Abstract
Infections caused by parasitic flatworms impose a considerable worldwide health burden. One of the most impactful is schistosomiasis, a disease caused by parasitic blood flukes. Treatment of schistosomiasis has relied on a single drug – praziquantel (PZQ) – for decades. The utility of PZQ as an essential medication is, however, intertwined with a stark gap in our knowledge as to how this drug works. No flatworm target has been identified that readily explains how PZQ paralyzes and damages schistosomes. Recently, a schistosome ion channel was discovered that is activated by PZQ and displays characteristics which mirror key features of PZQ action on schistosomes. Here, the journey to discovery of this target, properties of this ion channel, and remaining questions are reviewed.
Praziquantel – Still the Enigmatic Antiparasitic
The drug praziquantel (PZQ) (see Glossary) is an essential drug that has had a huge impact in tropical medicine. This is because PZQ is the key therapy for schistosomiasis (Bilharzia), as well as several other infections caused by parasitic flatworms. PZQ was first approved for human use in the 1980s and, despite being an ‘old’ drug, remains the antischistosomal agent of choice owing to a profile that combines high clinical efficacy with a low incidence of side effects. However, despite being used clinically for decades, the mechanism of action of PZQ still remains undefined. PZQ was handed the moniker of the ‘enigmatic’ antiparasitic over 25 years ago [1], and this tag has proved remarkably prescient. This is certainly unusual as few clinically approved drugs lack defined or predictable targets (<1%, [2]), and PZQ has been in use for a very long time [3]. Our lack of mechanistic knowledge can most likely be attributed to the ‘neglected’ nature of the tropical diseases that PZQ is used to treat, and that the drug’s target exists ‘in a worm’ not in a human (in reality within a worm in a human). Clearly, this important gap in our knowledge needs to be filled.
The recent discovery of a flatworm target for PZQ – a transient receptor potential (TRP) channel in Schistosoma mansoni (Sm.TRPPZQ, [4]) – is therefore an important breakthrough. Here, we review the journey that has culminated in the discovery of this target, starting by reflection on the importance of PZQ as an anthelmintic drug and key features of PZQ action on schistosomes. Data identifying host targets of PZQ that helped to narrow the search for flatworm targets are outlined. Finally, the likely properties of Sm.TRPPZQ are discussed and the new gaps in our understanding defined.
PZQ, an Essential Medication
Schistosomiasis is a neglected tropical disease caused by infection with parasitic blood flukes of the genus Schistosoma. The disease is endemic in over 70 countries worldwide, with greater than 750 million people estimated to be at risk [5]. The majority of infections occur in sub-Saharan Africa, and the morbidity of chronic schistosomiasis conveys a massive burden on the pediatric and adult populations impacted by this disease (estimated at ~70 million disability adjusted life years annually [6,7]). Chronic disabilities are driven by host responses to eggs laid by the adult worms and encompass gastrointestinal and liver pathologies, genitourinary disease, anemia, undernutrition, growth retardation, and a heightened risk for several comorbidities. For example, schistosomiasis is associated with increased risk of HIV infection in women, and treatment reduces HIV susceptibility [8]. Effective treatment of schistosomiasis is therefore a healthcare priority to mitigate the impact and inequity of this debilitating disease.
Thankfully, a highly effective therapy for schistosomiasis is available – PZQ. PZQ (Box 1) has been used for decades as the key agent for treating infections caused by each of the major species of schistosome worms. PZQ treatment can be associated with high cure rates at low cost (~US$0.20 drug cost to treat a child [7,9,10]), providing the foundation for mass drug administration (MDA) initiatives. PZQ is also effective for treating several other parasitic helminth infections and in veterinary applications. Overall, the impact of PZQ as a safe and efficacious ‘essential medication’ cannot be overstated – the WHO estimates that, in 2017, about 100 million people received prophylactic PZQ to prevent schistosomiasis (~80 million school-aged children and ~20 million adults). The need may be as great as ~2 billion tablets over 5 years for country-wide MDA initiatives [10]. Continued efficacy of PZQ in the field is therefore paramount. For these reasons, any vulnerabilities in the chemotherapeutic effectiveness of PZQ merit our attention. Major concerns are two-fold: first, the potential for emergence of PZQ resistance; second, our failure to define how PZQ actually works.
Box 1. PZQ: Small Molecule, Big Impact.
PZQ is a tetracyclic tetrahydroisoquinoline derivative (2-(cyclohexylcarbonyl)-1,2,3,6,7–11b-hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one) that emerged from a series of over 400 pyrazino-isoquinolines synthesized as potential tranquilizers by Merck and Bayer in the 1970s. PZQ was patented in Germany in 1973 and in the USA in 1977. Appreciation of the potent efficacy of PZQ against cestodes and trematodes prioritized its development first for veterinary applications and subsequently as a clinical therapy for schistosomiasis [3,83,84].
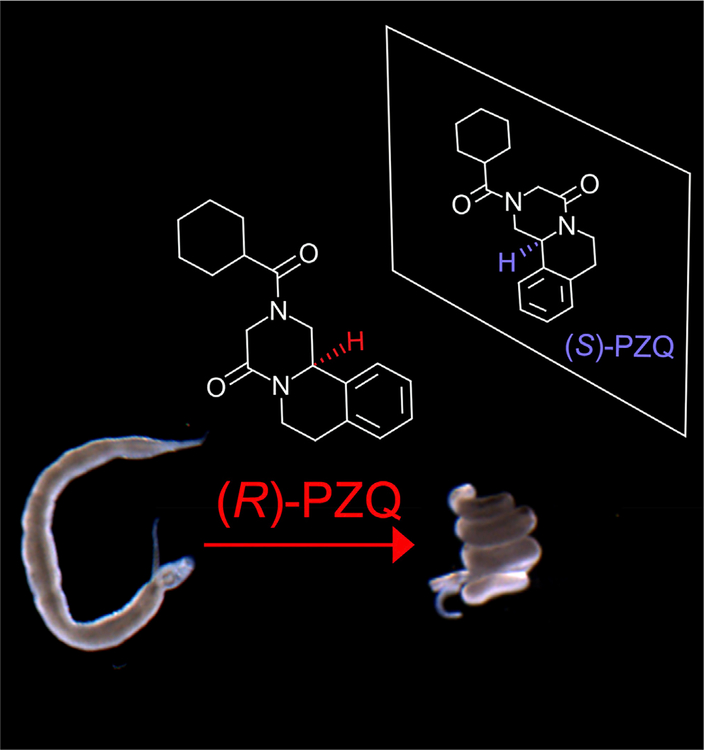
PZQ possesses asymmetry with two functionally unique stereoisomers (Figure I): (R)-PZQ (the biological active eutomer which causes rapid paralysis of schistosome worms at nanomolar concentrations) and (S)-PZQ (a less active – but not completely inactive – distomer). Clinically, PZQ is administered as a racemic mixture [(±)-PZQ, dosed orally at 40–60 mg/kg] in the form of a large pill (a consequence of the low solubility of PZQ) that has an unappealing bitter taste [attributed to (S)-PZQ [85]]. Methods for resolution of the two enantiomers have been developed with one goal being the development of an enantiopure formulation. PZQ is rapidly eliminated (half time, 60–90 min) and therefore provides little protection against reinfection.
Figure I. Praziquantel (PZQ) Stereochemistry.
PZQ is chiral, existing in two enantiomeric forms [laevo or (R)-PZQ (red); dextro or (S)-PZQ (blue, mirrored)]. (R)-PZQ causes rapid paralysis of adult schistosome worms (‘corkscrewed’ phenotype). (R)-PZQ also causes rapid damage to the worm’s surface.
The emergence of schistosome strains refractory to PZQ would be highly impactful given the paucity of broadly effective alternative drugs or an effective vaccine [11–13]. PZQ is well known to be less effective against juvenile worms in vitro and in vivo, a property which likely contributes to the failure of PZQ to cure schistosome infections completely. Sensitivity of worms to PZQ depends on the age of infection, the sex of the worms, and their state of pairing [14]. Lower sensitivities to PZQ can also be engineered by selective pressure in the laboratory, and refractoriness to standard dosing has been observed in the field [11,13,15]. Vigilance is merited if selective pressures enhanced by MDA initiatives coalesce with suboptimal cure rates in endemic areas.
The other critical roadblock is our lack of mechanistic understanding of how PZQ works – the flatworm target(S) of PZQ action have remained undefined for over 40 years. Although, multiple flatworm targets have been proposed over the years (reviewed in [13,16]), none convincingly explains the key features of PZQ action on schistosomes (outlined in the next section). This lack of an identified target precludes target-based screening for novel anthelmintics, or target-based modeling to engineer improved PZQ derivatives. Indeed, it is noteworthy that, from over 200 derivatives of PZQ synthesized to date, few match the efficacy of the parent molecule [17]. We therefore continue to rely on the anthelmintic activity of PZQ, discovered in 1972, as clinical monotherapy.
PZQ: Action on Schistosomes
What are the key facets of PZQ action on adult schistosome worms? First, an ability to cause a rapid, spastic paralysis of schistosome muscle. Second, an ability to cause a rapid vacuolation of the tegument, the syncytial surface of the worm. These dual actions are also apparent in other trematodes and cestodes exposed to PZQ. The characteristics of these responses are discussed in turn below.
Contractile Paralysis
Exposure of adult schistosome worms to PZQ (>200 nM, [3]) causes a rapid muscle contraction (onset within <30 s) that leads to a sustained spastic paralysis [18]. This response is caused by (R)-PZQ and is observed in vitro and in vivo. This effect is not irreversible, as paralysis is reversed after PZQ removal. As expected, contraction is Ca2+-dependent as omission of Ca2+ from the bathing medium abolishes both the PZQ-evoked 45Ca2+ influx and tetanus [18–22]. Early studies helped to define a limited pharmacological signature for this PZQ-evoked response in schistosomes. Elevation of medium Mg2+ resulted in a partial blockade of these effects, causing the PZQ-evoked contraction to be phasic rather than sustained [18, 23]. Other divalent cations (La3+) blocked PZQ action entirely [22]. PZQ-evoked 45Ca2+ uptake and/or contractions were not inhibited by established blockers of specific mammalian voltage-operated Ca2+ channel (Cav) subtypes, such as methoxyverapamil (D-600, [22]), nicardipine, or nifedipine [20]. Finally, a study of schistosome strains with varying sensitivities to PZQ revealed that PZQ-evoked contraction and 45Ca2+ uptake were attenuated in strains that display lower sensitivities to PZQ in vivo [21].
Tegument Damage
Exposure of adult schistosome worms to PZQ (>200 nM, [24]) rapidly initiates a vacuolization of the tegument (<30 s) This response is caused by (R)-PZQ [24] and occurs both in vitro and in vivo [25–27]. This effect is not irreversible, as worms can recover from exposure to submaximal doses of PZQ [25]. Initiation of this process is accompanied by a slower depolarization and eventually culminates in regionalized lesions, erosion of tegumental integrity, and exposure of worm antigens critical for ensuring host immunological engagement and worm clearance in vivo [28,29]. Importantly, PZQ-evoked vacuolization is also Ca2+-dependent and does not occur in Ca2+-free media [27]. These effects of PZQ on the worm tegument are also attenuated in strains that display lower sensitivity to PZQ [30].
The Ca2+-dependency of these dual PZQ-evoked effects (worm paralysis, tegument damage) provide the foundation for the ‘Ca2+ hypothesis’ underpinning PZQ action. This hypothesis spurred research into the molecular mediators of these effects, with initial suspicion falling upon schistosome voltage-operated Ca2+ channels as candidate targets [31,32]. Experimental analysis of hybrid Cav complexes provided early support for this idea [33]; however, additional structural, biochemical, and functional evidence for the hypothesis that PZQ directly engages Cav channels has not been forthcoming. This is not to say that schistosome Cav complexes are not enticing anthelmintic targets [32], and they are most certainly engaged as effectors of PZQ-evoked depolarization [34–36]. However, data demonstrating that they are ‘direct’ targets for PZQ is still lacking.
Many other flatworm targets for PZQ have subsequently been proposed over many years [13,16], although none readily manifests the required characteristics for PZQ engagement (nanomolar potency, stereoselectivity for (R)-PZQ, capacity to support a sustained and potentially toxic Ca2+ entry) that underpin these dual phenomenological pillars of PZQ action on worms.
PZQ Targets: Definitive in the Host
A critical breakthrough for identifying targets of ±PZQ came by taking a step away from working with the parasites themselves: schistosome life-cycle stages can be challenging to work with, and heterologous expression of flatworm gene products remains quite hit-or-miss. Instead, prospecting for PZQ interactions with human target(s), to capitalize upon well-optimized methodological screening platforms and preparations, was tantalizing as a more facile and less demoralizing endeavor. Screening for human targets of ±PZQ could, however, be regarded as counterintuitive given the premise that antiparasitics are de facto ‘selective’, having been optimized purposely to minimize host interactions, and thereby side effects, in those taking medications. However, selectivity in drug action is rarely absolute, and there existed a clearly evidenced ‘host’ activity of ±PZQ in muscle preparations studied in the laboratory [37,38], as well as known side effects of PZQ in the field.
Based on our own work studying PZQ in planarians (Box 2), which raised similarities between the action of PZQ and several ergot alkaloids, we were keen to explore the idea that PZQ acted as a bioaminergic G-protein-coupled receptor (GPCR) ligand [39]. Indeed, the ability of PZQ to block effects of 5-HT on schistosomes had been previously observed [40]. A virtual docking screen, as well as a functional screen of a human GPCR panel, both highlighted a specific serotoninergic GPCR (5-HT2BR) that exhibited stereoselective interaction with (R)-PZQ [41]. Other GPCRs were engaged at higher ±PZQ concentrations. (R)-PZQ elicited transient Ca2+ signals in cells heterologously expressing the human 5-HT2BR, a Gq-coupled receptor, and these signals were blocked by a 5-HT2BR antagonist [41]. These assays revealed that (R)-PZQ acted as a low-affinity partial agonist at 5-HT2BR (EC50 for ±PZQ, ~8 μM [41]). (R)-PZQ displaced 3H-lysergic acid diethylamide binding, and modeling analyses implicated involvement of residues within the orthosteric binding pocket and extracellular loop 2 (EL2). This includes a residue (L209EL2) critical for regulating ligand residence time [42], which was an intriguing observation given that brief exposure (minutes) to certain ergot alkaloids causes lengthy paralysis (hours) of flatworms [43,44].
Box 2. The Importance of Basic Science.
Our interest in the mechanism of action of PZQ came from a surprising result in a small-molecule drug screen on flatworm regeneration [34]. In experiments studying regeneration of Dugesia japonica, a free-living planarian, exposure of regenerating trunk fragments to PZQ (amputated pieces following removal of both head and tail structures) generated bipolar, two-headed worms (Figure II). This effect was highly penetrant and provided a visually striking phenotype for interrogating the mechanistic basis of PZQ action in vivo using chemical and functional genetic methods optimized in this model.
For example, knockdown of specific voltage-operated calcium channels (Cav) and their subunits using in vivo RNAi modulated PZQ-evoked bipolarity [34,35], drawing parallels with data from the foundational schistosome Cav expression work [33]. Impairment of the bioaminergic neurotransmission, through RNAi of tryptophan hydroxylase and tyrosine hydroxylase phenocopied the effects of Cav channel RNAi [86]. Such approaches helped to tease apart pathways and narrow focus on cell types relevant to PZQ action on regeneration in vivo. The amenability of Dugesia japonica for small-molecule screening also realized a key breakthrough that PZQ-evoked bipolarity was mimicked by certain ergot alkaloids [39]. Ergot alkaloid drugs cause smooth-muscle contraction via engagement of bioaminergic GPCRs. This is important in light of the remarkable finding that the ensemble of planarian polarity genes that control regenerative outcomes (‘position control genes’) are constitutively expressed in body wall muscle [87]. The ability of PZQ to cause a sustained Ca2+ signal in muscle likely provides a nonphysiological cue with the correct spatial and temporal signature to subvert normal regeneration. The shared structure–activity relationship between ligands effective at causing bipolarity and their action as serotoninergic blockers (as antagonists or partial agonists, of planarian 5-HT GPCRs) raised the possibility that PZQ acted as a serotoninergic ligand [39].
In short, the use of free-living planarians, a stalwart model for regenerative biology, provided key insights for shaping our understanding of how PZQ might work in parasitic schistosomes. This serves to underscore the essentiality of research in diverse model systems, often far removed from human disease processes, for catalyzing clinically relevant breakthroughs.
Figure II. Praziquantel (PZQ)-Evoked Bipolarity.
Example of a bipolar planarian produced by PZQ treatment during regeneration. Head structures can be seen at both ends of the worm. Data reproduced with permission from [34].
Separately, Babes et al. [45] were intrigued by the structural resemblance between the cyclohexylcarboxamide moiety of PZQ and a class of cooling agents known to act on the thermosensitive cation channel, transient receptor potential melastatin-8 (TRPM8, the ‘cold’ receptor, [46]). TRP channels are a superfamily of cation channels activated by diverse stimuli and notorious for their regulation by diverse chemical agents (‘polymodal’ regulation). This encompasses regulation of TRPM8 by tryptaminergic ligands [39]. In Ca2+-imaging studies, ±PZQ elicited transient Ca2+ signals in cells heterologously expressing human TRPM8, and these signals were blocked by a TRPM8 antagonist [45]. Assays suggested that ±PZQ acted as a low-affinity partial agonist at TRPM8 (EC50 for ±PZQ, ~25 μM [45]). No binding studies were performed, but mutational analyses were suggestive of ±PZQ interaction with residues defining the menthol-binding site [45], now known to be in a ligand-binding pocket shaped by residues from the voltage-sensor-like domain and TRP domain of human (h)TRPM8 [46,47]. Gunaratne et al. also demonstrated the ability of ±PZQ to activate hTRPM8, but resolved activity was solely attributable to (S)-PZQ interaction with hTRPM8 (EC50 for (S)-PZQ ~20 μM); (R)-PZQ had no effect [48]. Both studies demonstrated activation of additional TRP channels at higher ±PZQ concentrations [45,48], again underscoring the promiscuity of PZQ as a small-molecule ligand.
The importance of these recent findings was to define specific ‘host’ targets that stereoselectively engage either (R)-PZQ (5-HT2BR [41]) or (S)-PZQ (TRPM8, [48]), a rare example of enantiomers in a racemic mixture targeting unique classes of effector (GPCR vs TRP channel). The significance was two-fold: first, for understanding how PZQ might engage host signaling pathways relevant to its clinical efficacy; second, in providing a molecular toehold for homology searches aimed at uncovering similar PZQ-sensitive effectors in the parasite. A more speculative point of interest is whether these data could herald merit in repurposing PZQ toward other human disease indications (Box 3).
Box 3. Old Drug, New TRPs?
These new data reveal PZQ as a synthetic TRP ligand long approved for human use. Could PZQ harbor repurposing potential for treating other diseases? hTRPM8 has been proposed as a therapeutic target for pain, migraine, and cancer. hTRPM2 is also an emerging clinical target for nervous system and inflammatory disorders. Bitter-tasting compounds, a noted side-effect of (S)-PZQ [85], often produce stronger bronchodilation in asthmatic models than widely used β-receptor GPCR agonists. While (S)-PZQ has low affinity for the host target hTRPM8, and seemingly no regulatory action at hTRPM2 [48], there has been no effort to date optimizing PZQ-like derivatives at these targets. Docking studies using recently available hTRPM8 structures will aid understanding of the molecular basis for stereoselective PZQ binding at hTRPM8, facilitating identification of such ligands. Comparison of Sm.TRPMPZQ sequence with recently solved hTRPM2 structures may also help to catalyze drug design at this clinically important target. Known regulators of hTRPM2, including the endogenous agonist ADPR, act over the micromolar range such that the nanomolar affinity of PZQ at Sm.TRPMPZQ is very noteworthy. While interest has traditionally been focused on repurposing established drugs as antiparasitics, the road less traveled – repurposing antiparasitics toward noninfectious human disease indications – is garnering growing interest. Examples include evaluation of mebendazole and niclosamide as anticancer agents [88,89] and artemether as an antidiabetic therapy [90]. Repurposing of PZQ could yield a further example.
First, how are these data significant for understanding PZQ action in vivo? One example is that these data provide insight into the ‘hepatic shift’, a chemotherapeutic phenomenon in which PZQ (and other anthelmintics) cause displacement of worms from the mesenteric circulation to the liver, where they are eliminated. The ‘hepatic shift’ is a frequently used assay for antischistosomal drug efficacy, and acute PZQ-evoked changes in mesenteric blood flow mediated by host interactions may contribute to this phenomenon, helping to ‘flush’ PZQ-paralyzed worms toward the liver [41]. Acute administration of ±PZQ leads to high micromolar plasma concentrations of both enantiomers within the mesenteric vessels where adult worms reside. This mesenteric vascular compartment exists prior to circulation through the liver and therefore first-pass metabolism of PZQ. The concentration of PZQ enantiomers in the splanchnic beds in vivo is likely in the range of tens of micromolar [49–51], overlapping the concentration range for activation of 5-HT2BRs in vitro. Both (R)-PZQ and (S)-PZQ have been directly shown to be vasoactive on mesenteric vessel strips [41,48], with serotoninergic GPCRs (serotonin = the ‘tonic’ substance in ‘serum’) and TRP channels [52] being known mediators of vascular tone. (R)-PZQ activity is blocked by a 5-HT2BR antagonist [41], while the molecular basis of the (S)-PZQ is yet to be resolved as it persists in hTRPM8 knockout mice [48]. The vasoactivity of both PZQ enantiomers, mediated through interaction with specific host targets, may therefore optimize blood flow through splanchnic beds where the blood flukes reside, contributing to their displacement from these vessels on PZQ administration.
PZQ interactions with host receptors may also impact other responses during schistosome infection. For example, PZQ may impact immune responses independently from effects on worm and egg numbers by exerting direct regulatory effects on host immune cell function [53–56]. Host factors also regulate the progression of liver fibrosis in chronic infections [57], and strategies for engaging host responses that mitigate progression toward chronic pathologies hold considerable therapeutic interest. One example would be the use of drugs to repress the activation of hepatic stellate cells that drive fibrotic changes [58]. This could be done by coadministering antifibrotic adjuncts with anthelmintics, or potentially even with a single therapy. A proof of principle of this approach is exemplified by the ergot alkaloid ergotamine, which coalesces deleterious effects on the parasite (via parasite GPCRs) with beneficial actions on the host (via repression of hepatic stellate activation likely by engagement of host GPCRs, [56]). PZQ can also exhibit antifibrotic activity and repress hepatic stellate cell activation [59,60]. Therefore, rather than considering antiparasitics as ‘selective’ therapies with no host effects, these data underscore the potential contributions from exploiting the broader polypharmacology of PZQ in both host and parasite.
Most importantly, the discovery of host targets of PZQ [41,45,48] provided a clear structural blueprint to focus the search for homologous parasite targets of PZQ (Figure 1, Key Figure). A reasonable expectation based on discovery of the human targets would be the existence of schistosome 5-HT GPCRs with the ability to bind (R)-PZQ, and schistosome TRPM channels engaged by (S)-PZQ. Considerable recent progress has been made by studying both of these classes of targets in flat-worms, prioritized a priori by the long-known importance of serotonin in schistosome biology [61,62] and the role of TRP channels in sensory physiology [63,64].
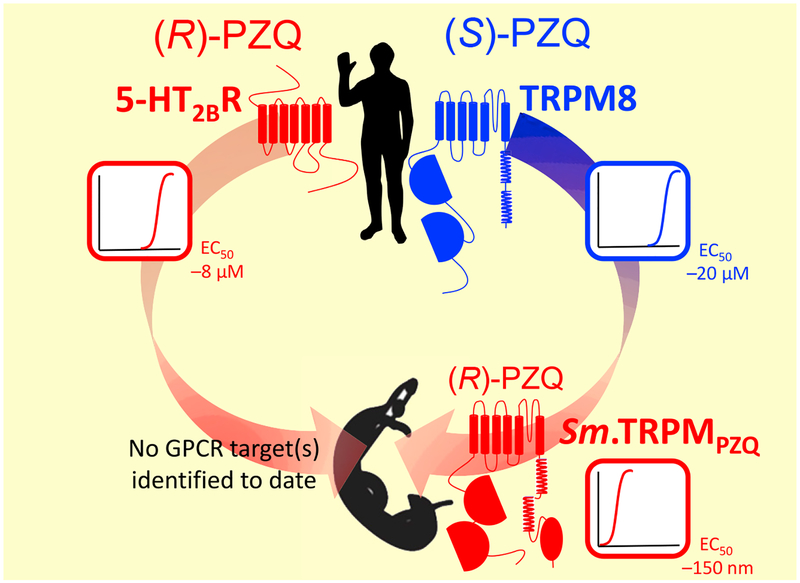
Figure 1.
Host targets for (R)-PZQ (red) and (S)-PZQ (blue) have been defined. These are a G-protein-coupled receptor (GPCR) [5-HT2BR for (R)-PZQ and a transient receptor potential (TRP) channel (transient receptor potential melastatin; TRPM8) for (S)-PZQ]. Recently, using a homology-based search, a TRPM channel in Schistosoma mansoni (Sm.TRPMPZQ) that is activated by (R)-PZQ in the nanomolar range has been identified [4]. Additional target(S) for PZQ in parasitic flatworms may yet be found. PZQ sensitivity of each of these targets is schematically illustrated by dose–response curves, with EC50s for activation by individual enantiomers taken from [4,41,48].
Schistosomes express a broad GPCR portfolio [65], and the predicted serotoninergic GPCRs are of high interest given the role of this neurotransmitter in parasite movement [62]. From the complement of predicted 5-HT GPCRs, only the pharmacological profile of a single schistosome 5-HTR (Sm.7aL [44,56,66], and orthologs Sj.7a, Sh.7a [56]) have been well studied to date, enabled by successful heterologous expression in mammalian cell lines. In vivo RNAi of Sm.7a [62] or a planarian homolog [39], and application of 5-HT ligands validated at these targets, all disrupted worm movement [39,43,44,56,67]. However, Sm.7a does not appear to be regulated by PZQ [44], and data from other schistosome 5-HT GPCRs have yet to be reported. Therefore, a GPCR target for PZQ has yet to be discovered (Figure 1). However, there is much work yet to be done comprehensively profiling schistosome bioaminergic GPCRs and understanding their in vivo roles throughout the parasitic life cycle. This has considerable merit as GPCRs are highly druggable targets, mediating the therapeutic effect of one-third of current FDA-approved small-molecule drugs [68].
Schistosome TRP channels have also been under scrutiny for their potential as anthelmintic targets [64,69]. Interest has again been heightened by data from free-living planarian flatworms that underscores the importance of TRP channels for transducing environmental cues into appropriate behavioral responses [70–72]. Schistosomes express representatives of five mammalian TRP subfamilies [63,64]: TRP-C (-canonical), TRP-A (-ankyrin), TRP-P (-polycystin), TRP-ML (-mucolipin), and TRP-M (-melastatin), but not TRP-V (-vanilloid). The schistosome TRP-A representative has been the best studied to date, with data underscoring two key themes in this review: first, the unique pharmacological profile of these targets compared with their mammalian ‘counterparts’ [73], and second, the continual interplay between worm and host responses as schistosome TRP-A1 may be activated by at least one host signaling molecule [73].
However, the ability of (S)-PZQ to activate hTRPM8 [48] prioritized attention on the schistosome TRPM subfamily. This subgroup is well represented in schistosomes, with seven predicted members. BLAST searches for worm TRPM channels resembling hTRPM8 prioritized candidates within this subfamily, and primary screens recently lead to the discovery of a TRPM candidate – christened Sm.TRPMPZQ – which was robustly activated by PZQ in Ca2+-imaging assays after heterologous expression [4]. Further characterization revealed that Sm.TRPMPZQ was stereoselectively activated by (R)-PZQ in the nanomolar range [EC50 for (R)-PZQ = 154 ±33 nM at 37oC] and mediated a sustained cellular Ca2+ signal on PZQ addition. The kinetics of this Ca2+ transient mirror the kinetics of the PZQ-evoked increase in schistosome muscle tension (Figure 2). Little desensitization to PZQ was apparent, with Ca2+ entry through Sm.TRPMPZQ subsiding only on PZQ removal [4]. The effectiveness of modulators of PZQ-evoked muscle contractions/45Ca2+ uptake in vivo was closely mirrored on Sm.TRPMPZQ [4]. Homologs of this channel are present in other flatworms that are PZQ-sensitive [4], but their properties have yet to be studied.
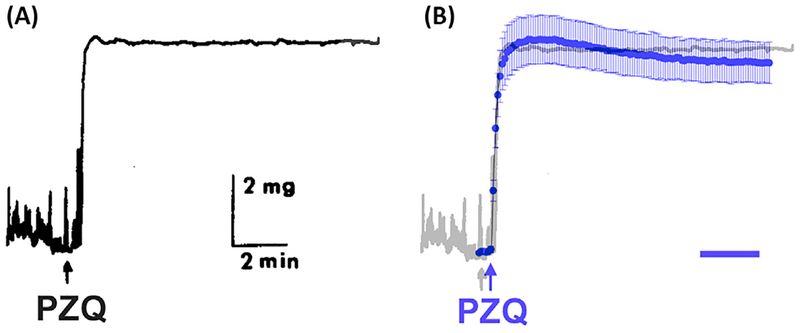
Figure 2. Kinetic Comparison of Responses to Praziquantel (PZQ) on Sm.TRPMPZQ and Schistosome Muscle.
(A) Chart recording of the effect of PZQ (1 μM) on schistosome muscle tension. Reproduced, with permission, from Figure 1 of Fetterer et al. [22]. (B) Response of Sm.TRPMPZQ to PZQ (blue) on the same timescale (bar, 2 min). Response is superimposed on the contraction trace. Data points indicate the increase in fluo-4 fluorescence on addition of PZQ (1 μM).
The characteristics of PZQ action on Sm.TRPMPZQ – nanomolar potency, stereoselectivity for (R)-PZQ, sustained and potentially cytotoxic Ca2+ entry – are highly intriguing as they parallel the key tenets of PZQ action on schistosome Ca2+ homeostasis (see ‘PZQ: Action on Schistosomes‘, above). Sm.TRPMPZQ is therefore the first flatworm target of PZQ to be identified exhibiting such properties [4]. Obviously, with any such breakthrough as many new questions are raised as answered, but identification of Sm.TRPMPZQ now provides the molecular grist to do so.
Finally, while this detour through human candidates provided the critical breakthrough in finding a worm target of PZQ, it was also fortuitous. Results underscore how little we know about flatworm receptor biology and the properties of their ligand-binding pockets – for example, the enantiomeric specificity for PZQ was inverted between hTRPM8 and Sm.TRPMPZQ and their sensitivities to PZQ very different (>100-fold difference in EC50, Figure 1). The cautionary note is one of care over the easy temptation to presume that mammalian pharmacological specificities are preserved at flatworm targets. Distinct pharmacological profiles of flatworm GPCRs and TRP channels have been consistently noted [44,56,73]. This is because flatworm binding pockets are customized to their unique neurochemical and host (e.g., molluscan, free-living, human) environments and having specialized along distinct evolutionary routes from their ‘counterparts’ in human pedigrees with which we have become overly familiar.
(R)-PZQ Activation of Sm.TRPMPZQ
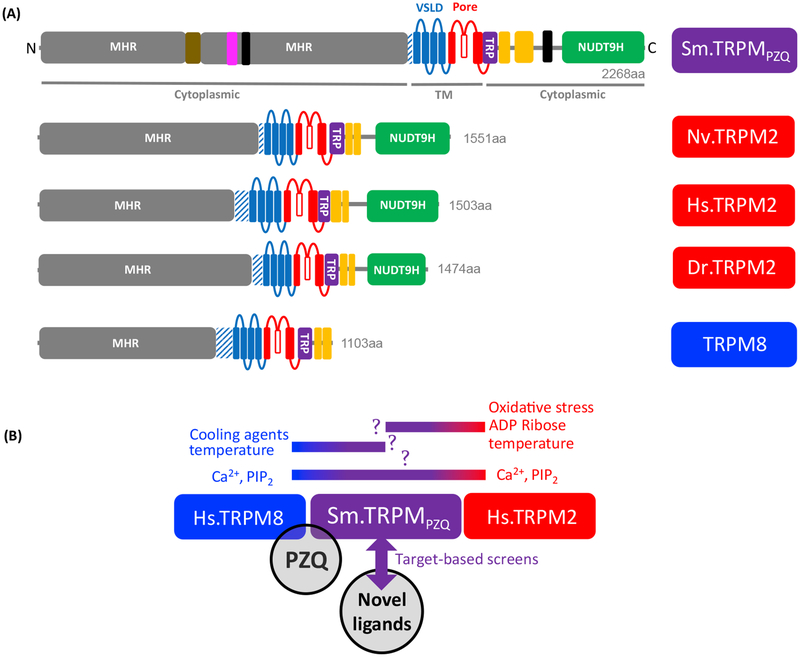
What sort of channel is Sm.TRPMPZQ? Sequence homology places Sm.TRPMPZQ in the TRPM subfamily, christened the ‘long TRPs’ as each human TRPM extends to >1000 amino acids in length. Sm.TRPMPZQ is longer than all human TRP channel sequences and exhibits the highest homology to the closely related channels hTRPM2 and hTRPM8. In terms of domain organization, TRPMs possess large cytoplasmic NH2-terminal TRPM homology regions (MHR) of unknown function that precede the voltage-sensor-like domain (transmembrane helices, S1–S4), the pore domain (S5,S6), the TRP box, and the cytoplasmic coiled-coil domains (Figure 3A). Importantly, the presence of a COOH terminal NUDT9 homology domain (NUDT9H) in Sm.TRPMPZQ identifies the PZQ target as a TRPM2-like channel. Human NUDT9 is an adenosine 5’-diphosphoribose (ADPR) pyrophosphatase, binding ADPR and catalyzing its conversion into adenosine monophosphate and ribose-5-phosphate. Consequently, TRPM2 is often referred to as a hybrid channel/enzyme (a ‘chanzyme’) irrespective of the actual catalytic activity of the NUDT9H domain in any particular species, with cytoplasmic ADP ribose (ADPR) binding serving to activate TRPM2. ADPR is considered the key physiological activator of TRPM2 channels, with elevations of resting cytoplasmic ADPR levels in response to reactive oxygen species (ROS) being the key transducer of environmental changes into TRPM2-mediated Ca2+ signals. The two other obligate coactivators are Ca2+ and PIP2.
Figure 3. Overview of Sm.TRPMPZQ and Related Transient Receptor Potential Melastatin (TRPM) Channel Architecture.
(A) Schematic of domain organization of Sm.TRPMPZQ (purple) and related TRPM2 (red) and TRPM8 (blue) channels for which structures are available. These are from starlet sea anemone (Nematostella vectensis TRPM2; Nv.TRPM2, [76]), human (Homo sapiens TRPM2; Hs.TRPM2 [74]), zebrafish (Danio rerio TRPM2; Dr.TRPM2, [75]), and collared flycatcher (Ficedula albicollis TRPM8; a mutant version listed for simplicity as TRPM8 [46]). Domain organization is drawn to scale (amino acid length listed) and highlights indicated regions: MHR (TRPM homology region, gray), pre-S1 domain (hatched blue), VSLD (voltage sensor-like domain S1–S4, blue), pore domain (S5,S6, red), transient receptor potential (TRP) helix region (purple), rib and pole helices (yellow), and NUDT9 homology domain (NUDT9H) enzyme-like domain (green, present in TRPM2 sequences). Additional predicted features are highlighted for Sm.TRPMPZQ and include an ankyrin-repeat domain (brown), and serine-rich (pink) and proline-rich (black) regions. (B) Potential regulators of Sm.TRPMPZQ. Sm.TRPMPZQ may present hybrid properties between TRPM2 and TRPM8 that became specialized in the vertebrate lineage. Aside from praziquantel (PZQ) (black) as a known ligand of TRPM8 and Sm.TRPMPZQ, other possible regulators of Sm.TRPMPZQ include other TRPM8 ligands (e.g., cold temperature and cooling agents, but see [4]) and TRPM2 ligands (e.g., reactive oxygen species, ADP ribose, and temperature). TRPM2 and TRPM8 channels are also coregulated by PIP2 and intracellular Ca2+. Target-based screening of Sm.TRPMPZQ may also generate novel scaffolds.
Recent cryo-electron microscopy structures of TRPM2 [74–76] and TRPM8 [46,47] channels from diverse organisms have furthered our understanding of how these channels operate. This new structural insight, coupled with the functional characterization of channel mutants from humans (Hs.TRPM2, [74]), zebrafish (Dr.TRPM2, [75]), and a cnidarian (Nematostella vectensis, Nv.TRPM2 [76]) has revealed considerable species-specific diversification in how these channels respond to similar stimuli. Like Nv.TRPM2, Sm.TRPMPZQ is an invertebrate representative and may exhibit features of multiple TRPM channels that have become diversified as specific human TRPM channel sub-types. For example, Sm.TRPMPZQ may retain a TRPM8-like ligand-binding pocket (there is no clear TRPM8-like channel in schistosomes) in the context of a broader TRPM2-like architecture. Given that there are seven predicted schistosome TRPM channels, these channels may have specialized in ways tailored to support the unique sensory requirements of the parasite. TRPM2 channels are known to be regulated by oxidative stress and elevated temperature [77], TRPM8 is regulated by cooling, cooling agents, and endogenous signaling molecules [46,47]. These channels may therefore act as sensors responsive to oxidative and thermal stresses experienced during transitions between the very different environments experienced through the parasite life cycle. The large, sustained Ca2+ signals mediated by Sm.TRPMPZQ in response to PZQ [4], are also reminiscent of properties shown by Nv.TRPM2, which shows substantial Ca2+ permeability (relative permeability of Ca2+ versus Na+, pCa/pNa = 35) and a lack of inactivation in cell-free patches in response to APDR [76]. The predicted pore filter of Sm.TRPMPZQ (‘FGD’) resembles that of Nv.TRPM2 (‘YGE’) with the negatively charged residue likely important for Ca2+ selectivity relative to other TRPM channels [76]. The post-filter motifs that regulate the kinetics of channel inactivation are also similar (‘LDA’ in Sm.TRPMPZQ versus ‘LDE’ in Nv.TRPM2, [76]). Such similarities merit detailed electrophysiological characterization.
Multiple alternatively spliced isoforms of Sm.TRPMPZQ exist, and these variants have yet to be functionally characterized. This is important as TRP channels are often customized in a species-specific and/or tissue-specific manner. Examples include the cell-specific splicing of TRPV1 channels to support infrared sensation in vampire bats [78], or customization of TRPA1 as a radiant heat sensor to support prey detection by snakes [79]. The TRPM subfamily is also subject to transcriptional regulation [80] – both TRPM2 and TRPM8 can be expressed as splice isoforms with unique properties, including isoforms that act as dominant negatives [81,82]. Characterization of such variants will also be important for understanding how Sm.TRPMPZQ activity is regulated throughout the parasitic life cycle.
From a drug design perspective, identification of the endogenous regulators of Sm.TRPMPZQ will be important for revealing new chemotherapeutic vulnerabilities. Our knowledge of human TRPM channels suggests possible candidates (Figure 3B). Identification of Sm.TRPPZQ regulators will also help to illuminate the physiological role of this channel, which in turn may provide insight as to why different life cycle stages show different sensitivities to PZQ. Notably, the lower sensitivity of juvenile worms to PZQ has important clinical consequences. Also important from a drug development perspective will be definition of the binding site for PZQ on Sm.TRPMPZQ if indeed the channel is directly activated by PZQ. There likely exist multiple ligand-binding pockets, encompassing those for ADP ribose (potentially within, or outside [75], the NUDT9H domain), the PIP2 coordination site, or within a hybrid TRPM8-like ligand-binding pocket. Understanding how PZQ potently activates Sm.TRPMPZQ and the basis for selectivity at this TRPM channel will aid the design of novel ligands. With a target in hand, target-based screening of Sm.TRPMPZQ is also a possibility to identify novel scaffolds that can be evaluated for anthelmintic potential (Figure 3B). Finally, identification of the cell types harboring Sm.TRPMPZQ, and the transcriptomic profile of these cells, could also highlight new opportunities for chemotherapy.
Concluding Remarks
While the road to discovery of Sm.TRPMPZQ has been long and indirect, much has been discovered about the fascinating biology of flatworms during this journey. Prioritizing the future characterization of Sm.TRPMPZQ and an understanding of how channel activity is dysregulated by PZQ will tease out additional secrets of this enigmatic anthelmintic drug. However, many important questions remain (see Outstanding Questions), most notably the significant distinction between Sm.TRPMPZQ being a schistosome target for (R)-PZQ and ‘the’ target of (R)-PZQ underpinning clinical efficacy (if indeed a sole target exists). While the new data [4] unambiguously identify Sm.TRPMPZQ as a flatworm target engaged by (R)-PZQ, is Sm.TRPMPZQ the clinically relevant target of PZQ? And if so, what are the implications for drug resistance, given that Sm.TRPMPZQ sensitivity to PZQ can be altered by point mutation [4]? Key data to address this question will derive from manipulation of Sm.TRPMPZQ activity in vivo (see Outstanding Questions). The sensitivity of other TRPM family members to PZQ will also need to be assessed – both in schistosomes and in other flatworms that show varied sensitivity to PZQ. While preliminary screening of other schistosome TRPM members suggests a lack of PZQ sensitivity [4], this lack of positive result is qualified by a lack of evidence for functional channel expression. There is clearly much yet to learn about other TRPM subfamily members and their physiological roles. The continuing journey to uncover their secrets will hopefully resolve new therapeutic strategies for combating schistosomiasis and other parasitic infections.
Outstanding Questions.
Is Sm.TRPMPZQ the clinical target for PZQ? Does in vivo RNAi of this channel inhibit PZQ action? Do Sm.TRPMPZQ antagonists impair and agonists phenocopy PZQ action? Or are there other (R)-PZQ targets yet to be discovered (e.g., bioaminergic GPCRs, other TRPs)?
What are the endogenous regulators of Sm.TRPMPZQ?
Where is Sm.TRPMPZQ expressed? What cell types express Sm.TRPMPZQ? Do these cell types afford other therapeutic vulnerabilities? How does Sm.TRPMPZQ expression and activity vary through the parasitic life cycle?
Are other flatworm TRPM channels sensitive to PZQ?
Can the host effects of PZQ be exploited for treatment of other diseases?
Highlights.
The anthelmintic drug PZQ is the key drug for treating schistosomiasis and several other diseases caused by parasitic flatworms.
Despite decades of clinical usage, no flatworm target for PZQ has been convincingly defined – a longstanding roadblock in this field.
A calcium-permeable ion channel in Schistosoma mansoni, activated by PZQ, has recently been identified. The characteristics of this channel mirror key features of PZQ action on schistosome muscle.
This ion channel is a member of the transient receptor potential melastatin (TRPM) channel subfamily which is broadly expressed in PZQ-sensitive flatworms.
This discovery will help to decipher how PZQ perturbs schistosome calcium homeostasis and the ensuing relevance to clinical activity.
Key Figure.
Discovery of Host Targets for Praziquantel (PZQ) Refined the Search for Parasite Targets
Acknowledgments
We thank members of the Marchant laboratory for helpful discussions. Owing to space limitations, we apologize to authors whose primary work we have been unable to cite. Work in the Marchant laboratory is supported by the National Science Foundation (MCB1615538), the National Institutes of Health (AI145871), and the Marcus Family.
Glossary
- Adenosine 5’-diphosphoribose (ADPR, ADP ribose)
a cellular metabolite and endogenous agonist of TRPM2 channels. ADPR production occurs by many pathways in the cell, including release from mitochondria during oxidative stress, or following apoptotic signaling and DNA damage.
- NUDT9 homology domain (NUDT9H)
the COOH terminus of TRPM2 channels contains a domain with homology to the mitochondrial pyrophosphatase NUDT9. This enzyme binds and cleaves ADPR, establishing the idea that the NUDT9H domain in TRPM2 mediates channel activation after binding ADPR. Recent data, however, implicate further complexities in where ADPR binds to TRPM2 channels, and the catalytic role of this domain.
- Praziquantel (PZQ)
the key drug used for treating schistosomiasis. It has been used clinically for decades owing to its effectiveness against all major Schistosoma species, and its low side-effect profile. Because of these advantages, it has largely supplanted the use of other anthelmintics, for example, oxamniquine, as the mainstay therapeutic.
- Reactive oxygen species (ROS)
by-products of cell metabolism that play important roles in signal transduction, cell function, and viability. TRPM2 channels act as sensors of cellular redox status via binding of ADPR to stimulate channel opening.
- Schistosomiasis (Biharzia, ‘snail fever’)
an infectious disease caused by parasitic flatworms of the genus Schistosoma. The disease afflicts over 200 million people worldwide in Africa, Asia, and South America. Praziquantel is the key drug therapy.
- Transient receptor potential (TRP) channels
a diverse family of cation channels that fulfill versatile roles in cell signaling and sensory physiology. Broadly expressed in excitable and nonexcitable cells, they display varied cation selectivity and modes of activation.
- Transient receptor potential melastatin (TRPM) channels
the largest of the six subfamilies of TRP channels, comprising eight distinct channels in humans (TRPM1–TRPM8). Their naming derives from the first member to be cloned (TRPM1) which was isolated from a melanoma cDNA library. TRPM channels can be activated by diverse stimuli (ligands, temperature, voltage) and display a range of permeabilities to Ca2+. TRPM channels are receiving increasing interest as a therapeutic target in a spectrum of human diseases.
References
- 1.Day TA et al. (1992) Praziquantel: The enigmatic antiparasitic. Parasitol. Today 8, 342–344 [DOI] [PubMed] [Google Scholar]
- 2.Gregori-Puigjane E et al. (2012) Identifying mechanism-of-action targets for drugs and probes. Proc. Natl Acad. Sci. U.S.A 109, 11178–11183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews P et al. (1983) Praziquantel. Med. Res. Rev 3, 147–200 [DOI] [PubMed] [Google Scholar]
- 4.Park SK et al. (2019) The anthelmintic drug praziquantel activates a schistosome transient receptor potential channel. J. Biol. Chem Published online October 25, 2019. 10.1074/jbc.AC119.011093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McManus DP et al. (2018) Schistosomiasis. Nat. Rev. Dis. Primers 4, 13. [DOI] [PubMed] [Google Scholar]
- 6.King CH and Dangerfield-Cha M (2008) The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 4, 65–79 [DOI] [PubMed] [Google Scholar]
- 7.Hotez PJ and Fenwick A (2009) Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl. Trop. Dis 3, e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yegorov S et al. (2019) Schistosoma mansoni treatment reduces HIV entry into cervical CD4+ T cells and induces IFN-I pathways. Nat. Commun 10, 2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenwick A et al. (2009) The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology 136, 1719–1730 [DOI] [PubMed] [Google Scholar]
- 10.Hotez PJ et al. (2010) Africa is desperate for praziquantel. Lancet 376, 496–498 [DOI] [PubMed] [Google Scholar]
- 11.Greenberg RM (2013) New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology 140, 1534–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergquist R et al. (2017) Controlling schistosomiasis with praziquantel: How much longer without a viable alternative? Infect. Dis. Poverty 6, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupit PM and Cunningham C (2015) What is the mechanism of action of praziquantel and how might resistance strike? Future Med. Chem 7, 701–705 [DOI] [PubMed] [Google Scholar]
- 14.Pica-Mattoccia L and Cioli D (2004) Sex- and age-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol 34, 527–533 [DOI] [PubMed] [Google Scholar]
- 15.Wang W et al. (2012) Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol. Res 111, 1871–1877 [DOI] [PubMed] [Google Scholar]
- 16.Thomas CM and Timson DJ (2018) The mechanism of action of praziquantel: six hypotheses. Curr. Top. Med. Chem 18, 1575–1584 [DOI] [PubMed] [Google Scholar]
- 17.da Silva VBR et al. (2017) Medicinal chemistry of antischistosomal drugs: Praziquantel and oxamniquine. Bioorg. Med. Chem 25, 3259–3277 [DOI] [PubMed] [Google Scholar]
- 18.Pax R et al. (1978) A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Arch. Pharmacol 304, 309–315 [DOI] [PubMed] [Google Scholar]
- 19.Wolde Mussie E et al. (1982) Schistosoma mansoni: calcium efflux and effects of calcium-free media on responses of the adult male musculature to praziquantel and other agents inducing contraction. Exp. Parasitol 53, 270–278 [DOI] [PubMed] [Google Scholar]
- 20.Pica-Mattoccia L et al. (2008) Schistosoma mansoni: Lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Exp. Parasitol 119, 332–335 [DOI] [PubMed] [Google Scholar]
- 21.William S and Botros S (2004) Validation of sensitivity to praziquantel using Schistosoma mansoni worm muscle tension and Ca2+-uptake as possible in vitro correlates to in vivo ED50 determination. Int. J. Parasitol 34, 971–977 [DOI] [PubMed] [Google Scholar]
- 22.Fetterer RH et al. (1980) Praziquantel, potassium and 2,4-dinitrophenol: analysis of their action on the musculature of Schistosoma mansoni. Eur. J. Pharmacol 64, 31–38 [DOI] [PubMed] [Google Scholar]
- 23.Blair KL et al. (1992) Praziquantel: physiological evidence for its site(S) of action in magnesium-paralysed Schistosoma mansoni. Parasitology 104, 59–66 [DOI] [PubMed] [Google Scholar]
- 24.Staudt U et al. (1992) Light and scanning electron microscopy studies on the effects of the enantiomers of praziquantel and its main metabolite on Schistosoma mansoni in vitro. Parasitol. Res 78, 392–397 [DOI] [PubMed] [Google Scholar]
- 25.Mehlhorn H et al. (1981) In vivo and in vitro experiments on the effects of praziquantel on Schistosoma mansoni. A light and electron microscopic study. Arzneimittelforschung 31, 544–554 [PubMed] [Google Scholar]
- 26.Bricker CS et al. (1983) The relationship between tegumental disruption and muscle-contraction in Schistosoma mansoni exposed to various compounds. Z. Parasitol 69, 61–71 [DOI] [PubMed] [Google Scholar]
- 27.Xiao SH et al. (1984) Praziquantel-induced vesicle formation in the tegument of male Schistosoma mansoni is calcium dependent. J. Parasitol 70, 177–179 [PubMed] [Google Scholar]
- 28.Becker B et al. (1980) Light and electron microscopic studies on the effect of praziquantel on Schistosoma mansoni, Dicrocoelium dendriticum, and Fasciola hepatica (Trematoda) in vitro. Z. Parasitenkd 63, 113–128 [DOI] [PubMed] [Google Scholar]
- 29.Brindley PJ and Sher A (1990) Immunological involvement in the efficacy of praziquantel. Exp. Parasitol 71, 245–248 [DOI] [PubMed] [Google Scholar]
- 30.William S et al. (2001) Praziquantel-induced tegumental damage in vitro is diminished in schistosomes derived from praziquantel-resistant infections. Parasitology 122, 63–66 [DOI] [PubMed] [Google Scholar]
- 31.Jeziorski MC and Greenberg RM (2006) Voltage-gated calcium channel subunits from platyhelminths: potential role in praziquantel action. Int. J. Parasitol 36, 625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan JD et al. (2013) Ca2+ channels and praziquantel: A view from the free world. Parasitol. Int 62, 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohn AB et al. (2001) Schistosome calcium channel b subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J. Biol. Chem 40, 36873–36876 [DOI] [PubMed] [Google Scholar]
- 34.Nogi T et al. (2009) A novel biological activity of praziquantel requiring voltage-operated Ca2+ channel beta subunits: subversion of flatworm regenerative polarity. PLoS Negl. Trop. Dis 3, e464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang D et al. (2011) Opposing roles of voltage-gated Ca2+ channels in neuronal control of stem cell differentiation in vivo. J. Neurosci 31, 15983–15995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You H et al. (2013) Transcriptional responses of in vivo praziquantel exposure in schistosomes identifies a functional role for calcium signalling pathway member CamKII. PLoS Pathog. 9, e1003254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chubb JM et al. (1978) Effects of praziquantel, a new anthelmintic, on electromechanical properties of isolated rat atria. J. Pharmacol. Exp. Ther 207, 284–293 [PubMed] [Google Scholar]
- 38.Jim K and Triggle DJ (1979) Actions of praziquantel and 1-methyladenine in guinea-pig ileal longitudinal muscle. Can. J. Physiol. Pharmacol 57, 1460–1462 [DOI] [PubMed] [Google Scholar]
- 39.Chan JD et al. (2015) Ergot alkaloids (re)generate new leads as antiparasitics. PLoS Negl. Trop. Dis 9, e0004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harder A et al. (1987) Praziquantel impairs the ability of exogenous serotonin to stimulate carbohydrate metabolism in intact Schistosoma mansoni. Parasitol. Res 73, 442–445 [DOI] [PubMed] [Google Scholar]
- 41.Chan JD et al. (2017) The anthelmintic praziquantel is a human serotoninergic G-protein-coupled receptor ligand. Nat. Commun 8, 1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wacker D et al. (2017) Crystal structure of an LSD-bound human serotonin receptor. Cell 168, 377–389 e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan JD et al. (2016) Kinetic profiling an abundantly expressed planarian serotonergic GPCR identifies bromocriptine as a perdurant antagonist. Int. J. Parasitol. Drugs Drug Resist 6, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan JD et al. (2016) A miniaturized screen of a Schistosoma mansoni serotonergic G protein-coupled receptor identifies novel classes of parasite-selective inhibitors. PLoS Pathog. 12, e1005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babes RM et al. (2017) The anthelminthic drug praziquantel is a selective agonist of the sensory transient receptor potential melastatin type 8 channel. Toxicol. Appl. Pharmacol 336, 55–65 [DOI] [PubMed] [Google Scholar]
- 46.Yin Y et al. (2019) Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 363, eaav9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diver MM et al. (2019) Structural insights into TRPM8 inhibition and desensitization. Science 365, 1434–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunaratne GS et al. (2018) Activation of host transient receptor potential (TRP) channels by praziquantel stereoisomers. PLoS Negl. Trop. Dis 12, e0006420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abla N et al. (2017) Evaluation of the pharmacokinetic-pharmacodynamic relationship of praziquantel in the Schistosoma mansoni mouse model. PLoS Negl. Trop. Dis 11, e0005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Botros SS et al. (2006) Drug-metabolizing enzymes and praziquantel bioavailability in mice harboring Schistosoma mansoni isolates of different drug susceptibilities. J. Parasitol 92, 1344–1349 [DOI] [PubMed] [Google Scholar]
- 51.Olliaro P et al. (2014) The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer).J. Antimicrob. Chemother 69, 863–870 [DOI] [PubMed] [Google Scholar]
- 52.Earley S and Brayden JE (2015) Transient receptor potential channels in the vasculature. Physiol. Rev 95, 645–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eyoh E et al. (2019) The anthelmintic drug praziquantel promotes human Tr1 differentiation. Immunol. Cell. Biol 97, 512–518 [DOI] [PubMed] [Google Scholar]
- 54.Kong D et al. (2018) Praziquantel targets M1 macrophages and ameliorates splenomegaly in chronic schistosomiasis. Antimicrob. Agents Chemother 62, e00005–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanchez MC et al. (2017) Effect of praziquantel on the differential expression of mouse hepatic genes and parasite ATP binding cassette transporter gene family members during Schistosoma mansoni infection. PLoS Negl. Trop. Dis 11, e0005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan JD et al. (2018) Coalescing beneficial host and deleterious antiparasitic actions as an antischistosomal strategy. eLife 7, e35755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamdem SD et al. (2018) Host regulators of liver fibrosis during human schistosomiasis. Front. Immunol 9, 2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carson JP et al. (2018) Schistosome-induced fibrotic disease: the role of hepatic stellate cells. Trends Parasitol. 34, 524–540 [DOI] [PubMed] [Google Scholar]
- 59.Liang YJ et al. (2011) New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum. PLoS One 6, e20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J et al. (2019) Praziquantel ameliorates CCl4-induced liver fibrosis in mice by inhibiting TGF-beta/Smad signalling via upregulating Smad7 in hepatic stellate cells. Br. J. Pharmacol Published online August 14, 2019. 10.1111/bph.14831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansour TE (1984) Serotonin receptors in parasitic worms. Adv. Parasitol 23, 1–36 [DOI] [PubMed] [Google Scholar]
- 62.Patocka N et al. (2014) Serotonin signaling in Schistosoma mansoni: a serotonin-activated G protein-coupled receptor controls parasite movement. PLoS Pathog. 10, e1003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bais S and Greenberg RM (2016) TRP channels in schistosomes. Int. J. Parasitol. Drugs Drug Resist 6, 335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bais S and Greenberg RM (2018) TRP channels as potential targets for antischistosomals. Int. J. Parasitol. Drugs Drug Resist 8, 511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zamanian M et al. (2011) The repertoire of G protein-coupled receptors in the human parasite Schistosoma mansoni and the model organism Schmidtea mediterranea. BMC Genom. 12, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchant JS et al. (2018) Structure-activity profiling of alkaloid natural product pharmacophores against a Schistosoma serotonin receptor. Int. J. Parasitol. Drugs Drug Resist 8, 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan JD et al. (2016) Pharmacological profiling an abundantly expressed schistosome serotonergic GPCR identifies nuciferine as a potent antagonist. Int. J. Parasitol. Drugs Drug Resist 6, 364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santos R et al. (2017) A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov 16, 19–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolstenholme AJ et al. (2011) TRP channels in parasites. Adv. Exp. Med. Biol 704, 359–371 [DOI] [PubMed] [Google Scholar]
- 70.Inoue T et al. (2014) Thermosensory signaling by TRPM is processed by brain serotonergic neurons to produce planarian thermotaxis. J. Neurosci 34, 15701–15714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arenas OM et al. (2017) Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat. Neurosci 20, 1686–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birkholz TR and Beane WS (2017) The planarian TRPA1 homolog mediates extraocular behavioral responses to near-ultraviolet light. J. Exp. Biol 220, 2616–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bais S et al. (2018) Atypical pharmacology of schistosome TRPA1-like ion channels. PLoS Negl. Trop. Dis 12, e0006495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L et al. (2018) Structures and gating mechanism of human TRPM2. Science 362, eaav4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y et al. (2018) Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature 562, 145–149 [DOI] [PubMed] [Google Scholar]
- 76.Zhang Z et al. (2018) Structure of a TRPM2 channel in complex with Ca(2+) explains unique gating regulation. eLife 7, 36409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan CH and McNaughton PA (2018) TRPM2 and warmth sensation. Pflugers Arch. 470, 787–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gracheva EO et al. (2011) Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature 476, 88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gracheva EO et al. (2010) Molecular basis of infrared detection by snakes. Nature 464, 1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lis A et al. (2005) Transcriptional regulation and processing increase the functional variability of TRPM channels. Naunyn Schmiedebergs Arch. Pharmacol 371, 315–324 [DOI] [PubMed] [Google Scholar]
- 81.Bidaux G et al. (2015) Epidermal TRPM8 channel isoform controls the balance between keratinocyte proliferation and differentiation in a cold-dependent manner. Proc. Natl Acad. Sci. U.S.A 112, E3345–E3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang W et al. (2003) A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death.J. Biol. Chem 278, 16222–16229 [DOI] [PubMed] [Google Scholar]
- 83.Thomas H and Gönnert R (1977) The efficacy of praziquantel against cestodes in animals. Z. Parasitenkd 52, 117–127 [DOI] [PubMed] [Google Scholar]
- 84.Gonnert R and Andrews P (1977) Praziquantel, a new board-spectrum antischistosomal agent. Z. Parasitenkd 52, 129–150 [DOI] [PubMed] [Google Scholar]
- 85.Meyer T et al. (2009) Taste, a new incentive to switch to (R)-praziquantel in schistosomiasis treatment. PLoS Negl. Trop. Dis 3, e357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan JD et al. (2014) ‘Death and Axes’: Unexpected Ca2+ entry phenologs predict new anti-schistosomal agents. PLoS Pathog. 10, e1003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witchley JN et al. (2013) Muscle cells provide instructions for planarian regeneration. Cell Rep. 4, 633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bai RY et al. (2011) Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro. Oncol 13, 974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen B et al. (2017) Computational discovery of niclosamide ethanolamine, a repurposed drug candidate that reduces growth of hepatocellular carcinoma cells in vitro and in mice by inhibiting cell division cycle 37 signaling. Gastroenterology 152, 2022–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li J et al. (2017) Artemisinins target GABAA receptor signaling and impair alpha cell identity. Cell 168, 86–100 e15 [DOI] [PMC free article] [PubMed] [Google Scholar]