Abstract
Coordinated brain activity between individuals, or inter-brain synchrony, has been shown to increase during cooperation and correlate with cooperation success. However, few studies have examined parent-child inter-brain synchrony and whether it is associated with meaningful aspects of the parent-child relationship. Here, we measured inter-brain synchrony in the right prefrontal (PFC) and temporal cortices in mother-child dyads while they engaged in a cooperative and independent task. We tested whether inter-brain synchrony in mother-child dyads (1) increases during cooperation, (2) differs in mother-son versus mother-daughter dyads, and (3) is related to cooperation performance and the attachment relationship. Overall inter-brain synchrony in the right hemisphere, and the right dorsolateral and frontopolar PFC in particular, was higher during cooperation. Mother-son dyads showed less inter-brain synchrony during the independent task and a stronger increase in synchrony in response to cooperation than mother-daughter dyads. Lastly, we did not find strong evidence for links between inter-brain synchrony and child attachment. Mother-child cooperation may increase overall inter-brain synchrony, although differently for mother-son versus mother-daughter dyads. More research is needed to better understand the potential role of overall inter-brain synchrony in mother-child cooperation, and the potential link between inter-brain synchrony and attachment.
Keywords: inter-brain synchrony, fNIRS hyperscanning, cooperation, mother-child attachment
Recent social neuroscience models have proposed moving beyond studying neural processes at the within-individual level to considering coordinated neural activity across multiple individuals during social encounters (Bilek et al., 2015; Schilbach et al., 2013). Thus, there is growing interest in studying when and how neural processes become synchronized, or temporally matched, between two or more people (Babiloni & Astolfi, 2014; Hasson et al., 2012), and whether this inter-brain synchrony may play a role in facilitating positive social interactions such as successful cooperation (Cui, Bryan, & Reiss, 2012; Tang et al., 2016). Research on inter-brain synchrony might be particularly important for understanding family relationships, as temporal matching in parent-child physiology and behavior is thought to underpin the attachment relationship (Feldman, 2017). However, few studies have directly examined inter-brain synchrony between parents and their children, potentially due to the difficulty of using some neuroimaging technologies with younger populations (e.g., functional magnetic resonance imaging - fMRI) and difficulty in pairing these technologies with social tasks that involve interaction with a partner. In addition, few studies have considered possible contexts that might modulate parent-child synchrony or investigated whether inter-brain synchrony is related to meaningful aspects of the parent-child relationship. In this study, we used functional near-infrared spectroscopy (fNIRS) to measure simultaneous brain activity (i.e., hyperscanning) in mother-child dyads during cooperation. We tested whether (1) inter-brain synchrony in mothers and their children increases during a cooperative relative to a non-cooperative task, (2) whether this increase differs for mother-son versus mother-daughter dyads, and (3) whether the synchrony is related to how well the dyad cooperates behaviorally and the quality of their attachment relationship.
Brain regions in the fronto-temporal network are critically involved in social cognitive processing (Frith & Frith, 2010; Hastings, Miller, Kahle, & Zahn-Waxler, 2014) and may be particularly prone to synchronization across individuals engaged in social interaction (Dikker, Silbert, Hasson, & Zevin, 2014; Jiang et al., 2012; Liu, Saito, Lin, & Saito, 2017; Tang et al., 2016). Adult dyads have been shown to increase synchrony in the right temporoparietal junction (TPJ) when participating in cooperative and face to face economic exchange games (Liu et al., 2016; Tang et al., 2016), and this right TPJ synchrony has been linked to greater shared intentionality between partners (Tang et al., 2016). In addition, inter-brain synchrony in prefrontal cortex (PFC) regions such as the superior and dorsolateral PFC has been linked to positive interpersonal outcomes including successful cooperation (Cui et al., 2012), effective communication (Stephens, Silbert, & Hasson, 2010), and perceived similarity between two individuals (Hu, Hu, Li, Pan, & Cheng, 2017). Taken together, there is increasing evidence that inter-brain synchrony in frontal and temporoparietal regions reflects a shared neural mechanism, or consequence, of prosocial interactions.
Biological and behavioral synchrony between humans first emerges in the context of the mother-child attachment relationship (Feldman, 2017). Mother-child dyads have been shown to coordinate their behaviors (e.g., social gaze), affect, and autonomic activity (e.g., heart rate), and the degree of this coordination is thought to consolidate their bond and promote a secure attachment relationship (Feldman, 2015, 2017; Leclere et al., 2014). Children develop secure attachment relationships when parents are sensitive and responsive to their needs, whereas harsh or inconsistent parenting contributes to the development of avoidant or anxious attachment orientations (Vrtička, 2017). To the degree that biological synchrony between individuals is an interpersonal marker of relationship quality, avoidant and anxious attachments should be linked to decreased biological matching between partners. For example, in a hyperscanning EEG study with romantic couples, Kinreich and colleagues (2017) found that anxious attachment status in males was negatively associated with TPJ gamma rhythm synchronization with a partner during naturalistic social interaction, suggesting that insecure attachment styles may interfere with inter-brain synchrony in the context of close relationships. Thus, it seems reasonable to expect that children with more avoidant or anxious attachments to their mothers would demonstrate less inter-brain synchrony.
Researchers have used several methodological tools to study the links between attachment-related constructs and behavioral (Leclere et al., 2014), autonomic (Ebisch et al., 2012), and hormonal (Papp, Pendry, & Adam, 2009; Saxbe et al., 2017) facets of parent-child synchrony across different temporal scales. Overall, however, studies of parent-child synchrony at the level of the brain have been rare, perhaps due in part to the physical and temporal constraints of popular neuroimaging technologies such as fMRI. fNIRS is one neuroimaging technique that allows for simultaneous acquisition of continuous brain activity across two individuals (i.e., hyperscanning), and is suitable for use with pediatric populations. Although fNIRS is increasingly being utilized in adult hyperscanning research (Baker et al., 2016; Cui et al., 2012; Nozawa et al., 2016), and has been proposed as a potentially useful tool for studying parent-child attachment (Vrtička, 2017), we are only aware of one recently published fNIRS study of parent-child inter-brain synchrony (Reindl, Gerlof, Scharke, & Konrad, 2018), which also focused on cooperation. In this study, inter-brain synchrony in the dorsolateral and frontopolar PFC regions was associated with cooperation and children’s emotion regulation skills (Reindl et al., 2018).
Lastly, some research suggests that the relationship and interactions between mothers and daughters versus mothers and sons may differ (Kerig, Cowan, & Cowan, 1993; Russel & Saebel, 1997). For example, parents have been found to encourage traditionally sex-typed activities (Lytton & Romney, 1991) and more risk-taking in sons and caution in daughters (Morrongiello & Dawber, 1999). In terms of synchrony, mother-son dyads have been found to be more coordinated in their behavioral interactions and autonomic activity than mother-daughter dyads (Helm, Miller, Kahle, Troxel, & Hastings, 2018; Tronick & Cohn, 1989). We were interested in examining whether mother-daughter and mother-son dyads might demonstrate differences in their inter-brain synchrony. Interestingly, recent research on adults has uncovered differences in inter-bran synchrony during cooperation in mixed-sex and same-sex dyads, indicating that males and females may rely on different strategies for cooperation. Cheng and colleagues (2015) found evidence for cooperation-related inter-brain synchrony in the PFC in mixed-sex dyads but not same-sex dyads. Conversely, Baker and colleagues (2016) did not find significant inter-brain synchrony during cooperation in mixed-sex dyads but found evidence that male-male and female-female dyads exhibited synchrony in different parts of the brain (right prefrontal and right temporal cortices, respectively). Similarly, Zhang and colleagues found that female, but not male, dyads demonstrated increased synchrony in temporoparietal cortices during social interaction (2017a, 2017b). Whether the observation of sex differences in inter-brain synchrony between adult strangers also extends to parent-child dyads, however, has not yet been tested.
The current study used fNIRS hyperscanning to assess mother-child inter-brain synchrony during a cooperative and non-cooperative computer-based task. We expected inter-brain synchrony to increase in the right temporoparietal cortex and right PFC during the cooperative task compared to the non-cooperative control task. In addition, given the mixed findings from recent research suggesting differences in inter-brain synchrony during cooperation in same-sex and mixed-sex dyads (Baker et al., 2016; Cheng et al., 2015), we explored whether increases in inter-brain synchrony differed in mother-son versus mother-daughter dyads. Finally, we hypothesized that stronger increases in inter-brain synchrony during cooperation would be related to better performance in the task and less insecure mother-child attachment.
Methods
Participants
This study initially included 30 mother-child dyads (16 girls, 14 boys) who were recruited from the local community to participate in a study of the neural correlates of attachment in late childhood. This study required children to undergo an fMRI scan, and mother-child dyads to participate in an fNIRS hyperscanning session. The present analyses focus on data from the mother-child fNIRS session. Two dyads were dropped from analyses due to unusable behavioral or fNIRS data. Thus, the final sample included 28 mother-child dyads (15 girls, 13 boys). Mothers’ age ranged from 38.89 to 56.82 years (M = 45.93, SD = 3.76). Children’s age ranged from 8.05 to 12.93 years (M = 11.17, SD = 1.27). In the final sample, two mother-child pairs were not biologically related (e.g., child was biological son of same-sex partner or was conceived through a donor egg), but in both cases the participating parent was involved in childcare from birth.
Cooperation and independent task conditions
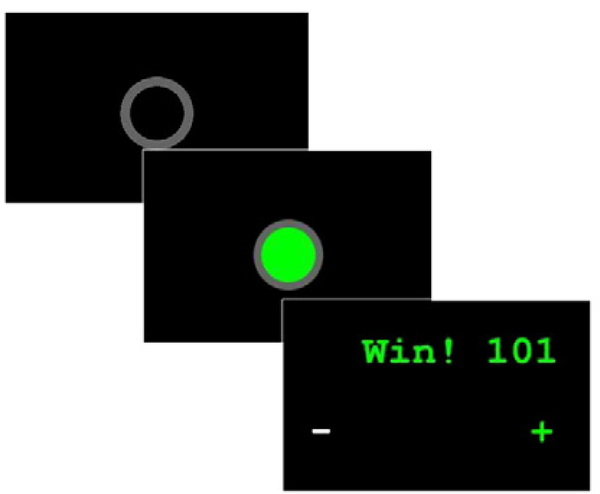
Mother-child dyads participated in a computer-based task to assess cooperation and inter-brain synchrony (Baker et al., 2016; Cui et al., 2012). Mothers and their children were seated in front of separate computer screens and keyboards placed on opposite sides of a table. Mothers and children could not see each other or make eye contact, and were instructed to not talk to each other during any part of the task. At the start of each trial, an empty gray circle against a black background was presented on both computer screens. After a random duration of 0.5 to 12 s, the empty gray circle filled with green, signaling to mothers and children to press a button on their keyboards. Mother-child dyads attempted to match their responses in time. If the difference in time between mothers’ and children’s responses was less than ½ of the sum of their response times, then the dyad earned a point. If the difference between response times exceeded this threshold, then the dyad lost a point. Each dyad started with 100 points and was instructed to maximize earned points. Following the slower participant’s response, a feedback screen was presented to both participants showing whether they won or lost, and a plus and minus sign on the left side of the screen indicated to participants whether they responded faster or slower than the other dyad member. Mother-child dyads could use this feedback to adjust the timing of their responses on subsequent task trials. Figure 1 presents the sequence of events for each trial. The cooperation condition consisted of 40 trials separated into two 20 trial blocks with a 30 s rest period in between. Behavioral performance for each mother-child dyad was calculated as the proportion of trials that were wins.
Figure 1. Trial Stimulus Sequence.
Screenshots of the ready signal, “go” signal to initiate mother and child response, and feedback window.
An independent, non-cooperative version of the task was used as a control condition. This task took place in the same room under the same conditions as the cooperative task. Prior to the task, participants were instructed that they would play the game independent of each other. In this version of the task, the screen with the green circle signaled to mothers and children to press their buttons as fast as possible. These cues were presented to both partners at the same time. Each partner started with 100 points and won a point for correct responses and lost a point for every early response (i.e., before seeing the green circle). Participants did not receive feedback regarding whether they were faster or slower than their partner, and their performance did not impact whether their partner gained or lost points.
The presentation of the cooperation and independent conditions were counterbalanced across dyads. Prior to starting the first task condition, participants were told that their overall performance on the cooperation and independent tasks would be scored and compared with other players.
fNIRS data acquisition
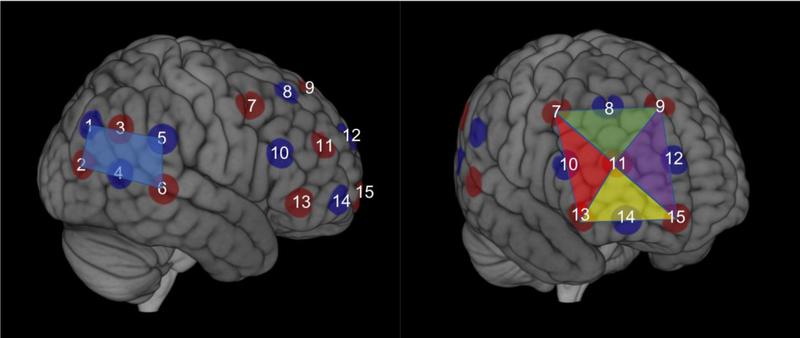
Changes in cortical oxygenated (HbO) and de-oxygenated (HbR) hemoglobin were measured in mothers and children simultaneously using an ETG-4000 (Hitachi, Japan) Optical Topography system. The optode arrangement was identical to those used in previous studies on inter-brain synchrony and cooperation from our group (Baker et al., 2016; Liu et al., 2016). More specifically, for each dyad member a 3 × 3 (i.e., 12 channels) optode patch was placed on the right forehead to measure right prefrontal cortex hemodynamic activity. The inside edge of the 3 × 3 patch was aligned to the midline (i.e., the arc running from the nasion to the inion) and the bottom edge was placed directly over the brow. In addition, a 2 × 3 (i.e., 7 channels) optode patch was placed on the right side of the head to measure right temporoparietal cortex hemodynamic activity. The front edge of the 2 × 3 patch was placed over the right ear and the bottom edge of the patch directly above T4 based on the 10–20 system. Within each mother-child dyad, placement of patches was examined and adjusted to ensure similar placement of optodes. Table 1 presents the average MNI coordinates of the optodes across all participants, which were estimated using data from a 3D digitizer and a probabilistic registration tool developed by Singh and colleagues (2005), and Figure 2 presents the average ROI and optode MNI locations across all participants. The fNIRS data were collected at a sampling rate of 10 Hz.
Table 1. Estimated MNI coordinates for optodes using probabilistic registration (Singh et al., 2005).
The optode numbers correspond with those presented in Figure 2.
| MNI Coordinates | |||
|---|---|---|---|
| Optode | X | Y | Z |
| 1 | 44 | −77 | 38 |
| 2 | 48 | −82 | 16 |
| 3 | 58 | −61 | 37 |
| 4 | 62 | −62 | 12 |
| 5 | 67 | −37 | 32 |
| 6 | 71 | −37 | 5 |
| 7 | 50 | 11 | 50 |
| 8 | 30 | 31 | 54 |
| 9 | 3 | 42 | 53 |
| 10 | 57 | 28 | 23 |
| 11 | 39 | 52 | 26 |
| 12 | 10 | 64 | 27 |
| 13 | 55 | 39 | −5 |
| 14 | 39 | 61 | −5 |
| 15 | 13 | 70 | −5 |
Figure 2. fNIRS Optodes and Regions of Interest.
The estimated average location of source (red circles) and detector (blue circles) optodes based on 3D digitizer data and plotted in MNI space. The average MNI coordinates for each optode are listed in Table 1. The ROIs were established a priori for the right inferior (yellow triangle), right dorsolateral (red triangle), right superior (green triangle), and right frontopolar prefrontal cortex (purple triangle), as well as right temporoparietal cortex (blue rectangle).
fNIRS data analysis
In accord with previous fNIRS hyperscanning studies from our group, we chose to examine inter-brain synchrony in a specific middle wavelength task frequency and filtered out data in low- and high-frequency bands (see below) (Baker et al., 2016; Cui et al., 2012). Motion artifacts were corrected using a wavelet-based artifact removal method (Molavi & Dumont, 2012). All data were visually inspected in time-series plots for excessive noise and identification of channels to be excluded from subsequent analyses. We chose to focus on the HbO data given that this signal is more robust than HbR and more comparable to BOLD signal captured using fMRI. We used the wavelet transform coherence (WTC) package in Matlab (Grinsted et al., 2004) to apply continuous wavelet transformation to the motion corrected HbO data and estimate inter-brain synchrony in mother-child dyads. WTC, which measures cross-correlations as a function of frequency and time, can identify locally phase-locked behavior between two HbO signals (Baker et al., 2016; Cui et al., 2012; Liu et al., 2016). Each channel was paired with the identical channel in the opposite member of the dyad. We were interested in inter-brain synchrony occurring at the frequency of the task. Thus, for each pair of channels, the mean of all inter-brain synchrony values between the periods of 3.2 and 12.8 s (our task frequency) was computed for each cooperation and independent task block. In other words, we interpreted task-related inter-brain synchrony as the magnitude of correlations between corresponding HbO signals that were specific to HbO fluctuations occurring at the frequency of our tasks. For a more in-depth explanation of WTC, please see Grinsted et al. (2004) and Chang and Glover (2010). For HbO data acquired form the 3 × 3 patch over the right forehead, we formed four regions of interest (ROIs) (i.e., each containing 3 channels) corresponding to the right inferior, right dorsolateral, right superior, and right frontopolar PFC. For HbO data acquired from the 2 × 3 patch, all channels were averaged together to create a ROI for the right temporoparietal cortex. Thus, our ROIs were defined a priori based on the location of optodes and channels in each patch (Baker et al., 2016). Within each of the five ROIs, inter-brain synchrony for each pair of channels was averaged together (Baker et al., 2016).
Attachment measures
Children reported on their attachment to their mothers using the Experiences in Close Relationships-Revised Child Questionnaire (ECR-RC; Brenning, Soenens, Braet, & Bosmans, 2011). The anxious and avoidant subscale items were averaged to form indices of anxious (e.g., “I’m worried that my mother doesn’t really love me”, α = .84) and avoidant attachment (e.g., “It’s not easy for me to tell my mother a lot about myself”, α = .91).
Analysis strategy
We used repeated measures analysis of variance (ANOVA) to examine whether inter-brain synchrony significantly increased from the independent to the cooperation task conditions. Given that previous research found evidence for cooperation-related synchrony increases in specific ROIs (Baker et al., 2016), we also conducted planned comparisons of the effects of task condition on inter-brain synchrony for each of the five ROIs. For planned comparisons (both within ROIs as well as post-hoc pairwise comparisons within ANOVAs), we included False Discovery Rate (FDR) correction to provide a more conservative estimate controlling for multiple comparisons. Given previous research suggesting sex-differences in inter-brain synchrony in specific ROIs during cooperation (Baker et al., 2016; Cheng et al., 2015), we furthermore tested for differences in synchrony increase in mother-son versus mother-daughter dyads by including child age and sex as a covariate and between-subjects factor, respectively, in the ANOVA, and performed planned comparisons for synchrony increases within specific ROIs. We subsequently used two approaches to examine whether inter-brain synchrony was related to dyadic behavioral performance during the cooperation task and measures of attachment. First, we included behavioral performance and attachment as covariates in an additional ANOVA model. Second, we conducted bivariate correlations. For this correlation analysis, cooperation-related increases in inter-brain synchrony in each ROI was defined as the average synchrony during the cooperation task condition minus the average synchrony during the independent task condition. Thus, positive values in the difference scores reflect increases in mother-child inter-brain synchrony from the independent to the cooperation task conditions. Lastly, we used an ANOVA approach to examine whether inter-brain synchrony changed over the course of the independent or cooperation task conditions.
Results
Behavioral Measures
Descriptive statistics for cooperation performance, anxious and avoidant attachment to mother, and response times in the cooperation and independent task conditions are presented in Table 2. Anxious and avoidant attachment scores were positively correlated (r = .59, p = .001), and older children reported less avoidant attachment (r = −.55, p = .002). Girls and boys did not differ in terms of attachment, and cooperation performance and response times did not differ between mother-son and mother-daughter dyads (all p > .175). Reaction times in the independent compared to cooperation task did not differ for mothers or children (both p > .243).
Table 2. Descriptive Statistics for Cooperation Performance and Attachment.
Statistics for response time variables (RT) are presented in seconds.
| Whole Sample | Mother-Son Dyads | Mother-Daughter Dyads | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | M | SD | Range | |
| Cooperation Performance | .59 | .14 | .30–.85 | .57 | .16 | .30–.80 | .61 | .13 | .38–.85 |
| Anxious Attachment | 2.04 | .81 | 1–4.06 | 2.27 | .82 | 1.11–3.78 | 1.85 | .77 | 1.00–4.06 |
| Avoidant Attachment | 2.52 | 1.07 | 1–4.61 | 2.76 | .96 | 1.28–4.61 | 2.31 | 1.15 | 1.00–4.61 |
| Child RT in Cooperation Task | .39 | .12 | .27–.91 | .41 | .08 | .28–.91 | .37 | .08 | .27–.56 |
| Child RT in Independent Task | .42 | .14 | .30–.95 | .46 | .08 | .31–.95 | .39 | .08 | .30–.63 |
| Mother RT in Cooperation Task | .39 | .14 | .27–1.02 | .35 | .07 | .29–1.02 | .35 | .07 | .27–.55 |
| Mother RT in Independent Task | .39 | .10 | .26–.71 | .39 | .10 | .26–.71 | .39 | .10 | .26–.61 |
Inter-brain synchrony in block 1 versus block 2 of the independent and cooperation task conditions
To test whether inter-brain synchrony significantly changed over the course of the independent condition, or over the course of the cooperation condition, we conducted a 2 (condition: cooperation versus independent) x 5 (ROI) x 2 (block) repeated measures ANOVA. We did not find any significant interactions with the factor block (all p > .432). Thus, inter-brain synchrony did not appear to differ across blocks within the independent and cooperation task conditions. We therefore collapsed the data from block 1 and block 2 for all remaining analyses.
Inter-brain synchrony increase during cooperation
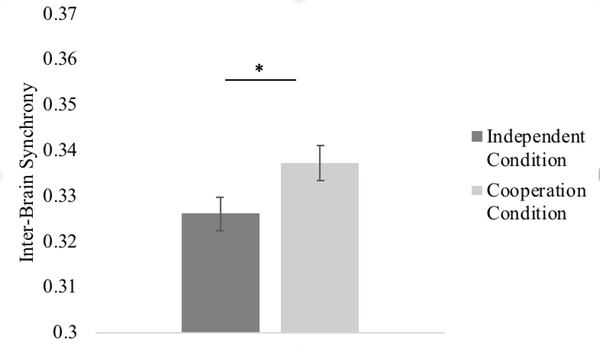
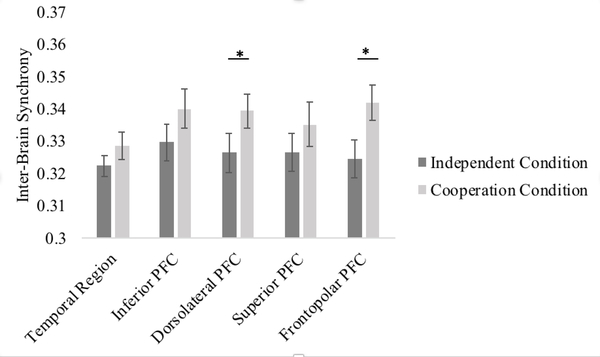
To analyze the effect of cooperation on inter-brain synchrony, we first conducted a 2 (condition: cooperation versus independent) x 5 (ROI) repeated measures ANOVA. There was a significant main effect of condition, F(1, 27) = 7.47, p = .011, partial η2 = .22, suggesting that, overall, mother-child dyads showed greater inter-brain synchrony during the cooperation condition than during the independent condition (see Figure 3). The main effect of ROI was not significant, F(1,27) = 0.89, p = .484. In addition, although the overall interaction between condition and ROI was not significant, F(1,27) = 0.77, p = .556, we conducted planned comparisons in accordance with previous literature using pairwise t-tests to analyze potential synchrony increase within specific ROIs (see Figure 4). Mother-child dyads showed greater inter-brain synchrony during the cooperation than independent conditions in the right dorsolateral, t(27) =2.15, p = .041, and right frontopolar PFC t(27) = 2.62, p = .014 (p = .103 and p = .070, respectively, after FDR correction). Inter-brain synchrony during the cooperation and independent conditions did not significantly differ in the right temporoparietal cortex, right inferior, or superior PFC (all p’s > .183, uncorrected).
Figure 3. Inter-brain Synchrony during the Independent versus Cooperation Conditions.
* p < .05. Error bars represent one standard error of the means.
Figure 4. ROI specific Inter-brain Synchrony during the Independent versus Cooperation Conditions.
* p < .05 uncorrected. Error bars represent one standard error of the means.
Sex-differences in inter-brain synchrony
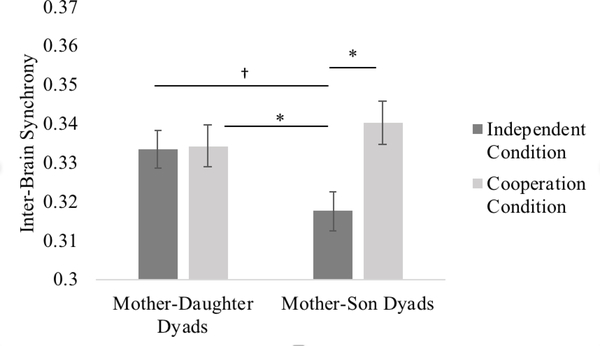
To test for differences in inter-brain synchrony between mother-son and mother-daughter dyads, child age and sex were added as a covariate and between-subject factor, respectively, to the ANOVA model. The main effect of condition (i.e., higher overall inter-brain synchrony during the cooperation than independent condition) remained statistically significant, F(1, 25) = 10.55, p = .001, partial η2 = .30, and there was a statistically significant interaction between child sex and condition, F(1, 25) = 7.57, p = .010, partial η2 = .24. Subsequent follow-up analyses revealed that the main effect of condition (i.e., higher overall inter-brain synchrony during the cooperation than independent condition) was only significant for mother-son dyads, mean difference = .02, p = .004, FDR p = .008, CI(95%) = [.01, .04], but not mother-daughter dyads, mean difference = .00, p = .702 , CI(95%) = [−.01, .01]. Furthermore, while mother-son and mother-daughter dyads differed in synchrony during the independent condition, mean difference = .02, p = .024 , FDR p = .048, CI(95%) = [.00, .03], no difference was present during cooperation, mean difference = .00, p = .506 , CI(95%) = [−.01, .02] (see Figure 5).
Figure 5. Inter-brain Synchrony during the Independent versus Cooperation Conditions for Mother-Daughter and Mother-Son Dyads separately.
† p = .05, * p < .05. Error bars represent one standard error of the means.
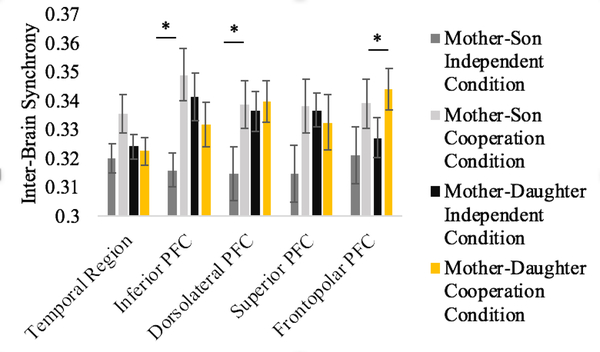
Previous research has found sex differences in cooperation-related inter-brain synchrony increase within specific ROIs (Baker et al., 2016). Thus, although the interaction between sex, ROI, and condition was not significant, F(4, 22) = 1.54, p = .211, we conducted planned comparisons to test sex-differences in synchrony increase within specific ROIs separately. Only the right inferior PFC showed a significant sex by condition interaction, F(1, 25) = 8.23, p = .006, FDR p = .024, partial η2 = .26. This interaction came about because (1) mother-son and mother-daughter dyads significantly differed in inter-brain synchrony during the independent condition, mean difference = .02, p = .024, FDR p = .048, CI(95%) = [.00, .05], while no such difference was present during the cooperation condition, mean difference = .02, p = .157, FDR p =.157, CI(95%) = [−.01, .04], and (2) mother-son dyads demonstrated a significant increase in inter-brain synchrony from the independent to the cooperation condition, mean difference = .03, p = .008, FDR p = .040, CI(95%) = [.01, .06], while no such effect was present for mother-daughter dyads, mean difference = .01, p = .365, FDR p = .365, CI(95%) = [−.02, 01].
We then examined inter-brain synchrony in mother-son and mother-daughter dyads separately (see Figure 6). In mother-son dyads, there was evidence for overall increased synchrony during cooperation in all 5 ROIs, but the effects only reached significance (uncorrected) in the right inferior PFC (t(12) =3.19, p = .008, FDR p = .016) and right dorsolateral PFC, (t(12) =2.22, p = .047, FDR p = .118). Conversely, in mother-daughter dyads, increased synchrony was only evident in the right frontopolar PFC (t(14) =2.87, p = .012, FDR p = .060).
Figure 6. ROI specific Inter-brain Synchrony during the Independent versus Cooperation Conditions for Mother-Son Dyads (left) and Mother-Daughter Dyads (right).
* p < .05 uncorrected. Error bars represent one standard error of the means.
Child age did not significantly moderate the effect of task condition on overall synchrony increase, the effect of ROI on overall synchrony level across task conditions, or the interaction between ROI and task condition (all p > .078).
Individual differences in behavior and inter-brain synchrony
We first examined whether there were any inter-individual differences related to child attachment avoidance and anxiety in behavioral cooperation performance. These analyses, however, revealed that dyads who performed better on the cooperation task did not differ in anxious (r = −.29, p = .128) or avoidant child attachment (r = −.31, p = .106). We subsequently conducted ANOVAs with cooperation performance, anxious attachment, and avoidant attachment as covariates to test for links with inter-brain synchrony. We did not find evidence that these variables moderated the effect of task condition on overall synchrony, the effect of ROI, or the interaction between task condition and ROI (all p > .213). Further correlation analyses are presented in the Supplement.
Discussion
This study extends previous hyperscanning research with adults by examining cooperation-related inter-brain synchrony in mother-child dyads. We found that mother-child dyads demonstrated overall increased inter-brain synchrony when playing an interactive cooperation game compared to a non-cooperative, single player game. Further analyses showed that increases in overall inter-brain synchrony were only significant in mother-son dyads, whereas an ROI-based approach suggested that cooperation increased synchrony in the inferior region of the PFC for mother-son dyads to a greater degree than mother-daughter dyads. Lastly, we found preliminary evidence that children’s avoidant attachment and poor behavioral performance were associated with less synchrony in the right frontopolar region of the PFC. However, it should be noted that many of the ROI-based findings and correlation analyses were only present in analyses using uncorrected thresholds for statistical significance. Taken together, these findings provide tentative insights into our understanding of inter-brain synchrony in mother-child dyads during cooperation and point to a need for further research on the potential link between inter-brain synchrony and attachment.
Our strongest finding was that overall inter-brain synchrony increased when mother-child dyads were engaged in a task that required cooperation compared to a similar game that did not. In other words, mothers and children appeared to engage similar brain regions in a temporally matched fashion more strongly during cooperation. Inter-brain synchrony may be particularly prominent during certain collaborative joint activities, which is in line with previous hyperscanning research with adults (Cui et al., 2012; Liu et al., 2016) and one study with parent-child dyads (Reindl et al., 2018) showing that inter-brain synchrony is heightened during cooperation. However, these studies focused on inter-brain synchrony at the ROI- or channel-based level. To our knowledge, the current study is the first to investigate and document general increases in inter-brain synchrony during cooperation. Coordinated activity in mother-child dyads in corresponding brain regions may reflect engagement in similar cognitive processes in both partners, but we cannot definitively conclude what overall inter-brain synchrony reflects in our study. We found some evidence that cooperation especially increased synchrony in the right dorsolateral and frontopolar PFC. Interestingly, Reindl and colleagues (2018) found these same regions to synchronize between parents (mothers or fathers) and their 5–9-year-old children during cooperation, and that synchrony in these regions was related to cooperative performance. These regions have been shown to be important for executive function and attention as well as theory of mind (Bzdok et al., 2012; Gilbert & Burgess, 2008). Thus, one interpretation of our findings is that the cooperation task elicited similar executive functioning and social cognitive processes in mother-child dyads to a greater degree than the independent comparison task.
Interestingly, mother-son dyads showed less inter-brain synchrony during the independent task than mother-daughter dyads, but mother-son and mother-daughter dyads did not differ in synchrony during the cooperation task. When we subsequently examined specific ROIs using uncorrected thresholds, we found evidence for less synchrony during the independent task compared to the cooperation task in mother-son dyads in all 5 ROIs, but particularly in the dorsolateral and inferior frontal PFC. In mother-daughter dyads, although there was no overall difference in synchrony during the independent and cooperation tasks, we found some evidence for a cooperation-related increase in synchrony in the frontopolar PFC. Our finding that mother-son dyads demonstrated overall greater differences in inter-brain synchrony during cooperation versus a control task is in line with some adult findings that cooperation elicits increased inter-brain synchrony in mixed-sex dyads (Cheng et al., 2015), but this effect was driven by mother-son dyads engaging in less synchrony during the independent task compared to mother-daughter dyads. These differences in inter-brain synchrony did not merely reflect differences in reaction time, and thus may be more indicative of a change in approach to the independent and cooperation tasks in mother-son dyads. One possibility is that the independent task elicited greater disengagement or deviation from strategies used during the cooperation task in mother-son relative to mother-daughter dyads. Boys have been shown to be more prone to engaging in competition whereas girls tend to be more eager to cooperate (Knight & Chao, 1989; Niederle & Vesterlund, 2011). Thus, perhaps boys switched to more competitive strategies during the independent task. Given that participants were told that their overall performance would be compared to other players, we believe it is plausible that some participants may have treated the task as a competition. However, further research that collects data on attitudes toward different task conditions is necessary to directly test this hypothesis and help tease apart the specific social, cognitive and behavioral processes related to overall versus region specific inter-brain synchrony.
Our findings for differences in inter-brain synchrony in mother-son versus mother-daughter dyads are interesting in light of recent work using the same task with mixed-sex adult dyads (Baker et al., 2016). Of course, the nature of mother-child interactions and relationships are very different from those of dyads of adult strangers. In addition, the social and/or cognitive demands of the independent and cooperation tasks may be different for mother-child versus adult dyads. Thus, it should be noted that the degree to which our findings are comparable to previous research on sex-differences in inter-brain synchrony is unclear.
Attachment has been the subject of considerable neuroscience research (Coan, 2008; Riem, Bakermans-Kranenburg, van Ijzendoorn, Out, & Rombouts, 2012; Vrtička, 2017; Vrtička & Vuilleumier, 2012), but the majority of this work has focused on within-individual neural processes in adults. To our knowledge, this study is the first to use a fNIRS hyperscanning paradigm to assess the link between inter-brain synchrony and attachment in mother-child dyads. However, we did not find robust evidence for a link between child attachment and inter-brain synchrony. It was only in our less conservative ROI-based correlation analysis (see Supplement) in which we found preliminary evidence that avoidant child attachment was associated with less cooperation-related synchrony in the right frontopolar PFC, but this effect was not present after statistical correction or after controlling for gender and age. We believe it is worth noting that a recent study of parent-child cooperation found that inter-brain synchrony in this same region mediated the link between parent and child emotion regulation skills (Reindl et al., 2018), and that securely attached children have consistently been shown to internalize effective emotion regulation strategies from their caregivers (Brumariu, 2015). Further studies with larger samples are needed to clearly identify whether mother-child attachment is manifested in inter-brain synchrony, especially in the right frontopolar PFC, during cooperation and in other contexts.
Limitations
One limitation of our findings is that they are specific to neural synchrony between mothers and 8–12-year-old children, but do not speak to earlier developmental periods thought to be particularly important for attachment. fNIRS is increasingly being used to study younger populations, and more work is needed to examine synchrony in infancy and early childhood using age-appropriate social tasks. In addition, we did not obtain data on children’s pubertal stage, which could have affected inter-brain synchrony and behavioral cooperation. Given that girls tend to advance to sexual maturation earlier than boys, sex differences in pubertal stage could have contributed to our observed sex differences in inter-brain synchrony. Future research should test this possibility. Furthermore, our sample size may have limited our ability to detect significant links between inter-brain synchrony and attachment and behavioral performance. As we note above, ROI specific effects were not robust, as they were reduced to statistical trends or non-significance in an FDR-corrected analysis. Future studies that include larger samples, and perhaps repeated measures over development, will be critical to better understand potential links between the ongoing development of attachment and parent-child synchrony over time. It should also be noted that due to the constraints of our fNIRS system, we were only able to examine inter-brain synchrony within a limited number of prefrontal and temporoparietal regions in the right hemisphere. Having access to more available recording channels to provide more extensive coverage of the cortex will be important for determining potential localization of inter-brain synchrony in mothers and children. In addition, two of our mother-child dyads were not biologically related, but these pairs were also not outliers on any of our variables of interest. Studies designed to assess the heritability of parent-child inter-brain synchrony (e.g., studies with identical and fraternal twins) would be an interesting avenue for future research. Lastly, although our computer game task was successful in eliciting increased inter-brain synchrony in this study and has been used and validated in previous work (Baker et al., 2016; Cui et al., 2012), the task does not mirror cooperative interactions that mother-child dyads might experience in their everyday life. One of the strengths of fNIRS is that it allows researchers to study cortical functioning in more naturalistic social environments (Lloyd-Fox, Blasi, & Elwell, 2010; McDonald & Perdue, 2018). Future fNIRS research on mother-child dyads should take advantage of this strength.
Supplementary Material
Acknowledgments
This study was supported by grants awarded to A.L. Reiss from the National Institute of Mental Health (T32MH019908) and Stanford Child Health Research Institute. We thank the families for their participation is this study.
References
- Babiloni F, & Astolfi L. (2014). Social neuroscience and hyperscanning techniques: Past, present and future. Neuroscience & Biobehavioral Reviews, 44, 76–93. 10.1016/j.neubiorev.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JM, Liu N, Cui X, Vrtička P, Saggar M, Hosseini SMH, & Reiss AL (2016). Sex differences in neural and behavioral signatures of cooperation revealed by fNIRS hyperscanning. Scientific Reports, 6, 26492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilek E, Ruf M, Schäfer A, Akdeniz C, Calhoun VD, Schmahl C, … Meyer-Lindenberg AM (2015). Information flow between interacting human brains: Identification, validation, and relationship to social expertise. Proceedings of the National Academy of Sciences of the United States of America, 112, 5207–5212. 10.1073/pnas.1421831112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenning K, Soenens B, Braet C, & Bosmans G. (2011). An adaptation of the Experiences in Close Relationships Scale-Revised for use with children and adolescents. Journal of Social and Personal Relationships, 28, 1048–1072. 10.1177/0265407511402418 [DOI] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, & Eickhoff SB (2012). Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure and Function, 217, 783–796. doi: 10.1007/s00429-012-0380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, & Glover GH (2010). Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage, 50, 81–98. doi: 10.1016/j.neuroimage.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Li X, & Hu Y. (2015). Synchronous brain activity during cooperative exchange depends on gender of partner: A fNIRS-based hyperscanning. Human Brain Mapping, 36, 2039–2048. 10.1002/hbm.22754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA (2008). Toward a neuroscience of attachment In Cassidy J. & Shaver PR (Eds.), Oxford Handbook of Social Neuroscience. New York: Oxford University Press. [Google Scholar]
- Cui X, Bryant DM, & Reiss AL (2012). NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage, 59, 2430–2437. doi: 10.1016/j.neuroimage.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikker S, Silbert LJ, Hasson U, & Zevin JD (2014). On the same wavelength: Predictable language enhances speaker-listener brain-to-brain synchrony in posterior superior temporal gyrus. Journal of Neuroscience, 34, 6267–6272. doi: 10.1523/JNEUROSCI.3796-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Aureli T. , Bafunno D, Cardone D, Romani GL, & Merla A. (2012). Mother and child synchrony: Thermal facial imprints of autonomic contagion. Biological Psychology, 89, 123–129. doi: 10.1016/j.biopsycho.2011.09.018 [DOI] [PubMed] [Google Scholar]
- Feldman R. (2015). Sensitive periods in human social development: New insights from research on oxytocin, synchrony, and high-risk parenting. Development and Psychopathology, 27, 369–395. 10.1017/S0954579415000048 [DOI] [PubMed] [Google Scholar]
- Feldman R. (2017). The neurobiology of human attachments. Trends in Cognitive Sciences, 21, 80–99. 10.1016/j.tics.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Frith U, & Frith C. (2010). The social brain: Allowing humans to boldly go where no other species has been. Philosophical Transactions of the Royal Society B, 365, 165–176. doi: 10.1098/rstb.2009.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted A, Moore JC, & Jevrejeva S. (2004). Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes in Geophysics, 11, 561–566. 10.5194/npg-11-561-2004 [DOI] [Google Scholar]
- Gilbert SJ, & Burgess PW (2008). Executive function. Current Biology, 18, 110–114. 10.1016/j.cub.2007.12.014 [DOI] [PubMed] [Google Scholar]
- Hasson U, Ghazanfar AA, Galantucci B, Garrod S, & Keysers C. (2012). Brain-to-brain coupling: A mechanism for creating and sharing a social world. Trends in Cognitive Sciences, 16, 114–121. doi: 10.1016/j.tics.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Miller JG, Kahle S, & Zahn-Waxler C. (2014). The neurobiological bases of empathic concern for others In Killen M. & Smetana J. (Eds.), Handbook of moral development (2nd ed, pp. 637–660). New York, NY: Guilford. [Google Scholar]
- Helm JL, Miller JG, Kahle S, Troxel NR, & Hastings PD (2018). On measuring and modeling physiological synchrony in dyads. Multivariate Behavioral Research. doi: 10.1080/00273171.2018.1459292 [DOI] [PubMed] [Google Scholar]
- Hu Y, Hu Y, Li X, Pan Y, & Cheng X. (2017). Brain-to-brain synchronization across two persons predicts mutual prosociality. Social Cognitive and Affective Neuroscience, 12, 1835–1844. doi: 10.1093/scan/nsx118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Dai B, Peng D, Zhu C, Liu L, & Lu C. (2012). Neural synchronization during face-to-face communication. Journal of Neuroscience, 32, 16064–16069. doi: 10.1523/JNEUROSCI.2926-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerig PK, Cowan PA, & Cowan CP (1993). Marital quality and gender differences in parent-child interaction. Developmental Psychology, 29, 931–939. doi: 10.1037/0012-1649.29.6.931 [DOI] [Google Scholar]
- Kinreich S, Djalovski A, Kraus L, Louzoun Y, & Feldman R. (2017). Brain-to-brain synchrony during naturalistic social interactions. Scientific Reports, 7, 17060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight GP, & Chao C-C (1989). Gender differences in the cooperative, competitive, and individualistic social values of children. Motivation and Emotion, 13, 125–141. 10.1007/BF00992958 [DOI] [Google Scholar]
- Leclere C, Viaux S, Avril M, Achard C, Chetouani M, Missonnier S, & Cohen D. (2014). Why synchrony matters during mother-child interactions: A systematic review. PLoS One, 9, e113571. 10.1371/journal.pone.0113571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Mok C, Witt EE, Pradhan AH, Chen JE, & Reiss AL (2016). NIRS-based hyperscanning reveals inter-brain neural synchronization during cooperative jenga game with face-to-face communication. Frontiers in Human Neuroscience, 10, 82 10.3389/fnhum.2016.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Saito G, Lin C, & Saito H. (2017). Inter-brain network underlying turn-based cooperation and competition: A hyperscanning study using near-infrared spectroscopy. Scientific Reports, 7: 8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, & Elwell CE (2010). Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neuroscience & Biobehavioral Reviews, 34, 269–284. 10.1016/j.neubiorev.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Lytton H, & Romney DM (1991). Parents’ differential socialization of boys and girls: A meta-analysis. Psychological Bulletin, 109, 267–296.doi: 10.1037/0033-2909.109.2.267 [DOI] [Google Scholar]
- McDonald NM, & Perdue KL (2018). The infant brain in the social world: Moving toward interactive social neuroscience with functional near-infrared spectroscopy. Neuroscience & Biobehavioral Reviews, 87, 38–49. 10.1016/j.neubiorev.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molavi B, & Dumont GA (2012). Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiological Measurement, 33, 259–270. doi: 10.1088/0967-3334/33/2/259 [DOI] [PubMed] [Google Scholar]
- Morrongiello BA, & Dawber T. (1999). Parental influences on toddlers’ injury-risk behaviors: Are sons and daughters socialized differently? Journal of Applied Developmental Psychology, 20, 227–251. doi: 10.1016/S0193-3973(99)00015-5 [DOI] [Google Scholar]
- Niederle M, & Vesterlund L. (2011). Gender and competition. Annual Review of Economics, 3, 601–630. 10.1146/annurev-economics-111809-125122 [DOI] [Google Scholar]
- Nozawa T, Sasaki Y, Sakaki K, Yokoyama R, & Kawashima R. (2016). Interpersonal frontopolar neural synchronization in group communication: An exploration toward fNIRS hyperscanning of natural interactions. Neuroimage, 133, 484–497. 10.1016/j.neuroimage.2016.03.059 [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry, & Adam EK (2009). Mother-adolescent physiological synchrony in naturalistic settings: Within-family cortisol associations and moderators. Journal of Family Psychology, 23, 882–894. doi: 10.1037/a0017147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl V, Gerloff C, Scharke W, & Konrad K. (2018). Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. Neuroimage, 178, 493–502. doi: 10.1016/j.neuroimage.2018.05.060 [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, van IJzendoorn MH, Out D, & Rombouts SA (2012). Attachment in the brain: Adult attachment representations predict amygdala and behavioral responses to infant crying. Attachment & Human Development, 14, 533–551. 10.1080/14616734.2012.727252 [DOI] [PubMed] [Google Scholar]
- Russell A, & Saebel J. (1997). Mother-son, mother-daughter, father-son, and father-daughter: Are they distinct relationships? Developmental Review, 17, 111–147. doi: 10.1006/drev.1996.0431 [DOI] [Google Scholar]
- Saxbe DE, Golan O, Ostfeld-Etzion S, Hirschler-Guttenberg Y, Zagoory-Sharon O, & Feldman R. (2017). HPA axis linkage in parent-child dyads: Effects of parent sex, autism spectrum diagnosis, and dyadic relationship behavior. Developmental Psychobiology, 59, 776–786. 10.1002/dev.21537 [DOI] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, & Vogeley K. (2013). Toward a second-person neuroscience. Behavioral and Brain Sciences, 36, 393–462. 10.1017/S0140525X12000660 [DOI] [PubMed] [Google Scholar]
- Singh AK, Okamoto M, Dan H, Jurcak V, & Dan I. (2005). Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage, 27, 842–851. doi: 10.1016/j.neuroimage.2005.05.019 [DOI] [PubMed] [Google Scholar]
- Stephen GJ, Silbert LJ, & Hasson U. (2010). Speaker-listener neural coupling underlies successful communication. Proceedings of the National Academy of Sciences of the United States of America, 107, 14425–14430. 10.1073/pnas.1008662107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Mai X, Wang S, Zhu C, Krueger F, & Liu C. (2016). Interpersonal brain synchronization in the right temporo-parietal junction during face-to-face economic exchange. Social Cognitive and Affective Neuroscience, 11, 23–32. 10.1093/scan/nsv092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick EZ, & Cohn JF (1989). Infant-mother face-to-face interaction: age and gender differences in coordination and the occurrence of miscoordination. Child Development, 60, 85–92. doi: 10.2307/1131074 [DOI] [PubMed] [Google Scholar]
- Vrtička P. (2017). The social neuroscience of attachment In Ibáñez A, Sedeño L, & Garcia AM (Eds.), Neuroscience and social science. Cham: Springer International Publishing. [Google Scholar]
- Vrtička P, & Vuilleumier P. (2012). Neuroscience of human social interactions and adult attachment style. Frontiers in Human Neuroscience, 6, 212 10.3389/fnhum.2012.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liu T, Dongchuan Y, & Pelowski M. (2017a). Gender differences in spontaneous deception: A hyperscanning study using functional near-infrared spectroscopy. Scientific Reports, 7, 7508. doi: 10.1038/s41598-017-06764-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liu T, Pelowski M, & Dongchuan Y. (2017b). Social risky decision-making reveals gender differences in the TPJ: A hyperscanning study using functional near-infrared spectroscopy. Brain & Cognition, 119, 54–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.