Abstract
Objective
To determine the frequency of high-convexity tight sulci (HCTS) in a population-based sample and whether the presence of HCTS and related features influenced participants' cognitive status and classification within the new Alzheimer-biomarker framework.
Methods
We analyzed 684 participants ≥50 years of age who were enrolled in the prospective population-based Mayo Clinic Study of Aging and underwent structural MRI, amyloid PET imaging, and tau PET imaging. A fully automated machine-learning algorithm that had been developed previously in house was used to detect neuroimaging features of HCTS. On the basis of PET and MRI measures, participants were classified as having normal (A−) or abnormal (A+) amyloid, normal (T−) or abnormal (T+) tau, and normal (N−) or abnormal (N+) neurodegeneration. The neuropsychological battery assessed domain-specific and global cognitive scores. Gait speed also was assessed. Analyses were adjusted for age and sex.
Results
Of 684 participants, 45 (6.6%) were classified with HCTS according to the automated algorithm. Patients with HCTS were older than patients without HCTS (mean [SD] 78.0 [8.3] vs 71.9 [10.8] years; p < 0.001). More were cognitively impaired after age and sex adjustment (27% vs 9%; p = 0.005). Amyloid PET status was similar with and without HCTS, but tau PET standard uptake value ratio (SUVR) was lower for those with HCTS after age and sex adjustment (p < 0.001). Despite a lower tau SUVR, patients with HCTS had lower Alzheimer disease (AD) signature cortical thickness. With the amyloid-tau-neurodegeneration framework, HCTS was overrepresented in the T−(N)+ group, regardless of amyloid status.
Conclusion
The HCTS pattern represents a definable subgroup of non-AD pathophysiology (i.e., T−[N]+) that is associated with cognitive impairment. HCTS may confound clinical and biomarker interpretation in AD clinical trials.
The combination of high-convexity tight sulci (HCTS), defined as compression of sulci at the vertex (figure 1), enlarged CSF spaces in the Sylvian fissure, and ventriculomegaly, is an established neuroimaging phenotype of a CSF dynamics disorder (CDD) called disproportionately enlarged subarachnoid space hydrocephalus (DESH).1 Although DESH was originally identified in individuals with symptomatic, normal-pressure hydrocephalus (NPH), recent population studies suggest that features of DESH are more common with advancing age and occur more frequently among persons without obvious symptoms than previously believed.2,3
Figure 1. Examples of high-convexity tight sulci (circled red areas) from individual participants.
We previously developed an automated algorithm to detect HCTS in MRIs.4 In this study, the HCTS is used as an indirect marker of a CDD. Unlike the other features of DESH (ventriculomegaly and widened sylvian fissure), which can be seen in neurodegenerative disease, compression of the sulci distinguishes HCTS from a neurodegenerative process such as AD,5 which would result in widening of the sulci. Furthermore, when HCTS occurs in NPH, it is often associated with improvement in symptoms after ventricular shunting.6 This supports the assumption that HCTS is indeed a disorder of CSF dynamics.
Whether HCTS influences neurodegenerative biomarkers is of particular importance, given the new research framework for Alzheimer disease (AD) published by the National Institute on Aging and Alzheimer's Association,7 that defines AD on the basis of both abnormal amyloid and tau biomarkers. The amyloid-tau-neurodegeneration [AT(N)] biomarker scheme classifies participants as having normal (A−) or abnormal (A+) amyloid, normal (T−) or abnormal (T+) tau, and normal (N−) or abnormal (N+) neurodegeneration.
In HCTS, the ventricles and Sylvian fissures can enlarge and mimic atrophy (i.e., neurodegeneration). We therefore hypothesized that this pattern could be overrepresented among persons with normal tau levels and abnormal neurodegeneration (T−[N]+). An additional objective therefore was to determine the frequency of HCTS and to categorize persons with the HCTS pattern of structural imaging abnormalities within the AT(N) biomarker framework.7
Methods
Participants
The Mayo Clinic Study of Aging (MCSA) is a population-based study that focuses on cognitive decline; details of the study design have been published previously.8 Using an age- and sex-stratified random sampling design, the MCSA initially enumerated residents of Olmsted County, Minnesota, who were 70 to 89 years of age on October 1, 2004, with the Rochester Epidemiology Project medical records linkage system.9 In 2012, the age range was expanded to enumerate and sample Olmsted residents 50 to 69 years of age. For the current study, MCSA participants ≥50 years of age were included if they had undergone structural MRI, amyloid PET imaging, and tau PET imaging.
Cognitive evaluation
The neuropsychological assessment battery included 9 tests covering 4 cognitive domains: (1) memory (Wechsler Memory Scale–Revised [Logical Memory II and Visual Reproduction II Tests]; Auditory-Verbal Learning Test [delayed-recall trial]), (2) attention-executive function (Wechsler Adult Intelligence Scale–Revised [Trail Making Test part B, digit symbol subtest]), (3) language (Boston Naming Test [fluency category]), and (4) visuospatial skills (Wechsler Adult Intelligent Scale–Revised [Block Design and Picture Completion subtests]). In addition, raw test scores were averaged and scaled to create a domain z score to facilitate comparisons across domains. The z scores from the 4 domains were averaged and scaled to calculate a global cognitive z score.8
Participant evaluations included a review of the medical history and an interview by a study coordinator. A physician performed a medical history review, mental status examination, and neurologic examination. A neuropsychological examination was also performed. Participants were then classified as cognitively impaired or unimpaired by a consensus panel consisting of the examining physician, the study coordinator, and a neuropsychologist, as previously described.8 Those with mild cognitive impairment or dementia were considered cognitively impaired. The diagnosis of cognitively unimpaired was established by the use previously published criteria.10,11
Other covariates
Demographics (age, sex, and years of education) were assessed by interview and electronic health record review. A nurse abstracted clinical data from the medical records linkage system.9 APOE status (ε4 carrier) was assessed at baseline.
Gait velocity (measured in meters per second) was assessed over 7.62 m (25 ft) at a self-selected pace. The full details of the gait evaluation have been previously published.12
Neuroimaging
Magnetic resonance imaging
Magnetization-prepared rapid gradient-echo (MPRAGE) and fluid-attenuated inversion recovery (FLAIR) images were acquired as part of the standard MCSA MRI protocol. Consistent imaging parameters were used throughout, and imaging was performed with 3T GE MRI scanners (GE Healthcare, Chicago, IL). Complete details of the acquisitions can be found elsewhere.13
The FLAIR MRI was used to ascertain vascular disease by assessing subcortical infarcts, cortical infarcts, and white matter hyperintensity load. All brain infarctions were assessed by a trained image analyst and confirmed by a vascular neurologist or radiologist (K.K., J.G.-R., C.R.J.) blinded to all clinical information.14 White matter hyperintensity load was assessed with a semiautomated FLAIR algorithm, as previously described.15
MPRAGE images were analyzed with probabilistic segmentation using SPM12 with the Mayo Clinic Adult Lifespan Template (MCALT)16 population-optimized priors and settings (nitrc.org/projects/mcalt/), and deformations were calculated by mapping the template grayscale image to each subject scan using the Advanced Normalization Tools17 toolkit. Two atlas parcellations, one with 122 tissue regions (MCALT_ADIR122) and another with 123 named sulcal regions (adapted from BrainVISA [brainvisa.info]), were propagated from MCALT coordinates to subject space.
Cortical thickness was estimated with FreeSurfer 5.3.18 Average cortical thickness measures in the entorhinal, fusiform, parahippocampal, midtemporal, inferior-temporal, and angular gyrus regions of interest were combined to create an AD signature measurement. An abnormal AD signature cortical thickness (N+) was defined as <2.68 mm.19
Using the segmentation procedures described in the following section, we calculated ventricular volumes and normalized them to total intracranial volume with FreeSurfer 5.3.
Measuring HCTS
We have developed a fully automated imaging pipeline to detect HCTS that is described in prior work.4 In brief, the algorithm begins with the SPM12 CSF segmentation output probability in each sulcal region that is normalized to total intracranial volume. In order to train the algorithm, manual grading for the presence of high-convexity tightness was previously completed by an expert, and this information was used as the standard against which a machine-learning algorithm was trained.4 CSF regions relevant to predicting the presence of high-convexity tightness were chosen by an area under the receiver operating characteristic curve analysis. A support vector machine model was then trained on the regions selected. Using the model, the methodology classified scans for HCTS with an area under the receiver operating characteristic curve >0.96 with respect to the expert evaluations.4 Figure 1 provides an example of 2 cases of ventriculomegaly and HCTS from our cohort. This machine-learning pipeline was previously validated on a dataset separate from the training data set.4
Validation study
Although the automated HCTS detection algorithm was previously validated,4 we performed an independent validation for the present study. We randomly selected 10 participants with an HCTS score >1, 10 participants with an HCTS score close to the overall median, and 10 participants without HCTS from the present cohort. These images were visually graded for presence or absence of an HCTS by a board-certified neuroradiologist (J.H.) blinded to the machine-learning categorization. The visual grading served as the gold standard for the independent validation study.
Positron emission tomography
Amyloid PET imaging was performed with Pittsburgh compound B (PiB), and tau PET was performed with AV1451, with precursor supplied by Avid Radiopharmaceuticals (Philadelphia, PA). Details of PET acquisition have been published previously.20,21 Participant PET scans were aligned to their corresponding MPRAGE images by using a rigid transformation computed with SPM12 and combined with the Advanced Normalization Tools MCALT-to-subject warps to place atlases in PET image space. The full details of amyloid and tau PET acquisition have been published elsewhere.22 An abnormal amyloid level (A+) was defined as a PiB standard uptake value ratio (SUVR) >1.48. An abnormal tau (T+) was defined as a tau SUVR >1.25.23
Statistical analysis
Continuous variables were compared with t tests, and differences in proportions were compared with χ2 tests. Additional testing was adjusted for age and sex. The tests for continuous variables were performed with analysis of covariance models, and categorical variables were assessed with logistic regression models. Values of p < 0.05 were considered significant.
Sensitivity analysis
As shown later, the cerebrovascular disease burden was increased among participants with HCTS. We therefore performed a sensitivity analysis evaluating associations with HCTS in only those without infarcts.
Standard protocol approvals, registrations, and patient consents
The Mayo Clinic Institutional Review Board and the Olmsted Medical Center Institutional Review Board approved all study protocols. Written informed consent was obtained from all participants. The reporting of this study is in compliance with Strengthening the Reporting of Observational Studies in Epidemiology guidelines.24
Data availability
Data from the MCSA, including data from this study, are available on request.
Results
Characteristics of participants
We included 684 participants in the study. Forty-five (6.6%) were classified as having HCTS. The demographic characteristics of the participants with and without HCTS are listed in table 1. Compared with those who did not have HCTS, those with HCTS were older (mean [SD] age, 78.0 [8.3] vs 71.9 [10.8] years; p < 0.001) and more often cognitively impaired after adjustment for age.
Table 1.
Characteristics of participants, stratified by the presence of CDD
Neuroimaging
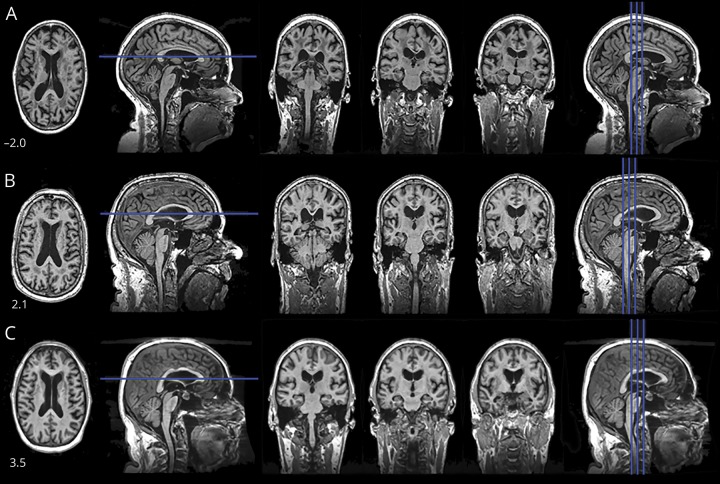
Figure 2 shows MRI features characteristic of HCTS. Table 2 compares the neuroimaging characteristics of those with and those without HCTS. The HCTS group had larger ventricles (p < 0.001) and a lower tau SUVR (p < 0.001), despite being older. There was no difference between the groups in PiB SUVR (p = 0.52). The HCTS group also had lower AD signature cortical thickness (p = 0.02), greater white matter hyperintensity volume (p < 0.001), and more infarctions (p = 0.03).
Figure 2. Images from 3 participants (A, B, C) are shown.
The number in the lower left corner is the score from the high-convexity tightness classifier (positive values = tighter). Top row represents those without high-convexity tight sulci. Overall, the CSF spaces are increasingly larger, with the exceptions of the high convexity and on the midline.
Table 2.
Neuroimaging characteristics, stratified by the presence of CDD
We next determined the difference in AT(N) biomarker profiles between the 2 groups. Because amyloid PET SUVR did not differ between the 2 groups, we did not differentiate between participants who were A+ or A− in the comparison (table 3). The HCTS group was more commonly T− and (N)+ (56%) compared with the no-HCTS group (23%) (p < 0.001). The AT(N) biomarker profiles of the individual patients are reported in table e-1 (available from Dryad, doi.org/10.5061/dryad.mn176dr).
Table 3.
AT(N) biomarker groupings, stratified by the presence of CDD
Cognitive testing and gait evaluation
Table 4 summarizes the cognitive profile and gait speed by HCTS status. The groups had similar scores for memory and visual-spatial function, but the HCTS group had lower scores in the attention (p = 0.02) and language domains (0.002) after adjustment for age. Gait velocity was slower in the HCTS group (p = 0.34), but the differences were not significant after adjustment for age and sex.
Table 4.
Cognitive and gait differences, stratified by the presence of CDD
Independent validation study
Of 30 images reviewed, the expert visual rater graded 8 as having an HCTS and 22 as not having an HCTS. The machine-learning method was 8 of 8 sensitive for the cases visually graded as positive and 20 of 22 specific for cases visually rated as negative or 100% sensitive and 90.9% specific relative to the expert visual grading. There were 2 cases that the algorithm classified as HCTS that the rater did not rate as HCTS.
Sensitivity analysis
Participants with HCTS were more likely to have cerebrovascular disease than those without an HCTS. Therefore, we performed a secondary analysis among participants without infarcts. The findings remained unchanged. Those with HCTS were more likely to have abnormal AD signature cortical thickness 69% (n = 18) compared to those without HCTS 36% (n = 179) (p = 0.037) after adjustment for age and sex. Participants with HCTS were similarly overrepresented among the T-N+ (65%) vs (22%) group. Participants with HCTS had lower scores in the language domain after adjustment for age (p = 0.022). The difference in the attention domain was no longer significant after the exclusion of individuals with infarcts.
Discussion
In the MCSA, of the participants ≥50 years of age who underwent brain MRI and PET imaging, ≈6.6% were classified as having HCTS. Those with HCTS were older, and a greater proportion had cognitive impairment, even after adjustment for age. In addition, the presence of HCTS appears to have an important influence on AD biomarker-profile classifications (i.e., AT[N]) currently being used in research and clinical trials.7
Those with HCTS more commonly were characterized as (N)+ on the basis of abnormal AD signature cortical thickness. Despite their older age, participants with HCTS had a lower tau burden (more often T−), indicating that the abnormal AD signature cortical thickness could not be explained by tauopathy. Whether the abnormal AD signature thickness is due to nonatrophy effects (e.g., compression, rather than destruction, of tissue) or whether it truly represents decreased cortical thickness is an important area of future investigation. The AD signature thickness region included areas in the basal-medial temporal lobe, not the parietal lobe, so the decreased cortical thickness could not be explained by focal sulcal effacement. This finding also indicates that HCTS represents a subpopulation of individuals who would be labeled suspected non-Alzheimer pathology by biomarkers.25 Because biomarkers are increasingly used to determine eligibility in clinical trials and to measure outcomes, it is important to recognize the HCTS pattern of cortical anatomy. For example, if a clinical trial aims to enroll cognitively normal individuals and includes a neurodegeneration biomarker, amyloid-positive individuals with a CDD may be erroneously enrolled, which may affect interpretation of the results. Those with HCTS were less likely to be T+. A possible explanation for this observation is that individuals with significant AD pathology (i.e., A + T + N+) will not have an HCTS because the sulci will be widened due to generalized neurodegenerative brain atrophy.
HCTS and idiopathic NPH are both felt to be manifestations of CDDs. NPH manifests with gait impairment, cognitive impairment, and urinary incontinence; these symptoms improve with a CSF-diverting shunt. In fact, the presence of high-convexity tightness is included in Japanese guidelines for the management of idiopathic NPH.26 Although the presence of HCTS is an established prognostic feature in patients who also have NPH, HCTS is also observed in the general population. Unlike ventriculomegaly and enlarged Sylvian fissures, which can occur with both neurodegenerative atrophy and CDDs, HCTS does not occur as a consequence of neurodegenerative disease, which is why it was the focus of the current analysis. The limitation of this approach is that it may underestimate the frequency of other manifestations of CDDs (e.g., focal sulcal dilation reflecting extraventricular hydrocephalus) not captured by the current approach.27
Recent population-based studies have shown that the neuroimaging features associated with the clinical phenotype of NPH are more common in the general population than previously recognized. For example, a Swedish study of persons >80 years of age showed that 5.4% of the population had HCTS (the term used in that study was occluded sulci).3 In a community-based Japanese study, ≈2.9% of elderly individuals had ventriculomegaly with high-convexity tightness.3 Longitudinal studies will determine whether individuals with HCTS in the general population represent a prodromal variant or an early imaging manifestation of NPH. The link between NPH and HCTS suggests that HCTS also represents a disorder of CSF dynamics, but the pathophysiology of HCTS remains uncertain at this time.
We observed no significant differences in gait velocity between the groups in our study, and currently, gait is the only feature of NPH reliably treated with shunt surgery. Whether gait differences between these 2 groups appear over time is an important area for future investigations.
The HCTS group had more neuroimaging characteristics consistent with vascular disease than the non-HCTS group. This difference was not surprising because individuals with neuroimaging features of NPH have increased white matter hyperintensity volume suggestive of extravasation of CSF, as well as an increased frequency of subcortical infarcts. Vascular risk factors may have a role in the development of hydrocephalus.28
CSF AD biomarkers are currently used interchangeably with amyloid and tau PET in the recent AD research criteria.7 However, unlike imaging, CSF biomarkers are susceptible to confounding with CDDs. In AD, β-amyloid42 (Aβ42) is low in the CSF, whereas total tau and phosphorylated tau are elevated. In NPH, however, many proteins, including Aβ42, total tau, and phosphorylated tau, are low, with protein levels normalizing after a shunt.29 The finding of low Aβ42 and tau in NPH has been confirmed in several studies.30,31 Because Aβ42 is low in NPH and AD, the utility of CSF biomarkers is limited for distinguishing AD from NPH. Moreover, individuals with imaging features of CDD may have low Aβ42 and tau, which can cause discrepant CSF and amyloid PET values. For example, an individual who was amyloid negative by PET may appear amyloid positive by CSF in the presence of a CDD because the Aβ42 in the CSF is low.
HCTS has been underrecognized as a contributor to biomarker abnormalities for several reasons. First, the association of high-convexity tightness with enlarged Sylvian fissures and parieto-occipital fissures is not recognized and is frequently mistaken as degenerative atrophy. Second, HCTS and DESH are detectable in vivo only. Therefore, pathologic studies cannot be used to investigate this phenomenon. Third, as research datasets increase in size, visual assessment of all scans becomes logistically impractical.
Strengths of this study include the large number of population-sampled participants, the use of automated MRI- and PET-based imaging biomarkers of AD, and our in-house automated HCTS classifier. The study has limitations, including that HCTS is an indirect marker of disturbed CSF dynamics. While HCTS was described in a subset of patients with NPH and is a predictor of shunt responsiveness,6 because it is an indirect marker of a CDD, it is possible that not all patients with an HCTS have a CDD. While the focus of this study was on AD PET biomarkers, because there can be a dissociation between AD PET and CSF biomarkers, future studies should determine whether those with HCTS are overrepresented in discrepant cases. Current tau PET ligands such AV-1451 have limited use in non-AD tauopathies; therefore, we cannot exclude that pathologies not measured by tau PET such as 4 R tau and TAR DNA-binding protein 43 were not present in the cases with HCTS. Future studies evaluating CSF flow directly or with neuroimaging will be important in confirming the findings of this study. Our current algorithm detects HCTS only; therefore, we likely underestimated the total impact of the commonly associated ventricular enlargement and sulcal widening elsewhere.
Although individuals with neuroimaging features consistent with HCTS are uncommon in the general population, the features of HCTS should be better characterized because they appear to have distinct clinical effects (i.e., cognitive impairment) and are associated with AD biomarkers. The longitudinal clinical implications of these biomarkers need to be studied.
Acknowledgment
The authors thank AVID Radiopharmaceuticals, Inc for supplying the AV-1451 precursor, chemistry production advice, and oversight, as well as the US Food and Drug Administration regulatory cross-filing permission and documentation needed for this work.
Glossary
- Aβ42
β-amyloid42
- AD
Alzheimer disease
- AT(N)
amyloid-tau-neurodegeneration
- CDD
CSF dynamics disorder
- DESH
disproportionately enlarged subarachnoid space hydrocephalus
- FLAIR
and fluid-attenuated inversion recovery
- HCTS
high-convexity tight sulci
- MCALT
Mayo Clinic Adult Lifespan Template
- MCSA
Mayo Clinic Study of Aging
- MPRAGE
magnetization-prepared rapid gradient-echo
- NPH
normal-pressure hydrocephalus
- PiB
Pittsburgh compound B
- SUVR
standard uptake value ratio
Appendix. Authors

Footnotes
CME Course: NPub.org/cmelist
Study funding
Funded by the NIH: K76 AG057015 (J. Graff-Radford), R37 AG011378 (Jack), R01 AG041851 (Jack and Knopman), U01 AG006786 (Petersen; MCSA), R01 NS097495 (Vemuri), and P50 AG016574 (Petersen). Other sources include the Elsie and Marvin Dekelboum Family Foundation, Alexander Family Professor of Alzheimer's Disease Research, Mayo Clinic, Liston Award, Schuler Foundation, GHR Foundation, and Mayo Foundation for Medical Education and Research. This study was made possible by the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the NIH under award R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
J. Graff-Radford receives research funding from National Institute on Aging/NIH. J. Gunter, D. Jones, S. Przybelski, and C. Schwarz report no disclosures relevant to the manuscript. J. Huston reports patents from the Mayo Foundation, royalties from Resoundant stock, and stock options in Resoundant. V. Lowe serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences, and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and NIH (National Institute on Aging, National Cancer Institute). B. Elder reports no disclosures relevant to the manuscript. M. Machulda receives NIH funding. N. Gunter reports no disclosures relevant to the manuscript. R. Petersen is a consultant for Roche, Biogen, Merck, Eli Lilly, and Genentech. He receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003) and research support from NIH. K. Kantarci serves on the data safety monitoring board for Takeda Global Research & Development Center, Inc and receives research support from the NIH. P. Vemuri receives NIH funding. M. Mielke is a consultant for Eli Lilly and Lysosomal Therapeutics and receives unrestricted research grants from Biogen, Lundbeck, and Roche and research funding from NIH/National Institute on Aging and the US Department of Defense. D. Knopman serves on a data safety monitoring board for the Dominantly Inherited Alzheimer's Disease study. He is an investigator in clinical trials sponsored by Lilly Pharmaceuticals, Biogen, and the Alzheimer's Treatment and Research Institute at the University of Southern California and receives research support from NIH. N. Graff-Radford serves on a scientific advisory board for Codman; serves on the editorial boards of The Neurologist and Alzheimer's Research & Therapy; has received publishing royalties from UpToDate; and receives research support from Biogen, Lilly, and Axovant. He has consulted for Cytox. C. Jack consults for Lily and serves on an independent data monitoring board for Roche. He receives research support from NIH/National Institute on Aging and the Alexander Family Professor of Alzheimer's Disease Research, Mayo Clinic. Go to Neurology.org/N for full disclosures.
References
- 1.Hashimoto M, Ishikawa M, Mori E, Kuwana N, Study of INPH on Neurological Improvement (SINPHONI). Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 2010;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiraoka K, Meguro K, Mori E. Prevalence of idiopathic normal-pressure hydrocephalus in the elderly population of a Japanese rural community. Neurol Med Chir (Tokyo) 2008;48:197–199. [DOI] [PubMed] [Google Scholar]
- 3.Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelsø C. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology 2014;82:1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunter NB, Schwarz CG, Graff-Radford J, et al. Automated detection of imaging features of disproportionately enlarged subarachnoid space hydrocephalus using machine learning methods. Neuroimage Clin 2019;21:101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita F, Sasaki M, Saito M, et al. Voxel-based morphometry of disproportionate cerebrospinal fluid space distribution for the differential diagnosis of idiopathic normal pressure hydrocephalus. J Neuroimaging 2014;24:359–365. [DOI] [PubMed] [Google Scholar]
- 6.Narita W, Nishio Y, Baba T, et al. High-convexity tightness predicts the shunt response in idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol 2016;37:1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 12.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci 2013;68:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatemi F, Kantarci K, Graff-Radford J, et al. Sex differences in cerebrovascular pathologies on FLAIR in cognitively unimpaired elderly. Neurology 2018;90:e466–e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raz L, Jayachandran M, Tosakulwong N, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology 2013;80:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarz CG, Gunter JL, Ward CP, et al. The Mayo clinic adult life span template: better quantification across the life span. Alzheimers Dement 2017;13:P93–P94. [Google Scholar]
- 17.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B. Freesurfer. Neuroimage 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz CG, Gunter JL, Wiste HJ, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage Clin 2016;11:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008;131:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knopman DS, Jack CR Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 2012;78:1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dementia 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age-specific and sex-specific prevalence of cerebral beta-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50-95 years: a cross-sectional study. Lancet Neurol 2017;16:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 25.Jack CR Jr, Knopman DS, Chetelat G, et al. Suspected non-Alzheimer disease pathophysiology: concept and controversy. Nat Rev Neurol 2016;12:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori E, Ishikawa M, Kato T, et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo) 2012;52:775–809. [DOI] [PubMed] [Google Scholar]
- 27.Holodny AI, George AE, de Leon MJ, Golomb J, Kalnin AJ, Cooper PR. Focal dilation and paradoxical collapse of cortical fissures and sulci in patients with normal-pressure hydrocephalus. J Neurosurg 1998;89:742–747. [DOI] [PubMed] [Google Scholar]
- 28.Jaraj D, Agerskov S, Rabiei K, et al. Vascular factors in suspected normal pressure hydrocephalus: a population-based study. Neurology 2016;86:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeppsson A, Zetterberg H, Blennow K, Wikkelsø C. Idiopathic normal-pressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology 2013;80:1385–1392. [DOI] [PubMed] [Google Scholar]
- 30.Graff-Radford NR. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology 2014;83:1573–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapaki EN, Paraskevas GP, Tzerakis NG, et al. Cerebrospinal fluid tau, phospho-tau181 and beta-amyloid1-42 in idiopathic normal pressure hydrocephalus: a discrimination from Alzheimer's disease. Eur J Neurol 2007;14:168–173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the MCSA, including data from this study, are available on request.