Abstract
Background
Monophosphoryl lipid A (MPLA) is a TLR4 agonist that has potent immunomodulatory properties and modulates innate immune function to improve host resistance to infection with common nosocomial pathogens in mice. The goal of this study was to assess the safety and efficacy of MPLA in a sheep model of burn injury and Pseudomonas aeruginosa pneumonia. The sheep provides a favorable model for pre-clinical testing as their response to TLR4 agonists closely mimics that of humans.
Methods
Twelve chronically instrumented adult female Merino sheep received 20% total body surface area, third-degree cutaneous burn under anesthesia and analgesia. At 24 hours after burn, sheep were randomly allocated to receive; 1) MPLA (2.5 μg/kg IV, n=6), or 2) vehicle (IV, n=6). At 24 hours after MPLA or vehicle treatment, Pseudomonas aeruginosa pneumonia was induced. Sheep were mechanically ventilated, fluid resuscitated and cardiopulmonary variables were monitored for 24 hours after induction of pneumonia. Cytokine production, vascular barrier function, and lung bacterial burden were also measured.
Results
MPLA infusion induced small and transient alterations in core body temperature, heart rate, pulmonary artery pressure, and pulmonary vascular resistance. Pulmonary mechanics were not altered. Vehicle-treated sheep developed severe acute lung injury during Pseudomonas aeruginosa pneumonia, which was attenuated by MPLA as indicated by improved PaO2/FiO2 ratio, oxygenation index, and shunt fraction. Sheep treated with MPLA also exhibited less vascular leak, lower blood lactate levels, and lower modified organ injury score. MPLA treatment attenuated systemic cytokine production and decreased lung bacterial burden.
Conclusions
MPLA was well tolerated in burned sheep and attenuated development of acute lung injury, lactatemia, cytokinemia, vascular leak, and hemodynamic changes caused by Pseudomonas aeruginosa pneumonia.
Keywords: sepsis, TLR4 agonists, pneumonia, burns, organ injury, immunomodulation, inflammation, vascular permeability
Background
Hospital-acquired infections are among of the most pressing threats facing modern healthcare facilities [1–3]. Critically ill, immunosuppressed and high-risk surgical patients are most vulnerable, although anyone receiving hospital care is at risk. Patients with large total body surface area (TBSA) cutaneous burns are particularly susceptible to hospital-acquired infections, especially pneumonia and wound infections, due to loss of the skin barrier, invasive monitoring, mechanical ventilation and immune dysfunction [4, 5]. Pseudomonas aeruginosa (P. aeruginosa) is the most common organism causing pneumonia in burn patients [6–9].
Other than infection control strategies such as hand washing, gowning and aseptic technique, there are not any proven ways to decrease or prevent infection in burn patients. In general, the evidence that systemic antibiotic prophylaxis reduces the incidence of wound and invasive infections or infection-associated mortality is weak [10–12]. Therefore, new strategies are needed to decrease the incidence and severity of infections in burn victims. Immunotherapy using toll-like receptor (TLR) agonists provides a means of achieving that goal. Monophosphoryl lipid A (MPLA) is a TLR4 agonist with negligible toxicity and pro-inflammatory effects but potent immunomodulatory properties [13, 14]. MPLA is employed as an adjuvant in the human papilloma virus (Cervarix) and shingles (Shingrix) vaccines and has been administered safely to more than a million people worldwide in that application [15, 16]. Our interest in MPLA is not as a vaccine adjuvant but a means of augmenting innate immunity against common opportunistic pathogens in vulnerable patients. Our published studies, in mice, show that treatment with MPLA confers resistance against P. aeruginosa, S. aureus and C. albicans infection as well as polymicrobial sepsis caused by cecal ligation and puncture [17–21]. MPLA-induced protection persists for at least 15 days and is independent of the adaptive immune system but highly dependent on innate immune function [19, 21, 22]. Thus, treatment with MPLA induces a state of innate immune memory that confers resistance against common hospital-acquired pathogens. However, to develop MPLA for clinical use, further work is needed in models that closely replicate the clinical environment.
The sheep model provides an ideal model for testing of MPLA because both physiological and genomic responses of sheep to TLR4 agonists are essentially identical to that of humans [23–25]. Consequently, sheep provide a model to test the systemic effects of MPLA and gain an understanding of how critically ill patients will respond to treatment. In this study, we evaluated the effect of MPLA infusion on hemodynamics and pulmonary function in sheep with skin burn. We then assessed the effect of MPLA on the response of those sheep to P. aeruginosa pneumonia. Our study shows that burned sheep tolerate MPLA infusion well and that MPLA prophylaxis attenuates acute lung injury and sepsis severity during post-burn P. aeruginosa pneumonia.
Methods
Animals
Twelve adult female Merino sheep (body weight [BW] 35.4 ± 1.0 kg) were studied. The study was approved by the Institutional Animal Care and Use Committee of The University of Texas Medical Branch and conducted in compliance with the guidelines of the National Institutes of Health (NIH) and the American Physiological Society for the care and use of laboratory animals.
Surgical Preparation of Sheep for Study
Fasted animals were surgically prepared for chronic study under isoflurane anesthesia. Pre- and post-surgical analgesia was provided with long acting (72 hours) buprenorphine SR (0.05 mg/kg, SR Veterinary Technologies, Windsor, CO). Catheters were placed into the right femoral artery and the left atrium for continuous measurement of blood pressure and left atrial pressure, respectively. A 7Fr Swan-Ganz thermal dilution catheter (Edwards Lifesciences LLC; Irvine, CA) was introduced through the right external jugular vein and advanced into the pulmonary artery for measurement of cardiac output, and pulmonary artery and central venous pressures. Following the operative procedures, the sheep were given five to seven days to recover. For admission into the protocol the animals must have: a PaO2 >100 mmHg on room air, a core body temperature greater than 38°C and less than 40°C, and a hematocrit >20%.
Induction of burn injury
Instrumented sheep were anesthetized with intravenous ketamine (800 mg) and inhaled isoflurane via mask (to effect) and tracheostomy was performed. Pre- and post-surgical analgesia was provided with long acting (72 hours) buprenorphine SR (0.05 mg/kg, SR Veterinary Technologies, Windsor, CO) via the subcutaneous route as previously described [26]. Anesthesia was maintained using inhaled isoflurane via tracheostomy. The 20% TBSA, third-degree flame burn was made on one flank by Bunsen burner as previously described [27, 28]. Afterwards, anesthesia was discontinued and sheep were placed on mechanical ventilation and monitored for 24 hours in a conscious state. Sheep were fluid resuscitated with lactated Ringer’s solution (LR) per protocol [29]. Sheep were studied in pairs to provide side-by-side assessment and were randomized to treatment with saline (control) or MPLA.
Monophosphoryl lipid A (MPLA) treatment
MPLA derived from Salmonella enterica serotype Minnesota Re 595 was purchased from Sigma-Aldrich Co. (Catalog #: L6895, St. Louis, MO), solubilized in sterile water containing 0.2% triethylamine solution (1 mg/ml) and sonicated for 1 hour at 40°C. For administration, MPLA was diluted in saline solution (25 ml) and administered by intravenous infusion (2.5 μg/kg) over 50 minutes. Physiologic measurements were performed prior to and at 5, 10, 20, 30, 45, 60, 75, 90, 105, and 120 minutes after initiation of MPLA infusion in awake sheep. During this and previous (burn induction) phases, sheep were fluid resuscitated with LR using our standard formula [29].
Induction of pneumonia
At 24 hours after MPLA or vehicle treatment, sheep were anesthetized (as described in Burn Induction Section) and P. aeruginosa (1.6 ~ 2.5 × 1010 colony-forming units in 30-mL solution, strain; 27317™, ATCC, Manassas, VA) was instilled into the airways through a bronchoscope as previously described [30–33]. After instillation, sheep were maintained on mechanical ventilation with a pressure-regulated volume control, assist-control (PRVC A/C) mode, a tidal volume (TV) of 12 mL/kg, and a positive end-expiratory pressure of 5 cmH2O and monitored in an awake condition throughout for 24 hours. Physiologic measurements were performed at baseline and at every 3 hours after infection out to 24 hours. Sheep were fluid resuscitated with LR to maintain hematocrit at baseline levels (±3%) [15, 16].
Multi-organ function assessment
To assess the severity of multi-organ dysfunctions during pneumonia, we modified the Sequential Organ Failure Assessment (SOFA) score [34]. The modified sheep SOFA (mSOFA) scores included the values of mean arterial pressure (MAP) and PaO2/FiO2 ratio, total platelet count measured by HEMAVET HV950FS (Drew Scientific Inc., Miami Lakes, FL), and total bilirubin and creatinine concentrations in plasma as measured at the institutional clinical chemistry laboratory through spectrophotometric assay (Supplemental Table 1, http://links.lww.com/SHK/A872). To assess mental status, a simplified sheep neurological/alertness assessment scale was developed (Supplemental Table 2, http://links.lww.com/SHK/A873).
Bacterial Clearance in Lung
Lung tissue (100 mg from the dorsal edge of right middle lobe) was taken during the necropsy (72 hours post-burn), homogenized with 1x PBS and plated (200 μL) onto soy agar plates. The plates were incubated for 24 hours at 37 °C and colony-forming units were counted.
Plasma Interleukin-6 Measurement
Arterial blood samples were collected into EDTA tubes (BD Vacutainer®, Ref# 367861, Franklin Lakes, NJ) before instillation of the P. aeruginosa into the lung, and at 6 and 24 hours after the instillation. Blood was centrifuged at 1,800 g at 4°C for 10 min and plasma was aliquoted and frozen at −20°C until the day of assay. Quantification of interleukin 6 (IL-6) levels was performed using enzyme-linked immuno-sorbent assays (ELISA) kit (RPA079Ov01, Cloud-Clone Corp., Katy, TX, US), according to the manufacturer’s instructions. All samples and standards were assayed in duplicate.
Trans-endothelial electrical resistance assay
Pooled human dermal microvascular endothelial cells (Lonza, Basel, Switzerland) were primed with MPLA (10μg/mL) or vehicle for 24 hours, washed, and plated on 24 well ThinCert inserts (0.4 μm pore diameter, Greiner Bio-One, Kremsmünster, Austria) at 4×105 cells/mL. Prior to seeding, ThinCerts were coated with 0.01% Poly-L-Lysine (EMD-Millipore, Burlington, MA), 50% glutaraldehyde (EMD- Millipore), and 0.25mg/mL gelatin (Sigma, St. Louis, MO). Inserts were placed inside pots containing 950μL culture media and allowed to equilibrate. Trans-endothelial electrical resistance (TEER, Ω•cm2) across the pots and inserts was measured using a cellZscope (nanoAnalytics, Münster, Germany), reading once an hour. After 24 hours, LPS (1 μg/ml) or vehicle control was added as indicated and TEER was measured for another 16 hours. TEER readings were normalized to a baseline value (the last reading prior to treatments) and percentage change from baseline for each group was plotted. The area under the curve (AUC) was calculated by taking the percent change in resistance (increase or positive and decrease or negative) relative to the baseline TEER measurement prior to LPS and adding them together to get the area of percent change over time.
Statistical Analysis
All data were analyzed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). Variables are reported as mean ± standard error of mean (SEM). Data were analyzed using Mann-Whitney U test or two-way ANOVA with repeated measures followed by Bonferroni or Tukey post-hoc tests. A p value of less than 0.05 was considered statistically significant.
Results
MPLA infusion caused transient physiologic alterations in burned sheep
Hemodynamics, pulmonary function and core body temperature were not different at baseline or during the 24 hour period following the cutaneous burn when comparing sheep ultimately randomized to vehicle or MPLA treatment (Supplemental Table 3, http://links.lww.com/SHK/A874).
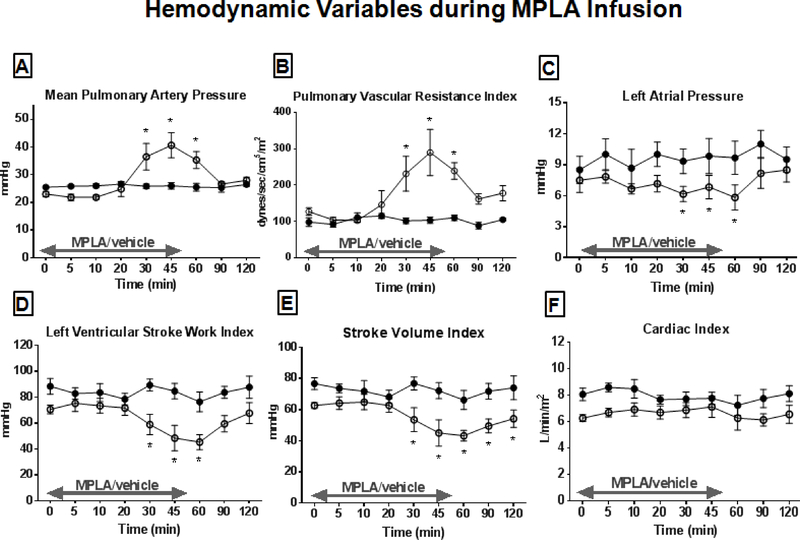
The MPLA infusion caused changes in core body temperature and heart rate (HR) but mean arterial pressure and systemic vascular resistance index were not affected (Figure 1). Mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance index (PVRI) were significantly elevated at 30–60 minutes following MPLA infusion and left atrial pressure, left ventricular stroke work index (LVSWI), and stroke volume index (SVI) were significantly decreased compared to control beginning 30 minutes after MPLA infusion (Figure 2). All parameters, with the exception of temperature and SVI, returned to baseline by 60 minutes after initiation of MPLA infusion.
Figure 1.
Hemodynamic variables during MPLA or vehicle infusion. MPLA (2.5 μg/kg) or vehicle were infused over 50 minutes. Hemodynamics variables were measured for 2 hours after initiation of infusion. (A) Core body temperature, (B) Heart rate, (C) Mean pulmonary artery pressure, (D) Systemic vascular resistance index during the MPLA infusion. Open circles represent MPLA-preconditioned treatment group animals. Closed circles represent control group. The animal numbers in both groups are n=6. Data are expressed as mean ± SEM (* p < 0.05 vs. control).
Figure 2.
Hemodynamic variables during MPLA or vehicle infusion. MPLA (2.5 μg/kg) or vehicle were infused over 50 minutes. Hemodynamics variables were measured for 2 hours after initiation of infusion. (A) Mean pulmonary artery pressure, (B) Pulmonary vascular resistance index, (C) Left atrial pressure, (D) Left ventricular stroke work index, (E) Stroke volume index, (F) Cardiac index. Open circles represent MPLA-preconditioned treatment group animals. Closed circles represent control group. The animal numbers in both groups are n=6. Data are expressed as mean ± SEM (* p < 0.05 vs. control).
The MPLA infusion transiently increased respiratory rate at 45 minutes after initiation of infusion but did not significantly affect peak/plateau airway pressures, and lung dynamic/static compliances (Figure 3).
Figure 3.
Respiratory variables during MPLA or vehicle infusion. MPLA (2.5 μg/kg) or vehicle were infused over 50 minutes. Respiratory variables were measured for 2 hours after initiation of infusion. (A) Respiratory rate, (B) Peak airway pressure, (C) plateau airway pressure, (D) Lung dynamic compliance, (E) Lung static compliance during the MPLA infusion period. Open circles represent MPLA-preconditioned treatment group animals. Closed circles represent control group. The animal numbers in both groups are n=6. Data are expressed as mean ± SEM (* p < 0.05 vs. control).
MPLA treatment improved oxygenation and pulmonary mechanics in sheep with P. aeruginosa pneumonia/sepsis
The PaO2/FiO2 ratio was decreased in sheep at 3 hours after P. aeruginosa instillation and reached levels below 300 mmHg (mild ARDS) at 9–24 hours after bacterial instillation in vehicle-treated sheep (Figure 4). Pulmonary oxygenation index and pulmonary shunt fraction were increased at 3 hours after P. aeruginosa instillation and remained increased throughout the study period in vehicle controls (Figure 4). Preconditioning with MPLA significantly attenuated the worsening PaO2/FiO2 ratio, pulmonary oxygenation index and shunt fraction after P. aeruginosa instillation. Peak and plateau airway pressures were significantly increased and static compliance decreased in vehicle-treated sheep with pneumonia (Figure 5). Those changes were attenuated in MPLA-treated sheep.
Figure 4.
Lung injury and gas exchange following P. aeruginosa instillation into lungs. Sheep underwent 20% TBSA cutaneous burn at time 0 followed by vehicle or MPLA (2.5 μg/kg) infusion at 24 hours after burn injury. P. aeruginosa was instilled into the lungs at 48 hours after burn injury. (A) PaO2/FiO2 ratio, (B) Oxygenation index, (C) Pulmonary shunt fraction. Open circles represent MPLA-preconditioned treatment group animals. Closed circles represent control group. The animal numbers in both groups are n=6. Data are expressed as mean ± SEM (* p < 0.05 vs. control).
Figure 5.
Pulmonary mechanics following P. aeruginosa instillation into lungs. Sheep underwent 20% TBSA cutaneous burn at time 0 followed by vehicle or MPLA (2.5 μg/kg) infusion at 24 hours after burn injury. P. aeruginosa was instilled into the lungs at 48 hours after burn injury. (A) Peak airway pressure, (B) Plateau airway pressure, and (C) Lung static compliance during the whole study period. Open circles represent MPLA-preconditioned treatment group animals. Closed circles represent control group. The animal numbers in both groups are n=6. Data are expressed as mean ± SEM (* p < 0.05 vs. control).
MPLA treatment attenuated pneumonia/sepsis-induced changes in hemodynamics, lactate production and organ injury during post-burn pneumonia
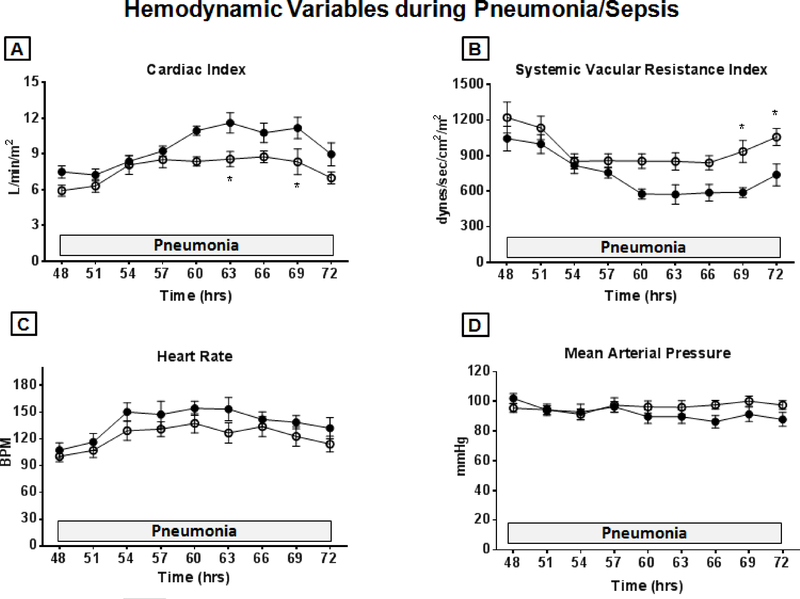
Increases in cardiac index and decreases in systemic vascular resistance index caused by intrapulmonary P. aeruginosa instillation were significantly attenuated by MPLA (Figure 6). No significant differences were noted in heart rate or mean arterial pressure. Core body temperature, mPAP, pulmonary artery wedge pressure, PVRI, LVSWI and right ventricular stroke work index were not different between groups (data not shown).
Figure 6.
Hemodynamic variables following P. aeruginosa instillation into lungs. Sheep underwent 20% TBSA cutaneous burn at time 0 followed by vehicle or MPLA (2.5 μg/kg) infusion at 24 hours after burn injury. P. aeruginosa was instilled into the lungs at 48 hours after burn injury and hemodynamics were measured for 24 hours. (A) Cardiac index, (B) Systemic vascular resistance index, (C) Heart rate, (D) Mean arterial pressure. Open circles represent MPLA-preconditioned treatment group animals. Closed circles represent control group. The animal numbers in both groups are n=6. Data are expressed as mean ± SEM (* p < 0.05 vs. control).
Instillation of bacteria significantly increased plasma lactate concentrations in control sheep, which were significantly lower in MPLA-preconditioned sheep (Figure 7). mSOFA score was increased during pneumonia in control sheep and significantly improved by MPLA preconditioning (Figure 7). The more favorable mSOFA score in MPLA-preconditioned sheep was related to improved PaO2/FiO2 ratio and less hypotension (Supplemental Table 4, http://links.lww.com/SHK/A875). Numbers of lung bacteria at 24 hours after P. aeruginosa instillation were significantly lower in MPLA-treated sheep compared to control (Figure 7).
Figure 7.
Plasma lactate, mSOFA score and lung P. aeruginosa CFU following P. aeruginosa instillation into lungs. Sheep underwent 20% TBSA cutaneous burn at time 0 followed by vehicle or MPLA (2.5 μg/kg) infusion at 24 hours after burn injury. P. aeruginosa was instilled into the lungs at 48 hours after burn injury The figure shows (A) Plasma lactate concentration and (B) Modified sheep Sequential Organ Failure Assessment (mSOFA) score during the whole study period and (C) Numbers of the bacteria in lung culture (at 24 hours after P. aeruginosa infection). Open circles represent MPLA preconditioned treatment group animals. Closed circles represent control group. The animal numbers in both groups are n=6. Data are expressed as mean ± SEM (* p < 0.05 vs. control).
MPLA treatment attenuates systemic cytokine production and vascular permeability during post-burn pneumonia and sepsis
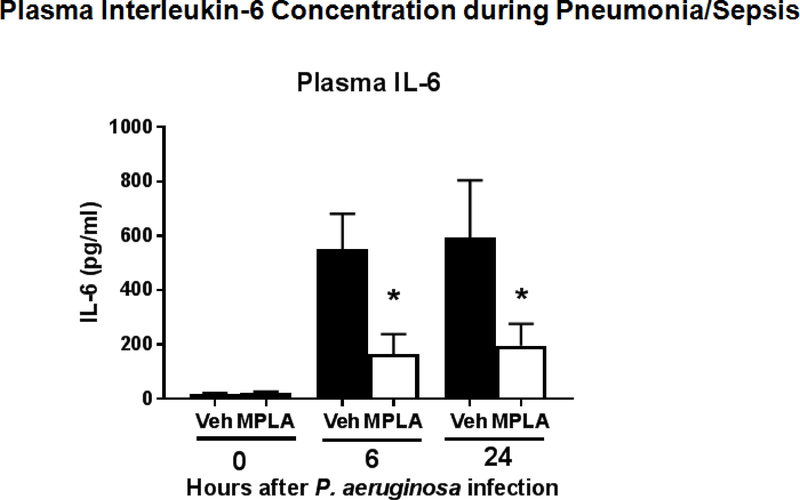
MPLA treatment significantly attenuated increases in IL-6 production at 6 and 24 hours after P. aeruginosa instillation (Figure 8). Preconditioning with MPLA reduced P. aeruginosa-induced plasma protein concentration changes and attenuated the increase in lung wet to dry weight ratio indicating less vascular leak (Figure 9). To further assess the impact of MPLA on endothelial barrier function, human endothelial cell monolayers were primed with MPLA or vehicle prior to LPS challenge. Measurement of barrier integrity by Trans-endothelial electrical resistance (TEER, Ω•cm2) showed significant improvement in human endothelial cell barrier function after MPLA-priming compared to control (Figure 9)
Figure 8:
Plasma interleukin-6 during post-burn pneumonia. Sheep underwent 20% TBSA cutaneous burn at time 0 followed by vehicle or MPLA (2.5 μg/kg) infusion at 24 hours after burn injury. P. aeruginosa was instilled into the lungs at 48 hours after burn injury Plasma IL-6 concentrations were measured at 0, 6, and 24 hours after P. aeruginosa challenge. Data are expressed as mean ± SEM (* p < 0.05 vs. vehicle (veh)).
Figure 9.
The graph shows that (A) Plasma protein concentration changes during pneumonia/sepsis period (48 – 72 hours), (B) Lung wet-to-dry weight ratio (at 72 hours), and (C) Trans-endothelial electrical resistance in human dermal microvascular endothelial cells. Open circles represent MPLA preconditioned treatment group animals. Closed circles represent control group. The animal numbers in both groups are n=6 in graph (A) and (B). Data are expressed as mean ± SEM (* p < 0.05 vs. control).
Discussion
The major findings of this study are that MPLA infusion is well tolerated in burned sheep and confers resistance to acute lung injury, lactatemia, and hemodynamic alterations during post-burn P. aeruginosa pneumonia and sepsis. These findings support the clinical relevance of applying MPLA, and other TLR4 agonists, to prevent and decrease the severity of serious infections and organ injury in high risk populations such as those suffering major burns. In the present study, MPLA treatment was initiated after burn injury but before microbial challenge. Our model is clinically relevant since one could envision treating burn victims with MPLA early during burn shock resuscitation, or shortly thereafter, to improve downstream resistance to organ injury and infection. Burn patients are particularly susceptible to infections and lung injury and represent a population that could benefit significantly from MPLA prophylaxis [6, 9, 35]. Sepsis and respiratory failure are the most common causes of morbidity and mortality in burn victims that survive the acute phase of injury [9]. Furthermore, the lungs are the most common site of serious infections in severely burned patients and P. aeruginosa is the most common pathogen [9, 36]. The Centers for Disease Control (CDC) defines P. aeruginosa as the most common cause of pneumonia in ICUs and the second most common Gram-negative pathogen causing hospital-acquired infections [36–38]. P. aeruginosa is of particular clinical concern because of its extraordinary resistance to antibiotics resulting in the CDC classifying it as a “SERIOUS” threat to public health [39]. Thus, the ability of MPLA to confer resistance to P. aeruginosa pneumonia has clinical implications beyond application in burn patients alone. Any patient group that is at risk of developing P. aeruginosa pneumonia could benefit including intubated critically ill patients and persons suffering major trauma, high risk surgery or prolonged hospitalization.
Sepsis is the leading cause of death in non-cardiac intensive care units (ICU) and accounts for 40% of ICU expenditures [40, 41]. Patients that survive sepsis suffer long term physical and cognitive disabilities and have a high 1-year mortality rate [42, 43]. Over the past two decades the incidence of sepsis has increased and that trend is likely to continue due to our aging population, increased use of immunosuppressive drugs and invasive procedures and the emergence of antibiotic resistant pathogens [41, 44]. Attempts at effectively treating sepsis have proven exceedingly difficult. No drugs are currently approved by the FDA for the treatment of sepsis due to the repeated failure of clinical trials [45, 46]. Therefore, new strategies are needed to decrease the burden of hospital-acquired infections and sepsis. The addition of immunoadjuvant therapy, such as that provided by treatment with TLR4 agonists, has significant potential to augment existing prophylactic approaches.
MPLA treatment attenuated acute lung injury during P. aeruginosa pneumonia in sheep preconditioned with MPLA compared to vehicle-treated controls as indicated by more favorable PaO2/FiO2 ratio, oxygenation index, shunt fraction, and airway pressures. Those findings were paralleled by lower systemic inflammation reflected by lower plasma IL-6 concentrations in MPLA-treated sheep. The results indicate that MPLA treatment lessened pulmonary and systemic inflammation, both of which can precipitate or contribute to acute lung injury. This is clinically important since acute lung injury is among the most common causes of mortality in severely burned patients [9]. Three factors are likely to contribute to the decreased inflammation and lung injury observed in MPLA-preconditioned sheep. Firstly, MPLA augmented clearance of bacteria from the lungs as indicated by lower P. aeruginosa CFU in lung cultures from MPLA-treated sheep. This is consistent with previous studies showing that MPLA facilitates clearance of bacteria at sites of infection and decreases the dissemination of bacteria to distant sites [19, 21, 22]. MPLA-induced protection persists for at least 15 days and is independent of the adaptive immune system but highly dependent on innate immune function [19, 21, 22]. The mechanisms of improved microbial clearance are multi-factorial and include expansion of myeloid cell numbers in bone marrow and blood in association with increased myeloid cell recruitment to sites of infection and augmented microbial phagocytosis and killing [18, 20, 21, 47]. Secondly, MPLA is known to induce endotoxin tolerance, a state of attenuated cytokine production during periods of inflammation and infection. Our cytokine measurements showed significantly decreased plasma IL-6 concentrations in MPLA-primed sheep compared to controls [18, 48, 49]. That finding connotes decreased local and systemic inflammation in sheep receiving MPLA treatment, which is likely to translate into attenuated lung injury. [50, 51]. Thirdly, it appears that MPLA treatment helps to maintain endothelial barrier integrity since plasma protein leak was attenuated in MPLA-treated mice. Loss of endothelial barrier function is a contributing factor to the development of pulmonary edema during acute lung injury and the acute respiratory distress syndrome. This is likely due to the ability of MPLA to modulate the endothelial cell response to microbial products and cytokines. Our previous studies, using human vascular endothelial cells, show that MPLA priming will attenuate TLR agonist-induced cytokine production by endothelial cells and improve barrier function through mechanisms activated via the MyD88-dependent signaling pathway [49, 52, 53].
The sheep model is advantageous for pre-clinical testing of TLR4 agonists because the physiologic and genomic response of sheep to TLR4 agonists is nearly identical to that of humans and sheep provide a model in which common clinical variables can be measured in real time [23, 54, 55]. Findings from the present study support the validity of sheep as a robust model for pre-clinical testing of TLR4 agonists. The response to MPLA observed in this study is highly similar to that observed in humans. The dose of MPLA chosen for our study was based on results published by Astiz and colleagues for normal human volunteers [48]. They reported that human subjects did not experience subjective side effects until MPLA was administered at doses of 10 μg/kg or greater. At 20 μg/kg, humans experienced mild to moderate symptomatology in association with increases in HR, temperature and elevated plasma TNFα, IL-6 and IL-8 concentrations [48]. Subjects in the 20 μg/kg group did not require therapy or intervention. We chose a dose of MPLA below the 10 μg/kg threshold because we employed burned, rather than normal, uninjured subjects in our study. Due to the inflammatory state induced by burns, we predicted that the physiologic and inflammatory responses to MPLA would be heightened. We observed that hemodynamics and respiratory function were minimally impacted by MPLA infusion at a dose of 2.5 μg/kg and that the response of sheep closely mimicked that seen in humans at similar doses. The most notable physiologic changes induced by MPLA infusion were increases in mean pulmonary artery pressure and pulmonary artery resistance. Those alterations persisted for about 30 minutes after MPLA perfusion and then returned to baseline. Although the burned sheep tolerated those alterations well, it is possible that burn victims suffering inhalation injury and a significant respiratory insult could respond unfavorably. Future studies using a burn and smoke inhalation model would be useful in assessing that possibility.
Our results provide important information about the host response to systemic MPLA infusion and the ability of MPLA to improve resistance pulmonary infection. However, further work is needed. We employed a single dose of MPLA that was guided by previous studies from humans. However, dose finding studies are needed to identify the dose of MPLA that is best tolerated and provides optimal immunomodulatory effect. The 2.5 μg/kg dose is a good starting point since MPLA was well tolerated and effective at that dose. Questions also remain about optimal timing of MPLA treatment. In this study, MPLA was administered 24 hours after burn injury and 24 hours prior to bacterial challenge. In previous studies in mice, we’ve initiated MPLA treatment 2–3 days after burn injury and 24 hours prior to P. aeruginosa burn wound infection [47]. Results of the present study indicate that it is generally safe to administer TLR4 agonists within 24 hours of a major burn injury. We do not know if administration of MPLA during early fluid resuscitation will have significant physiologic impact. We also don’t know if administration of MPLA at the time of infection initiation will be beneficial. Our previous studies suggest that prophylactic administration of MPLA 24 hours prior to infection provides optimal protection and that the beneficial effects persist for at least 15 days [18, 21]. Results of the present study provide proof of concept that MPLA, and other monophosphorylated TLR4 agonists, can be administered safely in a model that closely mimics human critical illness and induces protection from organ injury caused by pneumonia and sepsis.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants RO1 GM104306 to ERS and PE, K08 GM123345 to AH and K08 GM117367 to RS and Grant SHC84050 from the Shriners of North America to PE. The authors claim no conflicts of interest.
References
- 1.Dias VC, Resende JA, Bastos AN, De Andrade Bastos LQ, De Andrade Bastos VQ, Bastos RV, Diniz CG, Da Silva VL: Epidemiological, Physiological, and Molecular Characteristics of a Brazilian Collection of Carbapenem-Resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Microb Drug Resist 23(7):852–863, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel RP, Edmond MB: The impact of hospital-acquired bloodstream infections. Emerg Infect Dis 7(2):174–177, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G: Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 18(1):54–60, 2012, [DOI] [PubMed] [Google Scholar]
- 4.Jeschke MG, Patsouris D, Stanojcic M, Abdullahi A, Rehou S, Pinto R, Chen P, Burnett M, Amini-Nik S: Pathophysiologic Response to Burns in the Elderly. EBioMedicine 2(10):1536–1548, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheridan RL, Greenhalgh D: Special problems in burns. Surg Clin North Am 94(4):781–791, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Maillet M, Pelloux I, Forli A, Vancoetsem K, Cheong Sing JS, Marfaing S, Ducki S, Batailler P, Mallaret MR: Nosocomial transmission of carbapenem-resistant Pseudomonas aeruginosa among burn patients. Infection control and hospital epidemiology 35(5):597–599, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Glik J, Kawecki M, Gazdzik T, Nowak M: The impact of the types of microorganisms isolated from blood and wounds on the results of treatment in burn patients with sepsis. Pol Przegl Chir 84(1):6–16, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Geyik MF, Aldemir M, Hosoglu S, Tacyildiz HI: Epidemiology of burn unit infections in children. Am J Infect Control 31(6):342–346, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Williams FN, Herndon DN, Hawkins HK, Lee JO, Cox RA, Kulp GA, Finnerty CC, Chinkes DL, Jeschke MG: The leading causes of death after burn injury in a single pediatric burn center. Crit Care 13(6):R183, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barajas-Nava LA, Lopez-Alcalde J, Roque i Figuls M, Sola I, Bonfill Cosp X: Antibiotic prophylaxis for preventing burn wound infection. Cochrane Database Syst Rev (6):CD008738, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avni T, Levcovich A, Ad-El DD, Leibovici L, Paul M: Prophylactic antibiotics for burns patients: systematic review and meta-analysis. Bmj 340:c241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos G, Cornistein W, Cerino GT, Nacif G: Systemic antimicrobial prophylaxis in burn patients: systematic review. J Hosp Infect 97(2):105–114, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Bentala H, Verweij WR, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, Poelstra K: Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock 18(6):561–566, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Henricson BE, Benjamin WR, Vogel SN: Differential cytokine induction by doses of lipopolysaccharide and monophosphoryl lipid A that result in equivalent early endotoxin tolerance. Infect Immun 58(8):2429–2437, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monie A, Hung CF, Roden R, Wu TC: Cervarix: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics 2(1):97–105, 2008. [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch FX, de Sanjose S, Castellsague X: The prospects of HPV vaccination in cervical cancer prevention: results of a new independent trial. Cancer Discov 1(5):377–380. 2011. [DOI] [PubMed] [Google Scholar]
- 17.Bohannon JK, Hernandez A, Enkhbaatar P, Adams WL, Sherwood ER: The immunobiology of toll-like receptor 4 agonists: from endotoxin tolerance to immunoadjuvants. Shock 40(6):451–462, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fensterheim BA, Guo Y, Sherwood ER, Bohannon JK: The Cytokine Response to Lipopolysaccharide Does Not Predict the Host Response to Infection. J Immunol 198(8):3264–3273, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohannon JK, Luan L, Hernandez A, Afzal A, Guo Y, Patil NK, Fensterheim B, Sherwood ER: Role of G-CSF in monophosphoryl lipid A-mediated augmentation of neutrophil functions after burn injury. J Leukoc Biol 99(4):629–640, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez A, Bohannon JK, Luan L, Fensterheim BA, Guo Y, Patil NK, McAdams C, Wang J, Sherwood ER: The role of MyD88- and TRIF-dependent signaling in monophosphoryl lipid A-induced expansion and recruitment of innate immunocytes. J Leukoc Biol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fensterheim BA, Young JD, Luan L, Kleinbard RR, Stothers CL, Patil NK, McAtee-Pereira AG, Guo Y, Trenary I, Hernandez A et al. : The TLR4 Agonist Monophosphoryl Lipid A Drives Broad Resistance to Infection via Dynamic Reprogramming of Macrophage Metabolism. J Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER: The Toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect Immun 79(9):3576–3587, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traber DL, Redl H, Schlag G, Herndon DN, Kimura R, Prien T, Traber LD: Cardiopulmonary responses to continuous administration of endotoxin. Am J Physiol 254(5 Pt 2):H833–839, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Bohs CT, Fish JC, Miller TH, Traber DL: Pulmonary vascular response to endotoxin in normal and lymphocyte depleted sheep. Circ Shock 6(1):13–21, 1979. [PubMed] [Google Scholar]
- 25.Enkhbaatar P, Nelson C, Salsbury JR, Carmical JR, Torres KE, Herndon D, Prough DS, Luan L, Sherwood ER: Comparison of Gene Expression by Sheep and Human Blood Stimulated with the TLR4 Agonists Lipopolysaccharide and Monophosphoryl Lipid A. PLoS One 10(12):e0144345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito H, Malgerud E, Asmussen S, Lopez E, Salzman AL, Enkhbaatar P: R-100 improves pulmonary function and systemic fluid balance in sheep with combined smoke-inhalation injury and Pseudomonas aeruginosa sepsis. J Transl Med 15(1):266, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enkhbaatar P, Kikuchi Y, Traber LD, Westphal M, Morita N, Maybauer MO, Maybauer DM, Herndon DN, Traber DL: Effect of inhaled nitric oxide on pulmonary vascular hyperpermeability in sheep following smoke inhalation. Burns 31(8):1013–1019, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Traber MG, Shimoda K, Murakami K, Leonard SW, Enkhbaatar P, Traber LD, Traber DL: Burn and smoke inhalation injury in sheep depletes vitamin E: kinetic studies using deuterated tocopherols. Free Radic Biol Med 42(9):1421–1429, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez E, Fujiwara O, Lima-Lopez F, Suman OE, Mlcak RP, Hawkins HK, Cox RA, Herndon DN, Prough DS, Enkhbaatar P: Nebulized Epinephrine Limits Pulmonary Vascular Hyperpermeability to Water and Protein in Ovine With Burn and Smoke Inhalation Injury. Crit Care Med 44(2):e89–96, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Enkhbaatar P, Joncam C, Traber L, Nakano Y, Wang J, Lange M, Connelly R, Kulp G, Saunders F, Huda R et al. : Novel ovine model of methicillin-resistant Staphylococcus aureus-induced pneumonia and sepsis. Shock 29(5):642–649, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Lange M, Hamahata A, Traber DL, Esechie A, Jonkam C, Bansal K, Nakano Y, Traber LD, Enkhbaatar P: A murine model of sepsis following smoke inhalation injury. Biochem Biophys Res Commun 391(3):1555–1560, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Sousse LE, Jonkam CC, Traber DL, Hawkins HK, Rehberg SW, Traber LD, Herndon DN, Enkhbaatar P: Pseudomonas aeruginosa is associated with increased lung cytokines and asymmetric dimethylarginine compared with methicillin-resistant Staphylococcus aureus. Shock 36(5):466–470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonkam CC, Bansal K, Traber DL, Hamahata A, Maybauer MO, Maybauer DM, Cox RA, Lange M, Connelly RL, Traber LD et al. : Pulmonary vascular permeability changes in an ovine model of methicillin-resistant Staphylococcus aureus sepsis. Crit Care 13(1):R19, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S: Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26(11):1793–1800, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Gang RK, Bang RL, Sanyal SC, Mokaddas E, Lari AR: Pseudomonas aeruginosa septicaemia in burns. Burns 25(7):611–616, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Oncul O, Oksuz S, Acar A, Ulkur E, Turhan V, Uygur F, Ulcay A, Erdem H, Ozyurt M, Gorenek L: Nosocomial infection characteristics in a burn intensive care unit: analysis of an eleven-year active surveillance. Burns 40(5):835–841, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal VD, Al-Abdely HM, El-Kholy AA, AlKhawaja SAA, Leblebicioglu H, Mehta Y, Rai V, Hung NV, Kanj SS, Salama MF et al. : International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010–2015: Device-associated module. Am J Infect Control 44(12):1495–1504, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Klevens RM, Edwards JR, Gaynes RP: The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis 47(7):927–930, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Pena C, Suarez C, Gozalo M, Murillas J, Almirante B, Pomar V, Aguilar M, Granados A, Calbo E, Rodriguez-Bano J et al. : Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob Agents Chemother 56(3):1265–1272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K: Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 193(3):259–272, 2016. [DOI] [PubMed] [Google Scholar]
- 41.Mayr FB, Yende S, Angus DC: Epidemiology of severe sepsis. Virulence 5(1):4–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ: Late mortality after sepsis: propensity matched cohort study. Bmj 353:i2375, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angus DC, Opal S: Immunosuppression and Secondary Infection in Sepsis: Part, Not All, of the Story. JAMA 315(14):1457–1459, 2016. [DOI] [PubMed] [Google Scholar]
- 44.Kahn JM, Le T, Angus DC, Cox CE, Hough CL, White DB, Yende S, Carson SS: The epidemiology of chronic critical illness in the United States*. Crit Care Med 43(2):282–287, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall JC: Why have clinical trials in sepsis failed? Trends Mol Med 20(4):195–203, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Angus DC: The search for effective therapy for sepsis: back to the drawing board? JAMA 306(23):2614–2615, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Bohannon JK, Luan L, Hernandez A, Afzal A, Guo Y, Patil NK, Fensterheim B, Sherwood ER: Role of G-CSF in monophosphoryl lipid A-mediated augmentation of neutrophil functions after burn injury. J Leukoc Biol 99(4):629–40, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Astiz ME, Rackow EC, Still JG, Howell ST, Cato A, Von Eschen KB, Ulrich JT, Rudbach JA, McMahon G, Vargas R et al. : Pretreatment of normal humans with monophosphoryl lipid A induces tolerance to endotoxin: a prospective, double-blind, randomized, controlled trial. Crit Care Med 23(1):9–15, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Stark RJ, Choi H, Koch SR, Fensterheim BA, Lamb FS, Sherwood ER: Endothelial cell tolerance to lipopolysaccharide challenge is induced by monophosphoryl lipid A. Clin Sci (Lond) 130(6):451–461, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varma TK, Toliver-Kinsky TE, Lin CY, Koutrouvelis AP, Nichols JE, Sherwood ER: Cellular mechanisms that cause suppressed gamma interferon secretion in endotoxintolerant mice. Infect Immun 69(9):5249–5263, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan H, Cook JA: Molecular mechanisms of endotoxin tolerance. J Endotoxin Res 10(2):71–84, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Stark R, Choi H, Koch S, Lamb F, Sherwood E: Monophosphoryl lipid A inhibits the cytokine response of endothelial cells challenged with LPS. Innate Immun 21(6):565–74, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koch SR, Lamb FS, Hellman J, Sherwood ER, Stark RJ: Potentiation and tolerance of toll-like receptor priming in human endothelial cells. Transl Res 180:53–67 e54, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Redl H, Bahrami S, Schlag G, Traber DL: Clinical of LPS and animal models of endotoxemia. Immunobiology 187(3–5):330–345, 1993. [DOI] [PubMed] [Google Scholar]
- 55.Luan L, Patil NK, Guo Y, Hernandez A, Bohannon JK, Fensterheim BA, Wang J, Xu Y, Enkhbaatar P, Stark R et al. : Comparative Transcriptome Profiles of Human Blood in Response to the Toll-like Receptor 4 Ligands Lipopolysaccharide and Monophosphoryl Lipid A. Sci Rep 7:40050, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.