Abstract
An increasing number of patients are being referred to pancreatic centres around the world due to often incidentally discovered cystic neoplasms of the pancreas. The evaluation and management of pancreatic cystic neoplasms is a controversial topic and with existing guidelines based on a lack of strong evidence there is discordance between centres and guidelines with regard to when to offer surgery and when to favour surveillance. The frequency, duration and modality of surveillance is also controversial as this is resource-consuming and must be balanced against the perceived benefits and risks involved. While there is consensus that the risk of malignancy should be balanced against the life-expectancy and comorbidities, the indications for surgery and surveillance strategies vary among the guidelines. Thus, the tug of war between surveillance or resection continues. Here we discuss the recommendations from guidelines with further accumulating data and emerging reports on intraductal papillary mucinous neoplasm in the literature.
Keywords: Neoplasia, Pancreatic cancer, Pancreatic cyst, Diagnosis, Resection, Surveillance, Mutation, Biomarker
Core tip: For patients with intraductal papillary mucinous neoplasia detected in the pancreas, there is currently debate over the frequency, duration and modality of surveillance in the long-term. Surveillance is resource-consuming and must be balanced against the likely benefits and perceived risks for malignant transformation. Furthermore, the risk of malignancy should be balanced against the overall life-expectancy and comorbidities. Notably, the indications for either surgery or surveillance vary among the available guidelines. Thus, the tug of war between surveillance or resection continues. The recommendations from existing guidelines are highlighted with further accumulating data and emerging reports from the intraductal papillary mucinous neoplasm literature.
INTRODUCTION
An increasing number of patients are being referred to pancreatic centres around the world due to often incidentally discovered cystic neoplasms of the pancreas. High quality radiological imaging is increasingly utilised and although pancreatic cystic neoplasms have most likely always existed, it is only more recently that guidelines for follow-up, diagnosis and management have been issued. These guidelines themselves are based on the scarce data available. Intraductal papillary mucinous neoplasm (IPMN) was first described in 1982 by Ohashi and was, until the turn of the millennium, considered a rare entity but is now among the most commonly discovered and surgically resected pancreatic cystic lesions[2].
The evaluation and management of pancreatic cystic neoplasms is a controversial topic, and the existing guidelines are based on a lack of strong evidence. Thus, there is discordance between practice among centres and the available guidelines with regard to when and to whom surgery should be offered and when to favour surveillance. The frequency, duration and modality of surveillance is also controversial, as this strategy is resource-consuming and must be balanced against the perceived benefits and risks involved.
The first guidelines came after the turn of the millennium, with four guidelines now in place (Table 1), including the International Association of Pancreatology (IAP, also called “Fukuoka”) guidelines, European Study Group on Cystic Tumors of the Pancreas[4], American Gastroenterological Association (AGA) and American College of Gastroenterology (ACG) clinical guideline[6]. While there is consensus that the risk of malignancy should be balanced against life-expectancy and comorbidities, the indications for surgery and surveillance strategies vary among the guidelines. Thus, the tug of war between surveillance or resection continues (Figure 1). Here we discuss the recommendations from guidelines with further accumulating data and emerging reports on IPMN in the literature.
Table 1.
Comparison of existing guidelines for intraductal papillary mucinous neoplasia of the pancreas
| Guideline (yr) | IAP (2017) | European (2018) | AGA (2015) | ACG (2018) |
| Resection criteria | ≥ 1 high risk stigmata | ≥ 1 absolute indication | Solid component and dilated MPD and/or concerning features on EUS-FNA | Decided by multidisciplinary team. Refer if ≥ 1 high risk characteristics |
| ≥ 1 worrisome feature and ≥ 1 of: definitive mural nodule ≥ 5 mm, MPD involvement, suspicious or positive cytology. | ≥ 1 relative indication without significant co-morbidities | |||
| Consider surgery in young fit patients with cysts > 2 cm | ≥ 2 relative indications with significant co-morbidities | |||
| MD-/MT IPMN if ≥ 1 high risk stigmata | MD-/MT IPMN | |||
| High risk features/surgery indications | High risk stigmata: Jaundice; Enhancing mural nodule > 5 mm; MPD > 10 mm | Absolute criteria: Jaundice; Enhancing mural nodule ≥ 5 mm; MPD ≥ 10 mm; Solid mass; Positive cytology | High risk features: Cyst size ≥ 3 cm; Dilated MPD; Solid component | High-risk characteristics: Jaundice; Mural nodule/solid component; MPD > 5 mm; Abrupt pancreatic duct calibre change with distal atrophy; Cyst size ≥ 3 mm; Cyst growth 3 mm/yr; Positive cytology; Pancreatitis secondary to cyst; Elevated serum Ca19-9 |
| Worrisome features: Pancreatitis secondary to cyst; Cyst size ≥ 3 cm; Enhancing mural nodule < 5 mm; Enhancing thickened cyst wall; MPD 5-9 mm; Abrupt pancreatic duct calibre change with distal atrophy; Growth ≥ 5 mm/2 yr; Elevated serum Ca19-9 | Relative indications: Pancreatitis secondary to cyst; Cyst diameter ≥ 40 mm; Enhancing mural nodule < 5 mm; MPD 5-9 mm; Growth rate > 5 mm/yr; New onset diabetes mellitus; Elevated serum Ca19-9 | |||
| Surveillance intervals | < 1 cm: 6 mo, then every 2 yr; 1-2 cm: 6 mo 1st yr, yearly for 2 yr, then every 2 yr; 2-3 cm: 3-6 mo 1st yr, then yearly; > 3 cm 3-6 mo | 6 mo 1st yr, then yearly | 1, 3 and 5 yr | < 1 cm: Every 2 yr; 1-2 cm: Every 1 yr; 2-3 cm: Every 6-12 mo; > 3 cm Every 6 mo and consider referral to MDT |
| Surveillance modality | < 2 cm MRI or CT; 2 cm MRI and EUS | MRI and/or EUS Serum Ca19-9 | MRI | MRI and/or EUS |
IAP: International Association of Pancreatology; AGA: American Gastroenterology Association; ACG: American College of Gastroenterology; MPD: Main pancreatic duct; MRI, Magnetic resonance imaging; EUS: Endoscopic ultrasound; FNA: Fine needle aspiration; IPMN: Intraductal papillary mucinous neoplasm; CT: Computed tomography; MDT: Multidisciplinary team; MD: Main duct; MT: Mixed type.
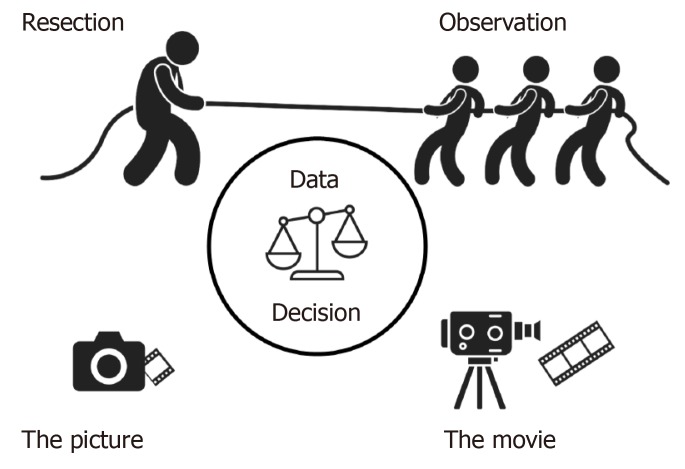
Figure 1.
Tug of war between resection and observation in intraductal papillary mucinous neoplasm. Depicted for illustration is the tug of war between strategies for intraductal papillary mucinous neoplasm, either resection or observation. Decision making is based on available data, which currently are conflicting. Reaction on first-event cross-sectional imaging (the Picture) may prevent information obtained from serial, temporal evaluation (the Movie) of how intraductal papillary mucinous neoplasm lesions may change in character, size and context.
IPMN IS A PREMALIGNANT CONDITION
IPMN harbours a malignant potential and is considered a premalignant condition of the pancreas. The underlying mechanisms of cancer progression and malignant evolution is poorly understood, but knowledge is emerging in this field[8-10]. Thus, the main rationale for surgery is to resect lesions that either harbour early invasive cancerous lesions or preferably to remove lesions that contain high-grade dysplasia but have yet to progress into invasive cancer. Based on recent data, approximately one in every four resected IPMN have histologically confirmed malignancy[11].
POPULATION AT RISK FOR IPMN
The exact incidence of IPMN is not known, but it is estimated to be present in 2%-45% of the general population[4]. Notably, many IPMNs are incidentally discovered during cross-sectional imaging performed for other unrelated medical reasons. This particularly holds true for the aging population when the prevalence of numerous other medical conditions increase[12], which leads to an increasing number of cross-sectional imaging being undertaken for work-up or surveillance. Thus, for transabdominal scans involving the pancreas, this often leads to the incidental detection of cystic lesions in the pancreas with a request for review by pancreatologists and surgeons. The influence of ageing on loss of functional reserve follows many of the same mechanisms that also lead to risk of premalignant and malignant disease. Thus, finding incidental IPMNs is likely to increase with the widespread use of cross-sectional imaging. Resection vs surveillance needs to be tailored to the likely clinical impact and outcome for the particular lesion and person. For one, extended surveillance needs to take into account the life-time risk of malignant development. Furthermore, when considering a need for resection, the type of surgery needs to be taken into account together with patient comorbidities. Notably, the risk profile, including mortality and morbidity is considerably different in pancreatoduodenectomy (mortality 3%-4%) compared to a distal resection (mortality < 1.5%)[14,15]. Furthermore, enucleations and central resections have their own morbidity profile. Lastly, a total pancreatectomy (sometimes indicated for disease affecting the entire gland) needs to be considered carefully as the risk, benefit and long-term consequences are considerable.
CONSIDERING PATIENT FITNESS
An important factor in limiting overtreatment and morbidity from surgery is careful patient selection by limiting surgery in highly comorbid surgical candidates. Progression to cancer, even in the presence of worrisome features, may be less common than previously thought, according to recent observational studies. In one observational group of IPMNs with worrisome features, the 5 year disease specific survival was 96% suggesting that conservative management was appropriate, but high-risk stigmata is associated with a 40% risk of IPMN-related death suggesting surgery as the treatment of choice in fit patients[18]. In a meta-analysis on patients unfit for surgery but with worrisome features and high risk stigmata, the IPMN related mortality was low, especially for branched duct IPMN[19], and death from other causes was much more common. In another observational study of low risk lesions, malignancy occurred rarely (4.2%), but the development of main pancreatic duct dilatation was strongly associated with malignant transformation[20].
RESECTION OR SURVEILLANCE–THE DILEMMA
Surgery is offered to a subset of patients with IPMN, yet pancreatic resection is associated with significant morbidity and even mortality. Thus, it is important to establish a better understanding of the impact of worrisome features so that surgery is offered when justified. The decision to resect an IPMN lesion is often based on any one of the mentioned guidelines, yet all current consensus guidelines are generated from low quality data. Also, even if based on low quality data, the recommendations vary considerably (Table 1). Furthermore, the indications may be “absolute” or “relative” (Table 1) with partial disagreement between guidelines and hence the ongoing debate among pancreatologists and pancreatic surgeons.
Both the European guidelines and the IAP guidelines justify resection if one or more is present: (A) Jaundice due to the IPMN; (B) Enhancing mural nodule over 5 mm; or (C) Main pancreatic duct dilatation over 10 mm (Table 1). These criteria are referred to as absolute indications in the European guidelines and high-risk stigmata in the IAP guidelines (Table 1). The European guidelines also opt for surgery if one or more of several relative indications are present in healthy individuals, which includes pancreatitis, cyst diameter over 40 mm, growth rate over 5 mm/year, new onset diabetes mellitus and elevated Ca19-9 among others. The IAP guidelines refer to many of the same criteria as worrisome features and also recommend surgery but only with some additional basic criteria present, namely main pancreatic duct involvement, mural nodule or positive/suspicious cytology. While the European and IAP guidelines are similar with regards to surgical indications, differences (such as cyst size) are present (Table 1). The European guidelines recommend surgery when cyst size is over 40 mm whereas the IAP guidelines opt for surgery if cyst size is over 20 mm in young fit individuals.
The AGA guidelines for surgical resection require both a solid component AND a dilated pancreatic duct OR a positive cytology on endoscopic ultrasound with fine-needle aspiration. AGA does not mention degree of dilatation of the main pancreatic duct.
The ACG guidelines fail to provide clear guidance on when to offer surgery but recommend referral to multidisciplinary teams if high risk characteristics are present. This provides opportunity for personalisation of treatment selection according to the teams and their members. For worrisome risk features, models have been proposed to predict risk of malignancy or high-grade dysplasia, with modest prediction values achieved[21].
In a retrospective study evaluating accuracy of resection criteria in patients with pancreatic cysts, the cohort included 75 patients with IPMN[22]. The investigators compared the final pathologic outcome of surgically removed pancreatic cysts with the recommended indications for resection according to three different guidelines (IAP, the European and AGA guidelines). For patients with suspected IPMN (n = 75), resection was justified in 36% of 67% (54%), 36% of 68% (53%) and 32% of 54% (59%) of patients based on the recommendations for surgery in the IAP, European and AGA guidelines, respectively[22]. The AGA guideline would have avoided resection in 21% of 75% (28%) patients, the IAP in 8% of 75% (11%) and the European in 7% of 75% (9%) if the guidelines would have been applied strictly. However, 4% of 33% patients (12%) with high-grade dysplasia or malignancy would have been missed with the AGA guidelines, compared with none if following the IAP or European guidelines[22]. This demonstrates that all guidelines will currently result in a degree of overtreatment, imposing a potential risk to the patient. The AGA guidelines, which represent the more conservative approach towards resection for IPMNs (Table 1), would have missed the surgical indication for two patients with malignancy and two patients with high-grade dysplasia[22]. This indicates that, based on the current guidelines, a degree of overtreatment is necessary in order to capture all malignant and high-grade dysplastic lesions.
IPMNS OF MAIN DUCT, BRANCH DUCT AND MIXED TYPE
Dilatation of the main duct is considered to be a strong risk feature for malignancy, yet controversy persists regarding optimal duct diameter cut-off for resection and the need for either observation or resection in main duct IPMNs[23,24]. The European guidelines recommend resection in all fit individuals, the IAP guidelines require the presence of any high-risk stigmata, the ACG recommends referral to a multidisciplinary team discussion whereas the AGA guidelines do not mention this group with main duct dilatation specifically. A recent study demonstrated a high risk for malignancy with main duct dilatation[23], while another study from Verona did not call main duct dilatation as a risk for malignancy for patients undergoing surveillance. Notably, the bi-institutional series[23] from Johns Hopkins and Karolinska was based on resected IPMNs, thus potentially biasing the results towards patients who underwent resection[23] rather than all patients diagnosed with a dilated main pancreatic duct. However, the Johns Hopkins/Karolinska series found an increased risk of malignancy even in the middle-ranged size of dilated main ducts (5-9 mm). In contrast, the Verona study included both resection and surveillance patients and did not find main duct dilatation alone to be a significant risk factor for malignancy, although it increased risk together with other worrisome features. Thus, we believe the jury is still out on the true risk associated with main duct dilatation in IPMN.
Branch duct IPMN are presumed to have a very low risk of malignancy, yet the risk is definite[20]. Controversy persists regarding criteria for continued observation or resection for these lesions, although the updated international guidelines aim to have better precision in predicting malignancy risk[25]. Initial cysts size (> 40 mm) and annual growth rate may be indicators for risk of malignancy in branch duct lesions observed over time[26]. A French study suggested that branch duct IPMN in men with recent onset diabetes should be considered for resection, as the incidence of malignancy and high-grade dysplasia was higher in this group[27]. This is in line with the evolving evidence that developing fasting blood glucose intolerance is associated with risk of pancreatic malignancy[28,29]. New onset diabetes associated with an IPMN is also a clear indication for resection in healthy individuals when following the European guidelines as it is a “relative indication” that should lead to resection in patients without comorbidities (Table 1). New onset diabetes is not a criterium for resection in the other guidelines, but the ACG guidelines acknowledges its importance and recommend further investigation and tighter surveillance.
INFORMED DECISION-MAKING: A STEPWISE APPROACH
The first step in the decision between surgery or surveillance of a pancreatic cystic lesion is making the correct diagnosis, which is not always straight forward in itself. In addition to evaluating the patient’s overall condition and fitness for surgery, information from a variety of modalities can be assimilated in order to enhance the decision-making process.
Transsectional body imaging by magnetic resonance imaging and computed tomography
Magnetic resonance imaging is usually the radiologic modality of choice for classification of IPMN. In the study by Lekkerkerker et al[22], preoperative diagnosis was correct in 80% of branch duct IPMNs and 89% of the main/mixed type IPMNs. In other high volume centres the preoperative diagnosis was incorrect in one third of the cases[30,31], and 20% of presumed branch duct IPMN had main duct involvement at postoperative histology. Among the conflicting and debated topics is the decision based on a cross-sectional image. Should one react on the “picture” or the “movie” (Figure 1)? While some argue that worrisome features present at first presentation would warrant resection, others uphold the view of further imaging to get a sense of the “movie” by depicting cystic progression (or stability) over time and thus make a decision to proceed with resection based on this time-dependent information. Obviously, the argument against such an approach is the risk of surveilling patients only to discover a lesion that has progressed to invasive (or even metastatic) carcinoma. Reports on progression to invasive cancer in some apparently innocuous lesions followed for many years is what concerns most pancreatic surgeons[32]. In one series, branch duct cysts that remained ≤ 1.5 cm after 5 years of follow up had a very low risk of progression to cancer[33]. However, in the population with cyst size > 1.5 cm, a reported 7.5% developed malignancy, thus suggesting these should be followed beyond 5 years of surveillance.
Endoscopic ultrasound
Contrast-enhanced endoscopic ultrasound has shown promising results with improved evaluation of mural nodules in cysts and subsequently the differentiation between malignant and non-malignant cysts[34]. Using the same contrast-enhanced endoscopic ultrasound with time intensity curves, blood flow and microvasculature density in IPMN cysts can be evaluated and this correlates with high-grade dysplasia/malignancy and has potential to differentiate them from low-grade dysplasia[35].
Tumour markers
Serum Ca19-9 and CEA are well-established tumour biomarkers that can help in decision making in some cases. Significantly elevated levels of these biomarkers in patients with worrisome lesions (without jaundice) would be suggestive of a higher risk of invasive cancer[36]. However, the overall sensitivity and specificity remains poor[37], and low values do not infer a benign condition. Thus, other biomarkers sampled from cyst fluid or other sources are emerging and of interest. Particular interest is expressed in next generation sequencing of pancreatic cystic fluid[38-41]. Investigators have reported KRAS/GNAS mutations to be present in 100% of IPMNs[39] and to be highly sensitive and specific (89% and 100%, respectively) for IPMNs and mucinous cystic lesions[39]. Furthermore, the addition of TP53/PIK3CA/PTEN evaluation provided an 88% sensitivity and 97% specificity for IPMNs with advanced neoplasia[39]. Further, evaluation of pancreatic juice mutation concentration by next generation sequencing may help distinguish high-grade from low-grade lesions, and mutant TP53/SMAD4 concentrations could distinguish patients with malignancy (e.g., invasive cancer or high-grade dysplasia) with a sensitivity and specificity of about 61% and 96%, respectively[42,43]. However, further validation is needed before these methods become integrated within the clinical setting.
In a large meta-analysis, a combination of cytology and immunohistochemical analysis of MUC1 and MUC2 in pancreatic juice samples identified malignant IPMNs with an area under the curve of 0.85 and sensitivity at 85% and specificity at 65%. In a test model, inclusion of cytologic analysis of pancreatic juice in the guideline algorithm significantly increased the specificity of detection of malignant IPMNs[44].
More advanced techniques including targeted mass spectrometry of peptides mucin-5AC, mucin 2 and prostate stem cell antigen could identify high-grade dysplasia/cancer with an accuracy of 96%. Thus, there are several ongoing studies and potential future tools for improved diagnostics and with potential to enhance surveillance algorithms in the future.
FUTURE PERSPECTIVES IN THE TUG OF WAR BETWEEN RESECTION AND OBSERVATION
The future strategy for IPMN treatment should be first and foremost to enhance the evidence that forms the basis of clinical guidelines currently used to guide cystic neoplasm management. Large-scale prospective registries of individuals undergoing cyst surveillance such as the PACYFIC-registry are required to accumulate unbiased data that will ultimately inform evidence-based guidelines. Furthermore, we must improve our understanding of the evolutionary biology underlying pancreatic cystic neoplasms, including IPMNs[9,46]. Improved understanding of the underlying biology will enhance our understanding of risk of malignant transformation beyond the stratification provided by traditional anatomical and radiological subsets of main duct and side branch IPMNs. In addition to the development of molecular assessment of biopsy specimens, the future role of non-invasive assessment techniques, including liquid biopsy and enhanced magnetic resonance imaging may be required in order to detect high-risk lesions early and simultaneously reduce the costs for life-time surveillance strategies. Alas, until this has been achieved the tug of war in management of IPMN lesions will continue.
CONCLUSION
IPMN of the pancreas is increasingly detected in the population, often as an incidental finding. Several guidelines have been proposed to provide criteria for observation or resection. The debate continues as to how to identify the most appropriate surgical candidate. Hence, the tug of war between surveillance and surgery continues until better evidence for decision making has been achieved.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: May 23, 2019
First decision: August 23, 2019
Article in press: September 26, 2019
Specialty type: Oncology
Country of origin: Norway
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cerwenka H S-Editor: Zhang L L-Editor: Filipodia E-Editor: Qi LL
Contributor Information
Jan Rune Aunan, Department of Gastrointestinal Surgery, HPB Unit, Stavanger University Hospital, Stavanger 4068, Norway.
Nigel B. Jamieson, Wolfson Wohl Cancer Research Centre, Institute of Cancer Sciences, University of Glasgow, Glasgow G61 1BD, United Kingdom West of Scotland Pancreatic Unit, Glasgow Royal Infirmary, Glasgow G4 0SF, United Kingdom.
Kjetil Søreide, Department of Gastrointestinal Surgery, HPB Unit, Stavanger University Hospital, Stavanger 4068, Norway; Gastrointestinal Translational Research Unit, Laboratory for Molecular Medicine, Stavanger University Hospital, Stavanger 4068, Norway; Department of Clinical Medicine, University of Bergen, Bergen 5003, Norway. ksoreide@mac.com.
References
- 1.Tollefson MK, Libsch KD, Sarr MG, Chari ST, DiMagno EP, Urrutia R, Smyrk TC. Intraductal papillary mucinous neoplasm: did it exist prior to 1980? Pancreas. 2003;26:e55–e58. doi: 10.1097/00006676-200304000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4–12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 4.European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. doi: 10.1136/gutjnl-2018-316027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vege SS, Ziring B, Jain R, Moayyedi P Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–22; quize12-3. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464–479. doi: 10.1038/ajg.2018.14. [DOI] [PubMed] [Google Scholar]
- 7.Levink I, Bruno MJ, Cahen DL. Management of Intraductal Papillary Mucinous Neoplasms: Controversies in Guidelines and Future Perspectives. Curr Treat Options Gastroenterol. 2018;16:316–332. doi: 10.1007/s11938-018-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka M. Intraductal Papillary Mucinous Neoplasm as the Focus for Early Detection of Pancreatic Cancer. Gastroenterology. 2018;154:475–478. doi: 10.1053/j.gastro.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Barco YG, Bardeesy N, Ting DT. No Cell Left Unturned: Intraductal Papillary Mucinous Neoplasm Heterogeneity. Clin Cancer Res. 2019;25:2027–2029. doi: 10.1158/1078-0432.CCR-18-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omori Y, Ono Y, Tanino M, Karasaki H, Yamaguchi H, Furukawa T, Enomoto K, Ueda J, Sumi A, Katayama J, Muraki M, Taniue K, Takahashi K, Ambo Y, Shinohara T, Nishihara H, Sasajima J, Maguchi H, Mizukami Y, Okumura T, Tanaka S. Pathways of Progression From Intraductal Papillary Mucinous Neoplasm to Pancreatic Ductal Adenocarcinoma Based on Molecular Features. Gastroenterology. 2019;156:647–661.e2. doi: 10.1053/j.gastro.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Khoury RE, Kabir C, Maker VK, Banulescu M, Wasserman M, Maker AV. What is the Incidence of Malignancy in Resected Intraductal Papillary Mucinous Neoplasms? An Analysis of Over 100 US Institutions in a Single Year. Ann Surg Oncol. 2018;25:1746–1751. doi: 10.1245/s10434-018-6425-6. [DOI] [PubMed] [Google Scholar]
- 12.Aunan JR, Watson MM, Hagland HR, Søreide K. Molecular and biological hallmarks of ageing. Br J Surg. 2016;103:e29–e46. doi: 10.1002/bjs.10053. [DOI] [PubMed] [Google Scholar]
- 13.Aunan JR, Cho WC, Søreide K. The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks. Aging Dis. 2017;8:628–642. doi: 10.14336/AD.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nymo LS, Søreide K, Kleive D, Olsen F, Lassen K. The effect of centralization on short term outcomes of pancreatoduodenectomy in a universal health care system. HPB (Oxford) 2019;21:319–327. doi: 10.1016/j.hpb.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Søreide K, Olsen F, Nymo LS, Kleive D, Lassen K. A nationwide cohort study of resection rates and short-term outcomes in open and laparoscopic distal pancreatectomy. HPB (Oxford) 2019;21:669–678. doi: 10.1016/j.hpb.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Pulvirenti A, Pea A, Rezaee N, Gasparini C, Malleo G, Weiss MJ, Cameron JL, Wolfgang CL, He J, Salvia R. Perioperative outcomes and long-term quality of life after total pancreatectomy. Br J Surg. 2019 doi: 10.1002/bjs.11185. [DOI] [PubMed] [Google Scholar]
- 17.Crinò SF, Frulloni L. Pancreatic cyst: What clinician needs? Endosc Ultrasound. 2018;7:293–296. doi: 10.4103/eus.eus_37_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crippa S, Bassi C, Salvia R, Malleo G, Marchegiani G, Rebours V, Levy P, Partelli S, Suleiman SL, Banks PA, Ahmed N, Chari ST, Fernández-Del Castillo C, Falconi M. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut. 2017;66:495–506. doi: 10.1136/gutjnl-2015-310162. [DOI] [PubMed] [Google Scholar]
- 19.Vanella G, Crippa S, Archibugi L, Arcidiacono PG, Delle Fave G, Falconi M, Capurso G. Meta-analysis of mortality in patients with high-risk intraductal papillary mucinous neoplasms under observation. Br J Surg. 2018;105:328–338. doi: 10.1002/bjs.10768. [DOI] [PubMed] [Google Scholar]
- 20.Petrone MC, Magnoni P, Pergolini I, Capurso G, Traini M, Doglioni C, Mariani A, Crippa S, Arcidiacono PG. Long-term follow-up of low-risk branch-duct IPMNs of the pancreas: is main pancreatic duct dilatation the most worrisome feature? Clin Transl Gastroenterol. 2018;9:158. doi: 10.1038/s41424-018-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu Y, Hijioka S, Hirono S, Kin T, Ohtsuka T, Kanno A, Koshita S, Hanada K, Kitano M, Inoue H, Itoi T, Ueki T, Matsuo K, Yanagisawa A, Yamaue H, Sugiyama M, Okazaki K. New Model for Predicting Malignancy in Patients With Intraductal Papillary Mucinous Neoplasm. Ann Surg. 2018 doi: 10.1097/SLA.0000000000003108. [DOI] [PubMed] [Google Scholar]
- 22.Lekkerkerker SJ, Besselink MG, Busch OR, Verheij J, Engelbrecht MR, Rauws EA, Fockens P, van Hooft JE. Comparing 3 guidelines on the management of surgically removed pancreatic cysts with regard to pathological outcome. Gastrointest Endosc. 2017;85:1025–1031. doi: 10.1016/j.gie.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Del Chiaro M, Beckman R, Ateeb Z, Orsini N, Rezaee N, Manos L, Valente R, Yuan C, Ding D, Margonis GA, Yin L, Cameron JL, Makary MA, Burkhart RA, Weiss MJ, He J, Arnelo U, Yu J, Wolfgang CL. Main Duct Dilatation Is the Best Predictor of High-grade Dysplasia or Invasion in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann Surg. 2019 doi: 10.1097/SLA.0000000000003174. [DOI] [PubMed] [Google Scholar]
- 24.Marchegiani G, Andrianello S, Morbin G, Secchettin E, D'Onofrio M, De Robertis R, Malleo G, Bassi C, Salvia R. Importance of main pancreatic duct dilatation in IPMN undergoing surveillance. Br J Surg. 2018;105:1825–1834. doi: 10.1002/bjs.10948. [DOI] [PubMed] [Google Scholar]
- 25.Jang JY, Park T, Lee S, Kang MJ, Lee SY, Lee KB, Chang YR, Kim SW. Validation of international consensus guidelines for the resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg. 2014;101:686–692. doi: 10.1002/bjs.9491. [DOI] [PubMed] [Google Scholar]
- 26.Han Y, Lee H, Kang JS, Kim JR, Kim HS, Lee JM, Lee KB, Kwon W, Kim SW, Jang JY. Progression of Pancreatic Branch Duct Intraductal Papillary Mucinous Neoplasm Associates With Cyst Size. Gastroenterology. 2018;154:576–584. doi: 10.1053/j.gastro.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Duconseil P, Adham M, Sauvanet A, Autret A, Périnel J, Chiche L, Mabrut JY, Tuech JJ, Mariette C, Turrini O. Fukuoka-Negative Branch-Duct IPMNs: When to Worry? A Study from the French Surgical Association (AFC) Ann Surg Oncol. 2018;25:1017–1025. doi: 10.1245/s10434-017-6318-0. [DOI] [PubMed] [Google Scholar]
- 28.Sharma A, Smyrk TC, Levy MJ, Topazian MA, Chari ST. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology. 2018;155:490–500.e2. doi: 10.1053/j.gastro.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soreide K. Sweet Predictions Speak Volumes for Early Detection of Pancreatic Cancer. Gastroenterology. 2018;155:265–268. doi: 10.1053/j.gastro.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 30.Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernández-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2010;10:144–150. doi: 10.1159/000243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Chiaro M, Segersvärd R, Pozzi Mucelli R, Rangelova E, Kartalis N, Ansorge C, Arnelo U, Blomberg J, Löhr M, Verbeke C. Comparison of preoperative conference-based diagnosis with histology of cystic tumors of the pancreas. Ann Surg Oncol. 2014;21:1539–1544. doi: 10.1245/s10434-013-3465-9. [DOI] [PubMed] [Google Scholar]
- 32.Honselmann KC, Patino M, Mino-Kenudson M, Ferrone C, Warshaw AL, Castillo CF, Lillemoe KD. Ductal Carcinoma Arising in a Largely Unchanged Presumed Branch-duct IPMN After 10 Years of Surveillance. Ann Surg. 2017;266:e38–e40. doi: 10.1097/SLA.0000000000002238. [DOI] [PubMed] [Google Scholar]
- 33.Pergolini I, Sahora K, Ferrone CR, Morales-Oyarvide V, Wolpin BM, Mucci LA, Brugge WR, Mino-Kenudson M, Patino M, Sahani DV, Warshaw AL, Lillemoe KD, Fernández-Del Castillo C. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology. 2017;153:1284–1294.e1. doi: 10.1053/j.gastro.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Kamata K, Kitano M, Omoto S, Kadosaka K, Miyata T, Yamao K, Imai H, Sakamoto H, Harwani Y, Chikugo T, Chiba Y, Matsumoto I, Takeyama Y, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy. 2016;48:35–41. doi: 10.1055/s-0034-1393564. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto N, Kato H, Tomoda T, Matsumoto K, Sakakihara I, Noma Y, Horiguchi S, Harada R, Tsutsumi K, Hori K, Tanaka T, Okada H, de Yamamoto K. Contrast-enhanced harmonic endoscopic ultrasonography with time-intensity curve analysis for intraductal papillary mucinous neoplasms of the pancreas. Endoscopy. 2016;48:26–34. doi: 10.1055/s-0034-1393563. [DOI] [PubMed] [Google Scholar]
- 36.Fritz S, Hackert T, Hinz U, Hartwig W, Büchler MW, Werner J. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br J Surg. 2011;98:104–110. doi: 10.1002/bjs.7280. [DOI] [PubMed] [Google Scholar]
- 37.Maker AV, Carrara S, Jamieson NB, Pelaez-Luna M, Lennon AM, Dal Molin M, Scarpa A, Frulloni L, Brugge WR. Cyst fluid biomarkers for intraductal papillary mucinous neoplasms of the pancreas: a critical review from the international expert meeting on pancreatic branch-duct-intraductal papillary mucinous neoplasms. J Am Coll Surg. 2015;220:243–253. doi: 10.1016/j.jamcollsurg.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volckmar AL, Endris V, Gaida MM, Leichsenring J, Stögbauer F, Allgäuer M, von Winterfeld M, Penzel R, Kirchner M, Brandt R, Neumann O, Sültmann H, Schirmacher P, Rudi J, Schmitz D, Stenzinger A. Next generation sequencing of the cellular and liquid fraction of pancreatic cyst fluid supports discrimination of IPMN from pseudocysts and reveals cases with multiple mutated driver clones: First findings from the prospective ZYSTEUS biomarker study. Genes Chromosomes Cancer. 2019;58:3–11. doi: 10.1002/gcc.22682. [DOI] [PubMed] [Google Scholar]
- 39.Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131–2141. doi: 10.1136/gutjnl-2016-313586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenbaum MW, Jones M, Dudley JC, Le LP, Iafrate AJ, Pitman MB. Next-generation sequencing adds value to the preoperative diagnosis of pancreatic cysts. Cancer Cytopathol. 2017;125:41–47. doi: 10.1002/cncy.21775. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Paris PL, Chen J, Ngo V, Yao H, Frazier ML, Killary AM, Liu CG, Liang H, Mathy C, Bondada S, Kirkwood K, Sen S. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett. 2015;356:404–409. doi: 10.1016/j.canlet.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suenaga M, Yu J, Shindo K, Tamura K, Almario JA, Zaykoski C, Witmer PD, Fesharakizadeh S, Borges M, Lennon AM, Shin EJ, Canto MI, Goggins M. Pancreatic Juice Mutation Concentrations Can Help Predict the Grade of Dysplasia in Patients Undergoing Pancreatic Surveillance. Clin Cancer Res. 2018;24:2963–2974. doi: 10.1158/1078-0432.CCR-17-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J, Sadakari Y, Shindo K, Suenaga M, Brant A, Almario JAN, Borges M, Barkley T, Fesharakizadeh S, Ford M, Hruban RH, Shin EJ, Lennon AM, Canto MI, Goggins M. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. 2017;66:1677–1687. doi: 10.1136/gutjnl-2015-311166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka M, Heckler M, Liu B, Heger U, Hackert T, Michalski CW. Cytologic Analysis of Pancreatic Juice Increases Specificity of Detection of Malignant IPMN-A Systematic Review. Clin Gastroenterol Hepatol. 2019 doi: 10.1016/j.cgh.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 45.Jabbar KS, Arike L, Verbeke CS, Sadik R, Hansson GC. Highly Accurate Identification of Cystic Precursor Lesions of Pancreatic Cancer Through Targeted Mass Spectrometry: A Phase IIc Diagnostic Study. J Clin Oncol. 2018;36:367–375. doi: 10.1200/JCO.2017.73.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong SM, Omura N, Vincent A, Li A, Knight S, Yu J, Hruban RH, Goggins M. Genome-wide CpG island profiling of intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2012;18:700–712. doi: 10.1158/1078-0432.CCR-11-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]