Abstract
BACKGROUND
Accurate assessment of the prognosis after colorectal cancer surgery is of great significance in patients with colorectal cancer. However, there is no systematic analysis of factors affecting the prognosis of colorectal cancer currently.
AIM
To systematically analyze the influence of clinical data and serological and histological indicators on the prognosis of patients with colorectal cancer, and to explore the indicators that can accurately assess the prognosis of patients with colorectal cancer.
METHODS
A total of 374 patients with colorectal cancer were enrolled. The clinical data, tumor-node-metastasis (TNM) stage, and Dukes stage were recorded. All patients received examinations including carcinoembryonic antigen (CEA), carbohydrate antigen 199, C-reactive protein, albumin, D-dimer, and fibrinogen as well as routine blood tests one week before surgery. The tumor location, size, depth of invasion, lymph node metastasis, and distant metastasis were recorded during surgery. The pathological tissue typing and expression of proliferating cell nuclear antigen (PCNA) and p53 were observed. All patients were followed for 3 years, and patients with endpoint events were defined as a poor prognosis group, and the remaining patients were defined as a good prognosis group. The differences in clinical data, serology, and histology were analyzed between the two groups. Multivariate COX regression was used to analyze the independent influencing factors for the prognosis of colorectal cancer. The receiver operating characteristic curve was used to evaluate the predictive value of each of the independent influencing factors and their combination for the prognosis of colorectal cancer.
RESULTS
The follow-up outcomes showed that 81 patients were in the good prognosis group and 274 patients in the poor prognosis group. The TNM stage, PCNA, Glasgow prognostic score (GPS), neutrophil-lymphocyte ratio (NLR), C-reactive protein/albumin ratio (CAR), D-dimer, and CEA were independent influencing factors for the prognosis of colorectal cancer (P = 0.000). NLR had the highest predictive power for colorectal cancer prognosis [area under the receiver operating characteristic curve (AUC) = 0.925], followed by D-dimer (AUC = 0.879) and GPS (AUC = 0.872). The accuracy of the combination of all indicators in predicting the prognosis of colorectal cancer was the highest (AUC = 0.973), which was significantly higher than that of any of the indicators alone (P < 0.05). The sensitivity and specificity of the combination were 92.59% and 90.51%, respectively.
CONCLUSION
The independent influence factors for the prognosis of colorectal cancer include TNM stage, PCNA, GPS, NLR, CAR, D-dimer, and CEA. The combined assessment of the independent factors is the most accurate predictor of the prognosis after colorectal cancer surgery.
Keywords: Colorectal cancer, Prognosis, Influencing factors, Combination assessment
Core tip: Accurate assessment of the prognosis after colorectal cancer surgery is of great importance in patients with colorectal cancer. This study systematically analyzed the influence of clinical data and serological and histological indicators on the prognosis of patients with colorectal cancer and the results revealed that the independent influence factors for the prognosis of colorectal cancer include tumor-node-metastasis stage, proliferating cell nuclear antigen, Glasgow prognostic score, neutrophil-lymphocyte ratio, C-reactive protein/albumin ratio, D-dimer, and carcinoembryonic antigen. The combined assessment of the independent factors is the most accurate predictor of the prognosis after colorectal cancer surgery.
INTRODUCTION
The prognosis of colorectal cancer is poor, and its mortality rate ranks third among all malignancies in the world[1-3]. Surgery is the main method of colorectal cancer treatment. Accurate assessment of surgical prognosis plays an important role in the treatment of colorectal cancer patients. Currently, studies on the factors affecting the prognosis of colorectal cancer are very popular. Studies have shown that high Glasgow prognostic score (GPS) in colorectal cancer is more likely to predict a poor prognosis[4-6]. The results of Arfa et al[7] showed that tumor-node-metastasis (TNM) stage is associated with the prognosis of colorectal cancer. Studies by Kanemitsu et al[8] have demonstrated that histological type and degree of differentiation of tumors can affect their biological behavior and further affect clinical outcomes. In addition, studies have found that in vivo inflammatory response can have an impact on the occurrence and development of tumors[9]. Serum C-reactive protein[10,11], albumin[12,13], C-reactive protein/albumin ratio (CAR)[14-16], and neutrophil-lymphocyte ratio (NLR)[17,18] reveal the possibility of inflammation-related complications, and have certain predictive value for prognosis[19]. However, there is no systematic analysis about the factors affecting the prognosis of colorectal cancer. Therefore, this study defined colorectal cancer patients as the research subjects. We statistically analyzed the influence of clinical data and serology and histology on the prognosis of patients with colorectal cancer, and assessed the accuracy of the combination of all indicators for the prognosis evaluation, aiming to find a more accurate assessment. This study is expected to provide a new method for predicting the prognosis of colorectal cancer in the early stage of clinical diagnosis and improve the prognosis of patients.
MATERIALS AND METHODS
Research subjects
A total of 374 patients with colorectal cancer who were admitted to Cangzhou Central Hospital from March 2012 to March 2015 were recruited. The inclusion criteria were: (1) Pathological diagnosis of colorectal cancer; (2) Patients who underwent radical surgery; (3) Complete clinical data, disease history, and family history data; and (4) Complete physical examination, routine blood tests, detection of tumor markers, and relevant laboratory examinations such as coagulation function within one week before surgery. The exclusion criteria were: (1) Other carcinomas combined; (2) Pre-operative infection or insufficient infection evidence but body temperature > 38 °C; (3) Patients combined with cardiovascular disease, cerebrovascular disease, and diseases of the liver, kidney, and other important organs; and (4) Undergoing radiotherapy, chemotherapy, biotherapy, or gene therapy before surgery. The study was approved by the Ethics Committee of Cangzhou Central Hospital. All patients included in the study had a detailed understanding of the research content and provided informed consent.
Research methods
Clinical data collection: After admission, complete clinical data, including age, gender, height, weight, family history (colorectal cancer in three generations of close relatives) and smoking history were collected. Body mass index (BMI) was calculated. All patients were staged by the TNM staging method and the improved Dukes staging method according to pathological findings[20].
Serological examination: All patients received carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA199), C-reactive protein, albumin, D-dimer, and fibrinogen detection as well as routine blood tests one week before surgery. CEA and CA199 were tested by chemiluminescence with a Roche kit. Routine blood tests were performed with a CC-3200 automatic blood tester and supporting kit. The numbers of neutrophil granulocytes, white blood cells, lymphocytes, and platelets were recorded, and NLR was calculated. C-reactive protein, albumin, D-dimer, and fibrinogen were detected using an Abbott C16000 automatic biochemical analyzer (Abbott Laboratories, Inc.). CAR was calculated. According to the results of C-reactive protein and albumin detection, GPS was calculated according to the following rules: (1) Increased C-reactive protein; and (2) Hypoproteinemia. Two points were recorded if both (1) and (2) were satisfied, 1 point if (1) or (2) was satisfied, and 0 points if normal C-reactive protein and no hypoproteinemia were found.
Surgery and histological examination: Under general anesthesia, colorectal cancer radical surgery was performed. Tumor location, size, depth of invasion, lymph node metastasis, and distant metastasis were recorded. The excised specimens were flattened and fixed, and pathological examination was performed. The pathological tissues were serially sectioned, fixed with 10% formalin, and embedded in paraffin, followed by hematoxylin-eosin and immunohistochemical staining. All the sections were observed by two experienced pathologists. The pathological tissue classification and expression of proliferating cell nuclear antigen (PCNA) and p53 were observed. After surgery, patients’ vital signs and wound oozing were closely observed. Anti-inflammatory drugs and intravenous nutrition were given regularly.
Follow-up
A total of 374 patients who participated in the study were followed for 3 years. The first follow-up was performed 1 mo after the end of treatment, followed by every 3 mo within 2 years. After 2 years, follow-up was performed every 6 mo. The endpoint events were defined as adverse prognostic events during follow-up, including recurrence of colorectal cancer, increased stage, other organ metastases, and death from colorectal cancer and its complications. Patients’ refusal to visit, halfway out, and death from other reasons unrelated to the study were defined as loss to follow-up. According to the follow-up results, patients with endpoint events were defined as a poor prognosis group, and the remaining patients were defined as a good prognosis group. Differences in clinical data, serology, and histology were analyzed.
Statistical analysis
Analyses were performed using Statistical Product and Service Solutions software. The measurement data are expressed as the mean ± standard deviation, and comparisons were performed using an independent sample t-test. The count data are expressed in case (percentage), and the chi-square test was used for comparison. Survival analysis was performed by the Kaplan-Meier method. The poor prognosis rate was calculated and the survival curve was drawn. Multivariate COX regression was used to analyze independent influencing factors for the prognosis of colorectal cancer. The receiver-operating characteristic (ROC) curve was used to evaluate the predictive value of each independent influencing factor and their combination for the prognosis of colorectal cancer. The difference was considered statistically significant at P < 0.05.
RESULTS
Patient follow-up results
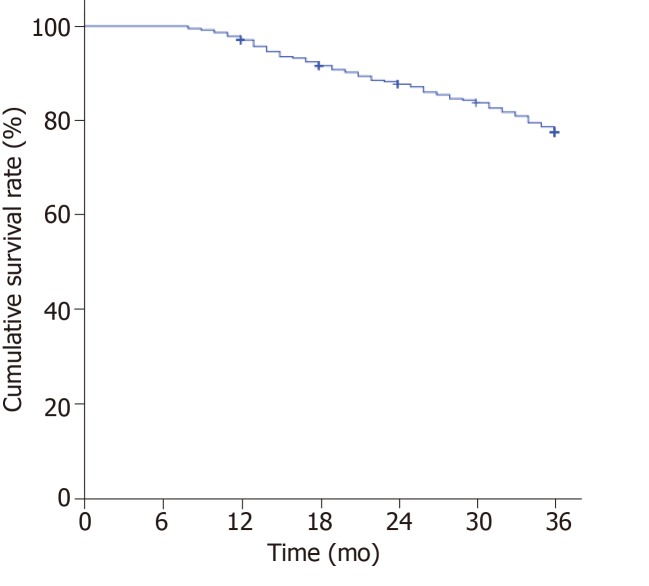
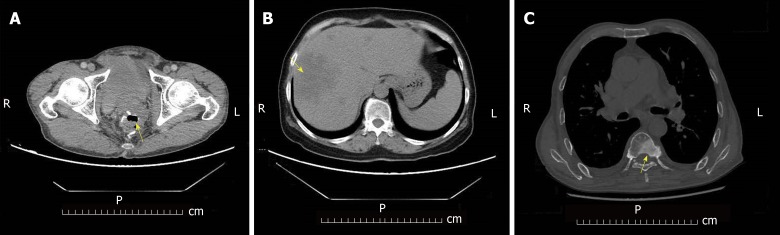
A total of 374 patients were enrolled in the study. The Kaplan-Meier survival curve showed an increase trend in the number of adverse prognosis cases over time (Figure 1). At the end of follow-up, 19 patients were lost to follow-up. There were a total of 355 patients with complete follow-up data, of whom 81 developed endpoint events. In these 81 patients, 40 had recurrence of colorectal cancer (Figure 2A), 26 had liver metastases (Figure 2B), and 15 had bone metastases (Figure 2C). The poor prognosis rate was 22.82%.
Figure 1.
Survival curve analysis of patients with colorectal cancer (Kaplan-Meier). The poor prognosis rate was 22.82%.
Figure 2.
Endpoint events during postoperative follow-up of colorectal cancer patients. A: Colorectal cancer recurrence (arrow); B: Colorectal cancer liver metastasis (arrow); C: Colorectal cancer bone metastasis (arrow).
Comparison of indicators between the prognosis group and poor prognosis group
Of the 355 patients with colorectal cancer who received complete follow-up data, 186 were male and 169 were female, with an average age of 59.83 ± 13.92 years and mean tumor size of 4.32 ± 2.56 cm. In terms of tumor location, there were 52 cases in the ascending colon, 21 in the transverse colon, 35 in the descending colon, 106 in the sigmoid colon, and 141 in the rectum. In terms of TNM stage, there were 16 cases of stage I, 251 stage II, 54 stage III, and 34 stage IV. In terms of Dukes stage, there were 17 cases of grade A, 225 grade B, and 112 grade C. Among the pathological types, there were 230 cases of adenocarcinoma and 125 cases of mucinous adenocarcinoma and signet ring cell carcinoma.
According to the prognosis results, the prognosis group included 274 patients and the poor prognosis group included 81 patients. The comparison of the indicators before treatment in the two groups of patients is shown in Table 1. The age, tumor diameter, TNM stage, PCNA index, GPS, C-reactive protein, albumin, white blood cell (WBC) count, NLR, CAR, D-dimer, fibrinogen, CEA, and CA199 in the poor prognosis group were significantly higher than those in the good prognosis group (P < 0.05). The gender, BMI, smoking proportion, alcohol abuse ratio, family history ratio, tumor location, tumor histology type, Dukes grade, proportion of positive P53, and platelet count were similar between the two groups (P > 0.05).
Table 1.
Comparison of indicators between the good prognosis group and poor prognosis group before treatment
| Good prognosis group (n = 274) | Poor prognosis group (n = 81) | t/χ2 value | P value | |
| Age | 53.64 ± 15.38 | 60.47 ± 8.34 | 3.831 | 0.000 |
| Gender (Male/Female) | 143/131 | 43/38 | 0.020 | 0.887 |
| BMI (kg/m2) | 26.49 ± 5.34 | 25.39 ± 5.91 | 1.589 | 0.113 |
| Smoking [n (%)] | 74 (27.01%) | 24 (29.63%) | 0.215 | 0.643 |
| History of alcohol abuse [n (%)] | 39 (14.23%) | 13 (16.05%) | 0.165 | 0.685 |
| Family history [n (%)] | 46 (16.79%) | 20 (24.69%) | 2.580 | 0.108 |
| Tumor diameter (cm) | 2.08 ± 1.47 | 5.69 ± 2.54 | 16.125 | 0.000 |
| Tumor location | ||||
| Ascending colon [n (%)] | 39 | 13 | 3.139 | 0.535 |
| Transverse colon [n (%)] | 14 | 7 | ||
| Lower colon [n (%)] | 25 | 10 | ||
| Sigmoid colon [n (%)] | 84 | 22 | ||
| Rectum [n (%)] | 112 | 29 | ||
| Tumor histology type | ||||
| Adenocarcinoma | 177 | 53 | 0.019 | 0.890 |
| Mucinous adenocarcinoma and signet ring cell carcinoma | 97 | 28 | ||
| TNM stage | ||||
| I | 10 (3.65%) | 6 (7.41%) | 29.292 | 0.000 |
| II | 213 (77.74%) | 38 (46.91%) | ||
| III | 30 (10.95%) | 24 (29.63%) | ||
| IV | 21 (7.66%) | 13 (16.05%) | ||
| Dukes stage | ||||
| A | 17 (6.20%) | 1 (1.23%) | 0.062 | |
| B | 177 (64.60%) | 48 (59.26%) | ||
| C | 80 (29.20%) | 32 (39.51%) | ||
| PCNA | ||||
| 1 | 98 | 4 | 114.291 | 0.000 |
| 2 | 122 | 11 | ||
| 3 | 45 | 43 | ||
| 4 | 9 | 23 | ||
| P53 | ||||
| - | 34 | 11 | 4.908 | 0.297 |
| + | 64 | 14 | ||
| ++ | 61 | 17 | ||
| +++ | 78 | 23 | ||
| ++++ | 37 | 19 | ||
| GPS | ||||
| 0 | 233 (85.04%) | 9 (11.11%) | 157.579 | 0.000 |
| 1 | 24 (8.76%) | 40 (49.38%) | ||
| 2 | 17 (6.20%) | 32 (39.51%) | ||
| C-reactive protein (mg/L) | 2.73 ± 0.64 | 21.83 ± 16.34 | 19.364 | 0.000 |
| Albumin (g/L) | 42.43 ± 7.93 | 33.74 ± 6.21 | 9.071 | 0.000 |
| WBC count (9 × 103/mm3) | 5.89 ± 1.98 | 8.53 ± 2.83 | 9.481 | 0.000 |
| Platelet count (9 × 104/mm3) | 237.43 ± 103.28 | 247.23 ± 116.39 | 0.728 | 0.467 |
| NLR | 2.68 ± 1.73 | 4.53 ± 3.29 | 6.699 | 0.000 |
| CAR | 0.089 ± 0.017 | 0.175 ± 0.092 | 14.693 | 0.000 |
| D-dimer (μg/mL) | 0.553 ± 0.207 | 0.943 ± 0.375 | 13.117 | 0.000 |
| Fibrinogen (g/L) | 3.121 ± 1.542 | 3.524 ± 1.053 | 2.204 | 0.028 |
| CEA (ng/mL) | 3.34 ± 1.82 | 6.13 ± 2.36 | 11.281 | 0.000 |
| CA199 (U/mL) | 186.82 ± 139.74 | 635.24 ± 284.38 | 15.067 | 0.000 |
BMI: Body mass index; TNM: Tumor-node-metastasis; PCNA: Proliferating cell nuclear antigen; GPS: Glasgow prognostic score; WBC: White blood cell; NLR: Neutrophil-lymphocyte ratio; CAR: C-reactive protein/albumin ratio; CEA: Carcinoembryonic antigen; CA199: Carbohydrate antigen 199.
Multivariate COX regression analysis of the prognosis in patients with colorectal cancer
Further multivariate COX regression analysis was performed on the different indicators between the two groups. The results showed that the impact of age, tumor diameter, C-reactive protein, albumin, WBC count, fibrinogen, and CA199 on the prognosis was not significant (P > 0.05). TNM stage, PCNA, GPS, NLR, CAR, D-dimer, and CEA were independent influencing factors for the prognosis of colorectal cancer (P = 0.000) (Table 2).
Table 2.
COX regression analysis of influence factors on the prognosis of colorectal cancer
| B | SE | Wald | P value | RR |
95%CI |
||
| Lower limit | Upper limit | ||||||
| Age | 0.241 | 0.223 | 1.328 | 0.154 | 1.273 | 0.822 | 1.971 |
| Tumor diameter | 0.160 | 0.315 | 1.142 | 0.328 | 1.173 | 0.633 | 2.175 |
| TNM Stage | 1.762 | 0.442 | 7.364 | 0.000 | 5.824 | 2.449 | 13.850 |
| PCNA | 1.534 | 0.382 | 6.338 | 0.000 | 4.635 | 2.192 | 9.800 |
| GPS | 2.238 | 0.448 | 8.927 | 0.000 | 9.377 | 3.897 | 22.564 |
| C-reactive protein | 0.454 | 0.355 | 2.773 | 0.058 | 1.574 | 0.785 | 3.156 |
| Albumin | -0.129 | 0.371 | 2.538 | 0.105 | 0.879 | 0.425 | 1.819 |
| WBC count | 0.149 | 0.433 | 1.292 | 0.183 | 1.161 | 0.497 | 2.713 |
| NLR | 0.816 | 0.233 | 6.792 | 0.000 | 2.261 | 1.432 | 3.570 |
| CAR | 2.678 | 0.341 | 9.338 | 0.000 | 14.552 | 7.459 | 28.391 |
| D-dimer | 2.498 | 0.636 | 8.923 | 0.000 | 12.153 | 3.494 | 42.272 |
| Fibrinogen | 0.603 | 0.386 | 0.751 | 0.625 | 1.827 | 0.857 | 3.893 |
| CEA | 1.342 | 0.492 | 6.877 | 0.000 | 3.827 | 1.459 | 10.038 |
| CA199 | 0.226 | 0.553 | 1.149 | 0.322 | 1.253 | 0.424 | 3.704 |
TNM: Tumor-node-metastasis; PCNA: Proliferating cell nuclear antigen; GPS: Glasgow prognostic score; WBC: White blood cell; NLR: Neutrophil-lymphocyte ratio; CAR: C-reactive protein/albumin ratio; CEA: Carcinoembryonic antigen; CA199: Carbohydrate antigen 199.
Receiver operating characteristic curve analysis of potential indicators for predicting colorectal cancer prognosis
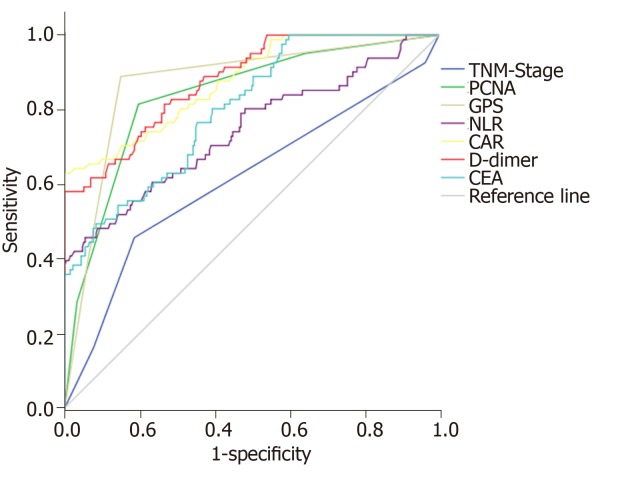
The ROC curve was used to further analyze the predictive value of TNM stage, PCNA, GPS, NLR, CAR, D-dimer, and CEA for the prognosis of colorectal cancer. The results showed that each indicator can predict the prognosis of colorectal cancer (AUC > 0.6 for all). Among them, NLR had the highest accuracy with an AUC of 0.925 (95%CI: 0.860-0.966), sensitivity of 94.12%, and slightly lower specificity of 80.25%. D-dimer was the second, and its AUC was 0.879 (95%CI: 0.841-0.911), with a specificity of 100% and sensitivity of only 58.02%. GPS was the third, and its AUC was 0.872 (95%CI: 0.832-0.905), with a sensitivity and specificity of 88.89% and 85.04%, respectively (Table 3 and Figure 3).
Table 3.
Receiver-operating characteristic (ROC) curve analysis of potential indicators for predicting the prognosis of colorectal cancer
| AUC | 95%CI | Best diagnostic point | Sensitivity (%) | Specificity (%) | |
| TNM Stage | 0.613 | 0.560-0.663 | 2 | 45.68 | 81.39 |
| PCNA | 0.837 | 0.794-0.874 | 2 | 81.48 | 80.29 |
| GPS | 0.872 | 0.832-0.905 | 1 | 88.89 | 85.04 |
| NLR | 0.925 | 0.860-0.966 | 0.206 | 94.12 | 80.25 |
| CAR | 0.743 | 0.695-0.788 | 4.81 | 45.68 | 94.53 |
| D-dimer | 0.879 | 0.841-0.911 | 0.795 (μg/mL) | 58.02 | 100 |
| CEA | 0.801 | 0.755-0.841 | 3.891 (ng/mL) | 76.54 | 64.60 |
TNM: Tumor-node-metastasis; PCNA: Proliferating cell nuclear antigen; GPS: Glasgow prognostic score; NLR: Neutrophil-lymphocyte ratio; CAR: C-reactive protein/albumin ratio; CEA: Carcinoembryonic antigen.
Figure 3.
Receiver operating characteristic curve analysis of potential indicators for predicting the prognosis of colorectal cancer.
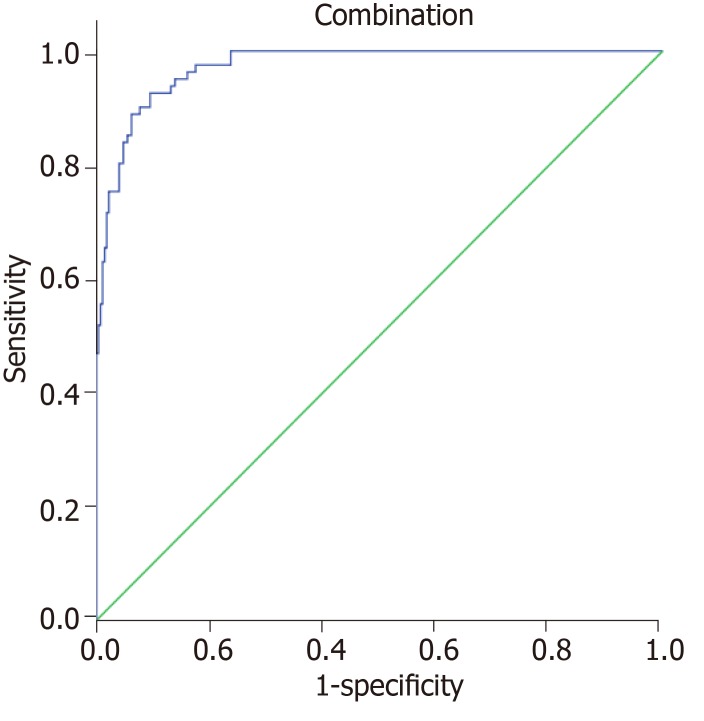
Each indicator had some limitations in predicting the prognosis of colorectal cancer, respectively. Therefore, this study attempted to establish a combination model based on logistic regression to predict the prognosis. The result showed that the accuracy of the combination of all indicators in predicting the prognosis of colorectal cancer was the highest (AUC = 0.973, 95%CI: 0.950-0.987), which was significantly higher than that of any indicator alone (P < 0.05). The best diagnostic point was 0.206, with a sensitivity of 92.59% and specificity of 90.51% (Figure 4).
Figure 4.
Receiver operating characteristic curve analysis of the combination assessment for predicting the prognosis of colorectal cancer.
DISCUSSION
The detection rate of colorectal cancer has increased recently[21]. Because of the hidden symptoms in early stage, the prognosis of colorectal cancer is poor[22]. Accurate assessment of patient outcomes is critical to the choice of clinical treatments. Currently, the prognosis of colorectal cancer is predicted mainly through CEA level, pathological typing, TNM staging, etc.[23-25], but the accuracy of prediction cannot be guaranteed due to different individuals[26]. Recent studies have shown that GPS, C-reactive protein, albumin, CAR, NLR and other inflammatory indicators can reveal the prognosis of malignant tumors, but the accuracy of different predictive indicators for the prognosis of colorectal cancer is uncertain[27]. Therefore, this study recruited colorectal cancer patients and screened the clinical data and serology and pathology data to find the indicators that can accurately evaluate the prognosis.
Survival analysis of patients with colorectal cancer
The incidences of postoperative recurrence, metastasis, and tumor-related death are very high[28]. A 3-year follow-up analysis in the study showed that the survival curves decreased significantly at 12-18 mo and 30-36 mo, respectively. It indicated that the number of patients with a poor prognosis increased rapidly at 12-18 mo and 30-36 mo. Among the patients with a poor prognosis, the incidence of colorectal cancer recurrence was the highest, accounting for 49.4% of all patients with a poor prognosis, followed by liver metastasis (32.1%) and bone metastasis (18.5%). The overall poor prognosis rate was 22.82%, which is similar to the recent finding of Shen et al[29] (24.3%), and lower than the result of Lujan et al[30] (38.3%-45.6%). The possible reason is that with the improvement of people's health awareness, the detection rate of early colorectal cancer is increased. More patients can receive timely treatment in early stage, in addition to the popularization of endoscopic and laparoscopic treatment techniques. Hence the prognosis of colorectal cancer is improved.
Multivariate regression analysis of the prognosis after colorectal cancer surgery
With the increase of age, the immune clearance ability and postoperative recovery ability of carcinoma are significantly reduced. The possibility of poor prognosis is gradually increased[31-33]. Studies have revealed that gender, smoking history, family history, etc. can affect the incidence and outcome of carcinoma[34-36]. Tumor location, size, TNM stage, Dukes stage, and pathological classification can indicate the progression of colorectal cancer and its invasion and metastasis ability. They are often utilized to predict the prognosis of colorectal cancer[37]. PCNA and p53 are common clinical tumor-related detection indicators, and their abnormal expression levels may be associated with colorectal cancer recurrence and poor prognosis[38-42]. Serological indicators such as neutrophils, lymphocytes, albumin, and C-reactive protein are commonly used indicators for monitoring inflammation in the body. Studies have shown that inflammation can promote tumor metastasis and recurrence[43-44]. In addition, inflammatory factors can inhibit the body’s immune response to tumors and stimulate tumor formation around the tumor, thereby promoting tumor growth and metastasis[45].
This study compared clinical, serological, and histological indicators of patients with different prognoses. It indicated that age, tumor diameter, TNM stage, PCNA index, GPS, C-reactive protein, albumin, WBC count, NLR, CAR, D-dimer, fibrinogen, CEA, and CA199 were possible risk factors for poor prognosis in patients with colorectal cancer. Further multivariate COX regression analysis of the above indicators showed that TNM stage, PCNA, GPS, NLR, CAR, D-dimer, and CEA were independent influencing factors for the prognosis of colorectal cancer. It suggested that when advanced TNM stage, high PCNA expression levels, GPS > 0, increase of NLR and CAR, or increase of D-dimer and CEA levels occurs, the possibility of poor prognosis should be guarded. In addition, early treatment, control of inflammation levels, and monitoring of D-dimer and CEA levels can prevent poor prognosis. It is worth noticing that NLR, CAR, and GPS are calculated by two serological indicators, which can simultaneously reveal changes of two indicators. Furthermore, TNM staging includes tumor diameter, lymph node metastasis, and distant metastasis. It indicated that multiple indicators are preferred when the accuracy of prognosis assessment based on single factor is poor to improve the accuracy of the evaluation results.
Potential indicators predicting the prognosis of colorectal cancer
Based on COX regression results, this study evaluated the ability of TNM stage, PCNA, GPS, NLR, CAR, D-dimer, and CEA to predict the prognosis of colorectal cancer. According to the evaluation results, the accuracy of NLR, D-dimer, and GPS was significantly higher than that of TNM stage, PCNA, and CAR. Among them, NLR had the highest accuracy. The possible reason may be that NLR is the ratio of neutrophils to lymphocytes. Both of them are reliable indicators for the inflammatory response in vivo. Neutrophils can release active factors to activate NF-κB, which is related to the formation of tumors. Also, it can inhibit the immunity of the body through the inflammatory reaction, and help tumor cells escape from immunization. In addition, it can promote tumor growth by promoting neovascularization. Hence, its increase is beneficial to tumorigenesis[43,44]. As one of the main cells of the body’s immunity, lymphocytes can produce an immune response to tumor cells, and the decrease of the lymphocytes leads to a decrease in the body’s ability to inhibit tumors. Therefore, increased NLR is beneficial to tumor survival and associated with a poor prognosis. The hypercoagulable state of the blood is beneficial for the metastasis of malignant tumors[46]. Studies have confirmed that preoperative D-dimer levels are associated with tumor prognosis[47-49]. In this study, D-dimer had a higher predictive ability for poor prognosis of colorectal cancer, second only to NLR. GPS is determined by the level of serum C-reactive protein and whether it has hypoproteinemia, which can simultaneously indicate the inflammatory condition and nutritional status of the body. In this study, GPS also had a high predictive ability for poor prognosis of colorectal cancer, which is similar to the study by Wind et al[50].
However, each indicator has a certain limitation in predicting the prognosis of colorectal cancer. Therefore, this study combined the independent risk factors to evaluate the accuracy for predicting the prognosis. It revealed that the accuracy of the combination in predicting the prognosis of colorectal cancer was significantly higher than the accuracy of individual indicators. It suggested that in the evaluation of surgical outcomes in patients with colorectal cancer, the combination of TNM stage, PCNA, GPS, NLR, CAR, D-dimer, and CEA has a higher accuracy.
Insufficient and prospects
This study is a single-center study and may have certain limitations. The follow-up time was short, and the indicators affecting long-term prognosis may be ignored. In future, multi-center research is considered to expand the sample size for improving the reliability of the research results. Meanwhile, the length of follow-up can be extended. The influencing factors on short-term and long-term prognosis of colorectal cancer should be analyzed respectively.
In conclusion, this study analyzed the clinical data and serological and pathological indicators that may affect the prognosis of colorectal cancer. It indicated that the independent influencing factors for the prognosis of colorectal cancer include TNM stage, PCNA, GPS, NLR, CAR, D-dimer, and CEA. Among them, NLR, D-dimer, and GPS have higher prediction capabilities. The combination of all independent factors can make a more accurate assessment of the prognosis of colorectal cancer.
ARTICLE HIGHLIGHTS
Research background
The prognosis of colorectal cancer is poor. Surgery is the main treatment for patients with colorectal cancer. Accurate assessment of surgical prognosis has an important impact on the choice of treatments for patients. Currently, there are many methods to evaluate the prognosis after colorectal cancer surgery, including tumor-node-metastasis (TNM) stage, Glasgow prognostic score (GPS) score and so on. However, the systematic analysis about the factors affecting the prognosis of colorectal cancer is still limited.
Research motivation
Currently, the prognosis of colorectal cancer is mainly predicted by carcinoembryonic antigen (CEA) level, pathological classification, and TNM stage. However, the accuracy of prediction cannot be guaranteed due to the influence of individual and environmental factors. Besides, studies have revealed that some inflammatory indicators are also related to the prognosis of cancer.
Research objectives
In this study, we analyzed the influence of clinical data, serology, and histology on the prognosis of patients with colorectal cancer, and assessed the accuracy of the combination of all indicators for the prognosis evaluation. The purpose of this study was to provide a new method for predicting the prognosis of colorectal cancer in the early stage.
Research methods
A total of 374 patients were recruited, and the patients were divided into a good prognosis group and a poor prognosis group. Relevant clinical indicators were recorded. The differences in clinical data, serology, and histology between the two groups were analyzed. Multivariate COX regression was used to analyze the independent influencing factors for the prognosis of colorectal cancer. The receiver operating characteristic curve was used to test the accuracy of different indicators and their combination for the prognostic evaluation of colorectal cancer.
Research results
The TNM stage, proliferating cell nuclear antigen (PCNA), GPS, neutrophil-lymphocyte ratio (NLR), C-reactive protein/albumin ratio (CAR), D-dimer, and CEA were independent influencing factors for the prognosis of colorectal cancer (P = 0.000). NLR, D-dimer, and GPS had the highest predictive power for colorectal cancer prognosis. But their accuracies were significantly lower than that of the combination of all indicators (AUC = 0.973; sensitivity, 92.59%; specificity, 90.51%).
Research conclusions
TNM stage, PCNA, GPS, NLR, CAR, D-dimer, and CEA are the independent influencing factors for the prognosis of colorectal cancer. Combined evaluation of independent factors is the most accurate method to predict the prognosis of colorectal cancer.
Research perspectives
On the purpose of avoiding the interference caused by the differences of individual and environmental factors, multi-center studies would be considered to enlarge the size of simple to improve the reliability of the research results. Besides that, long-term research is also planned to make up the ignorance of the factors affecting long-term prognosis in this study.
Footnotes
Institutional review board statement: The study was approved by the Ethics Committee of Cangzhou Central Hospital.
Informed consent statement: All patients gave informed consent.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: June 18, 2019
First decision: July 31, 2019
Article in press: August 26, 2019
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B,
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jorge AG, Orbell JH, Garcia SS S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu MY
Contributor Information
Li-Jun Jin, Department of Surgical Oncology (Division III), Cangzhou Central Hospital, Cangzhou 061001, Hebei Province, China.
Wei-Bin Chen, Department of Radiology, North China University of Science and Technology Affiliated Hospital, Tangshan 063000, Hebei Province, China.
Xiao-Yu Zhang, Department of Surgical Oncology (Division III), Cangzhou Central Hospital, Cangzhou 061001, Hebei Province, China.
Jie Bai, Department of Surgical Oncology (Division III), Cangzhou Central Hospital, Cangzhou 061001, Hebei Province, China.
Hao-Chen Zhao, Department of Anesthesiology (Division II), Cangzhou Central Hospital, Cangzhou 061001, Hebei Province, China.
Zun-Yi Wang, Department of Surgical Oncology (Division III), Cangzhou Central Hospital, Cangzhou 061001, Hebei Province, China. czwzy99@163.com.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Barontini J, Antinucci M, Tofanelli S, Cammalleri M, Dal Monte M, Gemignani F, Vodicka P, Marangoni R, Vodickova L, Kupcinskas J, Vymetalkova V, Forsti A, Canzian F, Stein A, Moreno V, Mastrodonato N, Tavano F, Panza A, Barale R, Landi S, Campa D. Association between polymorphisms of TAS2R16 and susceptibility to colorectal cancer. BMC Gastroenterol. 2017;17:104. doi: 10.1186/s12876-017-0659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan C, Quintero E, Dumonceau JM, Regula J, Brandão C, Chaussade S, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Gimeno-García A, Hazewinkel Y, Jover R, Kalager M, Loberg M, Pox C, Rembacken B, Lieberman D European Society of Gastrointestinal Endoscopy. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–851. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, He X, Pan J, Chen S, Wang L. Prognostic role of Glasgow prognostic score in patients with colorectal cancer: evidence from population studies. Sci Rep. 2017;7:6144. doi: 10.1038/s41598-017-06577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He L, Li H, Cai J, Chen L, Yao J, Zhang Y, Xu W, Geng L, Yang M, Chen P, Zheng J, Yang Y, Gong S. Prognostic Value of the Glasgow Prognostic Score or Modified Glasgow Prognostic Score for Patients with Colorectal Cancer Receiving Various Treatments: a Systematic Review and Meta-Analysis. Cell Physiol Biochem. 2018;51:1237–1249. doi: 10.1159/000495500. [DOI] [PubMed] [Google Scholar]
- 6.Eren T, Burcu B, Tombalak E, Ozdemir T, Leblebici M, Ozemir IA, Ziyade S, Alimoglu O. Clinical Significance of the Glasgow Prognostic Score for Survival after Colorectal Cancer Surgery. J Gastrointest Surg. 2016;20:1231–1238. doi: 10.1007/s11605-016-3114-2. [DOI] [PubMed] [Google Scholar]
- 7.Arfa N, Hamdani I, Gharbi L, Ben Abid S, Ghariani B, Mannai S, Mestiri H, Khalfallah MT, Mzabi SR. [Survival and prognostic factors of colorectal adenocarcinoma: analytic multifactor review of 150 cases] Ann Chir. 2006;131:104–111. doi: 10.1016/j.anchir.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Kanemitsu Y, Kato T, Hirai T, Yasui K, Morimoto T, Shimizu Y, Kodera Y, Yamamura Y. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum. 2003;46:160–167. doi: 10.1007/s10350-004-6518-0. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Remaileh M, Bender S, Raddatz G, Ansari I, Cohen D, Gutekunst J, Musch T, Linhart H, Breiling A, Pikarsky E, Bergman Y, Lyko F. Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Res. 2015;75:2120–2130. doi: 10.1158/0008-5472.CAN-14-3295. [DOI] [PubMed] [Google Scholar]
- 10.Deme D, Telekes A. [Prognostic importance of plasma C-reactive protein (CRP) in oncology] Orv Hetil. 2017;158:243–256. doi: 10.1556/650.2017.30646. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Xu Q, Chen L, Luo C, Ying J, Liu J. C-reactive protein (CRP) as a prognostic factor for colorectal cancer after surgical resection of pulmonary metastases. Bull Cancer. 2017;104:232–236. doi: 10.1016/j.bulcan.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Tian GW, Wang Y, Zhang H, Wang ZH, Li G. Prognostic Role of the Pretreatment C-Reactive Protein/Albumin Ratio in Solid Cancers: A Meta-Analysis. Sci Rep. 2017;7:41298. doi: 10.1038/srep41298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic Significance of the Preoperative Ratio of C-Reactive Protein to Albumin in Patients with Colorectal Cancer. Anticancer Res. 2016;36:995–1001. [PubMed] [Google Scholar]
- 14.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22:803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 15.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical Significance of the C-Reactive Protein to Albumin Ratio for Survival After Surgery for Colorectal Cancer. Ann Surg Oncol. 2016;23:900–907. doi: 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- 16.Koh YW, Lee HW. Prognostic impact of C-reactive protein/albumin ratio on the overall survival of patients with advanced nonsmall cell lung cancers receiving palliative chemotherapy. Medicine (Baltimore) 2017;96:e6848. doi: 10.1097/MD.0000000000006848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai PL, Su WJ, Leung WH, Lai CT, Liu CK. Neutrophil-lymphocyte ratio and CEA level as prognostic and predictive factors in colorectal cancer: A systematic review and meta-analysis. J Cancer Res Ther. 2016;12:582–589. doi: 10.4103/0973-1482.144356. [DOI] [PubMed] [Google Scholar]
- 18.Akinci Ozyurek B, Sahin Ozdemirel T, Buyukyaylaci Ozden S, Erdogan Y, Kaplan B, Kaplan T. Prognostic Value of the Neutrophil to Lymphocyte Ratio (NLR) in Lung Cancer Cases. Asian Pac J Cancer Prev. 2017;18:1417–1421. doi: 10.22034/APJCP.2017.18.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashtak S, Ruan X, Druliner BR, Liu H, Therneau T, Mouchli M, Boardman LA. Peripheral Neutrophil to Lymphocyte Ratio Improves Prognostication in Colon Cancer. Clin Colorectal Cancer. 2017;16:115–123.e3. doi: 10.1016/j.clcc.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kee KM, Wang JH, Lee CM, Chen CL, Changchien CS, Hu TH, Cheng YF, Hsu HC, Wang CC, Chen TY, Lin CY, Lu SN. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer. 2007;120:2650–2655. doi: 10.1002/ijc.22616. [DOI] [PubMed] [Google Scholar]
- 21.Jideh B, Bourke MJ. Colorectal cancer screening reduces incidence, mortality and morbidity. Med J Aust. 2018;208:483–484. doi: 10.5694/mja18.00279. [DOI] [PubMed] [Google Scholar]
- 22.Das V, Kalita J, Pal M. Predictive and prognostic biomarkers in colorectal cancer: A systematic review of recent advances and challenges. Biomed Pharmacother. 2017;87:8–19. doi: 10.1016/j.biopha.2016.12.064. [DOI] [PubMed] [Google Scholar]
- 23.Stojkovic Lalosevic M, Stankovic S, Stojkovic M, Markovic V, Dimitrijevic I, Lalosevic J, Petrovic J, Brankovic M, Pavlovic Markovic A, Krivokapic Z. Can preoperative CEA and CA19-9 serum concentrations suggest metastatic disease in colorectal cancer patients? Hell J Nucl Med. 2017;20:41–45. doi: 10.1967/s002449910505. [DOI] [PubMed] [Google Scholar]
- 24.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA. 1993;270:943–947. [PubMed] [Google Scholar]
- 25.Cao B, Zhou X, Yang W, Ma J, Zhou W, Fan D, Hong L. The role of cell-free DNA in predicting colorectal cancer prognosis. Expert Rev Gastroenterol Hepatol. 2018;12:39–48. doi: 10.1080/17474124.2017.1372191. [DOI] [PubMed] [Google Scholar]
- 26.Sun D, Chen J, Liu L, Zhao G, Dong P, Wu B, Wang J, Dong L. Establishment of a 12-gene expression signature to predict colon cancer prognosis. PeerJ. 2018;6:e4942. doi: 10.7717/peerj.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long AG, Lundsmith ET, Hamilton KE. Inflammation and Colorectal Cancer. Curr Colorectal Cancer Rep. 2017;13:341–351. doi: 10.1007/s11888-017-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei Z, Liu Y, Liu C, Cui L. Response to comment on 'Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis'. Br J Cancer. 2014;111:2372–2373. doi: 10.1038/bjc.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen L, Mo M, Jia L, Jia H, Li Q, Liang L, Shi D, Zhang Z, Cai S, Li X, Zhu J. Poorer prognosis in young female patients with non-metastatic colorectal cancer: a hospital-based analysis of 5,047 patients in China. Cancer Manag Res. 2018;10:653–661. doi: 10.2147/CMAR.S159901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lujan J, Valero G, Biondo S, Espin E, Parrilla P, Ortiz H. Laparoscopic versus open surgery for rectal cancer: results of a prospective multicentre analysis of 4,970 patients. Surg Endosc. 2013;27:295–302. doi: 10.1007/s00464-012-2444-8. [DOI] [PubMed] [Google Scholar]
- 31.McKay A, Donaleshen J, Helewa RM, Park J, Wirtzfeld D, Hochman D, Singh H, Turner D. Does young age influence the prognosis of colorectal cancer: a population-based analysis. World J Surg Oncol. 2014;12:370. doi: 10.1186/1477-7819-12-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg. 2004;187:343–348. doi: 10.1016/j.amjsurg.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Chou CL, Chang SC, Lin TC, Chen WS, Jiang JK, Wang HS, Yang SH, Liang WY, Lin JK. Differences in clinicopathological characteristics of colorectal cancer between younger and elderly patients: an analysis of 322 patients from a single institution. Am J Surg. 2011;202:574–582. doi: 10.1016/j.amjsurg.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Yeo SA, Chew MH, Koh PK, Tang CL. Young colorectal carcinoma patients do not have a poorer prognosis: a comparative review of 2,426 cases. Tech Coloproctol. 2013;17:653–661. doi: 10.1007/s10151-013-0977-z. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Cai G, Li D, Wang Y, Zhuo C, Cai S. Better long-term survival in young patients with non-metastatic colorectal cancer after surgery, an analysis of 69,835 patients in SEER database. PLoS One. 2014;9:e93756. doi: 10.1371/journal.pone.0093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le D, Holt CL, Pisu M, Brown-Galvan A, Fairley TL, Lee Smith J, White A, Hall IJ, Oster RA, Martin MY. The role of social support in posttreatment surveillance among African American survivors of colorectal cancer. J Psychosoc Oncol. 2014;32:245–263. doi: 10.1080/07347332.2014.897293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takii Y, Maruyama S, Nogami H. Can the prognosis of colorectal cancer be improved by surgery? World J Gastrointest Surg. 2016;8:574–577. doi: 10.4240/wjgs.v8.i8.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paradiso A, Rabinovich M, Vallejo C, Machiavelli M, Romero A, Perez J, Lacava J, Cuevas MA, Rodriquez R, Leone B, Sapia MG, Simone G, De Lena M. p53 and PCNA expression in advanced colorectal cancer: response to chemotherapy and long-term prognosis. Int J Cancer. 1996;69:437–441. doi: 10.1002/(SICI)1097-0215(19961220)69:6<437::AID-IJC2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Verma S, Das P, Kumar VL. Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chem Biol Interact. 2017;278:84–91. doi: 10.1016/j.cbi.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Risio M, Rocci MP. Intermediate biomarkers in the colorectal tumor progression. Tumori. 1995;81:16–18. [PubMed] [Google Scholar]
- 41.Lv Q, Zhang J, Yi Y, Huang Y, Wang Y, Wang Y, Zhang W. Proliferating Cell Nuclear Antigen Has an Association with Prognosis and Risks Factors of Cancer Patients: a Systematic Review. Mol Neurobiol. 2016;53:6209–6217. doi: 10.1007/s12035-015-9525-3. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka S, Haruma K, Tatsuta S, Hiraga Y, Teixeira CR, Shimamoto F, Yoshihara M, Sumii K, Kajiyama G. Proliferating cell nuclear antigen expression correlates with the metastatic potential of submucosal invasive colorectal carcinoma. Oncology. 1995;52:134–139. doi: 10.1159/000227444. [DOI] [PubMed] [Google Scholar]
- 43.Farhan-Alanie OM, McMahon J, McMillan DC. Systemic inflammatory response and survival in patients undergoing curative resection of oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2015;53:126–131. doi: 10.1016/j.bjoms.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brünner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487–495. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 45.Isella C, Terrasi A, Bellomo SE, Petti C, Galatola G, Muratore A, Mellano A, Senetta R, Cassenti A, Sonetto C, Inghirami G, Trusolino L, Fekete Z, De Ridder M, Cassoni P, Storme G, Bertotti A, Medico E. Stromal contribution to the colorectal cancer transcriptome. Nat Genet. 2015;47:312–319. doi: 10.1038/ng.3224. [DOI] [PubMed] [Google Scholar]
- 46.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11:223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 47.İnal T, Anar C, Polat G, Ünsal İ, Halilçolar H. The prognostic value of D-dimer in lung cancer. Clin Respir J. 2015;9:305–313. doi: 10.1111/crj.12144. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Zhang X, Yan B, Gu Q, Zhang X, Jiao J, Sun D, Wang N, Yue X. Elevated plasma D-dimer levels correlate with long term survival of gastric cancer patients. PLoS One. 2014;9:e90547. doi: 10.1371/journal.pone.0090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Man YN, Wang YN, Hao J, Liu X, Liu C, Zhu C, Wu XZ. Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patients with epithelial ovarian cancer independently of venous thromboembolism. Int J Gynecol Cancer. 2015;25:24–32. doi: 10.1097/IGC.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 50.Wind J, Duineveld LA, van der Heijden RP, van Asselt KM, Bemelman WA, van Weert HC. Follow-up after colon cancer treatment in the Netherlands; a survey of patients, GPs, and colorectal surgeons. Eur J Surg Oncol. 2013;39:837–843. doi: 10.1016/j.ejso.2013.04.001. [DOI] [PubMed] [Google Scholar]