Organic anion transporter 10 (OAT10), also known as SLC22A13, has hitherto been identified as a urate transporter by in vitro analyses.1 Despite the reported expression of OAT10 on the apical membrane of the renal proximal tubular cells,1 the physiological impact of OAT10 on urate handling in humans remains to be elucidated. Accumulating evidence suggests that functional variants of already-characterised, physiologically important urate transporters—URAT1/SLC22A12, GLUT9/SLC2A9, BCRP/ABCG2 and NPT1/SLC17A1—affect serum uric acid (SUA) levels and susceptibility of gout,2–6 the most common form of inflammatory arthritis. However, there are no reports on the association between OAT10 gene and either hyperuricaemia or gout. Here, for the first time, we reveal that a dysfunctional variant of OAT10 decreases both gout risk and SUA levels, suggesting OAT10 to be physiologically involved in urate reabsorption in the human kidney, as described below.
To explore exonic variants in OAT10 potentially associated with gout susceptibility, we sequenced all exons of OAT10 in 480 gout cases and 480 controls of Japanese male6 and conducted an association analysis (see online supplementary tables S1 and S2), followed by a replication study on 924 gout cases and 2113 controls (see online supplementary figure S1). In two identified OAT10 variants with minor allele frequency (MAF) >0.5%, only rs117371763 (c.1129C>T; p.Arg377Cys [R377C]) was significantly associated with gout susceptibility after Bonferroni correction (p=0.014). The significant association between rs117371763 and gout susceptibility was replicated, and our meta-analysis showed a significant protective effect of rs117371763 on gout susceptibility (OR=0.67; 95% CI 0.53 to 0.85; pmeta=7.8×10-4) (table 1). In addition, a quantitative trait locus analysis focusing on SUA levels in 3208 individuals (see online supplementary table S3) showed that the minor allele of rs117371763 significantly decreases SUA levels (β=–0.156 mg/dL, 95% CI –0.295 to –0.018 mg/dL, p=0.027). Results were similar even after adjustment for age.
annrheumdis-2019-216044supp001.pdf (1.4MB, pdf)
Table 1.
Association analysis of OAT10/SLC22A13 variant, rs117371763 [Arg377Cys (R377C)], with gout susceptibility
| Gout cases | Controls | p value | OR (95% CI) | |||||||
| C/C | C/T | T/T | MAF (%) | C/C | C/T | T/T | MAF (%) | |||
| Discovery phase | 447 | 31 | 2 | 3.65 | 427 | 46 | 6 | 6.05 | 0.014 | 0.59 (0.38 to 0.90) |
| Replication phase | 859 | 63 | 2 | 3.63 | 1900 | 203 | 5 | 5.02 | 0.015 | 0.71 (0.53 to 0.94) |
| Meta-analysis | 7.8×10-4 | 0.67 (0.53 to 0.85) | ||||||||
In the meta-analysis, no apparent heterogeneity was observed (p value for Cochran's Q test=0.48, I2=0%).
MAF, minor allele frequency.
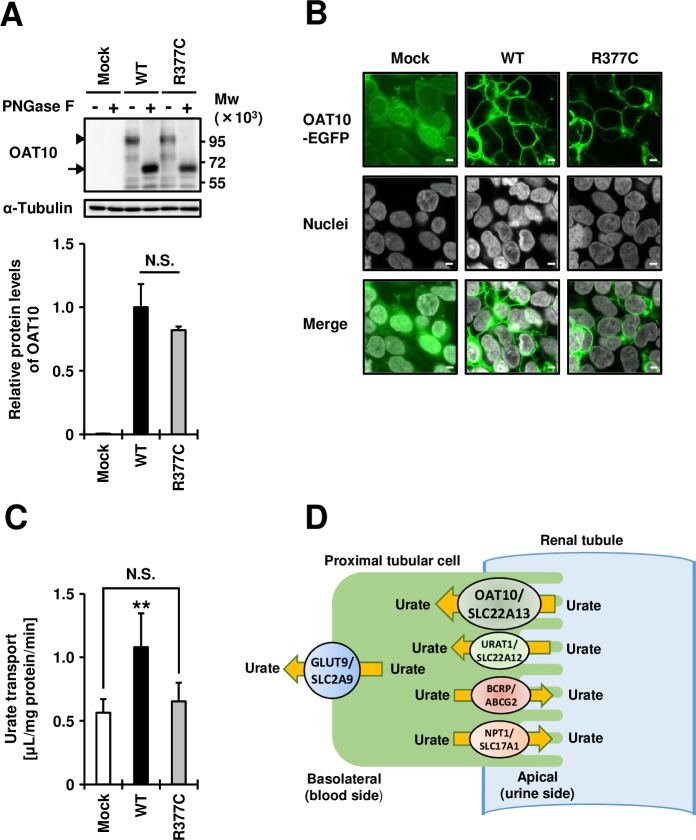
Furthermore, via a series of cell-based experiments, we identified the R377C variant as an almost null variant of OAT10 (figure 1A–C). Immunoblotting and confocal microscopic observations showed the R377C variant to have little effect on OAT10 protein levels (figure 1A) or its cellular localisation (figure 1B). Cell-based urate transport assay demonstrated that, consistent with a previous report,1 OAT10 wild-type can transport urate (figure 1C); however, the urate transport activity of R377C variant-expressing cells was close to that of mock cells, demonstrating that this variant disrupts OAT10’s function as a urate transporter. As it is conserved across different species (see online supplementary figure S2), R377 may be important for OAT10 function.
Figure 1.
Effects of Arg377Cys (R377C) on the expression, plasma membrane localisation, and function of the organic anion transporter 10 (OAT10) urate transporter transiently expressed in 293A cells. (A) (Upper) Immunoblot detection of OAT10/SLC22A13 protein in whole cell lysate samples. OAT10 fused with EGFP was detected by an anti-EGFP antibody. Arrowhead, matured OAT10 as a glycoprotein; arrow, non-glycosylated form of OAT10; α-tubulin, a loading control; (Lower) Relative protein levels of OAT10 wild-type (WT) and Arg377Cys (R377C) variant. Data are expressed as the mean±SD, n=3. N.S., not significantly different between groups (two sided t-test). (B) Confocal microscopic observation of cellular localisation. Nuclei were stained with TO-PRO-3 iodide (grey). Bars, 5 µm. (C) Functional analysis. OAT10-expressing 293A cells were incubated with 10 µM of [14C]-urate for 60 s, then the amount of urate incorporated into the cells was measured. Data are expressed as the mean±SD, n=7. **p<0.01 versus the other groups (Tukey-Kramer multiple-comparison test). All experiments were performed 48 hours after plasmid transfection. (D) Proposed physiological model of OAT10 in human kidney. OAT10 is expressed on the apical membrane of renal proximal tubules and mediates reabsorption of urate from urine to blood. Other previously characterised urate reabsorption transporters (URAT1/SLC22A12 and GLUT9/SLC2A9) and urate excretion transporters (BCRP/ABCG2 and NPT1/SLC17A1) are also described.
Considering the following three points, we conclude that OAT10 is a urate reabsorption transporter on the apical side of the renal proximal tubular cells (figure 1D). First, the R377C variant of OAT10 was almost null as a urate transporter (figure 1C). Second, this dysfunctional variant decreased SUA levels (see online supplementary table S3), suggesting that functional OAT10 is physiologically involved in a supply route of urate into the blood. Third, like URAT1/SLC22A12, which plays a pivotal role in urate transport from urine to the blood,2 OAT10 is reportedly expressed in the brush border membranes of the renal epithelium,1 therefore making it a potential target for urate-lowering therapy like URAT1. Although rs117371763 of OAT10 is common in Japanese (see online supplementary table S2), this variant is rare in other populations, including European Caucasians (see online supplementary table S4). Such populations, in which most people have functional OAT10, may offer a greater potential for OAT10 as a drug target for the treatment of gout/hyperuricaemia. Our findings will contribute to uncovering the physiological role of OAT10 as a renal urate reabsorber and its pathophysiological importance in urate-related disorders such as gout/hyperuricaemia.
Acknowledgments
We would like to thank all the participants for their generous involvement in this study. We also thank members of J-MICC Study Shizuoka Field and Daiko Field for supporting the study. We are indebted to K Gotanda, M Miyazawa, Y Aoyagi, Y Aoki and K Yokoi (National Defense Medical College) for genetic analysis. We are indebted to M Senda (Ryougoku East Gate Clinic), H Fujiwara (Midorigaoka Hospital), K Wakai and N Hamajima (Nagoya University) for sample collection.
Footnotes
Handling editor: Josef S Smolen
TH, KM, HNakaoka, YT, YKawamura and SS contributed equally.
Contributors: TH, HNakaoka, YT, TTakada and HM conceived and designed this study. TN, KH, AN, MU, TI, KI, KY, HS, NS and II assisted with research design. SS, KO, HO, TS, NS and HM collected and analysed clinical data of cases. YKawamura, SS, MU, TI, TTamura, MN, HNakashima, MK, MT and HM collected and analysed clinical data of controls. TH, HNakaoka, SS, NS, II and HM performed genetic analysis. HNakaoka, YKawamura, HNakashima, TN and II performed statistical analyses. KM, YT, HS and TTakada performed functional analysis. TTakada and HM organised this collaborative study as corresponding authors. KM, TN, KH, AN, YKawai, NO, KI and KY provided intellectual input and assisted with the preparation of the manuscripts. TH, YT, YKawamura, TTakada and HM wrote the manuscript. TH, KM, HNakaoka, YT, YKawamura and SS contributed equally to this work. All authors have read and approved the final version of the manuscript.
Funding: This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (Nos 17H04128, 19K22786, 25293145, 15K15227, 17015018, 221S0001, 221S0002, 15H05610, 16H06277, 16H06279, 16H01808, 18KK0247 and 22136015), the Ministry of Defense, the Uehara Memorial Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Takeda Medical Foundation, MSD Life Science Foundation, Public Interest Incorporated Foundation, the Kawano Masanori Memorial Foundation for Promotion of Pediatrics and the Gout Research Foundation of Japan.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the institutions’ Ethical Committees (National Defense Medical College, National Institute of Genetics, and Nagoya University). All procedures were performed in accordance with the Declaration of Helsinki, with written informed consent obtained from each participant.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Bahn A, Hagos Y, Reuter S, et al. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem 2008;283:16332–41. 10.1074/jbc.M800737200 [DOI] [PubMed] [Google Scholar]
- 2. Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002;417:447–52. 10.1038/nature742 [DOI] [PubMed] [Google Scholar]
- 3. Matsuo H, Takada T, Ichida K, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med 2009;1:5ra11 10.1126/scitranslmed.3000237 [DOI] [PubMed] [Google Scholar]
- 4. Woodward OM, Köttgen A, Coresh J, et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A 2009;106:10338–42. 10.1073/pnas.0901249106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakayama A, Nakaoka H, Yamamoto K, et al. GWAS of clinically defined gout and subtypes identifies multiple susceptibility loci that include urate transporter genes. Ann Rheum Dis 2017;76:869–77. 10.1136/annrheumdis-2016-209632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higashino T, Takada T, Nakaoka H, et al. Multiple common and rare variants of ABCG2 cause gout. RMD Open 2017;3:e000464 10.1136/rmdopen-2017-000464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2019-216044supp001.pdf (1.4MB, pdf)