Abstract
Objectives
Open-labelled clinical trials suggested that low-dose IL-2 might be effective in treatment of systemic lupus erythematosus (SLE). A double-blind and placebo-controlled trial is required to formally evaluate the safety and efficacy of low-dose IL-2 therapy.
Methods
A randomised, double-blind and placebo-controlled clinical trial was designed to treat 60 patients with active SLE. These patients received either IL-2 (n=30) or placebo (n=30) with standard treatment for 12 weeks, and were followed up for additional 12 weeks. IL-2 at a dose of 1 million IU or placebo was administered subcutaneously every other day for 2 weeks and followed by a 2-week break as one treatment cycle. The primary endpoint was the SLE Responder Index-4 (SRI-4) at week 12. The secondary endpoints were other clinical responses, safety and dynamics of immune cell subsets.
Results
At week 12, the SRI-4 response rates were 55.17% and 30.00% for IL-2 and placebo, respectively (p=0.052). At week 24, the SRI-4 response rate of IL-2 group was 65.52%, compared with 36.67% of the placebo group (p=0.027). The primary endpoint was not met at week 12. Low-dose IL-2 treatment resulted in 53.85% (7/13) complete remission in patients with lupus nephritis, compared with 16.67% (2/12) in the placebo group (p=0.036). No serious infection was observed in the IL-2 group, but two in placebo group. Besides expansion of regulatory T cells, low-dose IL-2 may also sustain cellular immunity with enhanced natural killer cells.
Conclusions
Low-dose IL-2 might be effective and tolerated in treatment of SLE.
Trial registration number
ClinicalTrials.gov Registries (NCT02465580 and NCT02932137).
Keywords: systemic lupus erythematosus, t cells, Autoimmune diseases, cytokines, treatment
Key messages.
What is already known about this subject?
Proof-of-concept studies and case reports suggested that low-dose IL-2 might be therapeutic in systemic lupus erythematosus (SLE).
What does this study add?
This is the first randomised, double-blind, placebo-controlled study of low-dose IL-2 in the treatment of SLE. The results suggest that low-dose IL-2 therapy may be effective and safe in SLE.
Immunological analysis revealed that low-dose IL-2 induced expansion of regulatory T cells and NK cells, which may contribute to the restoration of immune homeostasis in SLE patients.
How might this impact on clinical practice or future developments?
This study provides supportive data to confirm the therapeutic effects of low-dose IL-2 in SLE treatment.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with a wide range of clinical manifestations. Sustained remission is achieved in only a small fraction of patients.1–3 Current treatment regimens mainly rely on corticosteroids and immunosuppressive agents which are associated with substantial adverse effects including various infections.4–6 Defective IL-2 production contributes to the unbalanced immune system in SLE.7–10 Previous short term open-labelled trials showed that low-dose IL-2 treatment promoted regulatory T (Treg) cells and inhibited T helper 17 (TH17) cells and follicular helper T (TFH) cells. The immunological rebalancing was associated with the induction of remission in SLE patients.11 12 The benefits of low-dose IL-2 therapy were reported in case study and open-labelled trials for hepatitis C-associated vasculitis,13 graft-versus-host disease,14 15 type 1 diabetes,16 alopecia areata17 and SLE.11 12
In contrast to increased infection risk associated with standard therapies in SLE, we observed no serious infection in previous study,11 which was in line with the report showing that low-dose IL-2 ameliorated hepatitis C virus-induced vasculitis without increasing viral load.13 From an immunological perspective, IL-2 treatment may enhance virus-specific CD8+ T cell responses18 and promote the activity of NK cells against infections.19 20
To formally evaluate the safety and efficacy of low-dose IL-2 therapy in SLE, we carried out a randomised, double-blind, placebo-controlled trial in patients with active SLE, with response rate as the primary endpoint. Given that infection is a major cause of relapse, hospitalisation and death in patients with SLE,5 6 and that low-dose IL-2 might increase anti-infectious immune response,13 21 we determine whether low-dose IL-2 treatment benefits SLE patients by inducing clinical improvement without increasing the incidence of infection.
Methods
Patients
All SLE patients were diagnosed according to the 1997 revised classification criteria of the American College of Rheumatology,22 and had an inadequate response to standard treatment for ≥3 months. The background treatment with corticosteroids, antimalarials and immunosuppressants was shown in online supplementary appendix table S1 and S2. Exclusion criteria included: active severe neuropsychiatric manifestations of SLE; history of treatment with rituximab or other biologics; use of high-dose corticosteroids (≥1.0 mg/kg) in the preceding month; severe comorbidities including heart failure (≥grade III New York Heart Association), renal insufficiency (creatinine clearance ≤30 mL/min) or hepatic insufficiency (alanine aminotransferase or aspartate aminotransferase ≥2 times of the upper limit of the normal range); active infection (hepatitis B or C virus, Epstein-Barr virus, HIV or Mycobacterium tuberculosis); history of chronic infection; malignancy; pregnancy or lactation in females.
annrheumdis-2019-215396supp001.pdf (23.1MB, pdf)
Study design and blinding
We conducted a randomised, double-blind, placebo-controlled study to verify the clinical response and safety of low-dose IL-2 (recombinant human IL-2 from Escherichia coli, Beijing SL PHARM) for the treatment of active SLE (ClinicalTrials.gov number NCT02465580). Sixty patients with active SLE at 18–65 years of age were included. Patients were randomly assigned (in a 1:1 ratio) to one of the two arms (low-dose IL-2 or placebo) in the study.
All patients, investigators, sponsor and study staff were blinded to treatment. All clinical and laboratory assessments were performed by qualified, trained investigators who were blinded to the patient’s safety data, previous efficacy data and treatment randomisation. Placebo was provided as sterile and lyophilised vials completely matching those of IL-2 in appearance, which contained the same formulations as the study drug except without IL-2.
In addition to standard therapy, IL-2 (1 million IU) or placebo was administered subcutaneously every other day for 2 weeks (seven injections), followed by a 2-week break, as one treatment cycle of 4 weeks. All the patients were treated for the first 12 weeks which included three treatment cycles with IL-2 or placebo and followed up for further 12 weeks without study medicine. Patients were evaluated at screening, every 2 weeks to week 12, and every 4 weeks thereafter to week 24. Assessments included physical examination and laboratory tests for signs of effects. At each visit, patients were evaluated by investigators to determine whether it was necessary to adjust the dose of glucocorticoids. T cells and NK cells in peripheral blood mononuclear cells (PBMCs) were measured at each time point. Complete blood count (CBC), complete metabolic profile, urinalysis, serum immunoglobulin, C3 and C4, anti-dsDNA antibodies and antinucleosome antibodies were measured on the same schedule. Urine protein and serum albumin concentrations were also measured in patients with urinary protein excretion >0.5 g/day.
To evaluate the potential effects of low-dose IL-2 in anti-infectious immunity, another randomised, open-labelled trial was performed (ClinicalTrials.gov number NCT02932137). Twenty patients with active SLE were included and randomly assigned in a 1:1 ratio to one of the two arms (low-dose IL-2 or standard treatment) in the study.
These studies were performed in accordance with the protocol, the Declaration of Helsinki and Good Clinical Practice principles. All patients provided written informed consent.
Outcomes
The primary endpoint was the response measured by the SLE Responder Index-4 (SRI-4) at week 12.23 SRI response was defined as (1) a ≥4-point reduction in Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)-SLEDAI score,24 (2) no new British Isles Lupus Assessment Group (BILAG) A score or ≤1 new BILAG B score and (3) no deterioration from baseline in the physician’s global assessment (PGA) by ≥0.3 points. The secondary endpoints were other clinical responses, safety and dynamics of immune cell subsets including T cell and NK cell subsets.
Complete renal remission (CR) in this study was defined as (1) serum creatinine within the normal range with stable or improved values as compared with baseline (no >15% above baseline), (2) inactive urinary sediment and (3) normal range proteinuria <0.3 g/24 hours.25
Flow cytometry and intracellular cytokine assays
Relative proportions of CD4+ T cell, CD8+ T cell, Treg and NK cell subsets were analysed by flow cytometry using a FACSAria II (BD) instrument and FlowJo software (Tree Star). The detailed gating strategy for these subsets was outlined in online supplementary figure S1. The clone and catalogue numbers for all of the antibodies used in this study were provided in online supplementary table S6.
CD8+ T cell response to CMV-EBV-Flu (CEF) viral peptide pool stimulation was evaluated as described in previous studies.26 Briefly, one million PBMCs were incubated with 1 µg each of the co-stimulatory CD28 and CD49d monoclonal antibodies (BD Pharmingen, San Diego, California, USA) and 5 µg of CEF viral peptide poolin 1 mL of 1640 media containing 10% human AB serum. The cultures were incubated at 37°C in a 5% CO2 incubator for 1 hour, followed by an additional 5-hour incubation with 10 µg/mL of Brefeldin-A20. The cells were stained with antibodies staining CD3, CD8 and other cell surface markers, then fixed, permeabilised with BD FACS fixation and permeabilisation buffer set. Permeabilised cells were stained with fluorochrome-conjugated antibodies against cytotoxic cytokines for 30 min at 4°C.
For NK cell response evaluation, one million PBMCs were incubated at 37°C in a 5% CO2 incubator for 5 hours with 10 µg/mL of Brefeldin-A20. These cells were stained with antibodies staining CD3, CD56 and other cell surface markers. After fixation and permeabilisation, these cells were stained with fluorochrome-conjugated antibodies against cytotoxic cytokines for 30 min at 4°C.
Statistics
The protocol was designed as a superiority trial to demonstrate whether low-dose IL-2 was more efficacious than placebo at the background of standard treatment for active SLE. Power calculations using parameters from a previous open-labelled pilot trial11 had shown that 13 patients per group would provide 90% power, with two-sided p=0.05, to conclude that low-dose IL-2 was superior to placebo to achieve the primary endpoint.
For clinical characteristics and laboratory parameters, the primary efficacy analysis was a modified intention-to-treat (mITT) analysis that included all patients who were randomly assigned to this trial and underwent at least one efficacy assessment. Differences in the changes between baseline and the indicated time points were evaluated with paired-sample t-test for continuous variables and χ2 test for categorical variables. Differences between the two groups at indicated time points were compared with Mann-Whitney U test for continuous variables and χ2 test for categorical variables. The data were also analysed by perprotocol analysis which excluded patients who did not complete treatment and included only patients for whom outcome information was available. The safety population comprised all patients who received at least one cycle of study assessment. Safety variables were analysed descriptively with a between-group comparison of proportions of patients with adverse events. Statistical analysis was performed with the use of SPSS V.20.0. P value <0.05 was considered statistically significant.
Results
Patient characteristics
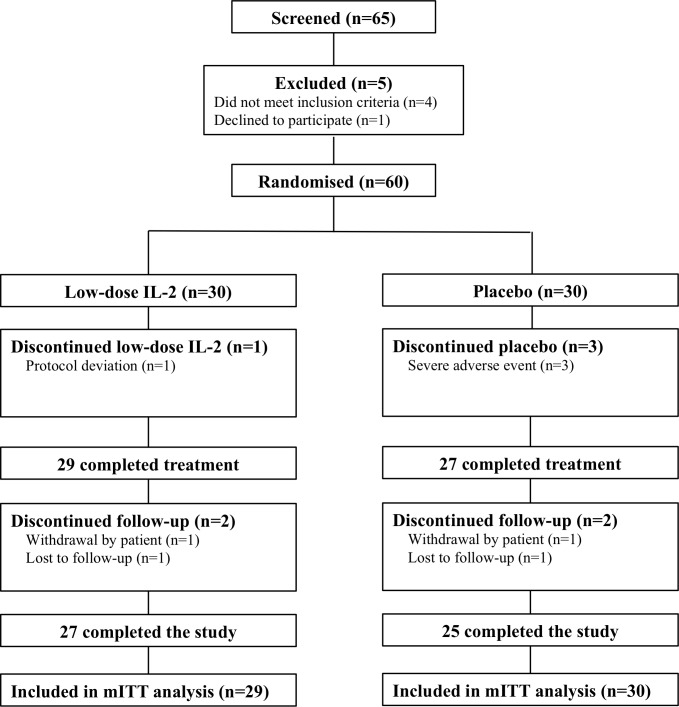
Between June 2015 and September 2017, 60 patients with active SLE at 18–65 years of age were included. The patients were randomly assigned in a 1:1 ratio to receive low-dose IL-2 or placebo (figure 1). The characteristics of the patients were shown in online supplementary table S1 and S2. Mean age was 31.6 and 29.8 years, mean body surface area was 1.57 and 1.62 m2, and mean disease duration was 66.7 and 63.6 in IL-2 and placebo arms, respectively. Baseline disease characteristics were summarised in table 1. Thirteen of 30 in IL-2 group and 12 of 30 in placebo group showed signs of lupus nephritis (LN), with a median 24-hour urinary protein of 1.37 and 1.55, respectively. Other symptoms including rash, oral ulceration, alopecia and so on were showed in table 2. All the baseline characteristics were comparable between two groups. Concomitant treatments were shown in Table 1, online supplementary table S1. Patients were carefully followed till week 24. The main reason for patient withdrawal in low-dose IL-2 group was inconvenience due to the required frequent hospital visits. In the placebo group, patient withdrawal mainly occurred because of unsatisfying therapeutic effects and development of SLE organ involvement (eg, neuropsychiatric systemic lupus erythematosus (NPSLE)) (figure 1).
Figure 1.
Patient enrolment and treatment assignments. Consolidated standards of reporting trials diagram was based on the 65 contacted SLE patients. Sixty of the patients were enrolled into two arms. Arm 1 (n=30), the IL-2 group, received three treatment cycles. Each cycle included subcutaneous IL-2 administration at a dose of 1 million IU every other day for 2 weeks (a total of seven doses) and a following 2-week break. Participants in arm 2 (n=30), the placebo group, started treatment with the same procedure as arm 1. mITT, modified intention-to-treat; N, no of patients.
Table 1.
Baseline characteristics of SLE patients in this study (n=60)
| Characteristics | IL-2 (n=30) | Placebo (n=30) | P value |
| Age, year, mean±SD | 31.58±9.25 | 29.83±9.72 | 0.474 |
| Female/male | 27/3 | 29/1 | 0.612 |
| Weight, kg, mean±SD | 54.81±8.33 | 58.69±8.87 | 0.117 |
| Height, cm, mean±SD | 162.23±6.81 | 162.67±5.41 | 0.743 |
| Area, m2, mean±SD | 1.57±0.140 | 1.62±0.13 | 0.708 |
| Duration, months, mean±SD | 66.7±57.4 | 63.6±59.9 | 0.652 |
| SLEDAI, median (range) | 12 (8–27) | 11 (8–22) | 0.351 |
| BILAG, median (range) | 10 (8–13) | 10.5 (8–13.75) | 0.372 |
| ≥1 BLIAG A or 2B score (%) | 21 (70) | 21 (70) | 1.000 |
| PGA, median (range) | 2.3 (1.55–2.75) | 2.2 (1–2.3) | 0.446 |
| Medications | |||
| Prednisone dose, mg/day, median (range) | 12.5 (0–50) | 15 (5–50) | 0.331 |
| Hydroxychloroquine | 29 (96.67) | 28 (93.33) | 1.000 |
| Cyclophosphamide | 4 (13.33) | 0 (0) | 0.112 |
| Azathioprine | 1 (3.33) | 4 (13.33) | 0.352 |
| Cyclosporine | 0 (0) | 5 (16.67) | 0.052 |
| Mycophenolate mofetil | 9 (30.00) | 8 (26.67) | 1.000 |
| Tacrolimus | 1 (3.33) | 1 (3.33) | 1.000 |
| Leflunomide | 3 (10.00) | 1 (3.33) | 0.611 |
| Thalidomide | 1 (3.33) | 0 (0) | 1.000 |
| Methotrexate | 1 (3.33) | 1 (3.33) | 1.000 |
Baseline information was collected at the time the systemic lupus erythematosus (SLE) patients entered the double-blind period of the study.
For a continuous variable, median (range) and mean±SD, for a categorical variable, count (percentage).
Area, body surface area; BILAG, British Isles Lupus Assessment Group; PGA, physician's global assessment; SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment version of the Systemic Lupus Erythematosus Disease Activity Index.
Table 2.
Responses of SLE patients to low-dose IL-2 treatment
| Characteristics | Baseline | Week 12 | Week 24 | P value (week 0 vs 12) | P value (week 0 vs 24) |
| SLEDAI, median (range) | |||||
| IL-2 | 12 (8–27) | 6 (0–16) | 4 (0–18) | <0.001 | <0.001 |
| Placebo | 11 (8–22) | 6 (0–25) | 8 (0–25) | <0.001 | 0.002 |
| BILAG, median (range) | |||||
| IL-2 | 10 (8–13) | 6 (4–11) | 6 (4–11) | 0.014 | <0.001 |
| Placebo | 10.5 (8–13.75) | 10 (4–11) | 8 (4–10.75) | 0.037 | 0.004 |
| ≥1 BLIAG A or 2B score (%) | |||||
| IL-2 | 21 (70.00) | 2 (6.67) | 1 (3.33) | <0.001 | <0.001 |
| Placebo | 21 (70.00) | 4 (13.33) | 2 (6.67) | <0.001 | <0.001 |
| PGA, median (range) | |||||
| IL-2 | 2.3 (1.55–2.75) | 0 (0–2) | 0 (0–1) | <0.001 | <0.001 |
| Placebo | 2.2 (1–2.3) | 1 (0–2) | 1 (0–2) | <0.001 | <0.001 |
| Rash, n (%) | |||||
| IL-2 | 13 (44.83) | 2 (6.90) | 2 (6.90) | 0.002 | 0.002 |
| Placebo | 16 (53.33) | 6 (20.0) | 6 (20.0) | 0.015 | 0.015 |
| Oral ulceration, n (%) | |||||
| IL-2 | 4 (13.79) | 0 (0) | 0 (0) | 0.112 | 0.112 |
| Placebo | 1 (3.33) | 0 (0) | 0 (0) | 1.000 | 1.000 |
| Arthritis, n (%) | |||||
| IL-2 | 14 (48.28) | 4 (13.79) | 3 (10.34) | 0.010 | 0.003 |
| Placebo | 15 (50.0) | 9 (30.00) | 8 (26.67) | 0.187 | 0.110 |
| Vasculitis, n (%) | |||||
| IL-2 | 4 (13.79) | 0 (0) | 0 (0) | 0.112 | 0.112 |
| Placebo | 2 (6.67) | 0 (0) | 0 (0) | 0.492 | 0.492 |
| Alopecia, n (%) | |||||
| IL-2 | 12 (41.38) | 6 (20.69) | 5 (17.24) | 0.158 | 0.082 |
| Placebo | 7 (23.33) | 2 (6.67) | 2 (6.67) | 0.144 | 0.144 |
| Fever, n (%) | |||||
| IL-2 | 3 (10.34) | 0 (0) | 0 (0) | 0.237 | 0.237 |
| Placebo | 4 (13.33) | 1 (3.33) | 0 (0) | 0.352 | 0.167 |
| Myositis, n (%) | |||||
| IL-2 | 1 (3.45) | 0 (0) | 0 (0) | 1.000 | 1.000 |
| Placebo | 2 (6.67) | 0 (0) | 0 (0) | 0.492 | 0.492 |
| Prednisone dose, mg/day, median (range) | |||||
| IL-2 | 15 (0–50) | 10 (0–25) | 10 (0–20) | 0.001 | <0.001 |
| Placebo | 15 (7.5–60) | 15 (5–40) | 10 (2.5–35) | 0.004 | <0.001 |
| ANA decreased, n (%) | |||||
| IL-2, n=29 | 0 (0) | 7 (24.14) | 8 (27.59) | 0.500 | 0.320 |
| Placebo, n=30 | 0 (0) | 11 (36.67) | 12 (40.0) | 0.036 | 0.036 |
| Anti-ds-DNA, IU/mL, median (range) | |||||
| IL-2, n=29 | 34.80 (1.0–1783.15) | 33.0 (7.0–876.21) | 29.0 (1.0–348.50) | 0.037 | 0.063 |
| Placebo, n=30 | 73.30 (1.0–2525.53) | 37.60 (1.40–3467.80) | 36.3 (1.0–3467.80) | 0.196 | 0.235 |
| AnuA, IU/mL, median (range) | |||||
| IL-2, n=29 | 14.45 (0.87–449.06) | 20.84 (1.28–287.07) | 16.72 (1.17–287.07) | 0.439 | 0.044 |
| Placebo, n=30 | 41.725 (0.0–315.80) | 16.03 (0.0–296.32) | 12.08 (0.0–266.740) | 0.282 | 0.149 |
| Albumin, g/L, median (range) | |||||
| IL-2, n=29 | 39.25 (27.60–44.70) | 43.90 (37.70–46.90) | 43.50 (39.80–47.20) | 0.046 | 0.017 |
| Placebo, n=30 | 39.80 (25.10–44.40) | 38.65 (31.90–43.60) | 40.40 (32.80–47.50) | 0.442 | 0.848 |
| LN complete remission, n (%) | |||||
| IL-2, n=13 | 0 (0) | 7 (53.85) | 7 (53.85) | 0.005 | 0.005 |
| Placebo, n=12 | 0 (0) | 1 (8.33) | 2 (16.67) | 1.000 | 0.478 |
| LN partial remission, n (%) | |||||
| IL-2, n=13 | 0 (0) | 10 (76.92) | 10 (76.92) | <0.001 | <0.001 |
| Placebo, n=12 | 0 (0) | 3 (25.0) | 6 (50.0) | 0.217 | 0.014 |
Data are median (IQR), n (%) or difference (95% CI).
ANA, antinuclear antibodies; AnuA, antinucleosome antibodies; BILAG, British Isles Lupus Assessment Group; LN, lupus nephritis; PGA, physician’s global assessment of disease activity;SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment version of the SLE Disease Activity Index; anti-dsDNA, anti–double-stranded DNA.
Efficacy
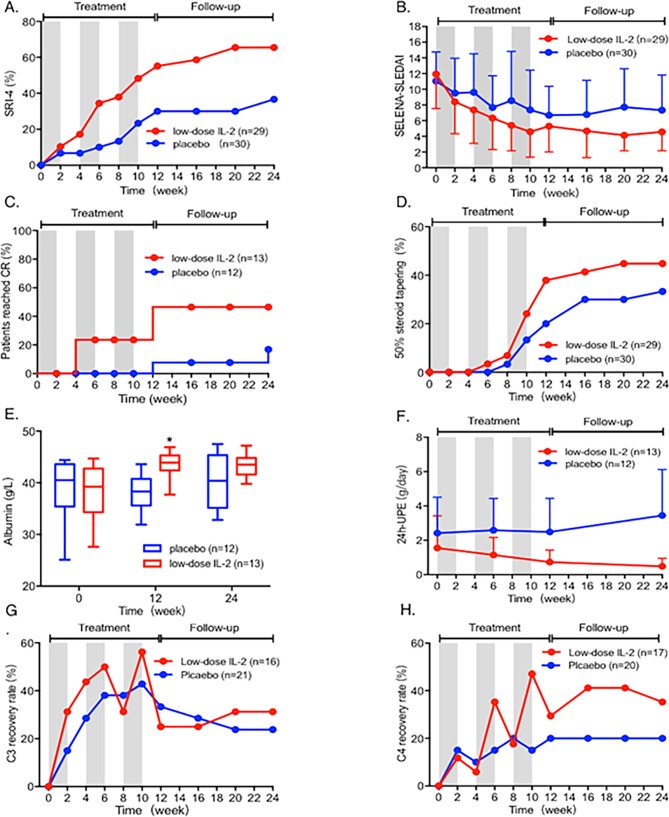
Improvements of clinical manifestations and laboratory parameters of patients with low-dose IL-2 therapy were shown in figure 2, table 2 and online supplementary table S3. The SRI-4 response rates of IL-2 and placebo groups at week 12 were 55.17% (16/29) and 30.00% (9/30), respectively. The primary endpoint on SRI-4 response was not achieved at week 12 (p=0.052). Notably, the response rates proceeded for further 12 weeks until the end of follow-up period at week 24. At this time point, the SRI-4 response rate was 65.52% (19/29) in low-dose IL-2 group and 36.67% (11/30) in the placebo group (p=0.027) (figure 2A). The response rate of IL-2 group was also significantly higher than that of placebo group at week 6, 8, 10 and 16 (p<0.05) and more reductions in SELENA-SLEDAI scores were observed in IL-2 group (figure 2A, online supplementary table S13, figure 2B).
Figure 2.
Clinical response of SLE patients after treatment with low-dose IL-2 and placebo. (A) The SRI-4 response rate of patients receiving low-dose IL-2 (red) or placebo (blue) treatment during the 12-week treatment and 12-week follow-up period. Grey areas indicate the periods on IL-2 or placebo therapy. (B) SELENA-SLEDAI scores during the 24 weeks. (C) Complete remission (CR) rate in patients with lupus nephritis. (D) Proportion of patients achieving corticosteroid reduction by ≥50% from baseline to 24 weeks. (E) Levels of albumin at week 0, 12 and 24. (F) Proteinuria per 24 hours (24-UPE) of patients with lupus nephritis from baseline to 24 weeks. (G, H) The percentages of patients achieving normal levels of C3 and C4 in the 24 weeks. *p<0.05. The actual data of the results are listed in online supplementary table S13–20 (online supplementary table S13 for (A), online supplementary table S14 for (B), online supplementary table S15 for (C), online supplementary file 1 for (D), online supplementary table S17 for (E), online supplementary table S18 for (F), online supplementary table S19 for (G) and online supplementary file 1 for (H)). SRI-4, SLE Responder Index-4.
As part of the clinical responses seen in the patients, the complete remission (CR) rate of LN was also significantly higher in the IL-2 group than the placebo group at both week 12 (53.85% vs 8.33%, p=0.013) and week 24 (53.85% vs 16.67%, p=0.036) (figure 2C, online supplementary table S5). Patients showed reduced 24-hour proteinuria in the low-dose IL-2 group from 1.55g at baseline to 0.48g at week 24 (p=0.002). In contrast, there was no significant change in 24-hour proteinuria in the placebo group (p=0.372) (figure 2F). Serum albumin was improved by IL-2 therapy in week 12 (p=0.046) and at the end of follow-up period in week 24 (p=0.017) (figure 2E). The levels of serum C3 and C4 were increased in the low-dose IL-2 group compared with the placebo group. And during the treatment period, more patients achieved normal levels of serum C3 and C4 in the low-dose IL-2 group than that in the placebo group (figure 2G and H).
As shown in table 2 and figure 2, clinical remission was accompanied by tapering corticosteroids in both groups. More reductions in corticosteroid were observed in the IL-2 group than in the placebo group. At week 24, 44.83% (13/29) of patients in the IL-2 group had reduced prednisone dose by ≥50%, compared with 33.33% (10/30) in the placebo group (figure 2D). Resolution of clinical manifestations present at baseline was observed in patients with IL-2 treatment, including rash (11/13), oral ulceration (4/4), arthritis (11/14), vasculitis (4/4), alopecia (7/12) and fever (3/3) (table 2, online supplementary table S4). In addition, anti-dsDNA antibody titres decreased in patients with IL-2 treatment, but not in the placebo group (table 2). Low-dose IL-2 treatment also resulted in improvements in the PGA and BILAG scores (table 2, online supplementary table S4). However, there was no significant difference between IL-2 group and placebo group (online supplementary table S4).
Perprotocol analysis was also performed with exclusion of patients lost in follow-up, and the results were similar to those obtained by mITT analysis.
Safety
Adverse events during the treatment period were shown in table 23. A lower incidence of infection was recorded in the IL-2 group (6.9%, 2/29) compared with the placebo group (20.0%, 6/30), but without statistical significance. No serious adverse events in IL-2 group were observed, while two patients in the placebo group had serious infections and were hospitalised (table 3). The most common adverse events were injection-site reactions, manifested as injection-site pain, redness and swelling, which were observed in 9 of 29 (31.0%) patients in the IL-2 group and 2 of 30 (6.7%) patients in the placebo group. Transient influenza-like symptoms and transient fever occurred in 3 (10.3%) and 4 (13.8%) patients in IL-2 group, respectively. These symptoms were resolved without intervention (table 3).
Table 3.
The adverse events during low-dose IL-2 treatment in SLE patients
| IL-2 (n=29) n (%) |
Placebo (n=30) n (%) |
|
| SAEs | 0 | 3 (10.0) |
| NPSLE | 0 | 1 (3.3) |
| Herpes zoster | 0 | 1 (3.3) |
| Pneumonia | 0 | 1 (3.3) |
| AEs | ||
| Infection | 2 (6.9) | 6 (20.0) |
| Upper respiratory infection | 2 (6.9) | 4 (13.3) |
| Severe infections | 0 | 2 (6.7) |
| Herpes zoster | 0 | 1 (3.3) |
| Pneumonia | 0 | 1 (3.3) |
| Injection site reactions | 9 (31.0) | 2 (6.7) |
| Fever | 4 (13.8) | 0 |
| Flu-like symptoms | 3 (10.3) | 0 |
.AE, adverse event; SAE, severe adverse events.
Immunological analysis
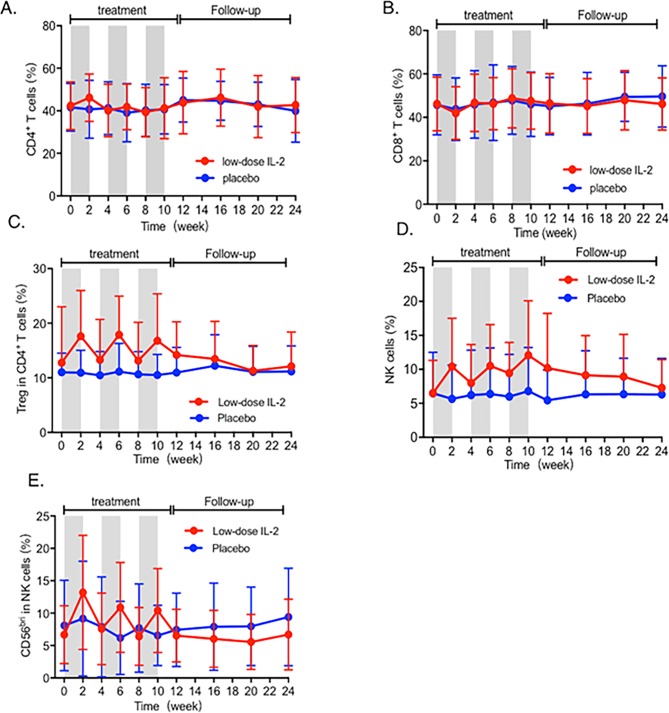
Flow cytometry analysis demonstrated low-dose IL-2 therapy induced a significant expansion of Treg cells (p<0.05), while total CD4+ and CD8+ T cells remained unchanged (figures 3A, B, C, online supplementary figure S2 and table S9). A significant increase of total NK cells was found after IL-2 therapy, from 6.48% at baseline to 12.07% at week 10 (p<0.01), while no obvious changes were detected in the placebo group (figure 3D, online supplementary figur S2 and table S9). Among NK cells, CD56bri NK subset increased with IL-2 therapy (p<0.05), which did not change significantly in the placebo group (figure 3E, online supplementary figure S2 and table S9).
Figure 3.
Dynamics of immune cell subsets in SLE during IL-2 treatment. (A, B, C) Changes in percentages of CD4+ T cell, CD8+ T cell and Treg cells at every visit. (D, E) Dynamics of total NK cells and CD56bri NK in SLE patients during 24 weeks. The actual data of the results are listed in online supplementary table S21. Treg, regulatory T.
To further verify the possible impact of IL-2 treatment on cell subsets involved in infectious immunity, a prospective, open-labelled observational study was conducted (NCT02932137). It showed that low-dose IL-2 activated NK cells and decreased viral titres in patients without antiviral therapy. The results of this study were described in online supplementary materials (online supplementary text; Online supplementary table S4, S6, S9, S11 and S12).
Discussion
After being molecularly cloned in 1983, IL-2 was utilised to treat patients with melanoma and other cancers. Due to its function in supporting T-cell proliferation, survival and effector differentiation, IL-2 treatment, when used in high doses, demonstrated efficacy in a fraction of patients. The approval of IL-2 therapy in certain types of solid tumours significantly contributed to the establishment of the concept of cancer immunotherapy.27
The management of active SLE is challenging due to the heterogeneous nature of the disease. Current therapy of active SLE relies primarily on corticosteroids and immunosuppressants to reduce disease activity. However, the incompletely effective outcomes achieved with these drugs are offset further by significant adverse effects, especially treatment-related infections.5 6 The concept of low-dose IL-2 therapy in autoimmunity and inflammation was inspired by the key role of IL-2 in the development and function of Treg cells, which are essential in maintaining immune tolerance. Instead of promoting immunity by high-dose IL-2, open-labelled trials suggested that low-dose IL-2 suppressed inflammation and autoimmunity in hepatitis C-associated vasculitis, graft-versus-host disease, type 1 diabetes, alopecia areata and SLE.11–13 17–20 Low-dose IL-2 treatment was reported safe and associated with clinical improvements in these studies. The benefit of low-dose IL-2 therapy is considered to be based on the expansion of immune tolerance-inducing Treg cells and suppression of effector T cells, including TFH cells and TH17 cells.11 Therefore, randomised and double-blind trials are expected clinically to formally evaluate the safety and efficacy of low-dose IL-2 treatment, which has the potential to become a new therapy to treat a broad range of inflammatory and autoimmune disorders refractory to current therapies.
In this study, we evaluated low-dose IL-2 therapy in a prospective, randomised, double-blind, placebo-controlled clinical trial in patients with active SLE despite standard therapy. The results showed that compared with the placebo group, SLE patients with active disease improved rapidly and significantly with low-dose IL-2 therapy. Remarkably, 65.52% of SLE patients reached SRI-4 response at the end of this study in low-dose IL-2 group, in comparison to 36.67% in the placebo group. Reduced SELENA-SLEDAI scores and resolution of clinical features were observed, along with decreased serological activities in the form of reduced autoantibodies and increased serum complements. The improvement of disease activities was observed across a wide range of SLE manifestations, including skin lesions, joint, fever, nephritis and permitted tapering of corticosteroid during the period of IL-2 treatment. It was clearly shown that the dose of corticosteroid could be reduced more during treatment with IL-2 than with placebo, which is clinically critical in SLE management.
We observed that 76.92% of patients achieved partial remission and 53.85% reached CR after IL-2 treatment (table 1) at week 12. In addition to reduced proteinuria, increased serum albumin was also observed. This placebo-controlled study also confirmed the therapeutic effects of low-dose IL-2 on LN reported in previous non-controlled studies.11 12 28 Since this study was not specifically designed to investigate the effects of IL-2 on LN and the number of patients with LN was limited, future studies on low-dose IL-2 treatment in LN or other autoimmune kidney diseases should be carried out.
One of the clinical observations in this study was the improvement in alopecia with the use of low-dose IL-2. The improvement of alopecia was of interest, given the recent report that low-dose IL-2 was effective in the treatment of severe alopecia areata.17Seven of 12 patients with alopecia in our study showed significant improvement with low-dose IL-2. Additionally, 11 of 13 patients with rash showed complete resolution of the skin lesion. This was in consistency with a recent study that demonstrated improvement in graft versus host disease (GVHD)-related erythema and scleroderma with low-dose IL-2.15 Therefore, low-dose IL-2 might be an option to treat autoimmune skin diseases.
Distinct from immunosuppressants and biologics which often increased infection incidence,29 low-dose IL-2 treatment was effective in SLE and was probably not accompanied with increased infection incidence. It has been reported that sustained expansion of Tregs by IL-2 inhibited autoimmunity in animal models without impairing immune responses to infection, vaccination and cancer.30 In this study, low-dose IL-2 was shown to expand Treg cells as well as NK cells, while total CD4+ and CD8+ T cells were not affected. Previous studies demonstrated that the function of NK cells was impaired in active SLE,31 and NK cells, especially the CD56bright NK subset, have been reported to be a regulatory controller of autoimmune responses, mainly by inhibiting T-cell proliferation through cytotoxic engagement and immunosuppressive cytokine expression.32 33 In this study, the CD56bright NK subset was preferentially expanded by low-dose IL-2, and might contribute to the alleviation of SLE autoimmunity together with expanded Treg cells. NK cells are also important in protection against viral infections.19 20 We observed significantly increased expression of IFNγ, NKp46 and NKG2D by NK cells in response to low-dose IL-2 treatment (online supplementary table S11), which implicated potential augmentation of anti-infectious cellular immunity. Clinically, in agreement with previous studies,11 we showed that low-dose IL-2 did not increase the incidence of infection, rather reduced the viral loads of BK and HPV viral loads in three SLE patients to undetectable level (online supplementary table S6). Whether low-dose IL-2 treatment could decrease viral loads in infected patients should be carried out in the future.
There were several limitations in this study, which might affect the outcome of the trial. First, no dose ranging comparison was designed. Although the current low-dose IL-2 dosage of 0.3–3 MIU/day was based on previous open-labelled trials,10 11 13–16 the optimal dosage of IL-2 therapy for individual disease remains to be determined. It is also highly possible that individuals might respond differently to dosage regimen. A better efficacy using optimal dosage regimen might be achieved, which can be tested in future clinical trials.
In this study, background treatments such as calcineurin inhibitors (CNIs) might affect the efficacy of low-dose IL-2 in our study. CNIs including cyclosporine or tacrolimus can impair the function of Treg cells while IL-2 was recently reported to restore the survival and suppressive properties of Tregs exposed to CNIs.34 Therefore, future clinical studies for low-dose IL-2 therapy should recruit a larger cohort and stratify patients based on background treatments, allowing analysing the therapeutic efficacy of low-dose IL-2 without the interference of background treatments.
We observed a more rapid disease improvement in IL-2 group. As the trial proceeded, more patients in IL-2 group improved significantly, and at week 24, the SRI-4 response rate of IL-2 group was 65.52%, compared with 36.67% of the placebo group (p=0.027). However, some severe manifestations such as nephritis were unable to be achieve complete remission in such a short period. Therefore, during the trial, neither of the groups achieved ‘Clinical Remission with No Treatment’ or ‘Clinical Remission On Treatment’ by the DORIS definition.35 Prolonged treatment should be considered in future studies to evaluate the efficacy of low-dose IL-2 to induce SLE disease remission by DORIS definition.
Collectively, the current study provided supportive evidence that low-dose IL-2 treatment might be effective and well tolerated in patients with SLE, which was supportive of further enlarged RCT studies with multiple patient cohorts from separate clinical centres.
Acknowledgments
We thank the patients for their participation in this study and their willingness to share the scientific data resulting from this clinical trial.
Footnotes
Handling editor: Josef S Smolen
JH, RZ and MS contributed equally.
Contributors: JH, DY, XS and ZL initiated the investigation, led the clinical experiments, and wrote, reviewed and edited the manuscript. RZ, MS, XS, JH, DY and YW obtained and analysed the data and wrote, edited and reviewed the manuscript. JA, JA, AJ obtained data and wrote, edited and reviewed the manuscript. YW provided statistical guidance prior to study implementation, conducted statistical analysis, and edited and reviewed the manuscript. XZ, JC, YJ, XL, XZ, CL, YZ, YY, HY, YL and YS implemented the double-controlled study and reviewed and edited the manuscript. SZ, LZ, RJ, XZ, NS, JG and XL contributed to the design and implemented FACS experiments of the study. NS reviewed and edited the manuscript. All authors gave final approval of the manuscript version to be published.
Funding: The work was supported by the National Natural Science Foundation of China (31530020,31570880,81471601,81601417 and 81701598), Peking-Tsinghua Center for Life Sciences to ZG LI, Beijing Sci-Tech Committee Z171100000417007, Clinical Medicine Plus X-Young Scholars Project of Peking University (PKU2019LCXQ013) supported by the Fundamental Research Funds for the Central Universities, Beijing Nova Program Z171100001117025, National Key Research and Development Program of China (2017YFC0909003 to DY), Bellberry-Viertel Senior Medical Research Fellowship to DY and Beijing SL PHARM.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: Peking University People’s Hospital Ethics Committee approved the protocol and all patients provided written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Ugarte-Gil MF, Wojdyla D, Pons-Estel GJ, et al. Remission and low disease activity status (LDAS) protect lupus patients from damage occurrence: data from a multiethnic, multinational Latin American lupus cohort (GLADEL). Ann Rheum Dis 2017;76:2071–4. 10.1136/annrheumdis-2017-211814 [DOI] [PubMed] [Google Scholar]
- 2. Morand EF, Mosca M. Treat to target, remission and low disease activity in SLE. Best Pract Res Clin Rheumatol 2017;31:342–50. 10.1016/j.berh.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 3. Franklyn K, Hoi A, Nikpour M, et al. The need to define treatment goals for systemic lupus erythematosus. Nat Rev Rheumatol 2014;10:567–71. 10.1038/nrrheum.2014.118 [DOI] [PubMed] [Google Scholar]
- 4. Bruce IN, Urowitz M, van Vollenhoven R, et al. Long-Term organ damage accrual and safety in patients with SLE treated with belimumab plus standard of care. Lupus 2016;25:699–709. 10.1177/0961203315625119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldblatt F, Chambers S, Rahman A, et al. Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus 2009;18:682–9. 10.1177/0961203308101019 [DOI] [PubMed] [Google Scholar]
- 6. Kang I, Park SH. Infectious complications in SLE after immunosuppressive therapies. Curr Opin Rheumatol 2003;15:528–34. 10.1097/00002281-200309000-00002 [DOI] [PubMed] [Google Scholar]
- 7. Lieberman LA, Tsokos GC. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. Journal of Biomedicine and Biotechnology 2010;2010:1–6. 10.1155/2010/740619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Humrich JY, Morbach H, Undeutsch R, et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci U S A 2010;107:204–9. 10.1073/pnas.0903158107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011;365:2110–21. 10.1056/NEJMra1100359 [DOI] [PubMed] [Google Scholar]
- 10. Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 2015;15:283–94. 10.1038/nri3823 [DOI] [PubMed] [Google Scholar]
- 11. He J, Zhang X, Wei Y, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med 2016;22:991–3. 10.1038/nm.4148 [DOI] [PubMed] [Google Scholar]
- 12. von Spee-Mayer C, Siegert E, Abdirama D, et al. Low-Dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis 2016;75:1407–15. 10.1136/annrheumdis-2015-207776 [DOI] [PubMed] [Google Scholar]
- 13. Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 2011;365:2067–77. 10.1056/NEJMoa1105143 [DOI] [PubMed] [Google Scholar]
- 14. Koreth J, Matsuoka K-ichi, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011;365:2055–66. 10.1056/NEJMoa1108188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuoka K-ichi, Koreth J, Kim HT, et al. Low-Dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 2013;5:179ra43–179r. 10.1126/scitranslmed.3005265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartemann A, Bensimon G, Payan CA, et al. Low-Dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. The Lancet Diabetes & Endocrinology 2013;1:295–305. 10.1016/S2213-8587(13)70113-X [DOI] [PubMed] [Google Scholar]
- 17. Castela E, Le Duff F, Butori C, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol 2014;150:748–51. 10.1001/jamadermatol.2014.504 [DOI] [PubMed] [Google Scholar]
- 18. Blattman JN, Grayson JM, Wherry EJ, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 2003;9:540–7. 10.1038/nm866 [DOI] [PubMed] [Google Scholar]
- 19. Zwirner NW, Domaica CI. Cytokine regulation of natural killer cell effector functions. Biofactors 2010;36:274–88. 10.1002/biof.107 [DOI] [PubMed] [Google Scholar]
- 20. Littwitz-Salomon E, Dittmer U, Sutter K. Insufficient natural killer cell responses against retroviruses: how to improve NK cell killing of retrovirus-infected cells. Retrovirology 2016;13:77 10.1186/s12977-016-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang R, Xi X, Wang C, et al. Therapeutic effects of recombinant human interleukin 2 as adjunctive immunotherapy against tuberculosis: a systematic review and meta-analysis. PLoS One 2018;13:e201025 10.1371/journal.pone.0201025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism 1997;40:1725 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 23. Furie RA, Petri MA, Wallace DJ, et al. Novel evidence-based systemic lupus erythematosus Responder index. Arthritis Rheum 2009;61:1143–51. 10.1002/art.24698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria The American College of rheumatology response criteria for systemic lupus erythematosus clinical trials: measures of overall disease activity. Arthritis Rheum 2004;50:3418–26. 10.1002/art.20628 [DOI] [PubMed] [Google Scholar]
- 25. Zavada J, Pesickova S, Ryšava R, et al. Cyclosporine A or intravenous cyclophosphamide for lupus nephritis: the Cyclofa-Lune study. Lupus 2010;19:1281–9. 10.1177/0961203310371155 [DOI] [PubMed] [Google Scholar]
- 26. Currier JR, Kuta EG, Turk E, et al. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J Immunol Methods 2002;260:157–72. 10.1016/S0022-1759(01)00535-X [DOI] [PubMed] [Google Scholar]
- 27. Rosenberg SA. Il-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451–8. 10.4049/jimmunol.1490019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suárez-Fueyo A, Bradley SJ, Klatzmann D, et al. T cells and autoimmune kidney disease. Nat Rev Nephrol 2017;13:329–43. 10.1038/nrneph.2017.34 [DOI] [PubMed] [Google Scholar]
- 29. He J, Li Z. An era of biological treatment in systemic lupus erythematosus. Clin Rheumatol 2018;37:1–3. 10.1007/s10067-017-3933-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Churlaud G, Jimenez V, Ruberte J, et al. Sustained stimulation and expansion of Tregs by IL2 control autoimmunity without impairing immune responses to infection, vaccination and cancer. Clin Immunol 2014;151:114–26. 10.1016/j.clim.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 31. Hervier B, Beziat V, Haroche J, et al. Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-γ production in patients with active disease. Arthritis Rheum 2011;63:1698–706. 10.1002/art.30313 [DOI] [PubMed] [Google Scholar]
- 32. Pallmer K, Oxenius A. Recognition and regulation of T cells by NK cells. Front Immunol 2016;7:251 10.3389/fimmu.2016.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gross CC, Schulte-Mecklenbeck A, Wiendl H, et al. Regulatory functions of natural killer cells in multiple sclerosis. Front Immunol 2016;7:606 10.3389/fimmu.2016.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whitehouse G, Gray E, Mastoridis S, et al. Il-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc Natl Acad Sci U S A 2017;114:7083–8. 10.1073/pnas.1620835114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Vollenhoven R, Voskuyl A, Bertsias G, et al. A framework for remission in SLE: consensus findings from a large international Task force on definitions of remission in SLE (DORIS). Ann Rheum Dis 2017;76:554–61. 10.1136/annrheumdis-2016-209519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2019-215396supp001.pdf (23.1MB, pdf)