Abstract
Alzheimer’s disease (AD) is historically difficult to treat, in part due the inaccessible nature of brain pathology. Amyloid-β (Aβ) and tau proteins drive pathology by forming toxic oligomers that eventually deposit as insoluble amyloid plaques and neurofibrillary tangles. Recent clinical studies suggest that effective drugs must specifically target oligomers, not native monomers or insoluble fibrils. Passive immunotherapy is one promising pharmaceutical strategy used to specifically target these oligomers in situ. Using the specificity of antibodies coupled with the natural power of the body’s immune response, this treatment provides an opportunity for safe clearance of pathogenic protein species from the brain. Passive immunotherapies against Aβ and tau oligomers have progressed to clinical trials, with many currently in progress. Biochemical studies of antibody-oligomer complexes have helped identify previously unknown toxic epitopes, thus providing knowledge to the AD field as a whole. This mini-review focuses on the efforts to develop passive immunotherapy treatments for AD and discusses the knowledge gained from recent failures and clinical trials in progress.
Keywords: Amyloid beta, tau, Alzheimer’s disease, protein oligomers, passive immunotherapy, protein aggregation, protein structure, blood brain barrier, clearance, CNS
Introduction
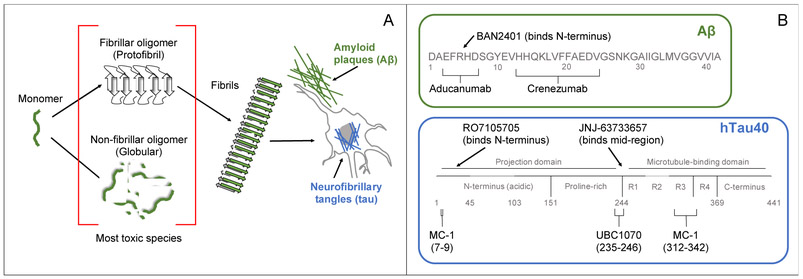
Alzheimer’s disease (AD) is historically difficult to treat, in part due to the challenge of delivering a drug across the blood brain barrier (BBB). Many neurodegenerative diseases share a common pathology of aberrant protein aggregation that results in neuronal toxicity, where native monomeric protein is converted to toxic oligomers and insoluble fibrillar deposits (Figure 1).1 According to the amyloid cascade hypothesis,2 AD is caused by abnormal aggregation of the amyloid beta (Aβ) peptide and the tau protein that results in neurotoxicity and leads to wide-spread neurodegeneration.
Figure 1:
(A) An AD, Aβ and tau aggregate from intrinsically disordered monomers into oligomers and finally end as insoluble β-sheet rich fibrils. Characteristic AD pathology includes amyloid plaques formed from extracellular Aβ deposits and neurofibrillary tangles formed from tau fibrils deposited around the nucleus. Oligomers are the most toxic species; they are observed as either β-sheet rich protofibrils or globular oligomers. (B) Aβ and tau sequences are labeled with binding sites for passive immunotherapy drugs in clinical trials that target oligomers. The sequence of full-length human tau, hTau40, is shown with alternative splicing domains colored dark gray. R1-R4 are four microtubule binding domain repeats that drive tau fibrillation.
Aggregation is thought to cause neurotoxicity through a variety of mechanisms. Insoluble protein deposits, such as amyloid plaques and neurofibrillary tangles (NFTs) made from Aβ and tau, respectively, are pathological hallmarks of AD.1 These proteinaceous deposits are observed in brain tissue during post-mortem analysis, and also through amyloid-binding PET probes in living patients. Amyloid plaques were originally thought to be the primary source of toxicity in AD, but they are now considered to be generally benign after discovering them in the brains of asymptomatic individuals.3 There is some evidence for plaque-mediated toxicity through inhibition of synaptic connections,4 however this is likely a secondary mode of toxicity.
Most researchers have turned to investigating oligomers made from Aβ and tau as the primary neurotoxic species. There are several hypothesized mechanisms of toxicity, the most prominent is that oligomers cause neuronal cell death through interactions with the cell membrane.5 Many studies have shown that Aβ and tau oligomers induce degradation of the membrane, resulting in dysregulation of cellular ion homeostasis.6 In AD, Aβ aggregates decades before the disease is apparent, followed by tau aggregation that coincides with the onset of symptoms.7 Since Aβ and tau oligomers are the most neurotoxic species,8 there is a significant focus and challenge in specifically targeting the toxic species while leaving the native monomeric proteins unperturbed. Most disease-modifying therapies in clinical trials aim to detoxify these oligomers or prevent their formation.
Passive immunotherapy9,10 is a promising pharmaceutical strategy for AD. Antibodies can be developed to specifically target Aβ and tau oligomers, and natural immune defenses can reach the brain and safely clear pathogenic species. These therapies invoke production-oriented host immune cells to generate monoclonal antibodies against a pre-formed population of antigens, Aβ and tau oligomers in this case. The monoclonal antibodies are collected and then administered to a patient with AD. The patient receives repeated doses of monoclonal antibodies and their own immune response clears the antibody-bound oligomers. Concurrent with passive immunotherapy treatment, there are also significant efforts to develop active immunotherapies, vaccines, for AD treatment.10 In contrast to passive immunotherapy, active strategies directly expose the patient to the antigen, thus soliciting antibody production from the patient’s own immune response. These strategies have their own unique challenges, including the risk of initiating a dangerous overactivated immune response, which is not the focus of the current minireview.
Passive immunotherapy has been wildly successful in AD-model animals, dramatically reversing cognitive impairment symptoms and brain pathology. These successes have led to large-scale clinical trials, many of which are currently ongoing. An history of passive immunotherapy has been reviewed for Aβ9 and tau10; however, early treatments mainly targeted monomers and insoluble fibrils. The failures of these early clinical trials point to a future of AD immunotherapy focused on oligomer-specific treatment. In light of recent advances,11,12 we chose to specifically focus on treatments targeting Aβ and tau oligomers.
In this minireview, we discuss passive immunotherapy treatments being developed to target Aβ and tau oligomers and explore some of the pharmaceutical challenges associated with these therapies. As epitope selection is critical in antibody design, we have included ongoing structural biology work that accompanies each therapy. We selected this topic for this dedicated issue in honor of John F. Carpenter and Theodore W. Randolph because of their pioneering work in two aspects protein therapeutics, driving forces and mechanisms of protein aggregation and the potential of protein aggregates to induce endogenous immunogenicity. Passive immunotherapies for AD are a timely marriage of these fields and we hope to provide an interesting background and perspective for readers of this dedicated issue.
Passive immunotherapy strategies for AD
There are two inherent challenges in using passive immunotherapy to treat AD. The first is to determine effective dosing protocols for the antibody to reach the neuropathology; an estimated <0.1% of immunoglobins dosed into the serum will penetrate the BBB.13 It has been proposed that pathology may instead be cleared through a peripheral sink mechanism. This involves an immune response that removes Aβ and tau from the serum, eventually drawing soluble pathological species out of the central nervous system by a concentration gradient.14 However, the predominant hypothesis is that the small percentage of antibodies that do cross the BBB are able to trigger microglial activation and phagocytosis of the antibody-bound antigens.15 Some additional strategies have been used to facilitate brain uptake, including the use of transferrin receptor (TfR)-mediated transcytosis in pre-clinical Aβ-targeted therapies.16,17
The second challenge is the high risk of unintended inflammatory reactions in the brain. Naturally, immune responses can include production of pro-inflammatory cytokines such as TNFα, with serious consequences in the brain. Strategies to minimize inflammation include specifically choosing IgG1 and IgG4 isotype subclasses, and avoiding pro-inflammatory subclasses such as IgG3.18 In AD clinical trials, brain inflammation likely occurs as a result of over-activated microglial cells that induce inappropriate proinflammatory responses.19 These inflammation impacts are generally classified as amyloid-related imaging abnormalities (ARIA).20 ARIA-E refers to vasogenic edema, where tight endothelial junctions of the BBB are broken down, eventually leading to fluid accumulation. ARIA-H refers to cerebral microhemorrhages, evidenced by accumulation of hemosiderin deposits in the brain. Clinical trial protocols include screening for ARIA-E and -H.
Although not currently applied in clinical trials, there are a number of clever passive immunotherapy strategies being tested in pre-clinical experiments targeting Aβ and tau.21 One approach is to use a single chain variable fragment (scFv), which contains only the variable domains of the heavy and light chain (VH and VL) connected with a short peptide linker.22 Another method for targeting Aβ and tau in situ23 is camelid single domain antibodies, which were discovered as a natural component of the camel immune system. These antibodies are ~15 kDa homodimers without light chains,24 and due to their small size they have better diffusion across the BBB. scFv’s and camelid single domain antibodies can also be used as intrabodies – antibodies recombinantly expressed inside the target cell.21 This gene-based approach uses either a virus or nanoparticle-based delivery system to introduce the intrabody gene into the host, and it has been applied for pre-clinical treatments targeting Aβ in mouse models.21,25,26
Antibodies targeting Aβ oligomers
It has been ca. two decades since researchers first began investigating Aβ as an immunotherapy target.27 The first generation of trials targeted monomeric Aβ, and antibodies for the fibrillar species soon followed. In 2003, Kayed et al. discovered a common epitope among non-fibrillar oligomers for a number of proteins involved in neurodegenerative diseases.28 His work was pivotal in naming non-fibrillar oligomers and protofibrils as the most toxic Aβ species, and many oligomer-based antibodies have since been developed. While there are many antibodies currently being investigated in pre-clinical contexts, we chose to focus specifically on those that have advanced to human clinical trials.
Crenezumab:
Developed by the company AC Immune SA and licensed by Genentech, crenezumab29 was the first antibody developed to target oligomeric Aβ. In early studies with hAPP(V717I)/PSI transgenic mice, crenezumab displayed higher affinity for oligomers over monomeric Aβ, while also binding to fibrillar species and plaques.19 Antibody treatment inhibited Aβ aggregation, and even promoted moderate disaggregation of oligomers and fibrils.19 The affinity for oligomers stems from the antibody’s recognition for amino acids 13-24 in an extended conformation, uniquely binding the mid-domain of the peptide.30,31 With this region bound, a hydrophobic section of the peptide was sequestered, thus inhibiting hydrophobicity-driven aggregation. The forced extended conformation consequently broke a salt bridge between Asp23 and Lys28 that is known to stabilize the β-hairpin in aggregated species.
The humanized antibody was developed with an IgG4 backbone, intended to limit inflammation by instead stimulating phagocytosis by microglia.19 Early clinical trial results showed that the drug displayed good BBB penetration.32 Secondary inflammation responses were minimal, with only an 11.4% increase in ARIA-H for the treatment group compared to the control.33-35 Analysis of this trial revealed non-significant trends of slowing symptoms and plaque accumulation in the highest-dose group,36 and was thus continued onto phase 3 trials in patients with prodromal to mild AD (CREAD1 and CREAD2 trials).37,38 In January 2019, both trials were halted due to a lack of efficacy. The drug was “unlikely to reach its primary endpoint” with no significant slowing in cognitive decline as measured by a Clinical Dementia Rating - Sum of Boxes (CDR-SB) test.11
One observation from the drug trial was that minimal ARIA may indicate minimal impact on clearing Aβ species in the brain. Inflammation is an expected side effect of microglial activation, so perhaps crenezumab was not successfully initiating the immune response needed to promote clearance.6 Another popular opinion is that treatment must be applied in pre-symptomatic patients to be effective. Patients who display symptoms already have significant neurodegeneration, so treatment even in prodromal AD cases may be ineffective. Accordingly, crenezumab continues to be tested as part of the Alzheimer’s Preventative Initiative39 in pre-symptomatic patients with the pre-senilin 1 (PS1) familial AD mutation E280A.40,41
Aducanumab:
Another promising treatment was the drug aducanumab,42 produced and tested by Biogen. Aducanumab has >10,000 fold increased selectivity for aggregated Aβ species compared to monomers.43,44 Crystal structures of the Fab revealed that the antibody binds residues 3-7 in an extended conformation, with Phe4 and His6 being critical to binding.45 Biochemical analyses showed that weak binding affinity to Aβ monomers, coupled with fast dissociation, contributed to high selectivity for aggregated species.45 Furthermore, in studies with artificial dimeric and tetrameric branched peptides, aducanumab had an EC50 of >1 μM for dimeric Aβ and ~7 nM for tetrameric Aβ. This indicates a large preference for Aβ assemblies with their N-termini in close proximity.
Early mouse studies showed that the murine precursor antibody entered the brain and reduced Aβ deposits by >70%, where microglia-mediated phagocytosis likely cleared the deposits.43 In 2016, reports from a Phase 1b trial of aducanumab (PRIME) showed no indications of toxicity.43 The trial contained 165 prodromal or mild AD patients with visually positive PET scans. There was however, a dose-dependent increase in ARIA-E, including up to 41% of patients (13 patients) treated with the highest dose (10 mg/kg). Florbetapir PET imaging results indicated that aducanumab was able to reduce Aβ plaques with dose- and time-dependency. It also appeared to slow cognitive decline, although the study was not powered to detect cognitive change.43 ENGAGE46 and EMERGE47 were two large phase 2 clinical trials, each aimed to enroll 1350 early-stage AD patients. In March 2019, both trials were halted due to a lack of efficacy because there was no slowing of cognitive decline over the 18-month treatment period.12
Complete analysis of the trial data has not been released, however there are some speculations about the reasons for aducanumab’s failure. Interestingly, the treatment was able to clear amyloid plaques, as measured by florbetapir PET imaging, but plaque clearance did not yield gains in cognitive function. Perhaps this is not entirely surprising, as the presence of plaques does not always correlate with neurodegeneration.8 AD clinical symptoms closely follow the appearance of tau pathology, so tau may be a more suitable target to stop disease progression. Aβ aggregation precedes clinical symptoms by several years, so drugs targeting Aβ will have the highest likelihood of success in pre-symptomatic patients.
BAN2401:
Developed by BioArctic AB and with clinical trials sponsored by Eisai Co., Ltd. and Biogen, the remaining antibody in clinical trials, BAN2401,48 has unique binding to soluble protofibrils. Soluble β-sheet rich protofibrils are a highly neurotoxic species, alongside non-fibrillar oligomers.49 The murine predecessor, mAb158, was shown to reduce protofibrils by 42% in the brains of tg-ArcSwe mice, with no change in monomeric Aβ42 50 or insoluble amyloid plaques.51 Through interactions with the N-terminus,52 the antibody displayed >1000-fold preference for binding protofibrils over monomeric Aβ, and 10-15 fold preference for binding protofibrils over mature fibrils.52,53 Cellular studies showed that mAb158 abolished Aβ accumulation in astrocytes and prevented toxicity in cultured neurons.54
The humanized IgG1 antibody was non-toxic in Phase 1 clinical trials, with no measurable increases in ARIA-E/H.55-57 Phase 2 trials were designed using a Bayesian adaptive proof-of-concept method to accelerate dosing decisions, thus shortening the trial and requiring fewer participants58. 18-month analysis of 856 early AD patients in the Phase 2b trial revealed statistically significant and dose-dependent slowing of disease markers, including amyloid accumulation in the brain and cognitive function as measured by the Alzheimer’s Disease Composite Score (ADCOMS).59-61 ARIA-E was observed in a <10% of patients. Phase 2b patients were re-enrolled for continued trials at the highest dose (10 mg/kg monthly), which are scheduled to continue until August 2021. Concurrently, a Phase 3 trial “Clarity AD” began in March 2019 with plans to enroll 1566 patients with early AD.62,63
Antibodies targeting tau oligomers
While Aβ pathology occurs years before onset of AD, tau pathology coincides with clinical symptoms. Cognitive ability is quickly reduced as tau pathology spreads through the brain, making tau an attractive candidate for slowing the disease progression.64,65 For targeting tau, there are additional considerations regarding the variety of splicing isoforms, truncations, and post-translational modifications naturally occurring in the brain. A single patient may have a heterogeneous population of pathogenic tau “seeds.” Another concern is the risk of perturbing native tau function of stabilizing microtubules, so antibodies must be purposely designed to avoid binding native monomeric tau in the cell.66 Choosing the right epitope, rather than achieving high binding affinity, is critical for the antibody’s ability to clear toxic tau species.67 In fact, antibodies against some epitopes can instead promote tau aggregation in vitro.68 A recent report by Courade et al. states that the central region of tau is the best target for passive immunotherapies, likely because antibody binding blocks key fibrillation-promoting regions.67 Furthermore, antibodies that target the N-terminus do not have access to all forms of tau due to frequent N-terminal truncations.67 Tau is a newer target for passive immunotherapy treatments in AD, and the biochemical knowledge of antibody-oligomer interactions is generally less developed. Most clinical studies are currently in Phase 1 or early Phase 2 trials with little data on efficacy.
UBC1070:
Tested by UBC, the antibody was developed to treat AD and progressive supranuclear palsy by specifically binding seed-capable tau oligomers. UBC1070 was designed to inhibit spread of pathogenic tau between cells, and it binds near the microtubule-binding domain at residues 235–246.69,70 In HEK293F cells expressing human Tau 2N4R with a P301S mutation, UCB1070 blocked tau seeding activity with an IC50 of 2.9 nM.67 Phase 1 trials were completed in December 2018,71,72 but no official publication has reported the safety or plans to progress to Phase 2 trials.
LY3303560:
Produced by Eli Lilly and Company, LY3303560 was designed to bind soluble pathological tau aggregates. The antibody was derived from MC-1, another antibody developed in 1997 to have affinity for paired helical filaments from AD brain homogenates.73 MC-1 interacts with both the N-terminus and the C-terminus of tau, including residues 7-9 and a location between residues 312-342.73 As these residues are conserved in all six isoforms of tau,74 the antibody is expected to bind regardless of splicing variant. Preclinical results show LY3303560 binds soluble tau aggregates with a Kd of <220 pM, while binding monomers with a Kd of 235 nM.75 The antibody binds the N-terminal region of tau, but more details have not yet been disclosed. Phase 1 trials illustrated safety and tolerability in healthy and AD patients,76,77 and Phase 2 trials began recruiting patients with early symptomatic AD in May 2018.78
RO 7105705:
This antibody was developed by Genentech in collaboration with AC Immune SA, with the intent to intercept extracellular tau and prevent pathogenic spread across the brain.79 RO7105705 is an IgG4 antibody that binds the N-terminus of tau, regardless of the protein’s isoform, phosphorylation, or oligomerization state. As the phosphorylation and oligomerization of pathogenic tau are poorly characterized, this treatment aims to clear all extracellular tau. The spread of tau pathology through the brain strongly correlates with clinical symptoms; thus, clearing all extracellular tau is proposed to preserve patient cognitive function.10,80 Phase 1 clinical trial results on safety and tolerability were presented at the 2017 Alzheimer’s Association International Conference.81,82 Two Phase 2 clinical trials began in October 2017 83 and February 2019 84, enrolling patients with prodromal to mild AD and moderate AD respectively.
JNJ-63733657:
Developed by Janssen Pharmaceutica, JNJ-63733657 was identified as a promising drug candidate because of its ability to eliminate pathogenic tau “seeds”.69,85 The antibody targets the mid-region of tau, following the hypothesis that this region, and not the N-terminus, should be the primary target for passive immunotherapy. JNJ-63733657 was reported to eliminate pathogenic tau "seeds" in cells expressing P301S tau, and mouse model experiments revealed decreased spread of tau pathology.69 Two phase 1 trials are actively recruiting, one testing the safety and tolerability in healthy Japanese adults,86 and another testing healthy or prodromal/mild AD adults in locations across Europe87.
Perspectives
Crenezumab and aducanumab clinical trials targeting Aβ oligomers were not successful, but Phase 3 trials with BAN2401 are ongoing. From these failures, we have gained the insight that Aβ is unlikely to be a good choice of target once a patient is already displaying clinical symptoms. Aβ aggregation drives the disease in the pre-symptomatic stage, so treatments targeting Aβ should be tested as early as possible in the disease trajectory. Of course, this creates a difficult problem of how to select patients for clinical trials before they are displaying symptoms. The results from the Alzheimer’s Preventative Initiative39 trials testing crenezumab in asymptomatic genetically pre-disposed patients will help to confirm this hypothesis. It is a newer idea to target tau, and specifically to target tau oligomers. As tau pathology coincides with clinical symptoms, we wait with anticipation for results from trials testing UBC1070, LY3303560, RO7105705, and JNJ-63733657. Despite the recent failures of passive immunotherapies targeting Aβ oligomers, there is hope for successful AD treatment by shifting the focus to the tau oligomers and by treating patients when they are in earlier disease stages.
The previous failures of passive immunotherapies raise a few general concerns about the methodology. An ever-present consideration is whether sufficient quantities of the antibody treatment are reaching their protein targets in the brain. Results from aducanumab trials show that enough of the drug reaches the brain to clear Aβ plaques,43 however perhaps increased BBB permeability would have resulted in additional aggregate clearance and improved cognitive function. It is important to continue research to improve BBB permeability, including alternative approaches such as transferrin receptor-mediated transcytosis11 and camelid antibodies24. Another concern is the applicability of pre-clinical tests using the current animal models for AD. The barrage of AD clinical trial failures, despite promising pre-clinical results, suggest that current animal models are failing to accurately portray the complexity of sporadic forms of the disease.
To better target the most toxic epitopes in AD, there is a critical need for atomic-level structural information for Aβ and tau oligomers. Due to their heterogenous and dynamic nature, this class of protein conformers eludes the efforts of crystallographers and NMR spectroscopists. Recent passive immunotherapy efforts highlight our current inability as a field to rationally target toxic epitopes on Aβ and tau, because the specific toxic epitopes are not known. By trial and error, we are learning information about which parts of these proteins should be targeted to neutralize toxicity. This seems especially important for tau, where mid-region epitopes are more effective targets in pre-clinical studies. These antibodies also provide an opportunity to obtain atomic-resolution structural information, as seen in the case of multiple crystal structures solved from Fabs co-crystallized with Aβ fragments.30,31,45 In a recent editorial by Novak et. al., he states “The greatest benefit to the field [of tau-targeted immunotherapy] would be a thorough characterization of the diseased tau proteome – what molecular properties are found in individual tau species, where these species are located (cellular compartment/extracellular), and what their effects are.”10 There is aspiration that antibody-tau crystallographic data will provide critical information about the toxic forms of tau in the coming years. Furthermore, high resolution fluorescent microscopy, performed with fluorescent-labeled Fab fragments, may be a powerful tool to track different tau species across the cell over time.
A final consideration is that there may not be one unique toxic protein species that is representative of all AD individuals.88 In vitro experiments have repeatedly shown that Aβ and tau adopt a wide variety of oligomeric conformations. The same variety of conformers may be present in an individual’s brain, or across AD patient populations depending on environmental and genetic factors. To this end, there may be hope in combination therapies; perhaps a cocktail of antibodies is required to target a heterogenous population of protein conformers. In conclusion, passive immunotherapy remains a promising pharmaceutical approach to targeting toxic Aβ and tau oligomers in the brain. The results from clinical trials and biochemical analyses of these antibodies will be highly informative about pathological epitopes of these oligomers in the coming years.
Acknowledgements
The authors express gratitude to Florencia Monge for her assistance in preparing the artwork for Figure 1. This work was supported by NSF grants 1150855 and 1605225, and NIH grant 1R21NS111267 to E.Y.C. and NIH grant NIGMS 5K12GM088021-09 supporting C.M.V.Z. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soto C Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4(1):49–60. doi: 10.1038/nrn1007 [DOI] [PubMed] [Google Scholar]
- 2.Cline EN, Assuncao Bicca M, Viola KL, Klein WL. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J Alzheimer’s Dis. 2018;64:S567–S610. doi: 10.3233/JAD-179941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chételat G, La Joie R, Villain N, et al. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. NeuroImage Clin. 2013;2(1):356–365. doi: 10.1016/j.nicl.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai J, Grutzendler J, Duff K, Gan W. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7(11):1181–1183. [DOI] [PubMed] [Google Scholar]
- 5.Kotler SA, Walsh P, Brender JR, Ramamoorthy A. Differences between amyloid-β aggregation in solution and on the membrane: insights into elucidation of the mechanistic details of Alzheimer’s disease. Chem Soc Rev. 2014;43(19):6692–6700. doi: 10.1039/C3CS60431D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams TL, Serpell LC. Membrane and surface interactions of Alzheimer’s Aβ peptide - Insights into the mechanism of cytotoxicity. FEBS J. 2011;278(20):3905–3917. doi: 10.1111/j.1742-4658.2011.08228.x [DOI] [PubMed] [Google Scholar]
- 7.Hamley IW. The Amyloid Beta Peptide: A Chemist’s Perspective. Role in Alzheimer’s and Fibrillization. Chem Rev. 2012;112(10):5147–5192. doi: 10.1021/cr3000994 [DOI] [PubMed] [Google Scholar]
- 8.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101 [DOI] [PubMed] [Google Scholar]
- 9.van Dyck CH. Anti-Amyloid-β Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biol Psychiatry. 2018;83(4):311–319. doi: 10.1016/j.biopsych.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak P, Kontsekova E, Zilka N, Novak M. Ten Years of Tau-Targeted Immunotherapy: The Path Walked and the Roads Ahead. Front Neurosci. 2018;12:1–14. doi: 10.3389/fnins.2018.00798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roche Pulls Plug on Two Phase 3 Trials of Crenezumab. Alzforum. https://www.alzforum.org/news/research-news/roche-pulls-plug-two-phase-3-trials-crenezumab. Published 2019.
- 12.Biogen/Eisai Halt Phase 3 Aducanumab Trials. Alzforum. https://www.alzforum.org/news/research-news/biogeneisai-halt-phase-3-aducanumab-trials. Published 2019.
- 13.Guyen MINHN, Oriano FES, Asquez NI V, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–919. doi: 10.1038/78682 [DOI] [PubMed] [Google Scholar]
- 14.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9(7):702–716. doi: 10.1016/S1474-4422(10)70119-8 [DOI] [PubMed] [Google Scholar]
- 15.Bournazos S, Dilillo DJ, Ravetch J V. The role of Fc-FcγR interactions in IgG-mediated microbial neutralization. J Exp Med. 2015;212(9):1361–1369. doi: 10.1084/jem.20151267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syvänen S, Hultqvist G, Gustavsson T, et al. Efficient clearance of Aβ protofibrils in AβPP-transgenic mice treated with a brain-penetrating bifunctional antibody. Alzheimers Res Ther. 2018;10(49):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niewoehner J, Bohrmann B, Collin L, et al. Increased Brain Penetration and Potency of a Therapeutic Antibody Using a Monovalent Molecular Shuttle. Neuron. 2014;81(1):49–60. doi: 10.1016/j.neuron.2013.10.061 [DOI] [PubMed] [Google Scholar]
- 18.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5(520):1–17. doi: 10.3389/fimmu.2014.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adolfsson O, Pihlgren M, Toni N, et al. An Effector-Reduced Anti-β-Amyloid (Aβ) Antibody with Unique Aβ Binding Properties Promotes Neuroprotection and Glial Engulfment of Aβ. Neurobiol Dis. 2012;32(28):9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities (ARIA) in Alzheimer’s disease patients treated with bapineuzumab: A retrospective analysis. Lancet Neurol. 2012;11(3):241–249. doi: 10.1016/S1474-4422(12)70015-7. Amyloid-related [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardinale A, Biocca S. The potential of intracellular antibodies for therapeutic targeting of protein-misfolding diseases. Trends Mol Med. 2008;14(9):373–380. doi: 10.1016/j.molmed.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Lobato MN, Rabbitts TH. Single domain intracellular antibodies: A minimal fragment for direct in vivo selection of antigen-specific intrabodies. J Mol Biol. 2003;331(5):1109–1120. doi: 10.1016/S0022-2836(03)00836-2 [DOI] [PubMed] [Google Scholar]
- 23.Li T, Vandesquille M, Koukouli F, et al. Camelid single-domain antibodies: A versatile tool for in vivo imaging of extracellular and intracellular brain targets. J Control Release. 2016;243:1–10. doi: 10.1016/j.jconrel.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 24.Hamer-Casterman Atarchouch,T C, Muyldermans S, Robinson G, et al. Naturally occurring antibodies devoid of light chains. Nature. 1998;363(June):446–448. https://www.nature.com/articles/363446a0.pdf. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Pollard A, Zhong J, et al. Intramuscular delivery of a single chain antibody gene reduces brain Aβ burden in a mouse model of Alzheimer’s disease. Neurobiol Aging 30. 2009;30:364–376. doi: 10.1016/j.neurobiolaging.2007.06.013 [DOI] [PubMed] [Google Scholar]
- 26.Kou J, Kim H, Pattanayak A, et al. Anti-Aβ single-chain antibody brain delivery via AAV reduces amyloid load but may increase cerebral hemorrhages in an Alzheimer mouse model. J Alzheimers Dis. 2013;27(1):23–38. doi: 10.3233/JAD-2011-110230.Anti-A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. [DOI] [PubMed] [Google Scholar]
- 28.Kayed R, Head E, Thompson JL, et al. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science (80-). 2003;300:486–489. doi: 10.1126/science.1079469 [DOI] [PubMed] [Google Scholar]
- 29.Crenezumab. Alzforum. https://www.alzforum.org/therapeutics/crenezumab.
- 30.Ultsch M, Li B, Maurer T, et al. Structure of Crenezumab Complex with Aβ Shows Loss of β-Hairpin. Nat Sci Reports. 2016;6(39374):1–11. doi: 10.1038/srep39374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crespi GAN, Hermans SJ, Parker MW, Miles LA. Molecular basis for mid-region amyloid-β capture by leading Alzheimer’s disease immunotherapies. Nat Sci Reports. 2015;5(9649):1–5. doi: 10.1038/srep09649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A Study to Evaluate the Efficacy and Safety of MABT5102A in Patients With Mild to Moderate Alzheimer’s Disease (ABBY). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT01343966?term=ABBY&rank=1.
- 33.Cummings J, Cho W, Ward M, et al. A randomized, double-blind, placebo-controlled phase 2 study to evaluate the efficacy and safety of crenezumab in patients with mild to moderate Alzheimer’s disease. In: Alzheimer’s Association International Conference Copenhagen, Denmark; 2014:p275. [Google Scholar]
- 34.Salloway S, Cho W, Clayton D, et al. Amyloid PET imaging results from a study to evaluate the impact of crenezumab on fibrillar amyloid in patients with mild-to- moderate Alzheimer’s disease In: Clinical Trials on Alzheimer’s Disease. Philadelphia, PA; 2014. [Google Scholar]
- 35.Honigberg L, Clayton D, Cho W, et al. Biomarker results from the crenezumab anti-Aβ phase 2 biomarker trial In: Clinical Trials on Alzheimer’s Disease. Philadelphia, PA; 2014. [Google Scholar]
- 36.Salloway S, Honigberg LA, Cho W, et al. Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer’s disease (BLAZE). Alzheimer’s Res Ther. 2018;10(1):1–13. doi: 10.1186/s13195-018-0424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CREAD Study: A Study of Crenezumab Versus Placebo to Evaluate the Efficacy and Safety in Participants With Prodromal to Mild Alzheimer’s Disease (AD). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02670083.
- 38.A Study of Crenezumab Versus Placebo to Evaluate the Efficacy and Safety in Participants With Prodromal to Mild Alzheimer’s Disease (AD) (CREAD 2). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03114657.
- 39.Reiman EM, Langbaum JBS, Fleisher AS, et al. Alzheimer’s Prevention Initiative: A Plan to Accelerate the Evaluation of Presymptomatic Treatments. J Alzheimers Dis. 2012;26:321–329. doi: 10.3233/JAD-2011-0059.Alzheimer [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.A Study of Crenezumab Versus Placebo in Preclinical Presenilin1 (PSEN1) E280A Mutation Carriers to Evaluate Efficacy and Safety in the Treatment of Autosomal-Dominant Alzheimer’s Disease (AD), Including a Placebo-Treated Non-Carrier Cohort. clinicaltrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT01998841. [DOI] [PMC free article] [PubMed]
- 41.NIH Director Announces $100M Prevention Trial of Genentech Antibody. Alzforum. https://www.alzforum.org/news/conference-coverage/nih-director-announces-100m-prevention-trial-genentech-antibody. Published 2012.
- 42.Aducanumab. Alzheimer’s News Today. https://alzheimersnewstoday.com/aducanumab/.
- 43.Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nat Publ Gr. 2016;537(7618):50–56. doi: 10.1038/nature19323 [DOI] [PubMed] [Google Scholar]
- 44.Sevigny J, Chiao P, Bussière T, et al. Addendum: The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nat Publ Gr. 2017;546(7659):564. doi: 10.1038/nature22809 [DOI] [PubMed] [Google Scholar]
- 45.Arndt JW, Qian F, Smith BA, et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid- β. Nat Sci Reports. 2018;8(6412):1–16. doi: 10.1038/s41598-018-24501-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.221AD301 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease (ENGAGE). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02477800.
- 47.221AD302 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease (EMERGE). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02484547. Published 2015.
- 48.BAN2401. Alzforum. https://www.alzforum.org/therapeutics/ban2401.
- 49.Hartley DM, Walsh DM, Ye CP, et al. Protofibrillar Intermediates of Amyloid β-Protein Induce Acute Electrophysiological Changes and Progressive Neurotoxicity in Cortical Neurons. J Neurosci. 2018;19(20):8876–8884. doi: 10.1523/jneurosci.19-20-08876.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucker S, Christer M, Tegerstedt K, et al. The Murine Version of BAN2401 (mAb158) Selectively Reduces Amyloid-β Protofibrils in Brain and Cerebrospinal Fluid of tg-ArcSwe Mice. J Alzheimer’s Dis. 2015;43:575–588. doi: 10.3233/JAD-140741 [DOI] [PubMed] [Google Scholar]
- 51.Lord A, Gumucio A, Englund H, et al. An amyloid-β protofibril-selective antibody prevents amyloid formation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;36(3):425–434. doi: 10.1016/j.nbd.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 52.Lannfelt L, Möller C, Basun H, et al. Perspectives on future Alzheimer therapies: Amyloid-β protofibrils-A new target for immunotherapy with BAN2401 in Alzheimer’s disease. Alzheimer’s Res Ther. 2014;6(1). doi: 10.1186/alzrt246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sehlin D, Englund H, Simu B, et al. Large aggregates are the major soluble Aβ species in AD brain fractionated with density gradient ultracentrifugation. PLoS One. 2012;7(2). doi: 10.1371/journal.pone.0032014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Söllvander S, Nikitidou E, Gallasch L, et al. The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death. J Neuroinflammation. 2018;15(1):1–15. doi: 10.1186/s12974-018-1134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Logovinsky V, Satlin A, Lai R, et al. Safety and tolerability of BAN2401 - A clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimer’s Res Ther. 2016;8(1):1–10. doi: 10.1186/s13195-016-0181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.A Randomized, Double-blind, Placebo-controlled, Combined Single Ascending Dose and Multiple Ascending Dose Study. clinicaltrials.gov. [Google Scholar]
- 57.A Randomized, Double-blind, Placebo-controlled Study to Assess Safety, Tolerability, Pharmacokinetics, Immunogenicity, and Pharmacodynamic Response of Repeated Intravenous Infusions of BAN2401 in Subjects With Mild Cognitive Impairment Due to Alzheimer’s. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02094729?term=BAN2401.
- 58.Satlin A, Wang J, Logovinsky V, et al. Design of a Bayesian adaptive phase 2 proof-of-concept trial for BAN2401, a putative disease-modifying monoclonal antibody for the treatment of Alzheimer’s disease. Alzheimer’s Dement Transl Res Clin Interv. 2016;2(1):1–12. doi: 10.1016/j.trci.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panza F, Lozupone M, Dibello V, et al. Are antibodies directed against amyloid- β (Aβ) oligomers the last call for the Aβ hypothesis of Alzheimer’s disease? Immunotherapy. 2019;11(1):3–6. [DOI] [PubMed] [Google Scholar]
- 60.A Study to Evaluate Safety, Tolerability, and Efficacy of BAN2401 in Subjects With Early Alzheimer’s Disease. clinicaltrials.gov.
- 61.BioArctic announces positive topline results of BAN2401 Phase 2b at 18 months in early Alzheimer’s Disease. BioArctic. https://www.bioarctic.se/en/bioarctic-announces-positive-topline-results-of-ban2401-phase-2b-at-18-months-in-early-alzheimers-disease-3600/. Published 2018.
- 62.A Study to Confirm Safety and Efficacy of BAN2401 in Participants With Early Alzheimer’s Disease (Clarity AD). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03887455.
- 63.Initiation Of Phase III Clinical Trial Of BAN2401 In Early Alzheimer’s Disease. Eisai. http://eisai.mediaroom.com/2019-03-22-Initiation-Of-Phase-III-Clinical-Trial-Of-BAN2401-In-Early-Alzheimers-Disease. Published 2019.
- 64.Sigurdsson EM. Tau Immunotherapies for Alzheimer’s Disease and Related Tauopathies: Progress and Potential Pitfalls. J Alzheimers Dis. 2018;64:S555–S565. doi: 10.3233/JAD-179937.Tau [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer’s Dement Transl Res Clin Interv. 2018;4(2018):195–214. doi: 10.1016/j.trci.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marciniak E, Leboucher A, Caron E, et al. Tau deletion promotes brain insulin resistance. J Exp Med. 2017;214(8):2257–2269. doi: 10.1084/jem.20161731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Courade J, Angers R, Mairet-Coello G, et al. Epitope determines efficacy of therapeutic anti-Tau antibodies in a functional assay with human Alzheimer Tau. Acta Neuropathol. 2018;136(5):729–745. doi: 10.1007/s00401-018-1911-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kontsekova E, Zilka N, Kovacech B, Skrabana R, Novak M. Identification of structural determinants on tau protein essential for its pathological function: Novel therapeutic target for tau immunotherapy in Alzheimer’s disease. Alzheimer’s Res Ther. 2014;6(4):1–16. doi: 10.1186/alzrt277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.To Block Tau’s Proteopathic Spread, Antibody Must Attack its Mid-Region. Alzforum. https://www.alzforum.org/news/conference-coverage/block-taus-proteopathic-spread-antibody-must-attack-its-mid-region. Published 2018.
- 70.UBC0107. Alzforum. https://www.alzforum.org/therapeutics/ucb0107.
- 71.A Study to Test the Safety, Pharmacokinetics, and Pharmacodynamics of Single Ascending Intravenous Doses of UCB0107 in Healthy Male Subjects. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03464227.
- 72.A Study to Test the Safety and Tolerability and Pharmacokinetics of Single Doses of UCB0107 in Healthy Japanese Subjects. clinicaltrials.gov.
- 73.Jicha GA, Bowser R, Kazam IG, Davies P. Alz-50 and MC-1, a New Monoclonal Antibody Raised to Paired Helical Filaments, Recognize Conformational Epitopes on Recombinant Tau. J Neurosci Res. 1997;48:128–132. [DOI] [PubMed] [Google Scholar]
- 74.Goedert M, Spillantini M, Jakes R, Rutherford D, Crowther R. Multiple isoforms of human microtubule-associated protein-tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–526. [DOI] [PubMed] [Google Scholar]
- 75.Alam R, Driver D, Wu S, et al. Preclinical Characterization of an Antibody [Ly3303560] Targeting Aggregated Tau. In: Alzheimer’s Association International Conference London, UK; 2017:P592–P593. doi: 10.1016/j.jalz.2017.07.227 [DOI] [Google Scholar]
- 76.A Study of LY3303560 in Healthy Participants and Participants With Alzheimer’s Disease (AD). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02754830.
- 77.A Study of LY3303560 in Participants With Mild Cognitive Impairment or Alzheimer’s Disease. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03019536.
- 78.A Study of LY3303560 in Participants With Early Symptomatic Alzheimer’s Disease. clinicaltrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT03518073.
- 79.RO7105705. Alzforum. https://www.alzforum.org/therapeutics/ro7105705.
- 80.Genentech to Discontinue Phase III CREAD 1 and 2 Clinical Studies of Crenezumab in Early Alzheimer’s Disease (AD) - Other Company Programs in AD Continue. Genentech Press Release. https://www.gene.com/media/press-releases/14774/2019-01-29/genentech-to-discontinue-phase-iii-cread. Published 2019.
- 81.A Study of RO7105705 in Healthy Participants and Participants With Mild-to-Moderate Alzheimer’s Disease. clinicaltrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02820896.
- 82.Kerchner GA, Ayalon G, Brunstein F, et al. A Phase I Study To Evaluate the Safety and Tolerability of RO7105705 in Healthy Volunteers and Patients With Mild-To-Moderate AD. In: Alzheimer’s Association International Conference London, UK: Elsevier; 2017:P601. doi: 10.1016/j.jalz.2017.07.243 [DOI] [Google Scholar]
- 83.A Study to Evaluate the Efficacy and Safety of RO7105705 in Patients With Prodromal to Mild Alzheimer’s Disease. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03289143.
- 84.A Study of RO7105705 in Patients With Moderate Alzheimer’s Disease. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03828747.
- 85.JNJ-63733657. Alzforum. https://www.alzforum.org/therapeutics/jnj-63733657.
- 86.A Study of JNJ-63733657 in Healthy Japanese Participants. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03689153.
- 87.A Study to Investigate Safety and Tolerability, Pharmacokinetics and Pharmacodynamics of JNJ-63733657 in Healthy Subjects and Subjects With Alzheimer’s Disease. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03375697.
- 88.Selkoe DJ. Alzheimer disease and aducanumab: adjusting our approach. Nat Rev Neurol. 2019;15:365–366. doi: 10.1038/s41582-019-0205-1 [DOI] [PubMed] [Google Scholar]