Abstract
Androgens, although traditionally thought to be male sex steroids, play important roles in female reproduction, both in healthy and pathological states. This mini-review focuses on recent advances in our knowledge of the role of androgens in the ovary. Androgen receptor (AR) is expressed in oocytes, granulosa cells, and theca cells, and is temporally regulated during follicular development. Mouse knockout studies have shown that AR expression in granulosa cells is critical for normal follicular development and subsequent ovulation. In addition, androgens are involved in regulating dynamic changes in ovarian steroidogenesis that are critical for normal cycling. Androgen effects on follicle development have been incorporated into clinical practice in women with diminished ovarian reserve, albeit with limited success in available literature. At the other extreme, androgen excess leads to disordered follicle development and anovulatory infertility known as polycystic ovary syndrome (PCOS), with studies suggesting that theca cell AR may mediate many of these negative effects. Finally, both prenatal and postnatal animal models of androgen excess have been developed and are being used to study the pathophysiology of PCOS both within the ovary and with regard to overall metabolic health. Taken together, current scientific consensus is that a careful balance of androgen activity in the ovary is necessary for reproductive health in women.
Sex steroids are crucial regulators of reproductive function. Although it was believed for a long time that androgens are strictly male hormones and estrogens are female hormones, we now know that both types of sex steroids are necessary for normal development and function of every human. Studies in androgen receptor (AR) knockout (ARKO) mice have shown that in the ovary, folliculogenesis does not reach its full potential in the absence of androgens. At the other extreme, follicle development is dysregulated in states of androgen excess and can lead to polycystic ovary syndrome (PCOS), a condition of hyperandrogenism and anovulatory infertility. Although much of the literature suggests that PCOS is caused by central defects in gonadotropin secretion, other studies point to a direct action of androgens in the ovary to alter folliculogenesis and ovulation. This mini-review focuses on recent advances in our knowledge of the role of androgens in cells that make up ovarian follicles, biological models of androgen deficiency and excess in the ovary, and the factors that regulate ovarian responsiveness to androgens.
Ovarian Cell-Specific AR Deletion Studies
Much of what we know about androgen actions in the ovary stems from mouse models lacking the AR gene. Before the gene was identified, its locus on the X chromosome was termed testicular feminization (Tfm) for its effects in males. Men and male animal models with absence of the Tfm locus were noted to have a female appearance and infertility due to lack of responsiveness to androgens. The infertility created a challenge in the development of a female homozygous Tfm mutant model. In the 1970s, Ohno et al. (1) managed to use chimeric Tfm male mice and XO female mice, which are fertile, to generate homozygous Tfm/O mutant females and were the first to describe their phenotype. These females appeared normal and were fully fertile shortly after puberty; however, their reproductive ability declined quickly and completely ceased much sooner than expected. Their ovarian morphology and follicle counts were normal at 36 days of age, but at day 56 and onward there was a reduction in the number of primordial follicles and premature degeneration of oocytes in early follicles with diffuse luteinization. XO mice have functioning ovaries but develop the same abnormalities starting much later, at day 210. This was the first piece of evidence that androgen action is necessary for maintaining the reproductive lifespan of a mouse ovary. Without it, the ovaries failed prematurely.
Later that decade, Lyon and Glenister (2) described the reproductive phenotype of homozygous Tfm/Tfm mutant females, which confirmed the initial observations in Tfm/O mice. The Tfm/Tfm females initially had normal fecundity but soon after started producing fewer pups per litter and stopped reproducing sooner than controls, resulting in an overall reduction of progeny numbers by approximately half. Their ovarian morphology findings were similar to those described by Ohno et al. (1), suggesting that androgens prevent atresia of early follicles.
With the advent of more sophisticated genetic technologies, the AR gene was identified and AR deletion studies were carried out beginning in the early 2000s. Several ARKO female mouse models were described and corroborated the findings of earlier Tfm mouse studies. Two global ARKO mouse models were created using Cre recombinase expression driven by the actin promoter (3, 4) or the cytomegalovirus promoter (5). Although the ARKO males had a femalelike appearance and were sterile due to arrest of spermatogenesis, the females were phenotypically normal and produced offspring. At a cursory glance, this may suggest that androgen is dispensable in females. However, as seen with the Tfm animals, over the course of their lifetimes, the AR-deficient female mice produced significantly smaller numbers of litters and fewer pups per litter, resulting in an overall reduction in fecundity of up to 70% compared with their littermates. This was due to premature depletion of follicles and early cessation of fertility, a phenotype resembling diminished ovarian reserve (DOR) in women. The ovaries of ARKO mice appeared normal at 4 weeks of age, but their response to gonadotropin stimulation in superovulation studies was already diminished at that time. By 8 weeks, the ovaries contained fewer corpora lutea and more atretic follicles than controls (3–5). Follicles were completely depleted by 40 weeks of age, resulting in infertility (5). Heterozygous AR-deficient mice produced slightly fewer pups than controls but were considerably more fertile than their homozygous ARKO counterparts. These studies proved that androgen action is not essential for survival and reproduction of female mice, but their reproductive potential is not fully realized in the absence of androgen.
The work of Hu et al. (3) showed that the loss of fecundity in female ARKO mice is due mostly to the reduced number of ovulated oocytes, with diminished endometrial thickness likely also contributing. Although AR is expressed at all levels of the reproductive hormone axis, the subnormal response to gonadotropin stimulation suggests that the primary defect in ARKO mice lies within the ovary. AR has been specifically knocked out in all three major ovarian cell types, and we have learned that androgens play very different roles in each of them. In 2010, Sen and Hammes (6) described the phenotypes of oocyte-specific and granulosa cell–specific ARKO mice. Although AR is expressed in the oocyte, its selective deletion by Cre-recombinase expressed from the growth differentiation factor 9 (Gdf9) gene promoter produced no effect on fertility or fecundity, and follicle counts at 8 to 9 weeks of age were the same as in heterozygous mice. On the contrary, granulosa cell–specific ARKO mice, generated by anti-Müllerian hormone receptor II–driven Cre expression, recapitulated the phenotype of the global ARKO. These mice had longer estrous cycles and reduced fertility, with premature ovarian failure. By 8 to 9 weeks of age, the knockout mice already were producing only one-quarter the number of offspring compared with wild-type controls, and this declined further to ∼10% by 24 to 25 weeks. Soon after this, FSH levels rose and the mice developed premature ovarian failure. Progression of small follicles to the antral stage was significantly reduced and apoptosis was increased. Interestingly, in this study the fecundity of heterozygous ARKO mice was unaffected, whereas global haploinsufficient mice had mildly reduced fecundity, suggesting that another site of full androgen activity, possibly the endometrium or centrally in the pituitary or hypothalamus, is required for optimal fertility. Still, this study was the first to prove that the main site of androgen activity in the ovary is granulosa cells, where AR is necessary for normal preantral follicle development. These findings were confirmed by Walters et al. (7), who further demonstrated that not only were the numbers of ovulated oocytes reduced when AR was deleted in anti-Müllerian hormone–expressing granulosa cells of preantral and antral follicles, but fewer of those ovulated oocytes were fertilized and survived to the two-cell stage, suggesting diminished oocyte quality in these mice.
Finally, a theca cell–specific ARKO mouse model was described by Ma et al. (8) in 2017, in which AR was deleted in Cyp17-expressing cells of the ovary. These mice were phenotypically normal, with histologically normal ovaries, and showed no reproductive deficit under standard conditions. However, when treated with prenatal dihydrotestosterone (PNA) to induce anovulation mimicking the PCOS phenotype (described in more detail below), mice lacking AR specifically in theca cells were protected from the reproductive deficits associated with the condition. Although PNA mice were completely acyclic and infertile, theca cell–specific ARKO-PNA mice maintained some cyclicity and produced approximately half the number of offspring compared with untreated controls. Thus, although the mechanisms of PCOS induction by PNA treatment remain obscure, it is now clear that androgen actions in theca cells are necessary at least in part in this disease model.
The above studies of mouse ARKO models have shed light on the pathogenesis of disorders related to androgen imbalance in women. Both excess and deficiency of androgens can cause female infertility. The proper balance of androgens in granulosa cells is necessary for optimal follicle progression and evading apoptosis, whereas an excess of androgen results in follicle arrest and anovulation mainly through its actions in theca cells.
Regulation of AR Gene Expression and Activity
As discussed above, ovarian AR mediates physiological and pathogenic effects of androgens on female fertility. AR expression levels in the ovary have been associated with ovarian dysfunction, namely PCOS, but the factors that regulate its expression in the ovary are not well known. Much of what we do know about the regulation of AR gene expression and activity comes from studies in the male, particularly in prostate cancer–derived cell lines. Factors at many levels of the gene expression paradigm have been implicated in AR gene regulation, including polymorphisms, splice variants, and cis- and trans-acting transcription regulators, including androgens themselves.
The human AR gene contains a poly-glutamine (CAG) repeat region within the N-terminal transcription activation region. Most people have between 8 and 35 repeats (9), and the shorter length is associated with increased activity of the receptor (10). Increased number of CAG repeats, conversely, is associated with reduced AR activity and has been linked to fertility problems in men (11). A number of studies have focused on the question of AR CAG repeat polymorphisms in women with PCOS, with the hypothesis that the shorter and more active form of AR could lead to hyperandrogenism, and the results have been variable. Some case-control studies support this association whereas others find no association (9, 12), possibly reflecting population differences in the pathogenesis of this complex and heterogeneous syndrome. In ovulatory women with longer CAG repeat length, intrafollicular fluid testosterone concentration was lower and aromatase expression was higher in one study (13), suggesting that AR may negatively regulate aromatase expression and reduce the conversion of testosterone to estradiol. However, serum testosterone measured in a different population was associated with longer CAG repeat length (14), so other factors are likely playing a role in this relationship.
Some studies, mostly in prostate cancer cells, suggest that androgen can induce or suppress AR gene expression. For instance, an enhancer element in the second intron of the AR gene binds AR and can either stimulate or repress AR gene transcription depending on the presence or absence of androgen (15). With androgen present, lysine-specific demethylase 1 is recruited to this location leading to transcription silencing. In the absence of androgen, this site recruits transcription coactivators FOXA1, OCT1, and GATA2 leading to H3K4 methylation and Pol II recruitment. In primates, but not in lower species, a nonconsensus androgen response element is found in the 5′ UTR of the AR gene and suppresses its transcription by approximately half when bound by AR with physiologically relevant (10 nM) concentration of DHT (16). However, there is no evidence to date that these regulatory sites are active in the ovary. In one study, 3- to 10-day testosterone treatment induced AR mRNA expression in granulosa cells of small and medium follicles of rhesus monkeys (17), but the treatment resulted in circulating testosterone levels that were more than 100-fold above normal, so the physiological significance of this finding is unclear. Overall, most evidence suggests that androgens can have a moderate suppressive effect on AR mRNA levels in the prostate but no substantial effect in the ovary, perhaps due to a difference in available transcription factors in these tissues.
In a recent study, Liu et al. (18) reported that AR mRNA splice variation in granulosa cells of patients with PCOS results in the presence of variant forms of AR (insertion-AR and deletion-AR), compared with ovulatory women. Both of these splice variants fail to translocate into the nucleus upon DHT stimulation due to altered interaction with cytoplasmic proteins HSP90 and importin-α. Although it is not clear whether these variants contribute to the pathogenesis of PCOS, it is intriguing to propose that extranuclear AR signaling pathways may be involved. Lastly, AR phosphorylation at multiple serine residues was shown by immunostaining in granulosa and theca cells of the marmoset ovary (19). This was not affected by treatment with testosterone or GnRH antagonist, and the physiological significance of these phosphorylation sites is presently unknown.
Molecular Actions of Androgens in the Ovary
As expected from ARKO studies in mice, multiple lines of evidence from cell and follicle culture show that androgens act via AR in the ovary to promote follicle growth and reduce granulosa cell apoptosis (Table 1). Microarray analysis of mRNA from global ARKO mouse ovaries revealed that many genes involved in folliculogenesis, including kit ligand (Kitl), bone morphogenic protein 15 (Bmp15), Gdf9, and hepatocyte growth factor (Hgf), are under control of AR (5). Still, the cell-specific ovarian transcriptome directly regulated by AR remains unknown. In addition, there are likely nongenomic effects of AR that are important for ovarian function and have yet to be elucidated.
Table 1.
Summary of Available Evidence From Studies of Androgen Actions in the Ovary
| Observed Effect | Experimental Species and Design | DHT Dose | Reference | |
|---|---|---|---|---|
| Increased preantral follicle growth | Rat | In vitro | 1 nM and higher | (20, 21) |
| In vivo | 83 µg continuous daily-release pellet | (21) | ||
| Monkey | In vivo | 145 µg/kg/d for 5 d | (22) | |
| Increased FSH receptor mRNA expression | Rat | In vitro | 1 nM and higher | (20) |
| Monkey | In vivo | 0.4 or 4 mg/kg of testosterone for 3 d | (23) | |
| Increased FSH receptor protein expression | Mouse, human | In vitro | 25 nM | (24) |
| Increased granulosa cell proliferation | Rat | In vitro | 100 nM | (25) |
| Pig | In vitro | 500 nM | (26, 27) | |
| Reduced apoptosis of follicles | Mouse | In vitro | 25 nM | (24, 28) |
| Increased expression of steroidogenic enzymes StAR, P450scc, and 3βHSD in mature granulosa cells | Rat | In vitro | 100 nM | (25) |
| Increased expression of cyclooxygenase-2 and amphiregulin in periovulatory granulosa cells | Mouse | In vivo | 5 µg/g 4 h prior to RNA isolation | (29) |
| Human | In vitro | 100 nM | ||
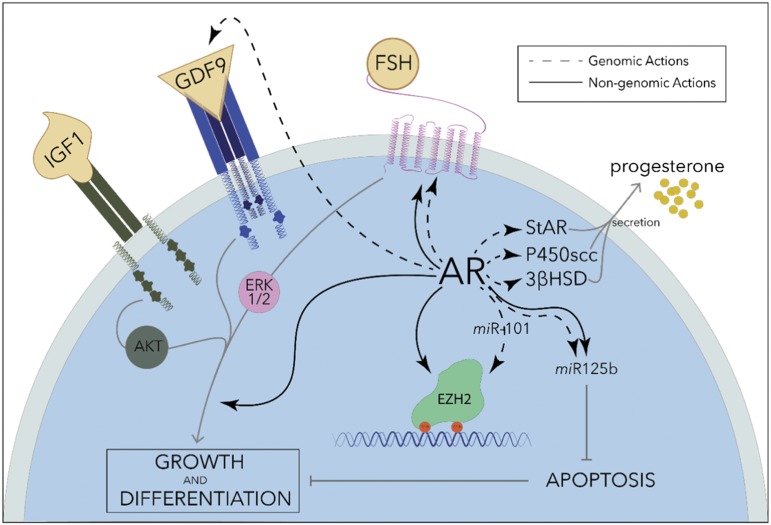
Preantral follicles isolated from rat ovaries grow faster in culture when DHT is present in the media in concentrations as low as 1 nM (20, 30), which is ∼10-fold higher than the reported EC50 of DHT for human AR (21). In addition, preantral follicles from rats that are chronically treated with DHT (83 µg continuous daily release) also grow faster in culture (20), and the same has been found in short-term DHT-treated monkeys (145 µg/kg/day for 5 days) (22). Thus, DHT has a trophic effect on preantral follicle growth both in vitro and in vivo. AR is most highly expressed in the granulosa cells of small follicles, and its abundance decreases as the follicles progress through development in rats (31) and in primates (32). Androgen actions in small follicles are likely mediated by enhanced FSH and growth factor signaling in granulosa cells. In fact, mRNA expression of FSH receptor, the major trophic factor in granulosa cells, was shown to be induced by androgen treatment of cultured rat follicles (20), as well as monkey granulosa cells (23) in vivo (0.4 or 4 mg/kg of testosterone for 3 days). In the latter study, AR and FSH receptor were also found to colocalize. Granulosa cell proliferation in response to stimulation with FSH, IGF-1, and the oocyte-secreted growth factor GDF-9, as well as intracellular messengers of these growth factors, are all augmented in the presence of androgens in nanomolar concentrations (25–27) (Fig. 1), and these effects seem more pronounced in smaller preantral follicles. Coincidentally, apoptosis of cultured follicles is suppressed by androgens (DHT, 25 nM) (24, 28) through mechanisms that may involve granulosa-specific miRNA miR-125b (Fig. 1). Thus, androgens appear most important in small follicles where they promote follicle development and growth through multiple pathways.
Figure 1.
AR actions in granulosa cells. AR works through genomic (dashed lines) and nongenomic (solid lines) pathways to promote growth and differentiation of granulosa cells, suppress apoptosis and, in dominant follicles, increase steroid synthesis. The effects of granulosa growth factors IGF1, GDF9, and FSH are all enhanced in the presence of androgens through extranuclear activity of AR. At the gene level, AR induces the expression of antiapoptotic miRNA miR125b, multiple steroidogenic enzymes, GDF9, and FSH receptor, and regulates the activity of DNA methyltransferase Ezh2 through modulation of Ezh2 phosphorylation as well as transcriptional regulation of the miRNA miR101 (24–28, 33).
Nongenomic pathways involved in the proliferative effects of androgens in the ovary have also been described, though these are better characterized in prostate cancer cells, where membrane-associated AR enhances epidermal growth factor signaling through a fast-onset, transcription-independent mechanism (34). In mouse primary granulosa cells, as well as in the immortalized granulosalike KGN cells, extranuclear AR induces FSH receptor protein expression independently of gene transcription, through an MAPK-dependent pathway (24) (Fig. 1). Wartalski et al. (35) found that extranuclear AR in porcine granulosa cells is modulated by environmental fungicide vinclozolin and activates ERK1/2 and AKT. In croaker granulosa cells, a membrane zinc transporter family protein identified as ZIP9, unrelated structurally to AR, is liganded by androgens and signals via Gsα and MAPK (36, 37). These pathways may act in concert with nuclear AR to stimulate granulosa cell proliferation.
Some studies suggest that androgens modulate ovarian steroidogenesis downstream of FSH signaling. For instance, the expression of key steroidogenic enzymes StAR, P450scc, and 3βHSD are amplified by 100 nM DHT treatment of cultured rat granulosa cells (25), resulting in increased progesterone synthesis (Fig. 1). This effect was not observed in cumulus granulosa cells (26), highlighting the specialization of granulosa cells throughout follicular development. Similarly, DHT has variable effects on the expression levels of aromatase and estrogen synthesis. In rat granulosa cells, testosterone stimulates aromatase gene expression. LRH-1 is a direct target gene of AR in this context and mediates the induction of aromatase and P450scc by testosterone (38). However, these findings were not substantiated in cultured human granulosa cells, which are typically obtained from later-stage follicles. Yang et al. (39) obtained granulosa cells from women without PCOS and then treated them in culture with testosterone at levels measured in the intrafollicular fluid of women with PCOS. In this study, aromatase gene expression and protein abundance was downregulated by testosterone via AR. Developmental stage differences in follicular response to androgens were highlighted in a study of the marmoset ovary by Harlow et al. (40): aromatase activity was significantly induced by DHT treatment of small follicles but suppressed by the same treatment of large follicles. Although there are differences between studies, which may be a function of transcription coregulators present in granulosa cells at different stages of development, it is clear that supra-physiological levels of testosterone can shift the steroid hormone balance within the ovary.
There are limited data suggesting that androgens may directly affect ovulation in certain circumstances. Ovulation-related genes cyclooxygenase-2 and amphiregulin are acutely induced by DHT (5 µg/g) in periovulatory granulosa cells in mice (29) and in KGN cells. Androgens can also promote oocyte meiotic competence and maturation in some species, although there are likely redundant pathways for this in the absence of androgens (41, 42). Conversely, chronic excess of androgens is detrimental to ovulation due to disordered follicular development, described in more detail below. Overall it appears that androgens play a larger role early in follicular development, and ovulatory dysfunction can result from defects in preantral follicles due to either insufficient or excessive androgen levels.
Clinical Use of Androgens in Female Infertility
The preponderance of evidence from animal studies has shown that insufficient androgen activity in the female leads to subfertility that very closely resembles the clinical presentation of DOR in women. Consequently, cautious use of dehydroepiandrosterone (DHEA), a less potent androgen than DHT, has been applied to women with DOR undergoing fertility treatments. Empirical evidence suggests there is little if any benefit of DHEA to fertility treatment success, but there is also no evidence of harm. DOR often presents as a time-sensitive infertility situation when the desire is to try every available agent to maximize chances for success, and DHEA may be added to the treatment strategy. For similar reasons, recruitment of subjects for placebo-controlled randomized trials of DHEA in DOR is likely challenging and only small studies are available.
In a 2015 meta-analysis, Li et al. (43) evaluated eight studies, half of which were randomized clinical trials or case-control trials, and concluded that DHEA (75 mg daily in one or three divided doses for 3 to 4 months) modestly improves clinical pregnancy rates in women with DOR undergoing treatment with assisted reproductive techniques. There was no difference in the number of oocytes retrieved, contradicting the expected effect of androgens in improving follicle recruitment. Overall, the effect was minor and was lost in smaller studies. In a subsequent randomized clinical trial, DHEA pretreatment in in vitro fertilization cycles (25 mg three times daily for 8 weeks) did not improve ovarian response to gonadotropin stimulation or clinical pregnancy rates in women with DOR, though AR and FSH receptor expression were increased in granulosa cells (44). It is possible that ovarian stimulation with supraphysiological levels of gonadotropins typically used in IVF protocols overcomes any additional effects of androgen treatment. To speculate, as personalized medicine advances, there may be a place for androgen supplementation in women with DOR early in their reproductive lives with the goal of decelerating follicle depletion before attempting to have children.
Hyperandrogenism in PCOS
Both androgen insufficiency and androgen excess cause ovarian dysfunction. Although lack of androgen activity in the ovary, particularly in granulosa cells, leads to ovarian insufficiency as described above, excess androgen is linked to PCOS. In the latter, follicles are increasingly recruited to the preantral and antral stage but fail to progress to the Graafian stage and ovulate, and can give the ovaries a characteristic polycystic appearance on imaging. The hypothalamic and pituitary state in PCOS is marked by increased pulse frequency of the GnRH and high LH secretion. This leads to increased testosterone production by the hypertrophic theca cell population, adding to the hyperandrogenic state. This syndrome is in some ways the opposite of ovarian insufficiency, but both are frequent causes of anovulatory infertility. In addition, PCOS is associated with metabolic syndrome.
Excess androgen appears to be both a cause and a consequence of PCOS in a vicious cycle. For example, chronic exposure to exogenous androgens is sufficient to induce polyfollicular anovulatory infertility in mice (45) and rats (46). Chronic DHT exposure of these females through a pellet inserted prepuberty (27.5 µg in mice or 83 µg in rats daily continuous release for 90 days) results in cessation of estrous cycles and the appearance of multiple cystic, atretic follicles. The mice also develop increased body fat and glucose intolerance, closely mimicking PCOS in women. Unlike classic PCOS, chronic exogenous androgen exposure in rodents is not associated with increased LH levels or hyperthecosis and their ovarian morphology is distinct with many atretic but few antral follicles. However, in other animal models the syndrome can be programmed through transient hormone disruption in utero, leading to hyperactive LH tone and hyperplastic follicles that in turn secrete abnormally high amounts of testosterone.
A very well-validated method to induce a PCOS-like phenotype in animal models is prenatal exposure to excess androgens. In many mammals, including rodents (47–50), sheep (51), and primates (52–54), in utero treatment with excess androgens during critical developmental windows produces remarkably consistent results in female offspring. Invariably, the females develop ovulatory dysfunction early during their reproductive life, characterized by polyfollicular ovaries, hyperandrogenemia, and LH hypersecretion. For instance, injection of pregnant rhesus monkeys with 10 mg of testosterone propionate daily during early (beginning days 40 to 44) or late (beginning days 100 to 115) gestation with the target circulating testosterone levels in the male range resulted in the PCOS phenotype in female offspring of both treatment groups (52). These females had ∼50% fewer menstrual cycles than controls, basal testosterone levels 50% above controls, and polyfollicular ovaries in 40% of treated animals compared with 14% of controls. In this model, the early exposure window coincided with the differentiation of the ovary and appearance of oogonia, whereas the late exposure occurred during ovarian maturation when follicles appear and begin to produce steroids and gain gonadotropin responsiveness. In some sheep and primate models of PCOS, particularly those exposed to androgens earlier in fetal development, increased follicle recruitment is associated with early follicle depletion and reduced ovarian reserve (55, 56). Studies using the AR antagonist flutamide and the nonaromatizable androgen DHT have demonstrated that prenatal testosterone in each of these animal models indeed acts via AR to program reproductive dysfunction. As mentioned above, AR in the theca cells may be specifically responsible for much of this effect (8).
It should be noted that, although prenatal androgen treatment is the most effective method to induce a PCOS-like phenotype in animal models, there are some important differences in treatment outcomes between experimental species. For example, PNA mice (typically injected with 250 µg DHT on days 16, 17, and 18 of gestation) develop oligocyclicity and glucose intolerance similar to women with PCOS (49), but lack the characteristic polyfollicular ovarian morphology, which in humans and primates is due to many persistent antral follicles. This is due to the inherent differences between the menstrual cycle in humans and the estrous cycle in rodents. In PNA mouse ovaries, a stark absence of corpora lutea compared with untreated controls signifies anovulation (57).
In addition to the reproductive defects, all prenatally androgenized animal models develop insulin resistance, impaired glucose tolerance, and increased visceral adiposity, similar to the metabolic syndrome observed with high frequency in women with PCOS (58, 59). Depending on the timing of prenatal androgen exposure, additional effects can include genital virilization and behavioral masculinization (52). Although the etiology of PCOS is still debated, the developmental origins hypothesis is clearly in the lead (60), though the precise mechanisms by which prenatal androgen excess programs this syndrome in animal models are still unclear.
In-depth characterization of the hypothalamic-pituitary-ovarian axis in prenatally androgenized mice and sheep has revealed hyperactive firing of GnRH neurons programmed by the treatment (47, 49), leading to increased LH secretion as well as reduced responsiveness to both negative and positive feedback of estradiol (61–63). These studies suggest that the pathogenesis of PCOS by prenatal androgens may be central in origin. However, intrinsic ovarian defects are evident in prenatally androgenized sheep as early as fetal days 90 to 140 (gestation length is 147 days). Although there is no effect on ovarian morphology or germ cell volume, the expression of steroidogenic enzymes and LH receptor is significantly reduced (64), and the expression of AR, but not estrogen or progesterone receptors, is selectively increased (65).
As with other models of developmental programming of adult disease, a proposed mechanism through which these acute cellular changes during tissue development can translate into dysfunction much later in life is through long-lasting epigenetic changes. In support of this, multiple studies have identified differences in promoter methylation of ovarian genes that regulate reproduction in prenatally androgenized rodents (66–68) and zebrafish (69) using genomic techniques. Genes with significant promoter hypomethylation include AR and steroidogenic enzymes Cyp11 and Cyp17 (67), Gata6 and StAR (68), whereas apoptosis-related genes Bcl2l1 and Scr5a1 are hypermethylated (66). It is not certain that these specific epigenetic alterations affect gene expression levels, but overall epigenetic modifications may be an important factor in the programming of ovarian dysfunction by prenatal androgen excess. As an example of androgen-induced changes in DNA methylation, acute treatment of mouse granulosa cells with DHT results in inactivation of the histone methyltransferase Polycomb group protein enhancer of zeste homolog 2 (Ezh2) by both rapid and long-acting mechanisms (33) (Fig. 1). Within minutes, DHT causes phosphorylation of Ezh2 via nonclassic extranuclear AR actions involving the PI3K/AKT pathway. Additionally, after 48 hours of DHT treatment, Ezh2 gene expression is silenced via the miRNA miR-101. This results in hypomethylation of the Runt-related transcription factor-1 (Runx1) gene, an LH-responsive factor important in ovulation, leading to its increased expression, and interruptions in this androgen-stimulated pathway can suppress ovulation in mice. Similar androgen-induced epigenetic changes may underlie the defects programmed by prenatal androgen excess.
Conclusions
To summarize, the proper balance of androgen activity is necessary to achieve and maintain normal ovarian function throughout the female reproductive lifespan. The mechanisms of androgen actions in the ovary are complex and dynamic, starting in the fetus and extending into adulthood, and require the cooperation of many cell-specific and follicle stage–specific factors, not all of which have been identified. Both excess and lack of androgens lead to ovarian dysfunction and are associated with the most common causes of infertility in women. With more studies, it is likely that clinicians will be able to better target disorders of androgen actions in women to improve their fertility.
Acknowledgments
Financial Support: O.A. was supported by National Institutes of Health Grant F32HD097939-01.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AR
androgen receptor
- ARKO
androgen receptor knockout
- Bmp15
bone morphogenic protein 15
- DHEA
dehydroepiandrosterone
- DOR
diminished ovarian reserve
- Ezh2
enhancer of zeste homolog 2
- Gdf9
growth differentiation factor 9
- Hgf
hepatocyte growth factor
- Kitl
kit ligand
- PCOS
polycystic ovary syndrome
- PNA
prenatal dihydrotestosterone
- Runx1
Runt-related transcription factor-1
- Tfm
testicular feminization
References
- 1. Ohno S, Christian L, Attardi B. Role of testosterone in normal female function. Nat New Biol. 1973;243(125):119–120. [PubMed] [Google Scholar]
- 2. Lyon MF, Glenister PH. Reduced reproductive performance in androgen-resistant Tfm/Tfm female mice. Proc R Soc Lond B Biol Sci. 1980;208(1170):1–12. [DOI] [PubMed] [Google Scholar]
- 3. Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci USA. 2004;101(31):11209–11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues [published correction appears in Proc Natl Acad Sci USA. 2002;99(23):15245]. Proc Natl Acad Sci USA. 2002;99(21):13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA. 2006;103(1):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24(7):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walters KA, Middleton LJ, Joseph SR, Hazra R, Jimenez M, Simanainen U, Allan CM, Handelsman DJ. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod. 2012;87(6):151. [DOI] [PubMed] [Google Scholar]
- 8. Ma Y, Andrisse S, Chen Y, Childress S, Xue P, Wang Z, Jones D, Ko C, Divall S, Wu S. Androgen receptor in the ovary theca cells plays a critical role in androgen-induced reproductive dysfunction. Endocrinology. 2017;158(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baculescu N. The role of androgen receptor activity mediated by the CAG repeat polymorphism in the pathogenesis of PCOS. J Med Life. 2013;6(1):18–25. [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22(15):3181–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab. 1997;82(11):3777–3782. [DOI] [PubMed] [Google Scholar]
- 12. Zhang T, Liang W, Fang M, Yu J, Ni Y, Li Z. Association of the CAG repeat polymorphisms in androgen receptor gene with polycystic ovary syndrome: a systemic review and meta-analysis. Gene. 2013;524(2):161–167. [DOI] [PubMed] [Google Scholar]
- 13. Borgbo T, Macek M Sr, Chrudimska J, Jeppesen JV, Hansen LL, Andersen CY. Size matters: associations between the androgen receptor CAG repeat length and the intrafollicular hormone milieu. Mol Cell Endocrinol. 2016;419:12–17. [DOI] [PubMed] [Google Scholar]
- 14. Skrgatic L, Baldani DP, Cerne JZ, Ferk P, Gersak K. CAG repeat polymorphism in androgen receptor gene is not directly associated with polycystic ovary syndrome but influences serum testosterone levels. J Steroid Biochem Mol Biol. 2012;128(3-5):107–112. [DOI] [PubMed] [Google Scholar]
- 15. Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z, Chen S, Nelson PS, Liu XS, Brown M, Balk SP. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20(4):457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hay CW, Watt K, Hunter I, Lavery DN, MacKenzie A, McEwan IJ. Negative regulation of the androgen receptor gene through a primate-specific androgen response element present in the 5′ UTR. Horm Cancer. 2014;5(5):299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83(7):2479–2485. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Wang Y, Wang F, Pan J, Xu J, Li J, Zhou C, Ding G, Wu Y, Liu X, Sheng J, Huang H. Mechanism underlying the retarded nuclear translocation of androgen receptor splice variants. Sci China Life Sci. 2019;62(2):257–267. [DOI] [PubMed] [Google Scholar]
- 19. McEwan IJ, McGuinness D, Hay CW, Millar RP, Saunders PT, Fraser HM. Identification of androgen receptor phosphorylation in the primate ovary in vivo. Reproduction. 2010;140(1):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xue K, Liu JY, Murphy BD, Tsang BK. Orphan nuclear receptor NR4A1 is a negative regulator of DHT-induced rat preantral follicular growth. Mol Endocrinol. 2012;26(12):2004–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sonneveld E, Jansen HJ, Riteco JA, Brouwer A, van der Burg B. Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol Sci. 2005;83(1):136–148. [DOI] [PubMed] [Google Scholar]
- 22. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101(12):2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84(8):2951–2956. [DOI] [PubMed] [Google Scholar]
- 24. Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, Barad D, Gleicher N, Hammes SR. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci USA. 2014;111(8):3008–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hasegawa T, Kamada Y, Hosoya T, Fujita S, Nishiyama Y, Iwata N, Hiramatsu Y, Otsuka F. A regulatory role of androgen in ovarian steroidogenesis by rat granulosa cells. J Steroid Biochem Mol Biol. 2017;172:160–165. [DOI] [PubMed] [Google Scholar]
- 26. Hickey TE, Marrocco DL, Gilchrist RB, Norman RJ, Armstrong DT. Interactions between androgen and growth factors in granulosa cell subtypes of porcine antral follicles. Biol Reprod. 2004;71(1):45–52. [DOI] [PubMed] [Google Scholar]
- 27. Hickey TE, Marrocco DL, Amato F, Ritter LJ, Norman RJ, Gilchrist RB, Armstrong DT. Androgens augment the mitogenic effects of oocyte-secreted factors and growth differentiation factor 9 on porcine granulosa cells. Biol Reprod. 2005;73(4):825–832. [DOI] [PubMed] [Google Scholar]
- 28. Otala M, Mäkinen S, Tuuri T, Sjöberg J, Pentikäinen V, Matikainen T, Dunkel L. Effects of testosterone, dihydrotestosterone, and 17beta-estradiol on human ovarian tissue survival in culture. Fertil Steril. 2004;82(Suppl 3):1077–1085. [DOI] [PubMed] [Google Scholar]
- 29. Yazawa T, Kawabe S, Kanno M, Mizutani T, Imamichi Y, Ju Y, Matsumura T, Yamazaki Y, Usami Y, Kuribayashi M, Shimada M, Kitano T, Umezawa A, Miyamoto K. Androgen/androgen receptor pathway regulates expression of the genes for cyclooxygenase-2 and amphiregulin in periovulatory granulosa cells. Mol Cell Endocrinol. 2013;369(1-2):42–51. [DOI] [PubMed] [Google Scholar]
- 30. Laird M, Thomson K, Fenwick M, Mora J, Franks S, Hardy K. Androgen stimulates growth of mouse preantral follicles in vitro: interaction with follicle-stimulating hormone and with growth factors of the TGFβ superfamily. Endocrinology. 2017;158(4):920–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tetsuka M, Whitelaw PF, Bremner WJ, Millar MR, Smyth CD, Hillier SG. Developmental regulation of androgen receptor in rat ovary. J Endocrinol. 1995;145(3):535–543. [DOI] [PubMed] [Google Scholar]
- 32. Hillier SG, Tetsuka M, Fraser HM. Location and developmental regulation of androgen receptor in primate ovary. Hum Reprod. 1997;12(1):107–111. [DOI] [PubMed] [Google Scholar]
- 33. Ma X, Hayes E, Biswas A, Seger C, Prizant H, Hammes SR, Sen A. Androgens regulate ovarian gene expression through modulation of Ezh2 expression and activity. Endocrinology. 2017;158(9):2944–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sen A, O’Malley K, Wang Z, Raj GV, Defranco DB, Hammes SR. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem. 2010;285(37):28787–28795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wartalski K, Knet-Seweryn M, Hoja-Lukowicz D, Tabarowski Z, Duda M. Androgen receptor-mediated non-genomic effects of vinclozolin on porcine ovarian follicles and isolated granulosa cells: vinclozolin and non-genomic effects in porcine ovarian follicles. Acta Histochem. 2016;118(4):377–386. [DOI] [PubMed] [Google Scholar]
- 36. Converse A, Zhang C, Thomas P. Membrane androgen receptor ZIP9 induces croaker ovarian cell apoptosis via stimulatory G protein alpha subunit and MAP kinase signaling. Endocrinology. 2017;158(9):3015–3029. [DOI] [PubMed] [Google Scholar]
- 37. Thomas P, Converse A, Berg HA. ZIP9, a novel membrane androgen receptor and zinc transporter protein. Gen Comp Endocrinol. 2018;257:130–136. [DOI] [PubMed] [Google Scholar]
- 38. Wu YG, Bennett J, Talla D, Stocco C. Testosterone, not 5α-dihydrotestosterone, stimulates LRH-1 leading to FSH-independent expression of Cyp19 and P450scc in granulosa cells. Mol Endocrinol. 2011;25(4):656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang F, Ruan YC, Yang YJ, Wang K, Liang SS, Han YB, Teng XM, Yang JZ. Follicular hyperandrogenism downregulates aromatase in luteinized granulosa cells in polycystic ovary syndrome women. Reproduction. 2015;150(4):289–296. [DOI] [PubMed] [Google Scholar]
- 40. Harlow CR, Shaw HJ, Hillier SG, Hodges JK. Factors influencing follicle-stimulating hormone-responsive steroidogenesis in marmoset granulosa cells: effects of androgens and the stage of follicular maturity. Endocrinology. 1988;122(6):2780–2787. [DOI] [PubMed] [Google Scholar]
- 41. Makita M, Miyano T. Androgens promote the acquisition of maturation competence in bovine oocytes. J Reprod Dev. 2015;61(3):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miedlich SU, Taya M, Young MR, Hammes SR. Paxillin and embryonic polyadenylation binding protein (ePABP) engage to regulate androgen-dependent xenopus laevis oocyte maturation - a model of kinase-dependent regulation of protein expression. Mol Cell Endocrinol. 2017;448:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li J, Yuan H, Chen Y, Wu H, Wu H, Li L. A meta-analysis of dehydroepiandrosterone supplementation among women with diminished ovarian reserve undergoing in vitro fertilization or intracytoplasmic sperm injection. Int J Gynaecol Obstet. 2015;131(3):240–245. [DOI] [PubMed] [Google Scholar]
- 44. Hu Q, Hong L, Nie M, Wang Q, Fang Y, Dai Y, Zhai Y, Wang S, Yin C, Yang X. The effect of dehydroepiandrosterone supplementation on ovarian response is associated with androgen receptor in diminished ovarian reserve women. J Ovarian Res. 2017;10(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Houten EL, Kramer P, McLuskey A, Karels B, Themmen AP, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153(6):2861–2869. [DOI] [PubMed] [Google Scholar]
- 46. Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148(8):3781–3791. [DOI] [PubMed] [Google Scholar]
- 47. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101(18):7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72(6):1475–1483. [DOI] [PubMed] [Google Scholar]
- 49. Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011;152(2):618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tehrani FR, Noroozzadeh M, Zahediasl S, Piryaei A, Azizi F. Introducing a rat model of prenatal androgen-induced polycystic ovary syndrome in adulthood. Exp Physiol. 2014;99(5):792–801. [DOI] [PubMed] [Google Scholar]
- 51. Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol. 2006;246(1-2):165–174. [DOI] [PubMed] [Google Scholar]
- 52. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11(4):357–374. [DOI] [PubMed] [Google Scholar]
- 53. Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9(2):62–67. [DOI] [PubMed] [Google Scholar]
- 54. Dumesic DA, Schramm RD, Abbott DH. Early origins of polycystic ovary syndrome. Reprod Fertil Dev. 2005;17(3):349–360. [DOI] [PubMed] [Google Scholar]
- 55. Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146(7):3185–3193. [DOI] [PubMed] [Google Scholar]
- 56. Dumesic DA, Patankar MS, Barnett DK, Lesnick TG, Hutcherson BA, Abbott DH. Early prenatal androgenization results in diminished ovarian reserve in adult female rhesus monkeys. Hum Reprod. 2009;24(12):3188–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Silva MS, Prescott M, Campbell RE. Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI Insight. 2018;3(7):99405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res. 2003;11(2):279–286. [DOI] [PubMed] [Google Scholar]
- 59. Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207(2):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Franks S, Berga SL. Does PCOS have developmental origins? Fertil Steril. 2012;97(1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sarma HN, Manikkam M, Herkimer C, Dell’Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology. 2005;146(10):4281–4291. [DOI] [PubMed] [Google Scholar]
- 62. Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796–806. [DOI] [PubMed] [Google Scholar]
- 63. Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol Reprod. 2005;72(3):619–627. [DOI] [PubMed] [Google Scholar]
- 64. Hogg K, McNeilly AS, Duncan WC. Prenatal androgen exposure leads to alterations in gene and protein expression in the ovine fetal ovary. Endocrinology. 2011;152(5):2048–2059. [DOI] [PubMed] [Google Scholar]
- 65. Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137(5):865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang D, Cong J, Shen H, Wu Q, Wu X. Genome-wide identification of aberrantly methylated promoters in ovarian tissue of prenatally androgenized rats. Fertil Steril. 2014;102(5):1458–1467. [DOI] [PubMed] [Google Scholar]
- 67. Xia Y, Shen S, Zhang X, Deng Z, Xiang Z, Wang H, Yi L, Gao Q, Wang Y. Epigenetic pattern changes in prenatal female Sprague-Dawley rats following exposure to androgen [published online ahead of print 31 March 2015]. Reprod Fertil Dev. doi: 10.1071/RD14292. [DOI] [PubMed] [Google Scholar]
- 68. Salehi Jahromi M, Hill JW, Ramezani Tehrani F, Zadeh-Vakili A. Hypomethylation of specific CpG sites in the promoter region of steroidogeneic genes (GATA6 and StAR) in prenatally androgenized rats. Life Sci. 2018;207:105–109. [DOI] [PubMed] [Google Scholar]
- 69. Xu N, Chua AK, Jiang H, Liu NA, Goodarzi MO. Early embryonic androgen exposure induces transgenerational epigenetic and metabolic changes. Mol Endocrinol. 2014;28(8):1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]