Abstract
Objective
To investigate the relationship between physical activity and prodromal features of Parkinson disease that often precede the clinical diagnosis.
Methods
Included are participants in 2 well-established cohorts: the Nurses' Health Study and the Health Professionals Follow-up Study. Physical activity was assessed using validated questionnaires at baseline (1986) and every 2 years until 2008. Prodromal features (e.g., constipation, hyposmia, and probable REM sleep behavior disorder [pRBD]) were assessed in 2012–2014.
Results
The multivariable-adjusted odds ratio (OR) for having ≥3 prodromal features vs none comparing the highest to the lowest quintile were 0.65 (95% confidence interval [CI] 0.53–0.79; ptrend = 0.0006) for baseline physical activity and 0.52 (95% CI 0.35–0.76; ptrend = 0.009) for cumulative average physical activity. Considering each feature independently, baseline physical activity was associated with lower odds of constipation (OR 0.78, 95% CI 0.73–0.83; ptrend < 0.0001), excessive daytime sleepiness (OR 0.72, 95% CI 0.60–0.86; ptrend = 0.002), depressive symptoms (OR 0.82, 95% CI 0.69–0.97; ptrend = 0.13), and bodily pain (OR 0.81, 95% CI 0.68–0.96; ptrend = 0.03). Similar or stronger associations were observed for cumulative average physical activity, which, in addition, was associated with pRBD (OR 0.85, 95% CI 0.77–0.95; ptrend = 0.02). In contrast, neither hyposmia nor impaired color vision was associated with physical activity. Early life physical activity was associated with constipation and, in men only, with the co-occurrence of ≥3 features.
Conclusions
The reduced prevalence of prodromal features associated with Parkinson disease in older individuals who were more physically active in midlife and beyond is consistent with the hypothesis that high levels of physical activity may reduce risk of Parkinson disease.
Individuals who are more physically active are less likely to develop Parkinson disease (PD).1–5 Even physical activity during high school and college1 or at age 35–394 appears to predict PD risk at old ages, a result consistent with a genuine protective effect, the mechanisms of which remain uncertain.6 With the purpose of better understanding the relation between physical activity and PD, we investigated the relation between physical activity and certain prodromal features that often precede the clinical diagnosis. It is well-known that clinical PD is preceded by a long prodromal period during which individuals may experience nonmotor and subtle motor signs before the onset of the classic motor features.7 These prodromal features are varied but can include hyposmia, constipation, REM sleep behavior disorder (RBD), depression, excessive daytime sleepiness, color vision impairments, and bodily pain.8–10 These features have been associated with PD risk,10–16 but it is their co-occurrence that most clearly predicts a future PD diagnosis.17
We report how physical activity in early and later life relates with prodromal features of PD, both individually and in combination, among participants in 2 large cohorts of men and women who were followed prospectively for over 25 years.
Methods
Population
Our study was conducted within 2 large cohort studies: the Nurses' Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). The NHS was established in 1976, when 121,700 female registered nurses aged 30–55 years living in the United States responded to a mailed questionnaire about their medical histories and health-related risk factors; the HPFS was established in 1986, when 51,529 male health professionals aged 40–75 years responded to a similar questionnaire. Follow-up questionnaires have been sent every 2 years to participants in both cohorts to collect updated disease and exposure data. All participants without diagnosed PD and under 85 years of age who completed the 2012 questionnaire and had nonmissing responses to the questions assessing constipation and probable RBD (pRBD) (number with missing constipation or pRBD data in NHS = 15,079 and in HPFS = 4,153) are included in the current study for analyses of constipation and pRBD (n = 46,272). However, for cost reasons, olfactory testing and a supplemental questionnaire to assess other prodromal features of PD were sent only to a subset comprising all men and women who screened positive for either pRBD or constipation (n = 5,500 men and n = 13,781 women) but only 23% of those with neither of these features (n = 7,762; 2,853 men and 4,873 women, randomly selected). Overall, 18,121 PD-free participants completed the olfactory assessment and supplemental questionnaire and are included in analyses including features other than constipation or pRBD. We examined baseline clinical and demographic variables among participants who provided data on other prodromal features compared to all other cohort members; overall participants with measured data on prodromal features were similar to all other participants (data not shown).
Standard protocol approvals, registrations, and patient consents
This study was approved by the Human Research Committees at the Brigham and Women's Hospital and the Harvard T. H. Chan School of Public Health.
Assessment of prodromal features
Methods for assessing prodromal features have been described previously.17 Briefly, constipation was defined as a bowel movement frequency of every other day or less or laxative use at least twice a week as reported in the 2012 questionnaire. pRBD was also assessed in 2012, using an RBD screening question from the validated Mayo Sleep Questionnaire (“Has your spouse [or sleep partner] told you that you appear to ‘act out your dreams’ while sleeping [punched or flailed arms in the air, shouted or screamed], which has occurred at least 3 times?”)18 This question, without the specification of dream enactment having occurred at least 3 times, was reported to have a sensitivity of 100% and a specificity of 95% for the diagnosis of polysomnography-confirmed RBD in a community-based sample.19 The presence of hyposmia or other prodromal features was assessed in 2014 for HPFS and 2015 for NHS. Hyposmia was measured using the Brief Smell Identification Test, a standardized, forced choice test booklet containing 12 odorants, which participants were asked to identify from a list of 4 possible alternatives.20 An olfactory score was calculated as the sum of correctly identified odors. Color discrimination was assessed using a mailed version of the Roth color discrimination test, which is an abridged version of the Farnsworth-Munsell Test.17,21 A color discrimination score was calculated by summing the number of incorrectly matched color hues. Excessive daytime sleepiness was assessed using the Epworth Sleepiness Scale.22 Depressive symptoms were assessed using the Mental Health Inventory,23 which is a subscale of the 36-item Short-Form Health Survey (SF-36). Bodily pain was also assessed using questions from the SF-36. We calculated a bodily pain score by summing the responses (0–6 and 0–5, respectively) to the first 2 questions (“How much bodily pain have you had during the past 4 weeks?” and “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?”, and set the score to 0 for individuals who responded that the pain was related to a recent injury or illness. For the olfactory, color discrimination, pain, and depression scales, participants were dichotomized as having the feature if their score was in the bottom 10th cohort-specific percentile of scores of individuals who screened negative for pRBD and constipation. Participants with a score of ≥10 on the Epworth Sleepiness Scale were considered to have excessive daytime sleepiness.
Assessment of physical activity
Physical activity was measured in both cohorts for the first time in 1986, and was updated every 2 years thereafter for HPFS, and every 2 years for NHS except for 1990, 2002, and 2006. At each measurement, participants reported the average time per week (in 7 categories, from 0 to ≥11 hours) that they spent during the previous year participating in specific activities, including walking or hiking outdoors; jogging (>10 min/mile); running (≤10 min/mile); bicycling; lap swimming; tennis, squash, or racquetball; and calisthenics or other aerobic exercise. In addition, participants reported their usual outdoor walking pace (easy [<2 miles per hour (mph)], average [2–2.9 mph], brisk [3–3.9 mph], very brisk [≥4 mph]) and average flights of stairs walked daily (in categories, from ≤2 to >15). Additional activities were included on subsequent questionnaires, including lower intensity exercise (e.g., yoga, stretching, or toning) and other vigorous activities (e.g., lawn mowing) from 1992, and weight training from 2000 in NHS, and heavy outdoor work (e.g., digging or chopping) from 1988, weightlifting from 1990, and moderate outdoor work (e.g., yard work or gardening) from 2004 in HPFS. Each specific activity was assigned a metabolic equivalent of task (MET) value,24 and MET hours per week were derived by multiplying the MET value for an activity by the average number of hours per week reported by the participant. Total physical activity was calculated by summing the MET hours per week across all activities reported by the participant. Activities assigned ≥6 METs per hour were considered vigorous (i.e., jogging, running, bicycling, lap swimming, tennis, squash, racquetball, stair climbing, and calisthenics or other aerobic exercise). Moderate activities (<6 METs per hour) included walking or hiking outdoors, weight lifting, and heavy outdoor work.25
In addition, early life physical activity was retrospectively assessed in HPFS in 1992 by asking participants to report retrospectively how many months of the year they participated in strenuous physical activity at least twice per week during high school, college, and between ages 30–40 (never, 1–3 mo/y, 4–6 mo/y, 7–9 mo/y, or 10–12 mo/y). In NHS, participants were asked in 1988 how often they participated in strenuous physical activity at least twice per week between the ages of 18 and 22 (same categories).
Our physical activity questionnaire was validated by comparison with four 1-week physical activity diaries among 238 randomly selected participants in the HPFS. Correlations for inactivity, vigorous activity, and total activity were 0.41, 0.58, and 0.65, respectively. The correlation between vigorous activity as assessed by the questionnaire and resting pulse rate was −0.45.25 The validity of the physical activity assessments is also indirectly supported by the fact that, in these cohorts, individuals who reported higher levels of physical activity had markedly reduced risk of coronary heart disease,26,27 stroke,28 and total mortality.29,30
Statistical analysis
In primary analyses, we used quintiles of MET h/wk reported on the baseline questionnaire (1986) as the physical activity exposure in order to minimize the possibility that prodromal features or subclinical prodromal PD could affect exercise behavior (i.e., reverse causality). We also conducted analyses of quintiles of cumulative average MET h/wk, using all available questionnaires from baseline (1986) through 2008, in order to examine the association between long-term physical activity and prodromal features. This approach also reduces random variation in reported physical activity levels. If a participant did not complete the 2008 questionnaire (11.1% of men and 4.2% of women in this study population), we carried forward their quintile assignment based on cumulative average physical activity through the most recent completed questionnaire. Among women with missing 2008 physical activity values, the median interval between last observation and 2008 was 4 years; in men, the median interval was 2 years. In a sensitivity analysis, we adjusted for missing questionnaire cycles using indicator variables. We also performed analyses of physical activity during early life (high school, college, and 30s in men and ages 18–22 in women; categorized as never, 1–3 mo/y, 4–9 mo/y, 10–12 mo/y). We used multinomial logistic regression to assess associations between physical activity and number of prodromal features (1, 2, or ≥3 vs 0). Because the association between physical activity and constipation may be due to a direct effect of exercise on gastrointestinal motility, these analyses were conducted with and without including constipation among the features of interest. We also used age and multivariate-adjusted logistic regression to estimate odds ratios (ORs) of individual prodromal features according to level of physical activity. Primary multivariate models were adjusted for age (continuous), caffeine intake (quintiles), alcohol intake (quintiles), total calorie intake (quintiles), body mass index (<25, 25 to <30, ≥30 kg/m2), pack-years (<5, 5 to <10, 10 to <15, 15 to <20, ≥20), and the Alternate Health Eating Index (quintiles) as a measure of adherence to a healthy diet. Because only a subset of our study population was invited to undergo olfactory testing and complete the supplementary questionnaire, we used inverse-probability weighting for analyses of all outcomes except pRBD and constipation to weight participants by the inverse of their probability of being selected to participate in secondary testing, conditional on pRBD and constipation status. All p values reported are based on 2-tailed statistical tests. All analyses were performed using SAS statistical software (SAS Institute, Inc., Cary, NC).
Data availability
Anonymized data will be shared by request from qualified investigators.
Results
Characteristics of the study population are shown in table 1. In both cohorts, individuals with higher physical activity levels had lower body mass, were less likely to smoke, consumed less caffeine, drank more alcohol, and had a healthier diet than less active individuals.
Table 1.
Age-adjusted characteristics of the study population by baseline physical activity level
Physical activity and combinations of prodromal PD features
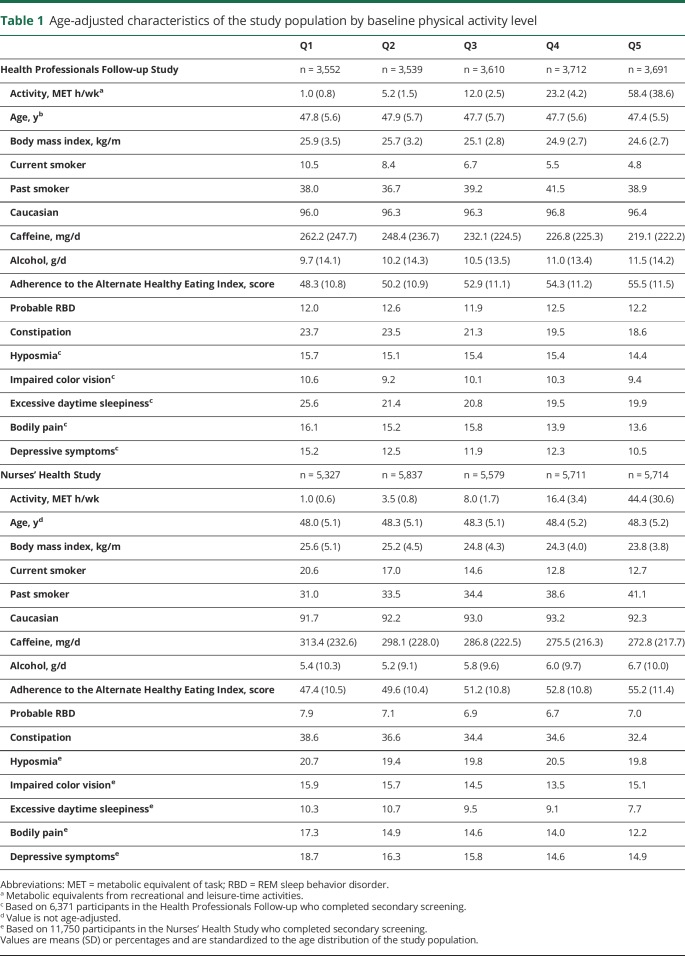
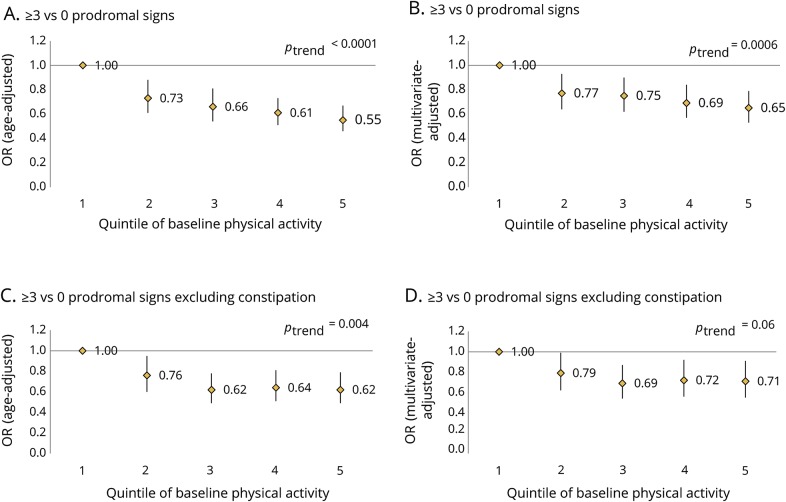
Baseline physical activity was associated with combinations of prodromal features (figure 1). In pooled analyses, the multivariate-adjusted OR for having ≥3 prodromal features vs none comparing the highest quintile of physical activity to the lowest was 0.65 (95% confidence interval [CI] 0.53–0.79; ptrend = 0.0006) (figure 1B). This association was weaker when constipation was not counted among the prodromal features (figure 1, C and D). In addition, cumulative physical activity was associated with prodromal features whether or not constipation was included (figure 2). Results were almost identical after additional adjustment for missing questionnaire cycles using indicator variables. We also repeated analyses considering physical activity on a continuous scale and found similar results (data not shown). Physical activity during college was also associated with having ≥3 prodromal features in men (OR comparing extreme categories 0.77, 95% CI 0.57–1.05; ptrend = 0.03). Results were similar for physical activity in high school (OR comparing extreme categories 0.80, 95% CI 0.59–1.07; ptrend = 0.09) and during the 30s (OR 0.76, 95% CI 0.57–1.03; ptrend = 0.26). However, number of prodromal features was no longer associated with early life physical activity when constipation was not included among the features (data not shown). Among women, strenuous activity at ages 18–22 was not associated with number of prodromal features (OR comparing extreme categories 1.02, 95% CI 0.74–1.41; ptrend = 0.70).
Figure 1. Pooled associations between baseline physical activity and presence of ≥3 prodromal signs.
(A) Age-adjusted association between baseline physical activity and presence of ≥3 vs 0 prodromal signs. (B) Multivariate-adjusted association between baseline physical activity and presence of ≥3 vs 0 prodromal signs. (C) Age-adjusted association between baseline physical activity and presence of ≥3 vs 0 prodromal signs, excluding constipation. (D) Multivariate-adjusted association between baseline physical activity and presence of ≥3 vs 0 prodromal signs, excluding constipation. OR = odds ratio.
Figure 2. Pooled associations between cumulative average physical activity and presence of ≥3 prodromal signs.
(A) Age-adjusted association between cumulative average physical activity and presence of ≥3 vs 0 prodromal signs. (B) Multivariate-adjusted association between cumulative average physical activity and presence of ≥3 vs 0 prodromal signs. (C) Age-adjusted association between cumulative average physical activity and presence of ≥3 vs 0 prodromal signs, excluding constipation. (D) Multivariate-adjusted association between cumulative average physical activity and presence of ≥3 vs 0 prodromal signs, excluding constipation. OR = odds ratio.
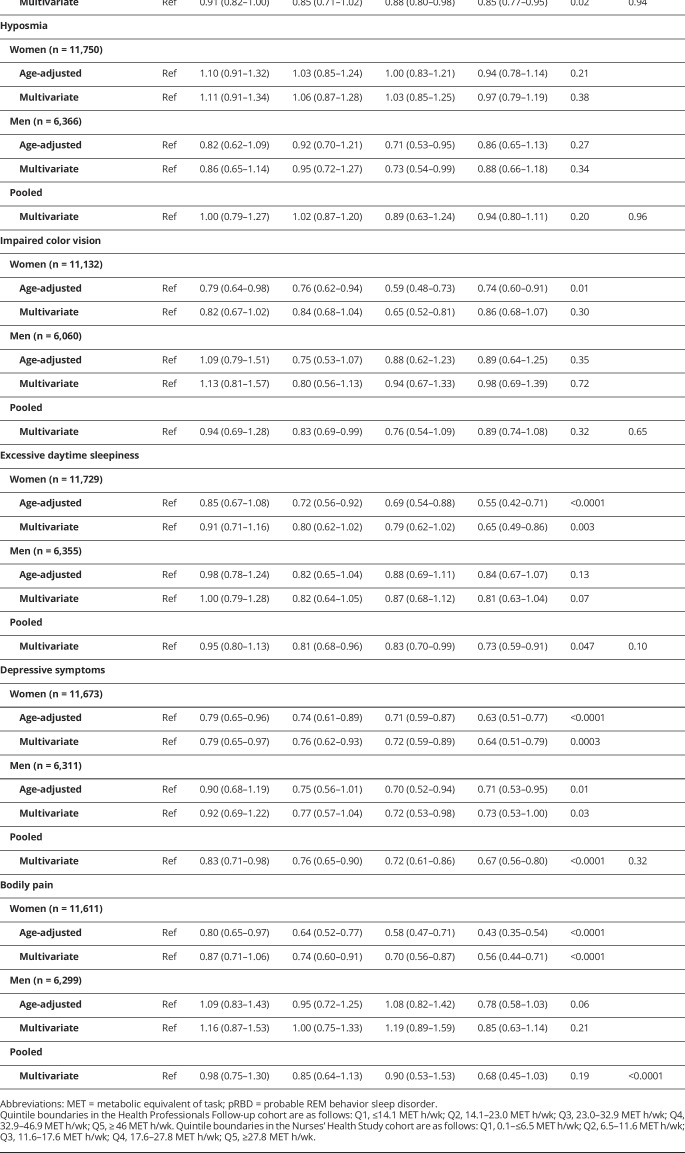
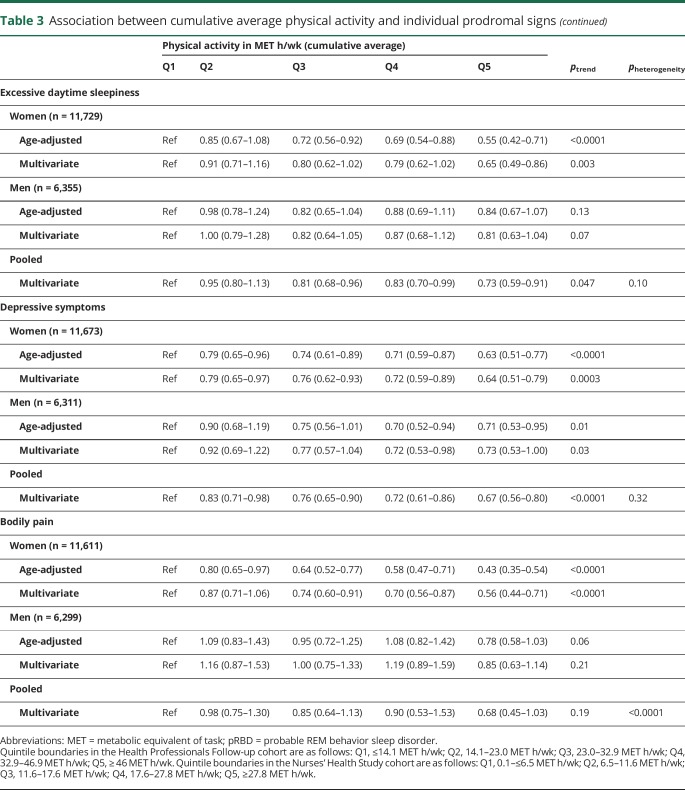
Physical activity and individual prodromal PD features
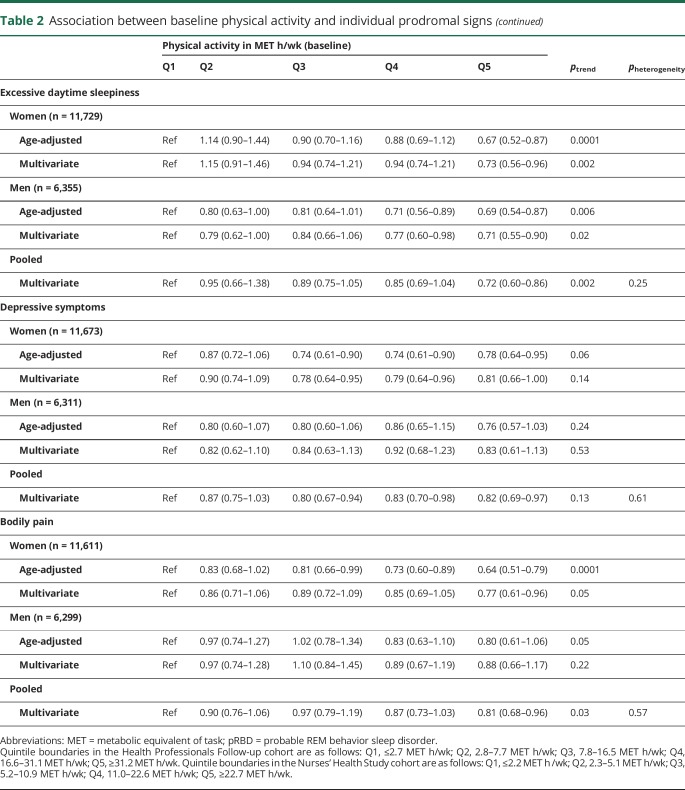
Overall, individuals with the highest levels of physical activity were less likely to have individual prodromal PD features, with the notable exception of hyposmia (tables 2 and 3). A strong association was found with constipation in both men and women (table 2); the pooled multivariate-adjusted OR comparing extreme quintiles of baseline physical activity was 0.78 (95% CI 0.73–0.83), and there was a clear dose-response relationship (ptrend < 0.0001). Analyses of cumulative average physical activity yielded even stronger associations (OR comparing extreme quintiles 0.71, 95% CI 0.66–0.77; ptrend < 0.0001). Baseline as well as cumulative physical activity were also associated with lowered odds of excessive daytime sleepiness, depressive symptoms, and bodily pain (table 2), whereas probable RBD was associated with cumulative average physical activity, but not baseline physical activity (table 3). In contrast, hyposmia was unassociated with physical activity in both baseline and cumulative average analyses. Overall, results were similar for vigorous and moderate physical activity. Some differences in the strengths of the associations were observed between men and women, but only the test for trend heterogeneity between sexes for bodily pain in the cumulative average analysis was significant.
Table 2.
Association between baseline physical activity and individual prodromal signs
Table 3.
Association between cumulative average physical activity and individual prodromal signs
In men, physical activity during early life (high school, college, and during thirties) was associated with lower odds of constipation in multivariate-adjusted analyses (ptrend for all <0.0001); no other features were significantly associated with early life physical activity (results not shown). In women, strenuous activity at ages 18–22 was associated with lower odds of constipation (multivariate-adjusted OR comparing extreme categories 0.72, 95% CI 0.64–0.81; ptrend < 0.0001) and depression (multivariate-adjusted OR comparing extreme categories 0.80, 95% CI 0.59–1.08; ptrend = 0.001). These associations remained after additional adjustment for baseline physical activity (ptrend for all <0.01). Unexpectedly, strenuous activity at ages 18–22 among women was associated with higher odds of hyposmia (multivariate-adjusted OR comparing extreme categories 1.40, 95% CI 1.08–1.81; ptrend = 0.007) and impaired color vision (multivariate-adjusted OR comparing extreme categories 1.65, 95% CI 1.24–2.19; ptrend < 0.0001).
Discussion
In this large investigation, we found that the odds of having 3 or more prodromal features that are often associated with PD were about 35% lower among individuals in the highest quintile of physical activity at baseline, and nearly 50% lower among those individuals in the highest quintile of overall physical activity during the follow-up. These odds are comparable to those relating physical activity to risk of PD, which according to a recent meta-analysis was 34% lower among individuals with the highest level of physical activity compared to those in the lowest.2 When individual prodromal symptoms were studied, participants with high levels of physical activity at recruitment were less likely to have constipation, excessive daytime sleepiness, depressive symptoms, and bodily pain more than 25 years later. Further, those individuals who maintained high levels of physical activity throughout the follow-up were also less likely to have pRBD. In contrast, we found no evidence that physical activity reduces risk of hyposmia.
A potential weakness of our study is that prodromal features were not assessed at baseline, thus it remains possible that, for some participants, prodromal features were present at the start of the study and could have affected their exercise patterns. This is particularly relevant for depressive symptoms and bodily pain, which have direct effects on physical activity and, in the case of depressive symptoms, often onset at relatively young ages. The associations between physical activity and these variables could therefore be at least in part due to reverse causation. There is also a possibility that early dopaminergic loss may have caused participants to exercise less, either because subtle rigidity or bradykinesia made exercise more difficult, or because of reduced responsivity of the dopamine-based reward system. To affect our results, these impairments would have to precede clinical PD by several decades. We controlled for several potential confounders, but as in any observational study, the possibility of unmeasured or residual confounding cannot be excluded. Some degree of measurement error in self-reported physical activity is inevitable; however, validation studies suggest that our questionnaire-based measurement captures physical activity reasonably well. In addition, all assessments were conducted using self-administered tests and questionnaires, including RBD, which ideally should be confirmed by polysomnography. Most participants in both cohorts are white, which may limit generalizability to more diverse populations. On the other hand, our study stands out because of the large sample size and the prospective design with collection over more than 2 decades of updated and detailed information on physical activity and potential confounding factors, using rigorously designed and well-validated instruments.
The strongest association that we found was between physical activity and intestinal constipation. Strikingly, even physical activity during adolescence and early adult life (high school, college, and at ages 30–39) was associated with a reduced prevalence of constipation 30 and more years later. An association between physical activity and constipation was reported in a previous investigation of women from the NHS cohort.31 Further, shorter transit in the gastrointestinal tract and increased stool frequency were reported during periods of training in a small group of athletes,32 and, in a separate study, randomization to a 12-week exercise program improved constipation.33 Other studies, however, have reported conflicting results.34,35 The strong associations found in our study in men and women, including for physical activity during early life, provide solid support for an effect of physical activity on the frequency of bowel movements, and suggest that such an effect could contribute to the beneficial effects of physical activity. Individuals with constipation have a markedly higher PD risk,12 are more likely to have Lewy bodies at autopsy in the locus ceruleus and substantia nigra,36 and have a lower density of neurons in the substantia nigra.37 It remains uncertain, however, whether constipation is an early consequence of PD, which is often accompanied by dopaminergic neuron depletion in the colon and presence of Lewy bodies in the myenteric plexus,38–40 or whether constipation itself may trigger or accelerate the pathologic process, perhaps by affecting the gut microbiome41 or otherwise promoting α-synuclein deposition.42 The latter hypotheses are intriguing and provide potential mechanisms by which physical activity may reduce PD risk.
Besides constipation, physical activity at baseline was also associated with reduced bodily pain, excessive daytime sleepiness, and depressive symptoms in our study. A previous study among over 2,400 community-based women found that higher baseline physical activity levels were associated with lower bodily pain over 3 years of follow-up,43 and a cross-sectional study found that leisure time physical activity was associated with a 30% lower prevalence of excessive daytime sleepiness.44 The design of these studies meant that temporality was difficult to determine. The extended follow-up in our study reduces the likelihood of reverse causation so that our results suggest that individuals who are regularly active in midlife are less likely to develop bodily pain and excessive sleepiness during aging.
An association between physical activity and depression or depressive symptoms has been reported in several investigations, including the TREND study, which found baseline physical activity was associated with lower depression scores over 6 years of follow-up.45 The results of a recent meta-analysis of prospective studies support the notion that physical activity is associated with reduced risk of depression.46 Because of the often insidious and recurring nature of depressive symptoms, however, and the likely effect of these symptoms on physical activity, reverse causation remains difficult to exclude.
Physical activity at baseline was not associated with other prodromal features. In contrast, cumulative average physical activity through 2008 was associated not only with constipation, bodily pain, excessive daytime sleepiness, and depressive symptoms, but also with pRBD. Previous results on the association between physical activity and pRBD are inconsistent. In a longitudinal study of over 600 adults over 50 years of age in Germany (TREND study), more physically active participants were less likely to have RBD assessed via a screening questionnaire at baseline, but not RBD assessed 6 years later.45 In a cross-sectional study in a community-based Chinese population, no association was found between physical activity and pRBD.47 However, in a different cross-sectional study, also in China, lower physical activity levels were associated with higher risk of having pRBD 6 years later.48 Patients diagnosed with idiopathic RBD at sleep disorder clinics have a very high risk of developing a neurodegenerative disease, most commonly PD or Lewy body dementia.49 Although these patients are selected for having severe disease and most likely do not represent the full spectrum of RBD in the general population, the results of these studies support the importance of RBD as a marker of neurodegeneration. The association that we found with physical activity is thus consistent with the hypothesis that physical activity may have neuroprotective effects.
A notable result of our study is the lack of association between physical activity and hyposmia or impaired color vision. The result on hyposmia is consistent with a previous report based on relatives of patients with PD (Parkinson at Risk Study), in which no association was found between recalled history of vigorous physical activity and University of Pennsylvania Smell Identification Test scores.50 Hyposmia is a nonspecific symptom that is common in older populations, so that the large majority of individuals with hyposmia do not have prodromal PD. This could lead to an attenuated association with physical activity, since physical activity may be unrelated to other causes of hyposmia in older adults. To our knowledge, the association between physical activity and impaired color vision among healthy individuals has not previously been investigated.
Important strengths of our study include the large sample size and the fact that we collected information on a range of possible prodromal features, which allowed us to investigate risk factors for combinations of prodromal features suggestive of prodromal PD. These features, when considered individually, are nonspecific for PD or for any neurodegenerative process, as they may well reflect idiopathic conditions or localized pathology, such as respiratory (hyposmia) or gastrointestinal (constipation) ailments. On the other hand, we have previously demonstrated that the co-occurrence of multiple features is strongly associated with having PD and parkinsonism.17 The lower risk of developing multiple prodromal features in regularly active individuals is thus consistent with a protective role of physical activity on risk of PD. Further, the fact that the lowest risk of prodromal features was observed among individuals who maintained a high degree of physical activity throughout the follow-up suggests physical activity during aging may have a cumulative protective effect. Future research could extend these findings by evaluating the mechanisms involved and investigating whether, among individuals with prodromal features of PD, higher levels of physical activity lead to lower rate of conversion to clinical PD.
Glossary
- CI
confidence interval
- HPFS
Health Professionals Follow-up
- MET
metabolic equivalent of task
- mph
miles per hour
- NHS
Nurses' Health Study
- OR
odds ratio
- PD
Parkinson disease
- pRBD
probable REM behavior sleep disorder
- RBD
REM sleep behavior disorder
- SF-36
36-item Short-Form Health Survey
Footnotes
Podcast: NPub.org/5j5nyl
Author contributions
K.C. Hughes: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis. X. Gao: drafting/revising the manuscript, data acquisition, accepts responsibility for conduct of research and final approval. S. Molsberry: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval. L. Valeri: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. M.A. Schwarzschild: drafting/revising the manuscript, data acquisition, accepts responsibility for conduct of research and final approval. A. Ascherio: drafting/revising the manuscript, data acquisition, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, obtaining funding.
Study funding
This study was supported by Department of Defense grant W81XWH-14-0131. The NHS cohort is funded by NIH grant UM1 CA186107 and the HPFS cohort is funded by NIH grant UM1 CA167552.
Disclosure
K.C. Hughes reports no disclosures relevant to the manuscript. X. Gao has served on a committee of the Parkinson Study Group and received funding from the NIH/National Institute of Neurologic Disorders and Stroke. S. Molsberry and L. Valeri report no disclosures relevant to the manuscript. M.A. Schwarzschild serves on scientific advisory boards of the Cure Parkinson's Trust, CBD Solutions Trust, the Michael J. Fox Foundation for Parkinson's Research, and Prevail Therapeutics; serves on a Data Monitoring Committee of an Eli Lilly and Company trial; served on a steering committee of Biotie Therapeutics, Inc./Acorda Therapeutics trial; serves as sponsor-investigator on a non-commercial IND (#100896; inosine for PD) to the FDA; and has received research support from the NIH, Parkinson's Disease Foundation, US Department of Defense, RJG Foundation, Target ALS Foundation, and Michael J. Fox Foundation. A. Ascherio serves on the Editorial Board of the Journal of Parkinson Disease, has received honoraria from Excemed (2016) and the Consortium of Multiple Sclerosis Centers (2018), and receives research support from the NIH, the US Department of Defense, the National Multiple Sclerosis Society, the ALS Association, and ALS Finding a Cure. Go to Neurology.org/N for full disclosures.
References
- 1.Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology 2005;64:664–669. [DOI] [PubMed] [Google Scholar]
- 2.Yang F, Trolle Lagerros Y, Bellocco R, et al. Physical activity and risk of Parkinson's disease in the Swedish National March Cohort. Brain 2015;138:269–275. [DOI] [PubMed] [Google Scholar]
- 3.Sääksjärvi K, Knekt P, Männistö S, et al. Reduced risk of Parkinson's disease associated with lower body mass index and heavy leisure-time physical activity. Eur J Epidemiol 2014;29:285–292. [DOI] [PubMed] [Google Scholar]
- 4.Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology 2010;75:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson's disease. Mov Disord 2008;23:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 2016;15:1257–1272. [DOI] [PubMed] [Google Scholar]
- 7.Mahlknecht P, Seppi K, Poewe W. The concept of prodromal Parkinson's disease. J Parkinsons Dis 2015;5:681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postuma RB, Aarsland D, Barone P, et al. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov Disord 2012;27:617–626. [DOI] [PubMed] [Google Scholar]
- 9.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord 2015;30:1600–1611. [DOI] [PubMed] [Google Scholar]
- 10.Lin CH, Wu RM, Chang HY, Chiang YT, Lin HH. Preceding pain symptoms and Parkinson's disease: a nationwide population-based cohort study. Eur J Neurol 2013;20:1398–1404. [DOI] [PubMed] [Google Scholar]
- 11.Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol 2008;63:167–173. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, Chen H, Schwarzschild MA, Ascherio A. A prospective study of bowel movement frequency and risk of Parkinson's disease. Am J Epidemiol 2011;174:546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boot BP, Boeve BF, Roberts RO, et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol 2012;71:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 2005;65:1442–1446. [DOI] [PubMed] [Google Scholar]
- 15.Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol 2011;69:811–818. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson H, Nordström A, Nordström P. Depression and subsequent risk of Parkinson disease: a nationwide cohort study. Neurology 2015;84:2422–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes KC, Gao X, Baker JM, et al. Non-motor features of Parkinson's disease in a nested case-control study of US men. J Neurol Neurosurg Psychiatry 2018;89:1288–1295. [DOI] [PubMed] [Google Scholar]
- 18.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med 2011;12:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med 2013;9:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 1996;106:353–356. [DOI] [PubMed] [Google Scholar]
- 21.Erb C, Adler M, Stübiger N, Wohlrab M, Zrenner E, Thiel HJ. Colour vision in normal subjects tested by the colour arrangement test “Roth 28-hue desaturated.” Vision Res 1998;38:3467–3471. [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 23.Berwick DM, Murphy JM, Goldman PA, Ware JE Jr, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care 1991;29:169–176. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- 25.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996;7:81–86. [DOI] [PubMed] [Google Scholar]
- 26.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000;343:16–22. [DOI] [PubMed] [Google Scholar]
- 27.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA 2002;288:1994–2000. [DOI] [PubMed] [Google Scholar]
- 28.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation 2008;118:947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ 2008;337:a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation 2003;107:2435–2439. [DOI] [PubMed] [Google Scholar]
- 31.Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol 2003;98:1790–1796. [DOI] [PubMed] [Google Scholar]
- 32.Strid H, Simrén M, Störsrud S, Stotzer PO, Sadik R. Effect of heavy exercise on gastrointestinal transit in endurance athletes. Scand J Gastroenterol 2011;46:673–677. [DOI] [PubMed] [Google Scholar]
- 33.De Schryver AM, Keulemans YC, Peters HP, et al. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scand J Gastroenterol 2005;40:422–429. [DOI] [PubMed] [Google Scholar]
- 34.Coenen C, Wegener M, Wedmann B, Schmidt G, Hoffmann S. Does physical exercise influence bowel transit time in healthy young men? Am J Gastroenterol 1992;87:292–295. [PubMed] [Google Scholar]
- 35.Tuteja AK, Talley NJ, Joos SK, Woehl JV, Hickam DH. Is constipation associated with decreased physical activity in normally active subjects? Am J Gastroenterol 2005;100:124–129. [DOI] [PubMed] [Google Scholar]
- 36.Abbott RD, Ross GW, Petrovitch H, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 2007;22:1581–1586. [DOI] [PubMed] [Google Scholar]
- 37.Petrovitch H, Abbott RD, Ross GW, et al. Bowel movement frequency in late-life and substantia nigra neuron density at death. Mov Disord 2009;24:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease: frequency and pathophysiology. Neurology 1992;42:726–732. [DOI] [PubMed] [Google Scholar]
- 39.Singaram C, Ashraf W, Gaumnitz EA, et al. Dopaminergic defect of enteric nervous system in Parkinson's disease patients with chronic constipation. Lancet 1995;346:861–864. [DOI] [PubMed] [Google Scholar]
- 40.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett 2006;396:67–72. [DOI] [PubMed] [Google Scholar]
- 41.Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord 2015;30:350–358. [DOI] [PubMed] [Google Scholar]
- 42.Sharma A, Kurek J, Morgan JC, Wakade C, Rao SSC. Constipation in Parkinson's disease: a nuisance or nuanced answer to the pathophysiological puzzle? Curr Gastroenterol Rep 2018;20:1. [DOI] [PubMed] [Google Scholar]
- 43.Dugan SA, Everson-Rose SA, Karavolos K, Sternfeld B, Wesley D, Powell LH. The impact of physical activity level on SF-36 role-physical and bodily pain indices in midlife women. J Phys Act Health 2009;6:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrianasolo RM, Menai M, Galan P, et al. Leisure-time physical activity and sedentary behavior and their cross-sectional associations with excessive daytime sleepiness in the French SU.VI.MAX-2 study. Int J Behav Med 2016;23:143–152. [DOI] [PubMed] [Google Scholar]
- 45.Lerche S, Gutfreund A, Brockmann K, et al. Effect of physical activity on cognitive flexibility, depression and RBD in healthy elderly. Clin Neurol Neurosurg 2018;165:88–93. [DOI] [PubMed] [Google Scholar]
- 46.Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med 2013;45:649–657. [DOI] [PubMed] [Google Scholar]
- 47.Ma JF, Qiao Y, Gao X, et al. A community-based study of risk factors for probable rapid eye movement sleep behavior disorder. Sleep Med 2017;30:71–76. [DOI] [PubMed] [Google Scholar]
- 48.Wong JC, Li J, Pavlova M, et al. Risk factors for probable REM sleep behavior disorder: a community-based study. Neurology 2016;86:1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009;72:1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siderowf A, Jennings D, Connolly J, Doty RL, Marek K, Stern MB. Risk factors for Parkinson's disease and impaired olfaction in relatives of patients with Parkinson's disease. Mov Disord 2007;22:2249–2255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from qualified investigators.