Abstract
Objective
To assess the association of cortical superficial siderosis (cSS) presence and extent with future bleeding risk in cerebral amyloid angiopathy (CAA).
Methods
This was a meta-analysis of clinical cohorts of symptomatic patients with CAA who had T2*-MRI at baseline and clinical follow-up for future intracerebral hemorrhage (ICH). We pooled data in a 2-stage meta-analysis using random effects models. Covariate-adjusted hazard ratios (adjHR) from multivariable Cox proportional hazard models were used.
Results
We included data from 6 eligible studies (n = 1,239). cSS pooled prevalence was 34% (95% confidence interval [CI] 26%–41%; I2 87.94%; p < 0.001): focal cSS prevalence was 14% (95% CI 12%–16%; I2 6.75%; p = 0.37), and disseminated cSS prevalence was 20% (95% CI 13%–26%; I2 90.39%; p < 0.001). During a mean follow-up of 3.1 years (range 1–4 years), 162/1,239 patients experienced a symptomatic ICH-pooled incidence rate 6.9% per year (95% CI 3.9%–9.8% per year; I2 83%; p < 0.001). ICH incidence rates per year according to cSS status were 3.9% (95% CI 1.7%–6.1%; I2 70%; p = 0.018) for patients without cSS, 11.1% (95% CI 7%–15.2%; I2 56.8%; p = 0.074) for cSS presence, 9.1% (95% CI 5.5%–12.8%; I2 0%; p = 0.994) for focal cSS, and 12.5% (95% CI 5.3%–19.7%; I2 73.2%; p = 0.011) for disseminated cSS. In adjusted pooled analysis, any cSS presence was independently associated with increased future ICH risk (adjHR 2.14; 95% CI 1.19–3.85; p < 0.0001). Focal cSS was linked with ICH risk (adjHR 2.11; 95% CI 1.31–2.41; p = 0.002), while disseminated cSS conferred the strongest bleeding risk (adjHR 4.28; 95% CI 2.91–6.30; p < 0.0001).
Conclusion
In patients with CAA, cSS presence and extent are the most important MRI prognostic risk factors for future ICH, likely useful in treatment planning.
Classification of evidence
This study provides Class III evidence that in symptomatic CAA survivors with baseline T2*-MRI, cSS (particularly if disseminated, i.e., affecting >3 sulci) increases the risk of future ICH.
Advanced cerebrovascular deposition of β-amyloid (Aβ), defined neuropathologically as cerebral amyloid angiopathy (CAA), is a prevalent small-vessel disease and a leading cause of spontaneous lobar intracerebral hemorrhage (ICH).1 The clinical management of symptomatic patients with CAA (presenting with or without ICH, in stroke or memory clinics) is thus centered around preventing future ICH, either first-ever or recurrent, since these are associated with substantial morbidity and mortality. Identifying strong risk factors of future CAA-related ICH, including hemorrhagic MRI biomarkers, is thus a crucial focus in the field.2
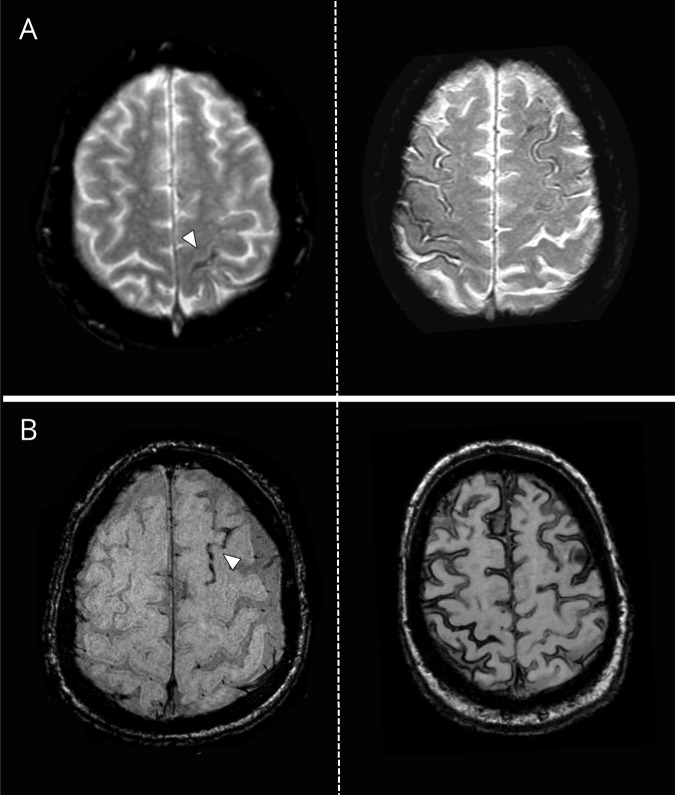
Putative hemorrhagic MRI biomarkers of small vessel damage in CAA include small, and typically silent, strictly lobar cerebral microbleeds (CMBs) and cortical superficial siderosis (cSS). While more recently described in relationship to CMBs, cSS has been implicated as a specific MRI footprint of a more aggressive CAA phenotype. cSS quite characteristically follows the curvilinear shape of the surrounding cerebral gyri on T2*-weighted MRI, reflecting blood breakdown products deposition that line the outermost surface of the cortex or the subarachnoid space (figure 1).3 cSS is thought to result from superficial cortical hemorrhages (designated as convexity subarachnoid hemorrhage, when acute), likely as a consequence of brittle superficial cortical penetrators or leptomeningeal vessels affected by advanced cerebrovascular Aβ deposition.3,4 cSS is rapidly gaining particular relevance to clinical practice as a marker for increased future CAA-related ICH risk in various different CAA patient populations and clinical settings.3,5,6
Figure 1. Representative examples of cortical superficial siderosis (cSS) on blood-sensitive MRI in patients with cerebral amyloid angiopathy (CAA).
(A) T2*-weighted gradient-recalled echo sequence shows focal cSS (i.e., affecting up to 3 sulci) on the left and disseminated cSS (i.e., affecting >3 sulci) on the right. (B) Susceptibility-weighted imaging sequences from 2 different patients with CAA with focal cSS (left) and disseminated cSS (right).
The aim of this work is to bring together the totality of evidence and obtain precise estimates on the effect sizes of cSS as an independent predictor of future ICH risk in patients with CAA, across the spectrum of different clinical presentations and settings. We investigate this clinical question in a systematic review and meta-analysis of published studies on the topic.
Methods
The study was conducted with reference to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),7 Meta-analysis of Observational Studies in Epidemiology (MOOSE)8 guidelines, and the Cochrane Handbook for Systematic Reviews of Interventions.
Standard protocol approvals, registrations, and patient consents
This study was performed in accordance with the guidelines at our institution and using a predefined summary protocol9 developed in September 2017 within our group (not published or registered as a stand-alone document). The protocol was reviewed by the authors' team and all details provided in the rest of the section reflect our prespecified decisions and strategy for the literature search, data extraction, outcome measures, and statistical approach. Of note, this protocol was identical to the one we developed in an earlier (preliminary) meta-analysis we have performed on the topic.9
Search strategy and study selection
We searched PubMed for potentially eligible studies published between January 1, 1990, and February 21, 2019, using a combination of keywords and Medical Subject Headings (“cortical superficial siderosis” or “convexity siderosis” or “convexal siderosis” or “cortical hemosiderosis”) and (“cerebral amyloid angiopathy” or “intracerebral hemorrhage” or “intracerebral haemorrhage”), without language restriction. We also used snowballing to screen the reference lists of all potentially eligible articles, relevant review articles, and author's own files (including regular weekly PubMed search updates on cSS for the last 6 years). Retrospective or prospective cohorts of symptomatic patients with CAA were eligible for inclusion if they characterized cSS presence and severity at baseline MRI with subsequent patient follow-up for the development of ICH. Specific inclusion criteria were (1) retrospective or prospective CAA patient cohorts (including ICH and non-ICH presentations, e.g., with cognitive impairment or transient focal neurologic episodes) defined according to the original or modified Boston criteria10,11 and including >50 adult patients; (2) baseline MRI (within 3 months of clinical presentations) with blood-sensitive sequences (T2*-weighted), incorporating cSS ratings for presence and severity using standard established criteria3,12; (3) clinical follow-up >6 months for new lobar ICH (either first-ever or recurrent) according to standardized criteria3; and (4) investigation of the association between cSS at baseline MRI and symptomatic ICH risk during clinical follow-up. We excluded case reports, cohorts selected by having isolated cSS at baseline, and cohorts on familial CAA or CAA-related inflammation. For studies with more than one publication describing results among overlapping cohorts and with the same outcome measure, we included the dataset with the longest follow-up, or the dataset with the largest number of participants if the follow-up period was identical.
The abstracts of all articles identified from online searches were reviewed by 2 authors, who also then reviewed the full text of all eligible studies independently. The final list of included studies was decided upon consensus among all coauthors.
Outcome measures
The primary outcome of interest was spontaneous symptomatic lobar ICH. This was defined as an acute onset focal neurologic deficit of presumed vascular cause lasting at least 24 hours or interrupted by death within 24 hours (definition of stroke), and diagnosed as spontaneous lobar ICH (presumed to be due to CAA-related small vessel disease) based on standardized criteria on brain imaging (either CT or MRI).13
Data extraction
We classified studies as being conducted in CAA-related lobar ICH, CAA presenting without lobar ICH at baseline (e.g., in memory clinics with cognitive symptoms or stroke clinics with transient focal neurologic episodes), a combination of the above, or spontaneous ICH including CAA-related lobar ICH patient populations. Where possible, in studies that included a combination of different CAA presentations (ICH, non-ICH), we extracted data for each subgroup separately.
For each study, we used a data collection pro-forma to extract information on study design, number and nature of patients (including mean age, sex, hypertension, antithrombotic drug use at baseline, history of ICH), inception point for inclusion in the study, MRI sequence measures and classification rules used for cSS rating, prevalence and severity of cSS and other MRI markers of CAA and small vessel disease (e.g., lobar CMBs, white matter hyperintensities), duration and methods of follow-up, and number of participants with the outcome of interest per cSS presence and severity category (focal: affecting up to 3 sulci and disseminated: affecting >3 sulci, figure 1).11 Where available, adjusted estimates from multivariable models of the independent association between cSS presence and severity (as well as other covariates included in the models from each individual) and the outcome of interest were extracted as hazard ratios (HRs) or odds ratios with corresponding 95% confidence intervals (CI). Two authors independently extracted data and disagreements were resolved by consensus.
Quality and risk of bias assessment
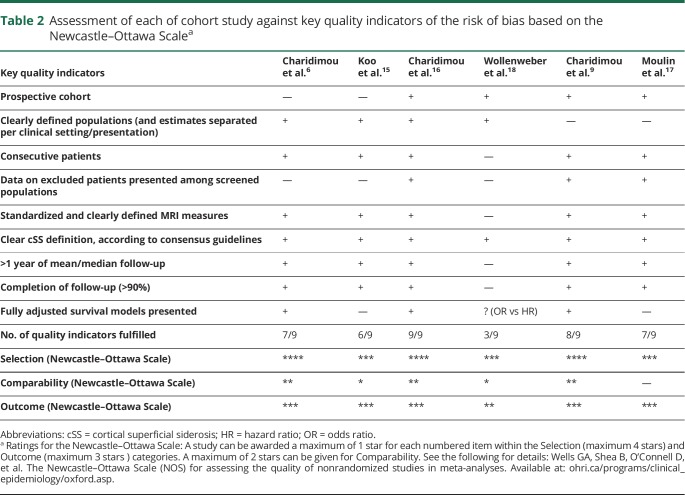
We assessed each study against a list of key quality criteria we devised based on study size, cohort recruitment method (prospective vs other), blinding between cSS ratings and outcome of interest, blood-sensitive MRI sequence type used, criteria of cSS assessment, and interrater agreement. These criteria were created using elements of the MOOSE8 recommendations and consensus standards for cSS assessment and rating.3 We assessed the risk of bias of each cohort according to the Newcastle–Ottawa Scale for assessing the quality of cohort (nonrandomized) studies.
Statistical analysis and data synthesis
We meta-analyzed data using a random effects model with DerSimonian-Laird weights.14 We quantified the strength of the association between cSS presence and severity and future ICH risk by pooling the covariate-adjusted HRs (adjHR). These HRs were extracted from relevant multivariable survival analysis models of all included studies (table 1, bottom row). Meta-analyses were first conducted across all eligible CAA cohorts (irrespective of clinical presentation with vs without ICH) and then stratified according to cohorts of patients with CAA-related ICH and patients presenting without ICH at baseline. We assessed statistical heterogeneity using I2 statistics and visually through inspection of the forest plot. Values of ≤25%, 25%–50%, and ≥50% were defined as low, moderate, and high degrees of heterogeneity, respectively. We explored publication bias with funnel plots. To provide estimates of absolute risks, often useful in clinical practice, we pooled and synthesized annualized symptomatic ICH risk (%/year) and corresponding 95% CIs where possible, using a Poisson regression model and exact Poisson intervals. We calculated pooled rates using the inverse variance method. All meta-analyses were performed using Stata 13.0 (StataCorp LP, College Station, TX) and considered a p value of <0.05 as evidence of statistical significance.
Table 1.
Basic elements of cohort design and patient population characteristics of included studies
Data availability policy
Data were deposited locally, but are not publicly available. All relevant data are included in the article.
Results
Characteristics, quality, and critical appraisal of included studies
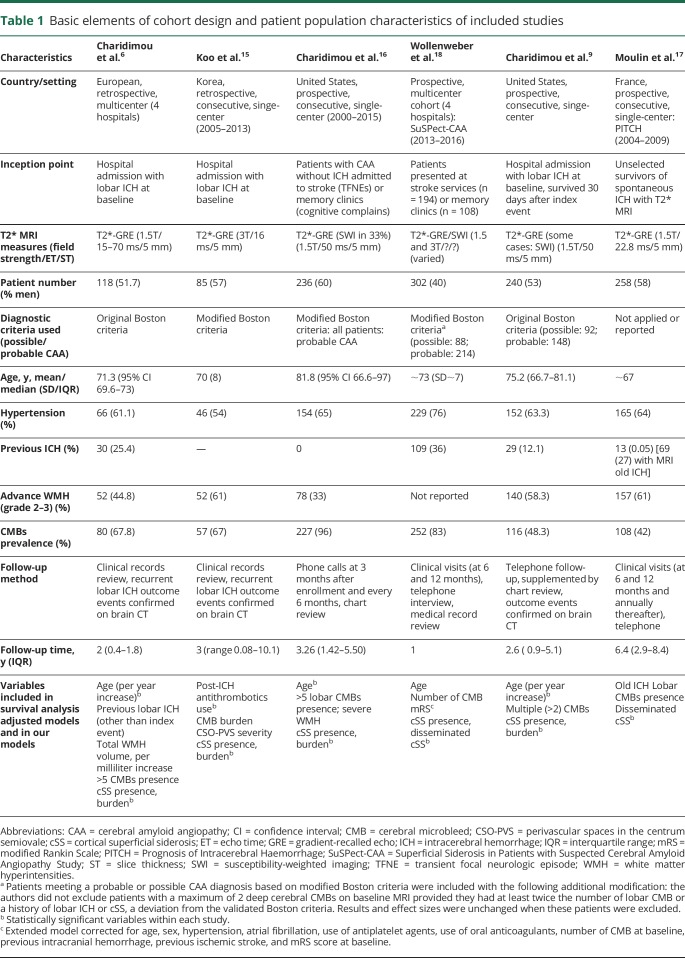
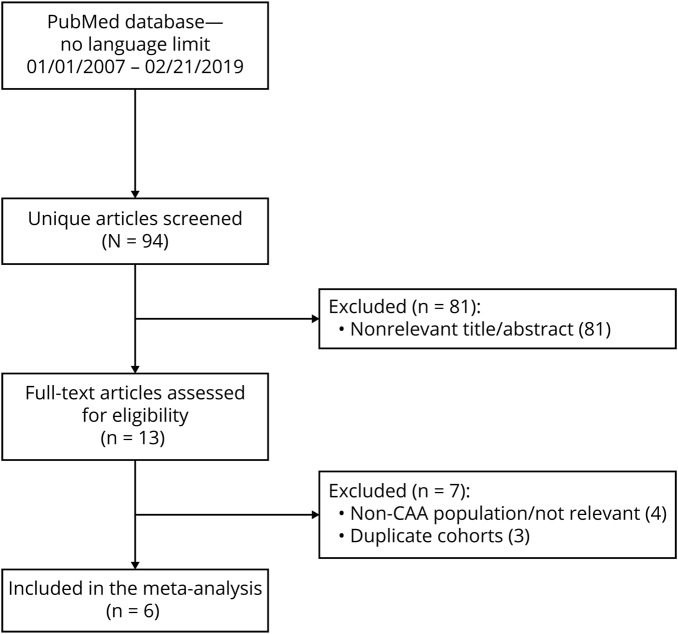
Based on our search criteria, we identified 94 publications, of which we retained 6 for the present meta-analysis, including a total of 1,239 patients.6,9,15–18 Figure 2 presents the flow chart for study selection. Table 1 summarizes basic study design elements and patient characteristics of all included studies. Study patient populations comprised 3 cohorts of CAA-related lobar ICH survivors,6,9,15 1 cohort of patients with probable CAA without ICH history presenting with cognitive impairment or transient focal neurologic episodes,16 and 1 cohort of patients with CAA presenting with both lobar ICH and non-ICH CAA syndromes.18 This latter study18 also included patients with incidental CAA diagnosed in patients with ischemic stroke/TIA (14%) and dizziness, headache, or other nonspecific neurologic complaints (4%), syndromes not typically caused by CAA. One of the studies we have identified included unselected survivors of spontaneous ICH with T2*-MRI available at baseline (i.e., both CAA-related lobar ICH and deep ICH).17 Since this was a prospective consecutive cohort, specifically investigating cSS in relation to ICH recurrence,17 for comprehensiveness and to increase the sample size, we have post hoc included it in our meta-analysis, but have also performed separate sensitivity analyses and reported estimates after removing this study. Of note, this applies only for the meta-analyses on disseminated cSS, since the study did not report estimates on any cSS presence and focal cSS. The outcome in this study was thus any recurrent ICH,17 but the majority of recurrences were lobar ICH in patients with CAA-ICH, thus not affecting the pooled estimates.
Figure 2. Flowchart of studies identification and selection.
CAA = cerebral amyloid angiopathy.
Included studies were somewhat different in their design and inception points, sample size, use of the Boston criteria (original vs modified), and presenting clinical features of patients. The MRI sequences used to assess cSS were comparable, though some studies used a combination of T2*–gradient-recalled echo (GRE) and susceptibility-weighted imaging (SWI) sequences. The criteria and instruments for cSS assessment were uniform across studies. All details can be reviewed in table 1. None of the studies have investigated (or incorporated in their adjusted models) blood pressure control and anticoagulation/antithrombotic treatment (only one study15) during follow-up. Of note, in all studies patients were treated according to current guidelines for blood pressure control after an ICH and a strategy of avoiding anticoagulation treatment in the setting of CAA-related ICH was followed.
Table 2 summarizes the results of the assessment of each cohort study against key quality indicators relevant for the clinical question under investigation and the risk of bias based on the Newcastle–Ottawa Scale. Studies were medium to high quality based on the total number of quality indicators fulfilled and had low risk of bias in the Newcastle–Ottawa Scale categories.
Table 2.
Assessment of each of cohort study against key quality indicators of the risk of bias based on the Newcastle–Ottawa Scalea
Pooled prevalence and severity of cSS in included studies
At baseline MRI, the pooled prevalence of cSS presence across all studies was 34% (95% CI 26%–41%; I2 87.94%; p < 0.001). Focal cSS (i.e., up to 3 affected sulci) prevalence was 14% (95% CI 12%–16%; I2 6.75%; p = 0.37) and disseminated cSS (i.e., >3 affected sulci) prevalence was 20% (95% CI 13%–26%; I2 90.39%; p < 0.001). When removing the single cohort that did not use the Boston criteria to report on cSS specifically in CAA-related ICH, the pooled prevalence for cSS presence was 37% (95% CI 34%–40%; I2 0%; p = 0.59), for focal cSS 15% (95% CI 12%–17%; I2 0%; p = 0.54), and for disseminated cSS 22% (95% CI 18%–26%; I2 60.5%; p = 0.04).
Meta-analyses: cSS and risk of future ICH
During a mean pooled follow-up time of 3.1 years (range 1–4 years), 162/1,239 patients overall experienced a symptomatic ICH, a pooled incidence rate of 6.9% per year (95% CI 3.9%–9.8% per year; I2 83%; p < 0.001). Assuming a uniform follow-up time and stable estimates of risk across time, the pooled symptomatic ICH incidence rates per year according to cSS presence and severity were 3.9% per year (95% CI 1.7%–6.1%; I2 70%; p = 0.018) for patients without cSS, 11.1% per year (95% CI 7%–15.2%; I2 56.8%; p = 0.074) in the presence of cSS, 9.1% per year (95% CI 5.5%–12.8%; I2 0%; p = 0.994) for focal cSS, and 12.5% (95% CI 5.3%–19.7%; I2 73.2%; p = 0.011) for disseminated cSS.
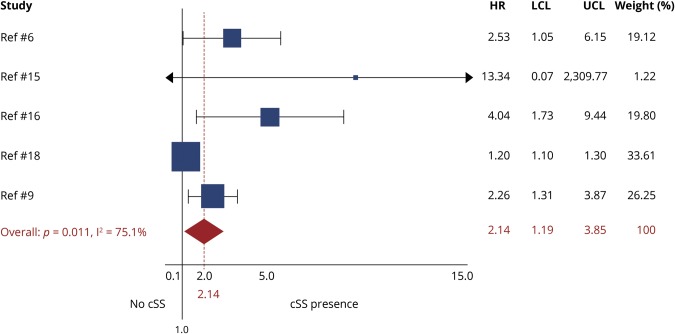
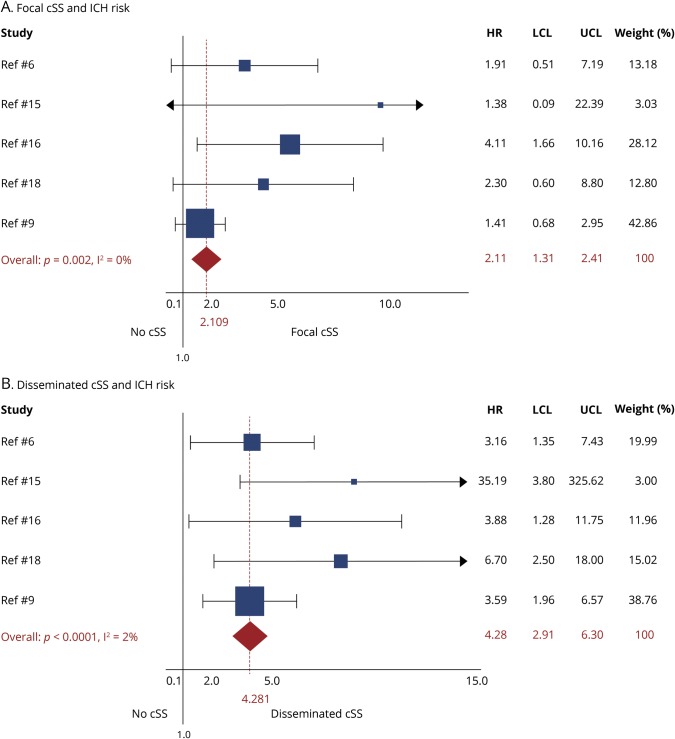
All studies, except one,17 provided adjusted estimates from survival analysis on cSS presence and future ICH risk. In meta-analysis of adjusted estimates from multivariable models, any cSS presence was independently associated with increased future ICH risk during follow-up (adjHR 2.14; 95% CI 1.19–3.85; p < 0.0001), compared to patients without cSS (figure 3). The association with future ICH risk showed a dose relationship with increasing cSS severity: focal cSS was linked with ICH risk (adjHR 2.11; 95% CI: 1.31–2.41; p = 0.002), while disseminated cSS conferred the strongest independent future bleeding risk (adjHR 4.28; 95% CI: 2.91–6.30; p < 0.0001) in patients with CAA during follow-up (figure 4). The results on disseminated cSS were consistent and of similar effect sizes in a subanalysis excluding the single study17 that did not present estimates for patients with CAA-ICH separately (adjHR 4.39; 95% CI 2.72–7.09; p < 0.0001). Among other common clinical and MRI variables included into multivariable models, increasing age (adjHR 1.04; 95% CI 1.01–1.06; p = 0.003; I2 0%; p = 0.597, per year increase), but not lobar CMBs (adjHR 1.05; 95% CI 0.76–1.44; p = 0.771; I2 27.4%; p = 0.229), were associated with ICH risk in CAA. Only one study incorporated MRI-visible centrum semiovale perivascular spaces severity in the survival models—no association with risk of ICH was found (table 1). Two studies included white matter hyperintensities severity in the multivariable survival models—no association was identified with the risk of ICH (table 1). In the rest of the studies, white matter hyperintensities severity was not even a univariable predictor of ICH risk and was hence not investigated further in adjusted models.
Figure 3. Forest plot of the association between the presence of cortical superficial siderosis (cSS) and risk of future intracerebral hemorrhage (ICH) during follow-up.
Meta-analysis was performed using a random effects model, pooling adjusted hazard ratios (HRs). The squares represent study-specific HRs, with their size proportional to their statistical weight (WGHT). LCL = lower confidence limit; UCL = upper confidence limit.
Figure 4. Forest plots of the association between focal cortical superficial siderosis (cSS) (A) and disseminated cSS (B) with risk of future lobar intracerebral hemorrhage (ICH) during follow-up.
Meta-analyses were performed using a random effects model, pooling adjusted hazard ratios (HRs). The squares represent study-specific HRs, with their size proportional to their statistical weight (WGHT). LCL = lower confidence limit; UCL = upper confidence limit.
Discussion
In this systematic review and meta-analysis, we have examined all currently available group-level data to establish a precise estimate of future ICH risk in patients with cSS across the spectrum of CAA presentations. Using data from more than 1,200 patients (the largest combined patient group to date), our pooled analysis provides compelling evidence that cSS presence and extent is the single strongest independent risk factor for CAA-related bleeding overall. Patients with cSS on MRI appear to have approximately 2 times the HR for future hemorrhage, compared to those without cSS. While focal cSS was also associated with doubling the bleeding hazards, the major driver of the elevated bleeding risk seems to be disseminated cSS (affecting more than 3 sulci), with pooled HRs 4 times greater than in patients without cSS. These estimates were independent of previously identified risk factors for CAA-related hemorrhage, including increasing age and lobar CMBs, and were stable irrespective of whether patients with CAA presented with lobar ICH or other non-ICH syndromes at baseline.
An important finding of this work, which should not be overlooked, is that the quality and methodologic aspects of studies on the topic vary and are not optimal. This partly reflects cSS being a relatively new imaging biomarker among CAA and small vessel disease MRI markers.2,13 Hence, our pooled estimates, while preliminary, do provide validity for earlier observations by synthesizing data from a larger number of patients from established CAA cohorts and more outcome events, conferring higher statistical power for multivariable survival models. These results add substantially to an increasing body of evidence supporting cSS (especially if disseminated) as a central and specific hemorrhagic footprint of advanced CAA.3 The major clinical relevance of current findings is that cSS should play a key part in the routine bleeding risk stratification in patients with symptomatic CAA, including decision-making around prognosis and treatment. This becomes particularly important for anticoagulation decisions in CAA—currently, one of the hotly debated topics in the field.19,20 Clinicians are often hesitant to prescribe oral anticoagulation in patients with suspected underlying CAA, who would otherwise have a strong indication for the medication (e.g., nonvalvular atrial fibrillation).20 Our results demonstrate that hemorrhagic risk, and hence the balance between hemorrhagic and cardioembolic stroke, is not uniform in patients with CAA. Instead, different CAA phenotypes with varying propensities towards bleeding can be dissected out based on the MRI presence and extent of cSS. The exact tipping point for when oral anticoagulation treatment should be avoided in patients with CAA in light of ICH risk and anticoagulation-related complications is difficult to calculate. However, a future incident/recurrent ICH rate of 12.5% per year (and HR of ∼4) in the presence of disseminated cSS in the current meta-analysis identifies a specific CAA patient group in which the benefits of oral anticoagulation for preventing ischemic strokes should be carefully balanced in order to outweigh the risks.20,21 On the other hand, the presence of atrial fibrillation might confer enough risk for ischemic stroke to offset the presumed ICH risk in the subset of patients with CAA without cSS and a CHA2DS2 score >1–2. We note that data on the true risk vs benefit in this scenario are not available. Taken together, our data solidify the argument for using T2*-MRI sequences in patients with CAA to guide treatment planning. Direct analyses of cSS and outcomes in patients with suspected CAA and comorbid conditions requiring antithrombotic treatment, such as atrial fibrillation, are needed.
The potential pathophysiologic mechanisms linking cSS to elevated ICH risk in CAA remain largely undefined. The prevailing hypothesis is that cSS results from repeated episodes of superficial cortical hemorrhage into the subarachnoid (following the curvilinear shape of the surrounding cerebral gyri) from brittle CAA-laden leptomeningeal arterioles.3 It is possible that ICH results from the same mechanisms22 but further evidence is required. In the absence of direct neuropathologic support, indirect evidence for this hypothesis is provided by specific associations with APOE alleles. APOE genotype is an important genetic determinant of CAA pathophysiology23,24: APOE ε4 seems to enhance vascular Aβ deposition in a dose-dependent fashion,25 while APOE ε2 is linked to CAA-related vasculopathic degenerative changes (vessel cracking, detachment and delamination of the outermost layer of the tunica media, and fibrinoid necrosis) that can lead to vessel rupture and ICH.26 APOE ε2 is known to be associated with CAA-related ICH, perhaps causally,27 and predisposes to larger volumes of CAA-related bleeding.28 In recent studies, the APOE ε2 allele had a higher prevalence among patients with CAA with cSS, especially when disseminated.29–31 These observations create some prospects in that the presence of cSS, especially if disseminated, and APOE ε2 could in fact allow for a better stratification of bleeding risk in CAA (for example, in the case of anticoagulation decisions). The presence of cSS is also associated with positive PET amyloid imaging in the general elderly population31 and higher amyloid PET deposition among memory clinic patients.32 Whether lobar ICHs at follow-up are observed in the vicinity of previously detected cSS remains an open question in the field, but has promise in improving our understanding of the pathogenic mechanisms that bind these 2 hemorrhagic lesions. There are currently no good quality data regarding the spatial location of new symptomatic lobar ICH found in patients with CAA during follow-up in relation to cSS. This hypothesis remains difficult to test just by visually correlating the area of ICH with previous cSS. Often these patients have cSS occurring in multiple brain locations and hence a large hematoma is very likely to be close to a cSS region without a real statistical topographic correlation. Future studies will need to come up with more sophisticated approaches to specifically test this hypothesis rigorously.
In included studies, and similarly in our meta-analysis, lobar CMBs were not an independent predictor of future ICH risk. This finding might appear counterintuitive. Previous clinical–MRI cohorts in CAA have, often disproportionally, focused on the role of lobar CMBs as a risk factor for future bleeding risk, showing that their presence and burden are associated with a higher risk of ICH recurrence.33–35 However, these older studies were performed prior to identification of cSS as an important CAA biomarker, and thus did not contain cSS in their analyses. When cSS is taken into account and included in adjusted survival models, lobar CMBs are no longer independently associated with the risk of future ICH,3,6 even in CAA cases with lobar ICH at baseline. This has led to an evolution of thinking concerning CMBs in CAA. CMBs likely represent good MRI markers of the presence of advanced disease (for example, multiple strictly lobar CMBs indicate underlying CAA with good sensitivity and specificity36), but are not necessarily strong predictors of the degenerative changes prone to ICH in amyloid-laden vessel wall segments. Corroborating this view, in a previous meta-analysis, strictly lobar microbleeds were related to APOE ε4 (OR 1.35, 95% CI 1.10–1.66; p = 0.005), but not APOE ε2.37 There is also direct neuropathologic evidence that microbleeds and larger symptomatic hemorrhages (e.g., macrobleeds) might be pathophysiologically distinct, and not in a continuum.38 These findings highlight the fact that cSS is more than just a cerebral sulcus equivalent of a microbleed—cSS is a distinct hemorrhagic signature of CAA and is strongly associated with risk of bleeding.
Strengths of our study include the increased sample size and number of outcome events by pooling all published data from retrospective and prospective MRI cohort studies investigating cSS. The combination of high statistical power and the homogeneity of effect sizes and direction across studies support the validity of the results. Some important limitations deserve careful consideration, and generally fall into 2 categories: (1) inherent limitations of CAA cohorts looking at baseline MRI markers and risk of future hemorrhage; and (2) limitations related to this type of aggregate data group-level meta-analysis. By definition, a potential limitation of any CAA clinical cohort is the selection bias due to the requirement for an MRI close to the baseline presentation, often as part of routine clinical care. Our results can thus only be generalized to symptomatic CAA survivors who get a brain MRI at baseline and fulfil the Boston criteria for CAA. This is often the clinical CAA population in which future ICH prevention is most relevant. Studies had varied follow-up times, ranging from 1 to 3 years, which are relatively short and might affect the reported future ICH estimates. In combination with the small sample sizes within individual cohorts, this led to wide CIs around risk estimates. Therefore, some of our meta-analyses were dominated in terms of statistical weighting by certain cohorts. The MRI protocols for cSS detection were not harmonized across, and often within studies, including the use of both T2*-GRE and SWI sequences at 1.5T or 3T, which might affect the sensitivity for grading cSS severity. This is an important limitation in synthesizing evidence for an MRI biomarker, in this case cSS, which might affect the rating for cSS presence and extent and affect our estimates. Of note, different MRI sequence measures and specifically SWI and T2*-GRE sequences have different sensitivities for blood product detection, as demonstrated in the CMB literature. However, there are no studies quantifying the difference in sensitivity of the 2 techniques for cSS classification. Given that cSS represents a much higher volume of blood breakdown products compared to CMBs, the differences in sensitivity might be less pronounced.
Despite using adjHR from multivariable survival models, a potential limitation of this study-level data approach is residual confounding of the estimates by other baseline variables related to future ICH. Our approach for this initial meta-analysis on cSS and risk of future ICH was to look at the clinical relevance of the biomarker across all symptomatic CAA presentations (ICH and non-ICH) in order to draw more definite conclusions. We also hypothesized based on prior studies that the effect of cSS in elevating future bleeding risk is independent of whether patients had a history of ICH.16,39 However, careful phenotyping of CAA based on MRI signatures in combination with the clinical setting would still be of interest to further stratify future ICH risk. Based on the meta-analysis design, it remains uncertain whether cSS is a “stronger” predictor than clinical presentation with ICH and ICH history (single or multiple). Only 2 studies16,18 in our meta-analysis have provided data on patients with CAA without lobar hemorrhage presenting with transient focal neurologic episodes (TFNEs) or cognitive impairment, a largely understudied group in the field. In one of these studies18 the authors have not presented estimates separately for patients with CAA with vs without ICH in relation to the outcomes of interest. Hence, a secondary analysis according to patient presentations was not possible based on available data. We acknowledge that different CAA clinical presentations (i.e., with ICH vs TFNEs or cognitive impairment) might have different baseline absolute risks for future ICH. However, we have hypothesized, and confirmed, that the effect of the marker—in this case cSS—has a consistent effect in increasing the relative risk for future ICH across the spectrum of CAA. In the absence of precise patient-years of follow-up, our pooled estimates on ICH incidence rate per year according to cSS status and severity are not stable, with moderate to severe statistical heterogeneity, and likely underestimate the true incidence rate, especially in patients with severe cSS.40 Finally, none of the included studies have investigated blood pressure control and antithrombotics use during follow-up, and how they might interact with MRI markers of CAA and ICH recurrence—a point for future investigation. The contribution of chronic arterial hypertension to lobar ICH risk is well-documented41–43 and blood pressure control in particular is a key target for reducing future ICH occurrence in CAA. The only way to circumvent the limitations discussed and further increase the sample size and generalizability of the findings, investigate the full range of interactions between different variables, and build more sophisticated multivariable models would be an individual patient data meta-analysis systematically exploring MRI and other predictors of CAA-related ICH recurrence. This analysis is currently planned under the umbrella of the International CAA Association (caaforum.org/).
Altogether, data reported here solidify cSS as a biomarker of increased cortical and leptomeningeal small-vessel fragility and high CAA disease activity, heralding a high risk for future ICH. Our findings therefore provide a strong argument for using blood-sensitive T2*-weighted MRI sequences to identify specific subgroups of patients with CAA at high risk for ICH. This could be helpful to inform and tailor treatment planning. Large international efforts in the field are underway to validate and expand these findings.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Glossary
- Aβ
β-amyloid
- adjHR
adjusted hazard ratio
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- CMB
cerebral microbleed
- cSS
cortical superficial siderosis
- GRE
gradient-recalled echo
- HR
hazard ratio
- ICH
intracerebral hemorrhage
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- SWI
susceptibility-weighted imaging
- TFNE
transient focal neurologic episode
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
This study was supported by NIH grant R01 AG26484.
References
- 1.Charidimou A, Boulouis G, Gurol ME, et al. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 2017;140:1829–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg SM, Al-Shahi Salman R, Biessels GJ, et al. Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol 2014;13:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charidimou A, Linn J, Vernooij MW, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015;138:2126–2139. [DOI] [PubMed] [Google Scholar]
- 4.Linn J, Herms J, Dichgans M, et al. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol 2008;29:184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linn J, Wollenweber FA, Lummel N, et al. Superficial siderosis is a warning sign for future intracranial hemorrhage. J Neurol 2013;260:176–181. [DOI] [PubMed] [Google Scholar]
- 6.Charidimou A, Peeters AP, Jager R, et al. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology 2013;81:1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 9.Charidimou A, Boulouis G, Roongpiboonsopit D, et al. Cortical superficial siderosis and recurrent intracerebral hemorrhage risk in cerebral amyloid angiopathy: large prospective cohort and preliminary meta-analysis. Int J Stroke Epub 2019 Feb 20. [DOI] [PubMed]
- 10.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539. [DOI] [PubMed] [Google Scholar]
- 11.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahneman. Thinking Fast, Thinking Slow. New York: Farrer Straus Giroux; 2011. [Google Scholar]
- 13.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 15.Koo HW, Jo KI, Yeon JY, Kim JS, Hong SC. Clinical features of high-degree centrum semiovale-perivascular spaces in cerebral amyloid angiopathy. J Neurol Sci 2016;367:89–94. [DOI] [PubMed] [Google Scholar]
- 16.Charidimou A, Boulouis G, Xiong L, et al. Cortical superficial siderosis and first-ever cerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2017;88:1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moulin S, Casolla B, Kuchcinski G, et al. Cortical superficial siderosis: a prospective observational cohort study. Neurology 2018;91:e132–e138. [DOI] [PubMed] [Google Scholar]
- 18.Wollenweber FA, Opherk C, Zedde M, et al. Prognostic relevance of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology 2019;92:e792–e801. [DOI] [PubMed] [Google Scholar]
- 19.Steiner T. Resumption of oral anticoagulation after warfarin-associated intracerebral hemorrhage: yes. Stroke a J Cereb Circ 2011;42:3661–3662. [DOI] [PubMed] [Google Scholar]
- 20.Charidimou A, Shoamanesh A, Al-Shahi Salman R, et al. Cerebral amyloid angiopathy, cerebral microbleeds and implications for anticoagulation decisions: the need for a balanced approach. Int J Stroke 2018;13:117–120. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg SM, Smith EE. Implications of cortical superficial siderosis in CAA: superficial relationships. Neurology 2019;92:360–361. [DOI] [PubMed] [Google Scholar]
- 22.Mandybur TI. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol 1986;45:79–90. [PubMed] [Google Scholar]
- 23.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol 1995;38:254–259. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg SM, Briggs ME, Hyman BT, et al. Apolipoprotein E epsilon 4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke 1996;27:1333–1337. [DOI] [PubMed] [Google Scholar]
- 25.Rannikmae K, Samarasekera N, Martinez-Gonzalez NA, Al-Shahi Salman R, Sudlow CL. Genetics of cerebral amyloid angiopathy: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2013;84:901–908. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg SM, Vonsattel JP, Segal AZ, et al. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology 1998;50:961–965. [DOI] [PubMed] [Google Scholar]
- 27.Biffi A, Anderson CD, Jagiella JM, et al. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. Lancet Neurol 2011;10:702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brouwers HB, Biffi A, Ayres AM, et al. Apolipoprotein E genotype predicts hematoma expansion in lobar intracerebral hemorrhage. Stroke 2012;43:1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charidimou A, Boulouis G, Roongpiboonsopit D, et al. Cortical superficial siderosis multifocality in cerebral amyloid angiopathy: a prospective study. Neurology 2017;89:2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoamanesh A, Martinez-Ramirez S, Oliveira-Filho J, et al. Interrelationship of superficial siderosis and microbleeds in cerebral amyloid angiopathy. Neurology 2014;83:1838–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichler M, Vemuri P, Rabinstein AA, et al. Prevalence and natural history of superficial siderosis: a population-based study. Stroke 2017;48:3210–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Na HK, Park JH, Kim JH, et al. Cortical superficial siderosis: a marker of vascular amyloid in patients with cognitive impairment. Neurology 2015;84:849–855. [DOI] [PubMed] [Google Scholar]
- 33.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010;75:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 2004;35:1415–1420. [DOI] [PubMed] [Google Scholar]
- 35.Charidimou A, Imaizumi T, Moulin S, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: a meta-analysis. Neurology 2017;89:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Ramirez S, Romero JR, Shoamanesh A, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement 2015;11:1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maxwell SS, Jackson CA, Paternoster L, et al. Genetic associations with brain microbleeds: systematic review and meta-analyses. Neurology 2011;77:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg SM, Nandigam RN, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke 2009;40:2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Etten ES, Auriel E, Haley KE, et al. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke 2014;45:2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roongpiboonsopit D, Charidimou A, William CM, et al. Cortical superficial siderosis predicts early recurrent lobar hemorrhage. Neurology 2016;87:1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biffi A, Rosand J. Blood pressure control and recurrence of intracerebral hemorrhage: reply. JAMA 2016;315:611–612. [DOI] [PubMed] [Google Scholar]
- 42.Broderick J, Brott T, Tomsick T, Leach A. Lobar hemorrhage in the elderly: the undiminishing importance of hypertension. Stroke 1993;24:49–51. [DOI] [PubMed] [Google Scholar]
- 43.Fischer U, Cooney MT, Bull LM, et al. Acute post-stroke blood pressure relative to premorbid levels in intracerebral haemorrhage versus major ischaemic stroke: a population-based study. Lancet Neurol 2014;13:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were deposited locally, but are not publicly available. All relevant data are included in the article.