ABSTRACT
Lateral medullary syndrome is a common brainstem stroke associated with a classical triad of Horner’s Syndrome, ipsilateral ataxia and hypalgesia and thermoanasthesia of ipsilateral face. We report a case of a 49-year-old diabetic, non-hypertensive, postmenopausal female who presented with symptoms involving the left dorsal medulla along with right sided hemiparesis and left UMN-type facial palsy. Contralateral hemiparesis was explained by caudal extension of infarct involving the pyramids before decussation at the medulla, known as Babinski–Nageotte Syndrome. UMN-type facial palsy was attributed to involvement of hypothetical supranuclear aberrant corticobulbar fibres of facial nerve which descend down in the contralateral ventromedial medulla, decussate at level of upper medulla and then ascend in the dorsolateral medulla to reach the facial nerve nucleus. Association of these two entities with Wallenberg’s Syndrome have been reported separately in literature, but not together as in this case.
Keywords: Wallenberg, Babinski-Nageotte, aberrant, corticobulbar
INTRODUCTION
Wallenberg’s syndrome or lateral medullary syndrome is associated with a variety of symptoms due to involvement of lateral segment of the medulla. It is caused most commonly due to atherothrombotic vertebral artery occlusion, followed by posterior inferior cerebellar artery (PICA) and medullary arteries. Hypertension, diabetes and smoking are the common risk factors. Amongst other causes, vertebral artery dissection due to neck manipulation/injury, Marfan’s syndrome, Ehler-Danlos syndrome and fibromusculoar dysplasia may be attributed to lateral medullary syndrome [1]. It typically presents with loss of pain, temperature sensation on ipsilateral half of face, hemisensory loss on contralateral trunk and extremities, ipsilateral Horner’s syndrome, vertigo, nausea, vomiting, diplopia, hiccups and ipsilateral cerebellar signs and symptoms. Apart from these classical clinical features, Wallenberg’s syndrome may present with some atypical manifestations as well.
CASE REPORT
A 49-year-old diabetic (poorly controlled, on oral antidiabetic agents), non-hypertensive postmenopausal female patient presented to us with vertigo, nausea, vomiting and a tendency to fall to her left following bouts of loose stool. The next morning, she developed a tingling sensation on her left half of face and gradually she noticed that she was unable to feel any pain or change in temperature on that half of face. Her symptoms aggravated over the next few hours, and she started to experience vertical diplopia. A day later, she developed a slowly evolving hemiparesis and hemisensory loss of pain and temperature on right half of body. An upper motor neuron (UMN)-type facial palsy with a partial ptosis on the left half of her face further complicated the scenario (Fig. 1). However, she did not experience any bulbar symptoms, namely nasal regurgitation/intonation, dysphagia, hoarseness of voice, hiccups, etc. A detailed neurological examination revealed a second degree jerky right horizontal nystagmus with a torsional upbeating component and a partial ptosis of left eye without any ophthalmoplegia (Fig. 2) Left sided cerebellar signs were positive with an abnormal vestibulo-ocular reflex (VOR) on the left. Pyramidal tract involvement was confirmed by extensor plantar response on the right.
Figure 1.

Facial deviation to right with preservation of forehead wrinkling suggestive of UMN facial palsy.
Figure 2.

Partial ptosis of left eye.
MRI Brain revealed diffusion restriction in left dorsal medulla suggestive of acute infarct in the DWI sequence and was supported by the ADC sequence (Figs. 3 and 4). CT Angiography of Brain (4 vessels) did not reveal any abnormality. ECG and Echocardiography findings were within normal limits.
Figure 3.
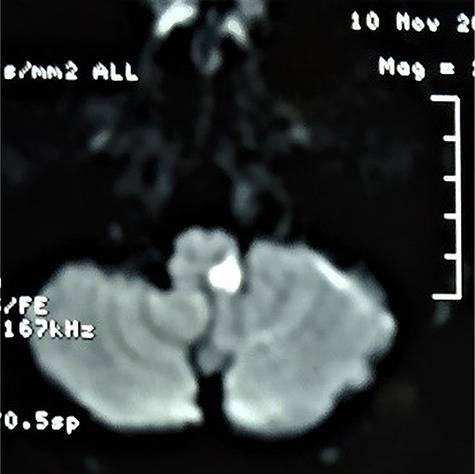
Diffusion weighted imaging showing diffusion restriction suggestive of an acute infarct in left dorsal medulla.
Figure 4.

A T2-weighted imaging showing infarct In left dorsal medulla.
The patient was started on antiplatelet therapy (aspirin), high-dose statin (atorvastatin) and rigorous physiotherapy. She recovered gradually over the next 2–3 months with residual hemiparesis.
DISCUSSION
Atypical features in our case, which questioned the diagnosis of a simple lateral medullary syndrome were as follows:
Contralateral hemiparesis.
Absence of bulbar symptoms.
Partial Horner’s syndrome.
UMN-type facial palsy.
Contralateral hemiparesis may be explained in this case by the caudal extension of infarct to involve the pyramidal tracts before decussation at the medulla [2]. However, some authors have attributed this weakness as a spinocerebellar hypotonic syndrome, but in our case, an extensor plantar response is suggestive of pyramidal tract lesion. Bulbar symptoms like dysphagia, nasal regurgitation/intonation, hoarseness of voice and hiccups occur due to involvement of nucleus ambiguus which is situated more deeply, hence a lesion involving the dorsal medulla may spare the nucleus ambiguus. In classical cases of lateral medullary syndrome, Horner’s syndrome is quite evident due to involvement of sympathetic tract. But in our case, only partial ptosis was present while other features of Horner’s syndrome (e.g. miosis, anhydrosis) were absent. This may be attributed to parts of the descending sympathetic tracts which are located more deeply, hence may be spared in a more dorsal medullary lesion leading to this differential involvement [3].
The patient had a vertical diplopia in absence of ophthalmoplegia, which could be attributed to skew deviation of eyes, due to involvement of vestibular nucleus, seen in lateral medullary stroke [4].
Ipsilateral UMN-type facial palsy may be attributed to interruption of hypothetical looping supranuclear corticobulbar fibres which descend down in the contralateral ventromedial medulla, decussate at the level of upper medulla and then ascend up in the dorsolateral medulla to reach the facial nerve nucleus (Fig. 5) [5].
Figure 5.

Diagram showing aberrant corticobulbar nerve fibres of facial nerve.
Though Wallenberg syndrome is commonly known as PICA (posterior inferior cerebellar artery) syndrome, the commonest cause of this syndrome is atherothrombotic occlusion of the vertebral artery [6]. Involvement of medullary penetrating arteries, branches of vertebral artery which supply the pyramidal fibres, may also lead to hemiparesis in these variants of brainstem stroke. Brainstem strokes, specially posterior circulation strokes have been reported after severe gastroenteritis, binge alcoholism, etc., due to watershed infarcts in the territory supplied by medullary penetrating arteries [2]. The mechanism of stroke in our case is arguable in the presence of poorly controlled diabetes as a risk factor.
So it is evident from the abovementioned scenario that lateral medullary syndrome may present with atypical findings like presence of hemiparesis, UMN facial palsy and absence of bulbar symptoms and classical Horner’s syndrome due to variation in the involvement of neuroanatomical structures. Neuroanatomical correlation is suggestive of a diagnosis of Babinski–Nageotte syndrome with UMN-type facial palsy. Hence, clinicians should always be aware of the atypical presentations of brainstem strokes.
REFERENCES
- 1. Ogawa K, Suzuki Y, Oishi M, Kamei S. Clinical study of 46 patients with lateral medullary infarction. J Stroke Cerebrovasc Dis. 2015 May;24:1065–74. [DOI] [PubMed] [Google Scholar]
- 2. Kk P, R K, P C, Aiyappan SK, N D. A rare variant of Wallenberg’s syndrome: Opalski syndrome. J Clin Diagn Res. 2014;8:MD05–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim JS. Pure lateral medullary infarction: clinical - radiological correlation of 130 acute, consecutive patients. Brain. 2003;126:1864–72. [DOI] [PubMed] [Google Scholar]
- 4. Nakazato Y, Shimazu T, Takei K, Sugawara K, Araki N, Tamura N. Shimazu K.[Diplopia in Wallenberg’s syndrome]. Rinsho Shinkeigaku 2004;44:1–6Japanese. PubMed PMID: 15199730. [PubMed] [Google Scholar]
- 5. Srinivasan M, Bindu B, Gobinathan S, Balasubramanian S, Nithyanandam A, Shanbhogue KR. An unusual presentation of lateral medullary syndrome with Ipsilateral UMN facial palsy - an anatomical postulate. Ann Ind Acad Neurol 2005;8:37–40. [Google Scholar]
- 6. Lui F, Tadi P, Anilkumar AC. Wallenberg Syndrome. [Updated 2019 Jun 4]. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2019-. Available from:https://www.ncbi.nlm.nih.gov/books/NBK470174 [PubMed] [Google Scholar]


