, to promote angiogenesis, or secrete MMPs, serine proteases, and cathepsins, leading to ECM alterations. MDSC, myeloid-derived suppressor cell; NK, natural killer; TAM, tumor-associated macrophage; Treg, regulatory T cells
Tumor-Associated Macrophages (TAMs)
Macrophages are terminally differentiated myeloid cells originating from monocytic precursors, whose functions are to eliminate infectious agents, promote wound healing and regulate adaptive immunity [22]. In mice, macrophages are characterized by the expression of markers such as CD11b, F4/80 and colony-stimulating factor-1 receptor (CSF-1R), and low levels of expression of Gr1, whereas, in humans, macrophages are identified by the expression of CD68, CD16, CD14, CD312, CD115, and other markers [23].
Macrophages have usually been classified into two subtypes according to their polarization state and their functional role. M1 or ‘classically activated’ macrophages are activated via T helper type I (Th1)-derived cytokines and/or bacterial products such as lipopolysaccharide (LPS). M1 macrophages secrete pro-inflammatory cytokines (IL-12, IL-1β, IL-6, IL-23 and tumor necrosis factor α (TNF-α)), generate reactive oxygen species and nitric oxide (NO) and express a high level of MHC class II. These cells have a tumoricidal function. By contrast, M2 or ‘alternatively activated’ macrophages are activated in response to T helper type II (Th2)-derived cytokines, such as IL-4, IL-10, IL-13, and glucocorticoid hormones. These cells are characterized by a high level of expression of scavenging, mannose and galactose receptors, activation of the arginase (ARG1) pathway, production of IL-10, vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and these cells facilitate tumor progression [24, 25]. Although the M1/M2 nomenclature has been useful for many years, it is now known that macrophages can change their polarity in response to environmental stimuli, acquiring intermediate plastic phenotypes. For this reason, it has been proposed that the TAM classification should be based on the anti-tumorigenic or pro-tumorigenic function of the macrophages [23].
The recruitment of monocytes into tumors is primarily regulated by tumor cell-derived cytokines, chemokines, and growth factors, such as CCL2, CCL7, CCL8, CCL3, CCL4, colony stimulating factor-1 (CSF-1), granulocyte-macrophage CSF (GM-CSF), macrophage-stimulating protein (MSP), platelet-derived growth factor (PDGF), VEGF-A, and transforming growth factor-β1 (TGF-β1). These cells may then differentiate into immunosuppressive M2-like macrophages in response to IL-4, IL-10, and IL-13 [10, 26].
In human colorectal, stomach and skin tumors, the presence of TAMs is associated with a favorable outcome, whereas in breast, prostate, ovarian, and cervical cancer, TAMs are linked to poor prognosis, suggesting that TAM infiltration may be a potentially prognostic marker of clinical outcomes. These disparate observations may be a consequence of the detection of macrophages with distinct differentiation features (pro-inflammatory M1-like vs. pro-tumoral M2-like cells). For instance, in colorectal cancer, pro-inflammatory TAMs attract T cells to the tumor site, promoting type-I T cell responses that lead to the inhibition of tumor-cell proliferation [27]. However, other studies demonstrated that TAMs support tumor growth and progression [23] by promoting angiogenesis [28], enhancing invasion and metastasis [29], and protecting tumor cells from apoptosis [30].
Immunosuppressive TAMs (M2-like) subvert anti-tumor immunity by eliminating M1-like immune responses and by impairing CD8+ T cell activation through direct interaction with these cells or by secreting immunosuppressive cytokines such as IL-10, TGF-β, ARG1, prostaglandins, and proteases [31] (Fig. 1). Macrophages express PD-L1/PD-L2 and CD80/CD86, which bind with the inhibitory receptors PD-1 and CTLA-4, respectively, resulting in the inhibition of T cell cytotoxic function. PD-L1 expression in TAMs is upregulated under hypoxic conditions, via hypoxia-inducible factor-1α (HIF-1α) signaling [32, 33]. In addition, TAMs can express PD-1, which is associated with a reduction of their phagocytosis activity. PD-1+ TAMs have a polarization M2-like and pro-tumorigenic function, and the blockade of PD-1/PD-L1 increases the phagocytosis activity of these cells, reducing tumor growth [34]. Therefore, anti-PD-1/PD-L1 therapy can block tumor growth by promoting CD8+ T cell activation and anti-tumor activity of TAMs.
Myeloid-Derived Suppressor Cells (MDSCs)
MDSCs play an important role in tumor progression by evading the host’s immune response. These cells arise as a consequence of the aberrant myelopoiesis associated with cancer, and are functionally defined as immunosuppressive immature myeloid cells [35]. Two main populations have been described: granulocytic or polymorphonuclear MDSCs (PMN-MDSCs), and monocytic MDSCs (M-MDSCs) [36]. PMN-MDSCs share phenotypic features with neutrophils, but are less phagocytic. By contrast, M-MDSCs exhibit a similar phenotype to that of inflammatory monocytes and differentiate into immunosuppressive macrophages and DCs under specific TME signals. In mice, PMN-MDSCs are identified by the expression of CD11b+Ly6G+Ly6Clow and M-MDSCs as CD11b+Ly6G−Ly6Chigh, whereas, in humans, PMN-MDSCs are CD11b+CD14−CD15+CD33+ cells, and M-MDSCs are CD11b+CD14+ CD15−CD33+HLA−DR−/lo cells.
M-MDSCs are recruited to tumor sites by tumor cell-derived cytokines, such as CCL2 and CCL5. In contrast, PMN-MDSCs are recruited by tumor cell-derived CXCL1, CXCL2, CXCL5, CXCL6, CXCL8, and CXCL12, as well as CCL2, CCL3, and CCL15 [37]. CXC chemokine receptor 2 (CXCR2) binds CXCL1, CXCL2, and CXCL5, among other chemokines, and plays an important role in the tumor recruitment of PMN-MDSCs. Several studies have shown that MDSCs disrupt immune surveillance mechanisms, such as T cell activation, DC antigen presentation, M1-like polarization, and NK cell cytotoxicity (Fig. 1). Factors implicated in MDSC suppressive activity include: i) the production of ARG1, which leads to the deprivation of arginine that is essential for several T cell functions; ii) inducible nitric oxide synthase (iNOS), which promotes NO synthesis; iii) production of reactive oxygen species (ROS); and iv) expression of cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), IL-10, and TGF-β, which induce severe anergy of effector immune cells. NO and ROS both induce T cell apoptosis and the nitration of cytokines and T cell receptor (TCR), which block T cell migration and cytotoxic activities against tumor cells. In addition, MDSCs upregulate the expression of PD-L1, blocking anti-tumor T cell-mediated activity via interaction with the PD-1 receptor of these cells [38, 39].
More recently, other MDSC functions have been described, such as the formation of a pre-metastatic niche [40], enhancement of tumor growth and invasion, and stimulation of angiogenesis [41] (see below). Furthermore, an elevated frequency of MDSCs is positively correlated with advanced disease and poor therapeutic response in patients with a range of cancers, including melanoma, colorectal, breast, bladder, thyroid and non-small-cell lung cancer (NSCLC) [42, 43]. Therefore, targeting MDSCs is emerging as an attractive approach to the design of new cancer treatments.
Regulatory T (Treg) Cells
Treg cells are characterized by the expression of markers such as CD4, CD25 and forkhead box P3 (FOXP3) that plays an important role in Treg-cell development and function [44]. Under physiological conditions, Treg cells regulate the activation of T and B cells and maintain the homeostasis of cytotoxic lymphocytes [45]. In contrast, Treg cells fulfill different functions during tumorigenesis, depending on the environmental stimuli, which means that they can be associated with poor prognosis, as in gastric, esophageal, pancreatic, liver, and breast carcinoma [46–50], or with improved survival, as in colorectal cancer [51]. Treg-cell recruitment in a range of human cancer types is mediated by tumor cell-derived CCL22 and CCL28, which attract CCR4- and CCR10-expressing Treg cells, respectively [52–54] (Fig. 1).
Similar to MDSCs, Treg cells facilitate tumor progression by interfering with the cytotoxic activity of T cells and suppressing tumor-antigen presentation [55]. In this regard, Treg cells secrete immunosuppressive cytokines, such as IL-10, IL-35, and TGF-β, and block the activity of T cells by direct interaction with these cells. In addition, Treg cells express CD39 and CD73, which induce metabolic alterations that suppress cytotoxic T cells and/or NK activity [56–58].
Impact of Tumor-Infiltrating Immunosuppressive Cells on Cancer-Cell Plasticity
Cancer-cell plasticity has been associated with the induction of the EMT program [4, 5], which promotes the acquisition of CSC properties. The induction of EMT is mediated by many growth factors and cytokines, such as TGF-β, hepatocyte growth factor (HGF), epidermal growth factor (EGF), NF-κB, Notch, and Wnt [59]. Growing evidence indicates that TAMs, MDSCs and Treg cells enhance tumor progression by promoting EMT and CSCs properties in tumor cells (Fig. 1). Indeed, M2-like TAMs, through TLR4/IL-10 signaling pathway [60] or via TGF-β1 [61], promote EMT and CSC-like properties in pancreatic and hepatocellular carcinoma, respectively. TNF-α secreted by pro-inflammatory TAMs induces a switch from a differentiated to a dedifferentiated phenotype in melanoma cells, which escape immune surveillance and contribute to tumor relapse [62]. Furthermore, mesenchymal-like breast cancer cells induce immunosuppressive TAM activation via GM-CSF. In turn, these activated macrophages secrete CCL18, creating a positive feedback loop that promotes EMT and metastasis [63]. TAM-derived IL-6 activates the STAT3 pathway to promote CSC self-renewal in breast and hepatocellular carcinoma [64]. Similarly, EGF released from TAMs activates the EGFR/STAT3 pathway of breast cancer cells, inducing the expression of SOX2 and other target genes involved in CSC state maintenance [65]. In addition, Lu and colleagues demonstrated that TAMs and tumor-associated monocytes physically interact with mouse mammary CSCs to support the maintenance of the stem-like state in this subset of tumor cells. EphA4 binding to its receptor on tumor cells resulted in the activation of Src and NF-κB signaling favoring the secretion of numerous cytokines involved in CSC maintenance [66].
In addition, CSCs can enhance the pro-tumor function of tumor-recruited macrophages. Hence, CSC-derived IFNβ induces the secretion of TAM-derived IFN-stimulated gene 15 (ISG15), which promotes CSC self-renewal and tumor-initiating features in pancreatic ductal adenocarcinomas (PDAC) [67]. Furthermore, PDAC CSCs secrete Nodal/Activin A and TGF-β1, which induces an M2 phenotype that favors CSC self-renewal and invasion through the secretion of hCAP-18/LL-37 [68].
Several studies showed that MDSCs are also involved in the regulation of EMT and CSC features [69, 70]. MDSC-induced EMT occurs through the upregulation of COX-2 and the activation of the β-catenin/TCF4 pathway in nasopharyngeal cells, or through the stimulation of miR-101 expression in ovarian cancer cells [69, 71]. Furthermore, tumor-infiltrated MDSCs, which show signal transducer and activator of transcription 3 (STAT3) signaling activation, promote the stemness of pancreatic cancer cells by increasing the expression of SNAIL, SLUG, ZEB1, NANOG, and OCT-4 [72]. In this regard, studies based on a mouse model of breast cancer implicated MDSC-derived IL-6 in increasing tumor cell stemness [73]. Zhu et al showed that CXCR2+ MDSCs are recruited and expanded during breast cancer progression, which promotes cancer-cell EMT via IL-6, and T cell exhaustion [74].
In ovarian cancer, the EMT-inducer transcription factor SNAIL induces CXCL1/2 expression through the NF-kB pathway and enhances MDSC tumor infiltration via CXCR2 [75]. CSCs stimulate the accumulation of MDSCs via the elevated production of G-CSF in mammary syngeneic tumor models. In turn, MDSCs trigger NOTCH signaling activation to enhance CSC properties in tumor cells, establishing a feedforward loop [76]. Accordingly, Peng and colleagues demonstrated that MDSCs endow breast cancer cells with stem-like features through IL6/STAT3 and NO/NOTCH crosstalk signaling, and their targeting may offer an opportunity to improve cancer therapies [77]. Although many studies have not distinguished between MDSC populations, a recent report demonstrated that M-MDSCs and PMN-MDSCs have opposite effects on tumor cells. Indeed, tumor-infiltrated M-MDSCs facilitate cancer-cell dissemination by inducing EMT/CSC properties. In contrast, pulmonary PMN-MDSCs support metastasis by reverting the EMT phenotype and promoting tumor-cell proliferation [78].
Finally, some reports have indicated that tumor-infiltrating Treg cells contribute to cancer-cell plasticity. Indeed, the activation of AKT and MAPK signaling pathways by FOXP3+ Treg-derived IL-17, and by PGE2-mediated NF-κB activation, induce the expansion of the CSC population in colorectal cancer [79–81].
CSC/EMT and Immune Evasion
A comprehensive study that immunophenotyped around 9000 samples of different solid cancers by their transcriptomic signature showed that tumor features may influence the characteristics of their immune infiltrate [82]. Tumors that are enriched for the EMT program, focal adhesion, ECM remodeling, angiogenesis, inflammation, and hypoxia genes, are associated with the infiltration of immunosuppressive cells, such as macrophages [82]. Several studies have revealed that CSC escape of immune surveillance occurs by several mechanisms. In this regard, CD44+ CSCs of head and neck carcinomas express PD-L1, which binds to PD-1 receptor on T cells, blocking their cytotoxic function [83]. Dongre et al showed that epithelial-like breast tumors exhibit high levels of CD8+ T cells and antitumor M1-like macrophages. In contrast, tumors arising from breast mesenchymal-like cells with an induced EMT, and a high level of expression of PD-L1, contain Treg cells, M2-like macrophages and exhausted CD8+ T cells. These findings indicate that tumor cells with an induced EMT program promote the recruitment of immunosuppressive cells and escape immune surveillance [84]. Accordingly, ZEB1 expression relieves microRNA-200 (miR-200) repression of PD-L1 on NSCLC cells, leading to CD8+ T cell immunosuppression and metastasis [85]. However, while epithelial tumor-bearing mice respond to anti-CTLA-4 immunotherapy, mesenchymal tumor-bearing mice are refractory to this treatment, suggesting that the immunosuppressive microenvironment of mesenchymal-like tumors makes them resistant to this therapy [84]. Accordingly, characterization of transcriptomic features in metastatic melanoma showed that resistant and non-responding melanomas to anti-PD-1 therapy display a gene signature characterized by the up-regulation of genes involved in EMT, angiogenesis and immunosuppression, characterized by monocytes and macrophages infiltration. These observations suggest that the mesenchymal and immunosuppressive phenotype is associated with innate anti-PD-1 resistance [86].
Another mechanism used by cancer cells to escape immune surveillance operates through alterations in the expression of MHC-I and MHC-II proteins. These proteins are required to elicit the immune responses by T lymphocytes, and MHC-I is downregulated in glioblastoma and breast CSCs [84, 87]. However, glioblastoma CSCs express various ligands of activating NK receptors, making them susceptible to NK-mediated cell cytotoxicity [88].
Impact of Tumor-Infiltrating Immune Cells on Tumor Progression and Metastasis
Several studies have associated immunosuppressive TAMs with tumor-cell invasion and metastasis. The induction of angiogenesis is crucial to the development and growth of most solid tumors. TAMs produce a plethora of pro-angiogenic factors, including VEGF, TNF-α, adrenomedullin (ADM), PDGF, TGF-β, and these cells are involved in promoting the formation of a vascular network during malignant progression in a mouse model of breast cancer [28]. In this regard, VEGF-A helps enhance vascularization and establishes an anti-inflammatory microenvironment, which is characterized by the presence of M2-polarized macrophages in a model of skin carcinogenesis [89]. Consistent with this, TAM depletion in Csf1-null mice or by using liposome-encapsulated clodronate reduces angiogenesis in various tumor models [90].
In addition, immunosuppressive TAMs promote tumor-cell invasion and metastasis via secretion of MMPs, serine proteases, and cathepsins, which alter the ECM, modify cell-cell contacts and induce basal membrane disruption (Fig. 1). In this regard, cancer cell-secreted IL-4 induces a M2 macrophage polarization state characterized by increased cathepsin activity, and synergizes with IL-6 and IL-10 to promote tumor growth and invasion in pancreatic cancer [91, 92]. This synergy is dependent on the STAT3 and STAT6 interaction, which activates inositol-requiring enzyme 1α (IRE1α), leading to enhanced cathepsin secretion [92].
CSF-1 promotes the development of invasive and metastatic carcinomas by regulating the recruitment of TAMs at the tumor site [93]. CSF-1R inhibition abrogates TAM infiltration and enhances CD8+ T cell recruitment, reducing cervical and mammary tumor growth and progression [94]. However, it was observed that glioma cell-supplied GM-CSF, IFNγ, and CXCL10 promote TAM survival upon CSF-1R inhibition, although these TAMs lose M2 polarization and show enhanced phagocytosis activity [95]. Similarly, the inhibition of CCL2-CCR2 signaling specifically blocks macrophage recruitment within the tumor, which is associated with reduced metastasis and enhanced mouse survival [26]. A paracrine signaling loop between tumor-derived CSF-1 and macrophage-derived EGF has been linked to increased carcinoma cell invasion [96, 97]. Accordingly, M2-polarized TAMs by IL-4-expressing TH2-CD4+ lymphocytes produced high levels of EGF, which enhance tumor-cell invasion, migration and lung metastasis by activating EGFR-signaling in breast cancer cells [98]. Therefore, these studies show that CD4+ T cells can enhance breast cancer-cell dissemination and metastasis through their ability to regulate the pro-tumor properties of TAMs.
Several studies have described the impact of TAMs on the efficacy of chemotherapeutic agents (reviewed in [99]). TAMs protect myeloma cells from chemotherapy-induced apoptosis by inhibiting the activation of caspase-dependent apoptotic signaling [30]. These immune cells contribute to cisplatin-chemoresistance of lung and colon CSCs through STAT3 and Hedgehog signaling by releasing milk-fat globulin epidermal growth factor-8 protein (MFG-E8) [100]. The juxtacrine activation of α4-integrin-expressing TAMs by vascular cell adhesion molecule-1 (VCAM-1)-positive breast cancer cells promotes tumor cell survival via the induction of PI3K/AKT signaling during lung metastasis development [101]. Interestingly, VCAM-1 also interacts with α4β1 in osteoclasts, contributing to bone metastasis [102]. Together, these findings demonstrate that tumor cells co-opt TAMs in order to survive therapies, and that the disruption of signaling axes between stromal and tumor cells may serve to prevent metastatic colonization.
On the other hand, several studies have also related MDSC function to tumor-cell migration, invasion and metastasis. The recruitment of MDSCs to the tumor site and to the pre-metastatic niche is induced by several cancer cell-derived chemokines, and is mediated by the expression CXCR2 in MDSCs, among others (see section 2.2). Hypoxia can enhance MDSC migration to the tumor site via HIF-1α-mediated production of chemokines [103]. VEGF-A secreted by colorectal carcinoma cells stimulated TAMs to produce CXCL1, which favored the recruitment of circulatory CXCR2+ MDSCs into pre-metastatic niche in order to promote liver metastasis [104]. Furthermore, hypoxia-induced lysyl oxidase (LOX), S100A8/A9, IL-6, and IL-10 are also implicated in the MDSC recruitment and the establishment of the pre-metastatic niche [105–107].
On the other hand, deletion of the gene encoding TGF-β receptor II (Tgfbr2) in mammary carcinoma cells increases MDSC infiltration into tumors through SDF-1/CXCR4 and CXCL5/CXCR2 axes. These tumor-recruited MDSCs directly promoted tumor-cell invasion and metastasis through enhanced MMP and TGF-β secretion [108]. Kumar and colleagues demonstrated that the upregulated expression of ΔNp63 transcription factor is correlated with an increased number of MDSCs in triple-negative breast cancer (TNBC) patients. MDSC recruitment to the primary tumor and metastatic sites occurs through the direct ΔNp63-dependent activation of the chemokines CXCL2 and CCL22. Likewise, CXCR2/CCR4 inhibitors reduce MDSC recruitment, angiogenesis and metastasis, and MDSCs secrete pro-metastatic factors, such as MMP9 and chitinase-3-like 1 to promote TNBC CSC function [109]. All these studies provide a rationale for the development of CXCR2 antagonists to prevent metastatic spread of tumor cells.
Finally, MDSCs are known to play a role in promoting angiogenesis. Hence, MDSC recruitment inhibition mediated by stem-cell factor (SCF) mRNA interference in breast tumor cells restores proliferation of tumor-infiltrating T cells, and the blockade of SCF receptor (cKit) prevents tumor-specific T cell anergy, Treg development, and angiogenesis [110]. In addition, MDSCs secrete Bombina variegata peptide 8 (Bv8), whose expression is upregulated by STAT3 signaling. STAT3 activation can also directly induce the secretion of VEGF and bFGF by MDSCs [111]. Blockade of Bv8 in combination with VEGF antibody inhibits angiogenesis and tumor growth [112]. Although VEGF antibody-mediated therapy has had some success in the clinic setting, tumors eventually become refractory to this treatment. MDSC recruitment could be a key mechanism mediating this resistance, as MDSCs can promote new vessel growth even in the presence of VEGF antibody [113, 114].
Therapeutic Strategies for Targeting Tumor-Immune Microenvironment
Some therapeutic strategies have been directed towards targeting stromal components rather than tumor cells. Stromal cells have a relatively low mutation rate [13] and may be less susceptible to developing therapeutic resistance. In addition, taking advantage of the characteristic of the TME to display anti- or pro-tumoral properties, it has been suggested that their re-education may be an effective therapeutic strategy [115, 116]. As TAMs, MDSCs, and Treg cells play an important role in tumor progression and metastasis and their tumor infiltration is associated with poor prognosis in various tumor types, targeting these populations is proving to be an attractive therapeutic strategy [117–123] (Table 1).
Table 1.
Therapeutic strategies to target tumor microenvironment
| Strategy | Target | Agent | Biological function | Disease | Refs |
|---|---|---|---|---|---|
| Immune activation | CTLA-4 | Ipilimumab | T-cell activation |
Melanoma* Preclinical trials: NSCLC, breast cancer |
[125–128] |
| PD-1 | Nivolumab | T-cell activation |
Metastatic melanoma*, NSCLC* and RCC* |
[129–133] | |
| Pembrolizumab | Metastatic HNSCC*, Hodgkin lymphoma* | [124] | |||
| Cemiplimab | Advanced and metastatic cutaneous SCC* | [134, 135] | |||
| PD-L1 | Atezolizumab |
T-cell activation Amplify anti-tumor immunity |
Metastatic NSCLC* and UC* | [136, 137] | |
| Avelumab | Metastatic Merkel-cell* and UC* | [138] | |||
| Durvalumab | Advanced bladder cancer* | [139] | |||
| TIM3 |
Sym023 TSR-022 LY3321367 MBG453 |
T-cell activation | Phase I trials: advanced solid tumors and lymphomas | [124] | |
| LAG3 |
Sym022 TSR-033 |
T-cell activation | Phase I trials: advanced solid tumors and lymphomas | [124] | |
| BMS-986016 | Phase I trials: recurrent GBM and hematologic neoplasms | ||||
| Re-education | CD40 | CD40 mAb |
APCs and T-cell activation Re-educating cytotoxic myeloid cells |
Lymphoma, melanoma, pancreatic carcinoma | [142] |
| T cells | CAR-T | Ex vivo genetic modification of T cells | Leukemia, large B cell lymphoma, neuroblastoma, sarcoma | [144–147] | |
| Macrophage-targeting | CSF-1R | PLX3397 | Macrophage infiltration reduction | Breast and prostate cancer, melanoma, GBM | [118, 149–151] |
| CCR2 |
CCX872-B MLN1202 BMS-813160 |
Phase I/II trials: PDAC, CRC and bone metastasis | [118, 149] | ||
| PI3Kγ in M2-like TAMs |
IPI-549 TG100–115 |
T-cell activation | HNSCC, PDAC, lung and breast cancer, melanoma | [118, 152] | |
| HRG | – | Macrophage polarization and angiogenesis | Fibrosarcoma, pancreatic and breast cancer | [118, 155] | |
| HDAC | TMP195 inhibitor | Repolarizes TAMs. Synergizes with PD-1 | Breast cancer | [118, 156] | |
| MDSCs-targeting | Class I HDAC | Entinostat | Inhibition of MDSC activity | LLC and RCC | [119–121] |
| STAT3 | AZD9150 |
Phase I trials: advanced HCC Phase II trials: pancreatic cancer, HNSCC, NSCLC and CRC |
[119] | ||
| CXCR2 | SX-682 | Blockade of MDSC recruitment | Oral cancer and LLC | [119, 122] | |
| Treg-targeting | CD25 | Daclizumab | Treg depletion | Breast cancer and melanoma | [123] |
| CCR4 | Mogamulizumab | Leukemia, lymphoma, lung and oesophageal cancer | [123] | ||
| OX40 |
PF-04518600 MEDI6383 |
Reduction of immuno-suppressive activity | Melanoma, RCC, B cell lymphoma, advanced HNSCC and metastatic breast cancer | [123] | |
| GITR |
MEDI1873 TRX518 MK-1248 |
Advanced solid tumors | [123] | ||
| PI3Kδ | Parsaclisib | Increased CD8+ T-cell activity | Phase I trial: advanced solid tumors | [123] |
*, FDA-approval; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; HNSCC, head and neck squamous cell carcinoma; UC, urothelial carcinoma; GBM, glioblastoma; PDAC, pancreatic ductal adenocarcinoma; CRC, colorectal cancer; LLC, Lewis lung carcinoma; HCC, hepatocellular carcinoma
Immune checkpoint inhibitors such as anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies, which suppress the function of T cell-inhibitory receptors, have been developed as therapeutic strategies that increase the content of activated tumor-specific cytotoxic T cells [124] (Table 1). The first clinical trial with ipilimumab, an antibody that targets CTLA-4, showed longer overall survival to ~10 months in metastatic melanoma patients compared with patients not receiving ipilimumab therapy [125]. Additional clinical trials using CTLA-4 blocking drugs, either alone or in combination therapy are being performed on patients with advanced melanoma, NSCLC and breast cancer [126–128]. For instance, nivolumab, an anti-PD-1 receptor antibody, has been used alone or in combination with ipilimumab to treat patients with advanced melanoma, osteosarcoma, colorectal and renal carcinomas [129–133]. The 53% of melanoma patients had an objective response to combinatory therapy, all with tumor reduction of at least 80% [129] and longer overall survival compared with monotherapy [130]. Recently, FDA has approved a PD-1 blockade treatment for unresectable locally advanced and metastatic cutaneous SCCs with Cemiplimab [134]. However, most patients’ clinical response was partial or short-lived, and a considerable percentage of patients suffered disease progression [135]. Checkpoint inhibitors that target PD-L1 have also been approved by FDA for the treatment of metastatic NSCLC, urothelial [136, 137], Merkel-cell carcinoma and advanced bladder cancer [138–140]. As some patients are intrinsically resistant or develop adaptive resistance after these treatments in several tumor types, inhibitors of additional immune checkpoint receptors, such as lymphocyte activation gene 3 (LAG3; CD223), and T cell immunoglobulin and mucin-containing molecule-3 (TIM3; CD366) [141] are currently being tested, alone or in combination with a variety of drugs, as new targets in cancer immunotherapy (Table 1).
Clinical trials with agonistic monoclonal antibody against CD40, a TNF receptor superfamily member that is expressed in DCs, B cells, monocytes and many nonimmune and tumor cells, have shown encouraging results in monotherapy and in combination with chemotherapy. The CD40 monoclonal antibody reverses immune suppression by activating APCs, promoting anti-tumor T cell responses and re-educating cytotoxic myeloid cells in lymphomas, melanomas, and pancreatic carcinomas [142] (Table 1).
On the other hand, chimeric antigen receptor-T cell (CAR-T) therapy is based on the ex vivo genetic modification of T cells in order to recognize tumor-associated antigens and kill target cells expressing these antigens [143]. CAR-T therapy has shown considerable success in treating leukemia but limited efficacy in solid tumors [143], except for neuroblastoma [144] and sarcoma [145]. These observations suggest that TME may block immune-driven tumor clearance by impairing CAR-T infiltration, activation or survival. Therefore, the removal of TME barriers to immune clearance may improve the efficiency of CAR-T-based therapies in solid tumors [146, 147].
Other TME-directed therapies are based on the neutralization of tumor-associated chronic inflammation [148]. The inhibition of the NF-κB pathway or immune-cell recruitment and/or function blockade through CSF-1R, CCR2 or CXCR2 inhibitors is being investigated in clinical trials [149]. For example, several CCR2 inhibitors (CCX872-B, PF-04136309, MLN1202, and BMS-813160) are currently undergoing clinical trials for the treatment of solid tumors (Table 1) [118]. In addition, CSF-1R inhibitors decrease tumor growth and increase survival in gliomas in preclinical trials, which are associated with macrophage reprogramming, but not with the depletion of these immune cells [95]. In contrast, treatment with CSF-1R inhibitor results in TAM depletion in preclinical models of breast cancer, without changes in the growth of primary tumors [149]. Moreover, PLX3397, a CSF-1R tyrosine kinase inhibitor, reduces macrophage infiltration and enhances the efficacy of radiotherapy and immunotherapy [150, 151]. This drug is currently under clinical development for the treatment of glioblastoma and breast cancer patients. Given that different tumor types show distinct responses to CSF-1R inhibition, it has been suggested that TME-targeted therapies should be administered depending on tumor type and location in order to block tumor progression and metastasis development.
Strategies to promote the polarization switch of M2 macrophages to an anti-tumor M1 phenotype are also emerging as new therapeutic approaches (Table 1). In this regard, the inhibition of PI3Kγ in M2-like TAMs gives rise to T cell activation that suppresses growth of head and neck SCC (HNSCC), PDAC, lung and breast tumor [152]. Furthermore, activation of TLR3/Toll-IL-1 receptor domain-containing adaptor molecule 1 by Poly (I:C) accelerates M1-like macrophage polarization [153]. Polarization from an M2 to an M1 phenotype suppresses mammary tumor growth and angiogenesis [154], indicating that the re-education of TAMs could restore normal vasculature and block the pro-tumorigenic effects of TAMs. In accordance, histidine-rich glycoprotein (HRG) inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization via downregulation of the placental growth factor (PIGF) [118, 155]. TMP195, a histone deacetylase (HDAC) inhibitor, repolarizes TAMs and synergizes with PD-1 to reduce tumor burden and metastasis in a breast cancer model [156].
Taken together, these studies suggest that a combination of therapies targeting immune evasion of tumor cells and those activating anti-tumor immune cell responses may provide optimal benefits for patients. However, these trials are still in the early stages and we do not fully understand how these drugs contribute to efficacy and overall survival. The long-term impact of these therapies on patient safety and survival also remains to be evaluated.
Concluding Remarks
Studies in recent years have significantly advanced our understanding of the cytokines and molecular pathways regulating the crosstalk between TME components such as TAMs, MDSCs, Treg, and cancer cells. These immunosuppressive cells and derived cytokines contribute to the generation of a tumor-immunosuppressive network that promotes tumor progression and negatively influences immunotherapy in a range of cancer types. Signals derived from these tumor-infiltrating immune cells help sustain cancer-cell proliferation, survival, and invasion, as well as metastasis and angiogenesis. In addition, cancer cells have evolved different mechanisms of immune evasion to avoid their recognition and/or attack by cytotoxic T cells or NK cells. These mechanisms include the recruitment of immunosuppressive cells that block the activity and proliferation of cytotoxic T cells, the downregulation of MHC-I protein expression, and/or the induction of PD-1 and CTLA-4 ligands, among others, for the activation of T cell-inhibitory immune checkpoint pathways.
Several studies have also shown that immune and cancer-cell crosstalk plays an important role in promoting the acquisition of CSC features, EMT, CSC self-renewal and maintenance. Likewise, CSC-derived factors stimulate the recruitment of these immunosuppressive cells into tumors. Given the important role of CSCs in driving long-term tumor growth, metastasis, and relapse after therapy, a thorough understanding of CSC-TME crosstalk is fundamental to the development of innovative therapeutic strategies. In this regard, therapies based on disrupting the pro-tumorigenic TME by blocking the recruitment of immunosuppressive TAMs and MDSCs to tumors have been developed. In addition, many studies have suggested that re-education of stromal cells, rather than their targeted ablation, may be an effective strategy for treating cancer. Future studies will allow us to establish combinatory therapies that block tumor growth and metastasis by targeting TME and cancer cells, and to increase the efficiency of currently immune checkpoint inhibitors.
Acknowledgments
LLS received an IDIBELL Fellowship. P. Muñoz’s laboratory is supported by the Spanish Ministry of Science and Innovation (SAF2017-84976R; co-funded by FEDER funds/European Regional Development Fund (ERDF) - a way to build Europe) and by the Catalan Department of Health (CERCA; Generalitat de Catalunya, 2017/SGR565).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merrell AJ, Stanger BZ. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat Rev Mol Cell Biol. 2016;17:413–425. doi: 10.1038/nrm.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge Y, Fuchs E. Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat Rev Genet. 2018;19:311–325. doi: 10.1038/nrg.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva-Diz V, Lorenzo-Sanz L, Bernat-Peguera A, Lopez-Cerda M, Muñoz P. Cancer cell plasticity: impact on tumor progression and therapy response. Semin Cancer Biol. 2018;53:48–58. doi: 10.1016/j.semcancer.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;25:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 10.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 12.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 13.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells -what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 18.Sica A, Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. J Autoimmun. 2017;85:117–125. doi: 10.1016/j.jaut.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 21.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 25.Huang YC, Feng ZP. The good and bad of microglia/macrophages: new hope in stroke therapeutics. Acta Pharmacol Sin. 2013;34:6–7. doi: 10.1038/aps.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong SM, Tan YC, Beretta O, Jiang D, Yeap WH, Tai JJY, Wong WC, Yang H, Schwarz H, Lim KH, Koh PK, Ling KL, Wong SC. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur J Immunol. 2012;42:89–100. doi: 10.1002/eji.201141825. [DOI] [PubMed] [Google Scholar]
- 28.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 29.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, Connolly AJ, Weissman IL. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 43.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3 + regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 45.Gasteiger G, Hemmers S, Firth MA, Le Floc’h A, et al. IL-2–dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med. 2013;210:1167–1178. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 47.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 48.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 50.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+) CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–2643. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 52.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 53.Maruyama T, Kono K, Izawa S, Mizukami Y, Kawaguchi Y, Mimura K, Watanabe M, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to infiltration of regulatory T cells in esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:422–429. doi: 10.1111/j.1442-2050.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 54.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 55.von Boehmer H, Daniel C. Therapeutic opportunities for manipulating TReg cells in autoimmunity and cancer. Nat Re Drug Disco. 2013;12:51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- 56.Bopp T, Becker C, Klein M, Klein-Heßling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, Stoll S, Schild H, Staege MS, Stassen M, Jonuleit H, Schmitt E. Cyclic adenosine monophosphate is a key component of regulatory T cell mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J, Yao H, Song G, Liao X, Xian Y, Li W. Regulation of epithelial-mesenchymal transition by tumor-associated macrophages in cancer. Am J Transl Res. 2015;7:1699–1711. [PMC free article] [PubMed] [Google Scholar]
- 60.Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Investig. 2013;93:844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 61.Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, Wei LX. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 62.Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M, Tüting T. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490:412–416. doi: 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- 63.Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 64.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, Markowitz D, Reisfeld RA, Luo Y. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31:248–258. doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- 66.Lu H, Clauser KR, Tam WL, Fröse J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, Weinberg RA. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 2014;16:1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sainz Bruno, Martín Beatriz, Tatari Marianthi, Heeschen Christopher, Guerra Susana. ISG15 Is a Critical Microenvironmental Factor for Pancreatic Cancer Stem Cells. Cancer Research. 2014;74(24):7309–7320. doi: 10.1158/0008-5472.CAN-14-1354. [DOI] [PubMed] [Google Scholar]
- 68.Sainz Bruno, Alcala Sonia, Garcia Elena, Sanchez-Ripoll Yolanda, Azevedo Maria M, Cioffi Michele, Tatari Marianthi, Miranda-Lorenzo Irene, Hidalgo Manuel, Gomez-Lopez Gonzalo, Cañamero Marta, Erkan Mert, Kleeff Jörg, García-Silva Susana, Sancho Patricia, Hermann Patrick C, Heeschen Christopher. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut. 2015;64(12):1921–1935. doi: 10.1136/gutjnl-2014-308935. [DOI] [PubMed] [Google Scholar]
- 69.Cui TX, Kryczek I, Zhao E, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39:611–621. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li ZL, Ye SB, OuYang LY, et al. COX-2 promotes metastasis in nasopharyngeal carcinoma by mediating interactions between cancer cells and myeloid-derived suppressor cells. OncoImmunology. 2015;4:e1044712. doi: 10.1080/2162402X.2015.1044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panni RZ, Sanford DE, Belt BA, Mitchem JB, Worley LA, Goetz BD, Mukherjee P, Wang-Gillam A, Link DC, DeNardo DG, Goedegebuure SP, Linehan DC. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother. 2014;63:513–528. doi: 10.1007/s00262-014-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, Lee DS. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013;15:R79. doi: 10.1186/bcr3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu H, Gu Y, Xue M, et al. CXCR2+ MDSCs promote breast cancer progression by inducing EMT and activated T cell exhaustion. Oncotarget. 2017;8:114554–114567. doi: 10.18632/oncotarget.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taki M, Abiko K, Baba T, Hamanishi J, Yamaguchi K, Murakami R, Yamanoi K, Horikawa N, Hosoe Y, Nakamura E, Sugiyama A, Mandai M, Konishi I, Matsumura N. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat Commun. 2018;9:1685. doi: 10.1038/s41467-018-03966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Welte T, Kim IS, Tian L, Gao X, Wang H, Li J, Holdman XB, Herschkowitz JI, Pond A, Xie G, Kurley S, Nguyen T, Liao L, Dobrolecki LE, Pang L, Mo Q, Edwards DP, Huang S, Xin L, Xu J, Li Y, Lewis MT, Wang T, Westbrook TF, Rosen JM, Zhang XHF. Oncogenic mTOR signaling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat Cell Biol. 2016;18:632–644. doi: 10.1038/ncb3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng D, Tanikawa T, Li W, Zhao L, Vatan L, Szeliga W, Wan S, Wei S, Wang Y, Liu Y, Staroslawska E, Szubstarski F, Rolinski J, Grywalska E, Stanis awek A, Polkowski W, Kurylcio A, Kleer C, Chang AE, Wicha M, Sabel M, Zou W, Kryczek I. Myeloid-derived suppressor cells endow stem-like qualities to breast cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res. 2016;76:3156–3165. doi: 10.1158/0008-5472.CAN-15-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ouzounova M, Lee E, Piranlioglu R, el Andaloussi A, Kolhe R, Demirci MF, Marasco D, Asm I, Chadli A, Hassan KA, Thangaraju M, Zhou G, Arbab AS, Cowell JK, Korkaya H. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumor plasticity during metastatic cascade. Nat Commun. 2017;8:14979. doi: 10.1038/ncomms14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, Zhang B, Liu T, Yang P. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. 2011;89:85–91. doi: 10.1189/jlb.0910506. [DOI] [PubMed] [Google Scholar]
- 80.Mahic M, Yaqub S, Johansson CC, Taskén K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–254. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 81.Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 2015;149:1884–1895. doi: 10.1053/j.gastro.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamborero D, Rubio-Perez C, Muinos F, et al. A pan-cancer landscape of interactions between solid tumors and infiltrating immune cell populations. Clin Cancer Res. 2018;24:3717–3728. doi: 10.1158/1078-0432.CCR-17-3509. [DOI] [PubMed] [Google Scholar]
- 83.Lee Y, Shin JH, Longmire M, Wang H, Kohrt HE, Chang HY, Sunwoo JB. CD44+ cells in head and neck squamous cell carcinoma suppress t-cell-mediated immunity by selective constitutive and inducible expression of PD-L1. Clin Cancer Res. 2016;22:3571–3581. doi: 10.1158/1078-0432.CCR-15-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, Weinberg RA. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 2017;77:3982–3989. doi: 10.1158/0008-5472.CAN-16-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal JD, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Jones S, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Peng D, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FXF. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hugo W, Zaretslky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Tomaso T, Mazzoleni S, Wang E, et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res. 2010;16:800–813. doi: 10.1158/1078-0432.CCR-09-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, Griffero F, Marubbi D, Spaziante R, Bellora F, Moretta L, Moretta A, Corte G, Bottino C. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 89.Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012;227:17–28. doi: 10.1002/path.3989. [DOI] [PubMed] [Google Scholar]
- 90.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 91.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yan D, Wang HW, Bowman RL, Joyce JA. STAT3 and STAT6 signaling pathways synergize to promote cathepsin secretion from macrophages via IRE1alpha activation. Cell Rep. 2016;16:2914–2927. doi: 10.1016/j.celrep.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, Daniel D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8+ T cells. Oncoimmunology. 2013;2:e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pyonteck SM, Akkari L, Schumacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 97.Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, Segall JE. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18:519–527. doi: 10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 100.Jinushi M, Sato M, Kanamoto A, Itoh A, Nagai S, Koyasu S, Dranoff G, Tahara H. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206:1317–1326. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen Q, Zhang XH, Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu X, Mu E, Wei Y, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging a4b1-positive osteoclast progenitors. Cancer Cell. 2011;20:701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ye XZ, Yu SC, Bian XW. Contribution of myeloid-derived suppressor cells to tumor-induced immune suppression, angiogenesis, invasion and metastasis. J Genet Genomics. 2010;37:423–430. doi: 10.1016/S1673-8527(09)60061-8. [DOI] [PubMed] [Google Scholar]
- 104.Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77:3655–3665. doi: 10.1158/0008-5472.CAN-16-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 106.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deng J, Liu Y, Lee H, et al. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012;21:642–654. doi: 10.1016/j.ccr.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumar S, Wilkes DW, Samuel N, Blanco MA, Nayak A, Alicea-Torres K, Gluck C, Sinha S, Gabrilovich D, Chakrabarti R. ΔNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J Clin Invest. 2018;128:5095–5109. doi: 10.1172/JCI99673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, Ho C, Ross J, Tan M, Carano RAD, Meng YG, Ferrara N. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 113.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML, Wu L. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 115.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10:58. doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in Cancer. Trends Immunol. 2019;40(4):310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 119.Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, Utikal J, Umansky V. Targeting myeloid-derived suppressor cells to BypassTumor-induced immunosuppression. Front Immunol. 2018;9:398. doi: 10.3389/fimmu.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, Chintala S, Ordentlich P, Kao C, Elzey B, Gabrilovich D, Pili R. Entinostat neutralizes myeloid derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res. 2017;23:5187–5201. doi: 10.1158/1078-0432.CCR-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111:11774–11779. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, Das R, Silvin C, van Waes C, Horn LA, Schlom J, Palena C, Maeda D, Zebala J, Allen CT (2019) Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight 4(7) [DOI] [PMC free article] [PubMed]
- 123.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression -implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 124.Isakov N. Cancer immunotherapy by targeting immune checkpoint receptors. World J Immunol. 2018;8(1):1–11. [Google Scholar]
- 125.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sckisel GD, Mirsoian A, Bouchlaka MN, Tietze JK, Chen M, Blazar BR, Murphy WJ. Late administration of murine CTLA-4 blockade prolongs CD8-mediated anti-tumor effects following stimulatory cancer immunotherapy. Cancer Immunol Immunother. 2015;64:1541–1552. doi: 10.1007/s00262-015-1759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sacco PC, Maione P, et al. The combination of new immunotherapy and radiotherapy: a new potential treatment for locally advanced non-small cell lung Cancer. Curr Clin Pharmacol. 2017;12:4–10. doi: 10.2174/1574884711666161201123439. [DOI] [PubMed] [Google Scholar]
- 128.Hu ZI, Ho AY, McArthur HL. Combined radiation therapy and immune checkpoint blockade therapy for breast Cancer. Int J Radiat Oncol Biol Phys. 2017;99:153–164. doi: 10.1016/j.ijrobp.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 129.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 131.Lussier DM, Johnson JL, Hingorani P, Blattman JN. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer. 2015;3:21. doi: 10.1186/s40425-015-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable clinical Benedit with Nivolumab plus Ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal Cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 133.Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA, Kollmannsberger C, Heng DYC, Spratlin J, McHenry MB, Amin A. Safety and efficacy of Nivolumab in combination with Ipilimumab in metastatic renal cell Carnicoma: the CheckMate 016 study. J Clin Oncol. 2017;35:3851–3858. doi: 10.1200/JCO.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG. PD-1 blockade with Cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 135.Pandey A, Liaukovich M, Joshi K, Avezbakiyev BI, O\'Donnell JE. Uncommon presentation of metastatic squamous cell carcinoma of the skin and treatment challenges. Am J Case Rep. 2019;20:294–299. doi: 10.12659/AJCR.913488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 137.Sidaway P. Urological cancer: Atezolizumab: an alternative to cisplatin? Nat Rev Clin Oncol. 2017;14:139. doi: 10.1038/nrclinonc.2016.222. [DOI] [PubMed] [Google Scholar]
- 138.McKay RR, Bossé D, et al. The clinical activity of PD-1/PD-L1 inhibitors in metastatic non-clear cell renal carcinoma. Cancer Immunol Res. 2018;6:758–765. doi: 10.1158/2326-6066.CIR-17-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brower V. Anti-PD-L1 inhibitor durvalumab in bladder cancer. Lancet Oncol. 2016;17:e275. doi: 10.1016/S1470-2045(16)30242-X. [DOI] [PubMed] [Google Scholar]
- 140.Luo W, Wang Z, Tian P, Li W. Safety and tolerability of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2018;144:1851–1859. doi: 10.1007/s00432-018-2707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 142.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370:85–90. doi: 10.1016/j.canlet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu MF, Gee AP, Mei Z, Rooney CM, Heslop HE, Brenner MK. Antitumor activity and long-term fate of chimeric antigen receptor-positive t cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, Gray T, Wu MF, Liu H, Hicks J, Rainusso N, Dotti G, Mei Z, Grilley B, Gee A, Rooney CM, Brenner MK, Heslop HE, Wels WS, Wang LL, Anderson P, Gottschalk S. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor- modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pahl J, Cerwenka A. Tricking the balance: Nk cells in anti-cancer immunity. Immunobiology. 2017;222:11–20. doi: 10.1016/j.imbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 147.Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics. 2016;3:16006. doi: 10.1038/mto.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L, Graeber TG, West BL, Bollag G, Ribas A. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 2014;74:153–161. doi: 10.1158/0008-5472.CAN-13-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. Abrogating the protumorigenic influences of tumor-infiltrating myeloid cells by CSF1R signaling blockade improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, Schmid MC, Pink M, Winkler DG, Rausch M, Palombella VJ, Kutok J, McGovern K, Frazer KA, Wu X, Karin M, Sasik R, Cohen EEW, Varner JA. PI3Kgamma is a molecular switch that controls immune suppression. Nature. 2016;539:437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shime H, Matsumoto M, Oshiumi H, Tanaka S, Nakane A, Iwakura Y, Tahara H, Inoue N, Seya T. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci U S A. 2012;109:2066–2071. doi: 10.1073/pnas.1113099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang X, Tian W, Cai X, Wang X, Dang W, Tang H, Cao H, Wang L, Chen T. Hydrazinocurcumin encapsuled nanoparticles “re-educate” tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following STAT3 suppression. PLoS One. 2013;8(6):e65896. doi: 10.1371/journal.pone.0065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rolny C, Mazzone M, Tugues S, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 156.Guerriero JL. Macrophages: the road less traveled, changing anticancer therapy. Trends Mol Med. 2018;24:472–489. doi: 10.1016/j.molmed.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]