Abstract
Statins, a common drug class for treatment of dyslipidemia, may be neuroprotective for spontaneous intracerebral hemorrhage (ICH) by targeting secondary brain injury pathways in the surrounding brain parenchyma. Statin-mediated neuroprotection may stem from downregulation of mevalonate and its derivatives, targeting key cell signaling pathways that control proliferation, adhesion, migration, cytokine production, and reactive oxygen species generation. Preclinical studies have consistently demonstrated the neuroprotective and recovery enhancement effects of statins, including improved neurologic function, reduced cerebral edema, increased angiogenesis and neurogenesis, accelerated hematoma clearance, and decreased inflammatory cell infiltration. Retrospective clinical studies have reported reduced perihematomal edema, lower mortality rates, and improved functional outcomes in patients who were taking statins before ICH. Several clinical studies have also observed lower mortality rates and improved functional outcomes in patients who were continued or initiated on statins after ICH. Subgroup analysis of a previous randomized trial has raised concerns of a potentially elevated risk of recurrent ICH in patients with previous hemorrhagic stroke who are administered statins. However, most statin trials failed to show an association between statin use and increased hemorrhagic stroke risk. Variable statin dosing, statin use in the pre-ICH setting, and selection biases have limited rigorous investigation of the effects of statins on post-ICH outcomes. Future prospective trials are needed to investigate the association between statin use and outcomes in ICH.
Brain injury after spontaneous intracerebral hemorrhage (ICH) results from pathophysiologic responses in the brain parenchyma due to hematoma formation, release of clot components, and surrounding edema.1 Inflammatory cascade activation in the perihematomal brain parenchyma has been implicated in the pathogenesis of secondary brain injury.1–3 Statins have been identified as a potential neuroprotective agent that targets the inflammatory response to ICH. In preclinical studies, statin treatment in animal ICH models has consistently demonstrated neuroprotective and recovery enhancement effects.4–13 Clinical investigations in humans have largely been limited to retrospective studies that reported better patient outcomes associated with pre-ICH statin use, including reduced perihematomal edema (PHE), lower mortality rates, and improved functional outcomes.14–30
Several observational studies have also found that patients who were continued or initiated on statins following ICH had lower mortality rates and improved functional outcomes.31–37 However, meaningful conclusions from these studies are limited by variable statin dosing, statin use in the pre-ICH setting, and selection bias. Herein, we reviewed the putative mechanism of action and preclinical and clinical data suggesting a neuroprotective effect of statins and provided rationale for a prospective, randomized trial of statin therapy in patients with ICH.
Potential mechanisms of action
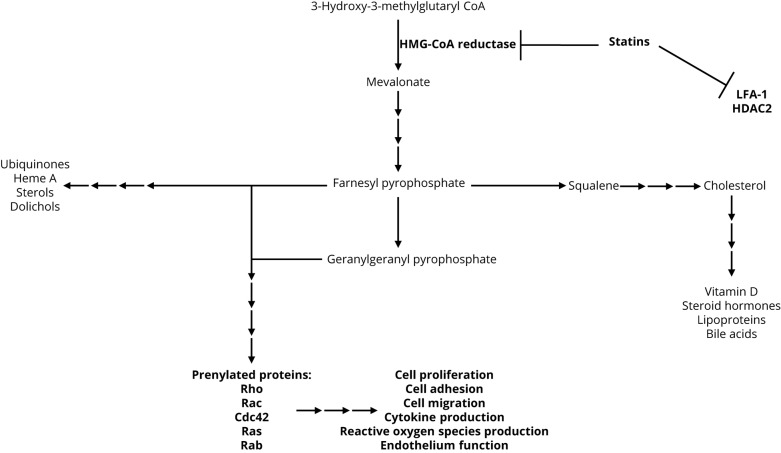
Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is the enzyme that catalyzes the conversion of HMG-CoA to mevalonate. HMG-CoA reductase is the rate-limiting step of cholesterol production (figure 1). In addition, statins may alter gene expression and lymphocyte migration and function via direct inhibition of histone deacetylase 2 and lymphocyte function-associated antigen-1/intercellular adhesion molecule interactions, respectively.38,39 However, the majority of statin-mediated effects act through downregulation of mevalonate and its derivatives, including farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). FPP and GGPP serve as important lipid attachments and membrane anchors for post-translation modification (prenylation) of many intracellular proteins. These proteins comprise heterotrimeric G-proteins and small GTP-binding proteins involved in key cell signaling pathways that control proliferation, adhesion, migration, cytokine production, and reactive oxygen species generation.40–42
Figure 1. Statins competitively inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting step of cholesterol production.
The majority of the effects of statins are mediated through downregulation of mevalonate and its derivatives.
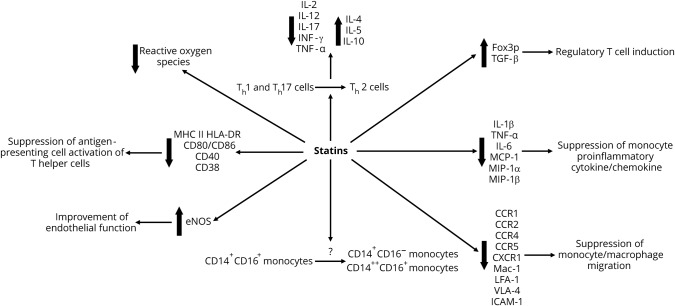
Figure 2 summarizes the multiple effects of statins. Statin-mediated actions on circulating monocytes are controversial. Some studies have suggested that statins induce a shift away from the antigen-presenting, proinflammatory, and cytokine-producing CD14+CD16+ monocyte phenotype.43,44 Meanwhile, others have not found statins to significantly change the relative or absolute distributions of monocyte phenotypes.45 Statins suppress monocyte/macrophage migration through downregulation of chemokine receptors (e.g., CCR1, CCR2, CCR4, CCR5, and CX3CR1) and adhesion molecules (e.g., Mac-1, lymphocyte function-associated antigen-1, VLA-4, and ICAM-1).46–51 In monocyte derivatives, statins have also been shown to reduce proinflammatory cytokine (e.g., interleukin [IL]-1β, tumor necrosis factor [TNF]-α, and IL-6) and chemokine (e.g., monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and macrophage inflammatory protein-1β) production.46,52–55 In addition, statins impair the ability of monocyte-derived dendritic cells (DCs) to activate T helper cells by inhibiting DC maturation and downregulating major histocompatibility complex class II receptor human leukocyte antigen-DR isotype and costimulatory molecules (e.g., CD80/CD86, CD40, and CD83).56–59
Figure 2. Effects of statins.
The anti-inflammatory effects of statins on lymphocytes are also mediated by direct T-cell targeting. Statins modulate T helper cell differentiation by promoting a shift in Th1/Th2 balance toward the Th2 phenotype.e-60—e-66 Upregulation of IL-4, IL-5, and IL-10 expression and downregulation of IL-2, IL-12, IL-17, INF-γ, and TNF-α expression lend credence to this pro-Th2 and anti-Th1 shift.60,e-61—e-66 Statins also upregulate Foxp3 and enhance TGF-β–mediated regulatory T-cell function.e-67 In addition, statins improve endothelial function by upregulating endothelial nitric oxide synthase (eNOS). Statins enhance eNOS activity by a combination of Rho GTPase inhibition, PI3K/Akt activation, caveolin-1 reduction, Rac1 inhibition, and nicotinamide adenine dinucleotide phosphate oxidase inhibition.e-68
Investigational therapies that target secondary brain injury after ICH focus on the brain parenchyma adjacent to the hematoma, which is otherwise known as the ICH boundary zone. The pathophysiologic responses to the hematoma and its breakdown components in the ICH boundary zone have been implicated in patient outcomes. These underlying cellular processes include apoptosis, necrosis, edema, blood-brain barrier (BBB) disruption, cerebral blood flow reduction, and inflammatory cascade activation.e-69 The impetus to investigate the beneficial effects of statins in patients with ICH derives from their potential interactions with some of the ICH-induced secondary brain injury pathways.
Preclinical studies of statins in animal ICH models
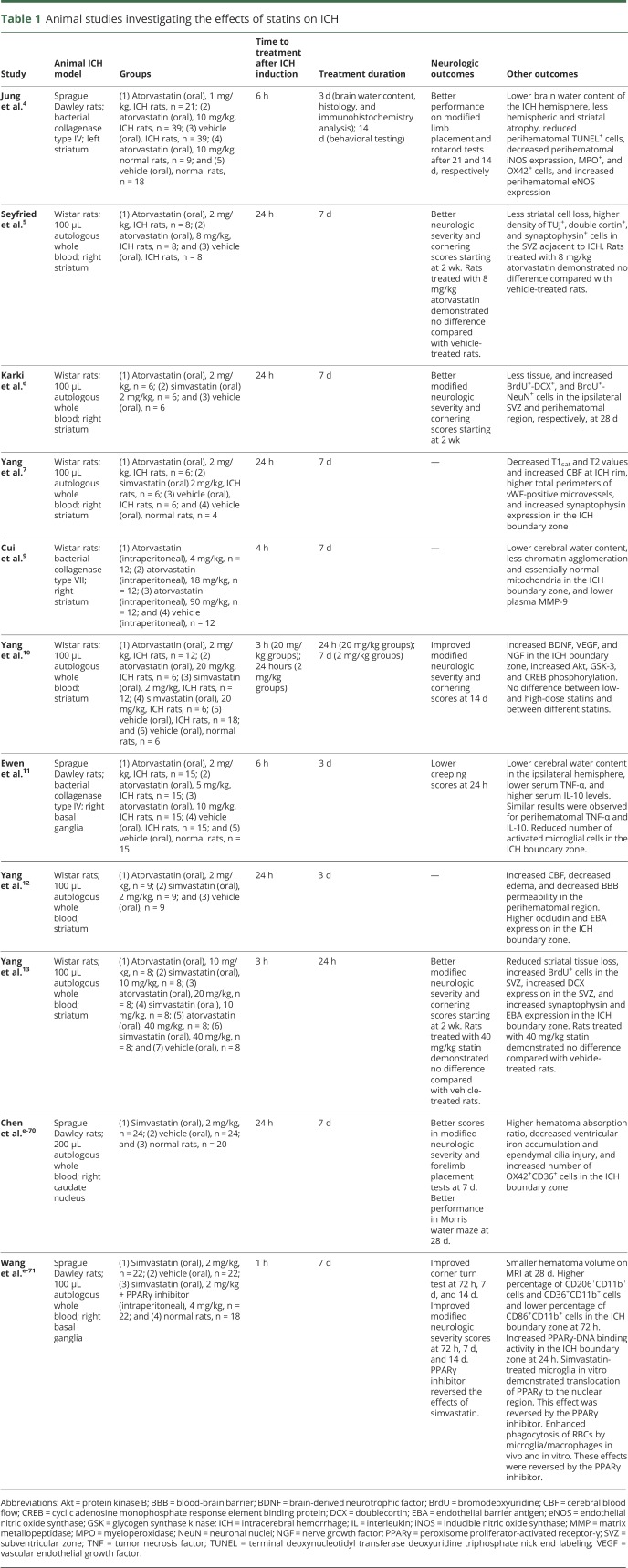
Statin treatment in animal ICH models has consistently been shown to improve neurologic function, reduce cerebral edema, increase angiogenesis and neurogenesis, accelerate hematoma resolution, and decrease inflammatory cell infiltration (table 1). The effects of atorvastatin and simvastatin were studied in rat models using autologous whole-blood injection and collagenase-induced ICH. The initial statin doses in these models were delivered 1–24 hours after ICH induction. The duration of statin therapy ranged between 24 hours and 14 days. These studies found statin treatment to improve neurologic outcomes beginning at approximately 2 weeks after ICH.4–6,10,11,13,e-70,e-71
Table 1.
Animal studies investigating the effects of statins on ICH
Statins demonstrated potential neuroprotective mechanisms, including antioxidative, anti-inflammatory, antiapoptotic, neurogenic, and angiogenic effects, in animal ICH models. Statin treatment increased eNOS expression in the ICH boundary zone, which is consistent with other non-ICH studies.4,e-68 Increased nitric oxide production and microvascular proliferation may contribute to improved brain tissue perfusion in the ICH boundary zone.7,12
Consistent with other non-ICH studies, statin treatment inhibits apoptosis by activating the PI3K/Akt pathway in the ICH boundary zone.10,e-72 Statin-treated rats showed decreased apoptosis and increased neurogenesis in the ICH boundary zone.4,9 In addition, statins may enhance recovery after injury by increasing growth factor (e.g., brain-derived neurotrophic factor, vascular endothelial growth factor, and nerve growth factor) expression and neurogenesis (indicated by TUJ1+, DCX+, synaptophysin+, NeuN+, and BrdU+ cells) in the ICH boundary zone and adjacent subventricular zone.4–7,10,13 These effects may reduce perihematomal brain tissue loss. Similar effects of statins were observed in ischemic stroke animal models. Statin-treated animals with acute ischemic stroke demonstrated improvements in neurologic outcome and enhanced brain plasticity.e-73—e-75
Statin treatment may also decrease inflammatory cell infiltration in the ICH boundary zone. Rats that received atorvastatin have reduced perihematomal inducible NOS, MPO+ cells, and OX42+ cells.4 Atorvastatin-treated rats also had lower serum/perihematomal TNF-α levels, higher serum/perihematomal IL-10 levels, and fewer perihematomal activated microglial cells.11 In contrast, 1 study showed that simvastatin increased the levels of OX42+ and CD36+ cells in the ICH boundary zone and raised the hematoma absorption ratio.e-70 The authors postulated that statin treatment resulted in activated microglia or macrophage recruitment, which facilitated red blood cell (RBC) phagocytosis and hematoma resorption.e-70 A subsequent study by the same group demonstrated that simvastatin accelerated RBC phagocytosis and hematoma resolution via peroxisome proliferator-activated receptor-γ–dependent mechanisms.e-71 Statins have been shown to increase perihematomal occludin and endothelial barrier antigen expression and to decrease plasma MMP-9 levels. These molecular alterations may decrease BBB permeability in the ICH boundary zone, thereby reducing cerebral water content and PHE.4,7,9,11–13 Overall, in vivo findings have consistently supported statins as a potential neuroprotective and recovery enhancement agent in acute ICH.
Clinical studies of statins in patients with ICH
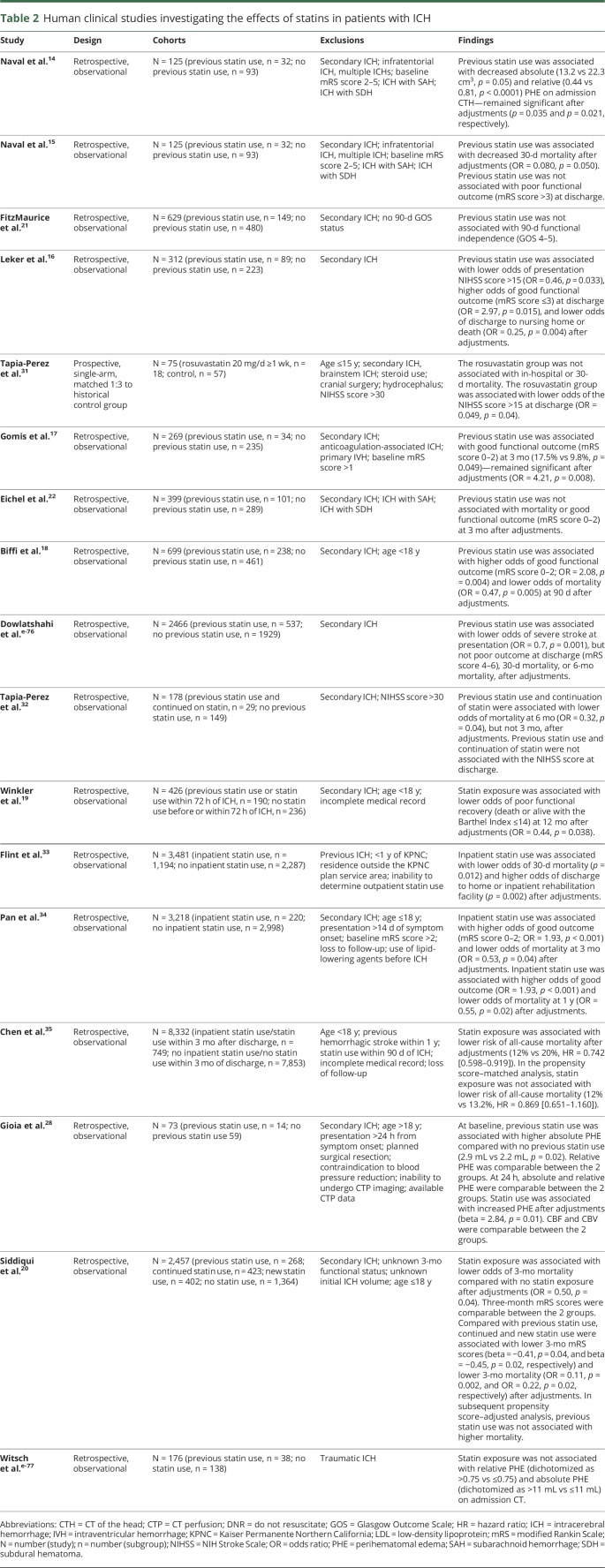
In light of the potential therapeutic effects of statins in ICH, a number of human clinical studies have investigated statin exposure and ICH outcomes (table 2). In a single-center study comprising 125 consecutive patients with supratentorial ICH, the investigators observed an independent association between previous statin use and lower 30-day mortality.15 In the same patient cohort, patients with previous statin use had smaller absolute and relative PHE volumes.14 Previous statin use remained an independent predictor of lower absolute and relative PHE volumes in multivariable models.
Table 2.
Human clinical studies investigating the effects of statins in patients with ICH
A study of 312 patients with ICH from the National Acute Stroke Surgery in Israel Study also showed improved ICH outcomes in statin users.16 In this study, previous statin use was independently associated with lower odds of presenting NIH Stroke Scale (NIHSS) score >15, higher odds of discharge modified Rankin Scale (mRS) score ≤3, and lower odds of discharge to nursing home or death. A retrospective analysis of a prospective stroke registry comprising 269 patients with ICH corroborated this finding.17 The investigators observed an independent association between previous statin use and a greater likelihood of functional independence (mRS score 0–2) at 3 months. In a larger study of 699 patients with ICH, previous statin use was also associated with higher odds of functional independence and lower odds of mortality at 90 days in multivariable analyses.18 In their meta-analysis comprising 7 studies (previous statin use n = 698; no previous statin use n = 1,823), previous statin remained a predictor of functional independence and reduced mortality.
A study comprising 3,481 patients with ICH admitted to the Kaiser Permanente Northern California health care system found inpatient statin use to be associated with lower 30-day mortality.33 Inpatient statin use was also associated with higher rates of discharge to home or inpatient rehabilitation facility. In the same study, statin discontinuation in previous statin users was associated with higher 30-day mortality rates and lower rates of discharge to home or inpatient rehabilitation facility. Similarly, in-hospital initiation of statins in 3,218 statin-naive patients with ICH from the China National Stroke Registry was associated with higher odds of good outcome (mRS score 0–2) and lower odds of mortality at 3 months and 1 year.34 Statin use after ICH was also associated with lower rates of all-cause mortality in a population-based cohort study from the Taiwan's National Health Insurance Research Database comprising 8,332 patients with ICH without previous statin use within 90 days of presentation.35 In a more recent analysis from the Ethnic/Racial Variation of Intracerebral Hemorrhage study comprising 2,457 patients, the investigators reported an independent association between statin use (including previous statin use, previous statin use and continued use, and new statin use) and lower odds of 3-month mortality.20 Although most studies have shown improved functional outcomes and reduced mortality in statin users with ICH, this beneficial effect has not been universally reported.21,22,e-76 Improvements in PHE metrics among statin users have also not been consistently observed.28, e-77
One of the major limitations of the clinical literature pertaining to statin use in patients with ICH is the preponderance of retrospective studies and the paucity of prospective data. In a prospective, single-arm trial (ClinicalTrials.gov NCT00364559) of rosuvastatin (n = 18, 20 mg daily for 7 days) in patients with ICH matched in a 1:3 ratio to a retrospective cohort, the rosuvastatin cohort had lower rates of in-hospital and 30-day mortality.31 Rosuvastatin treatment was also associated with lower odds of discharge NIHSS score >15. However, after adjustments for baseline risk factors, the between-cohort differences in in-hospital and 30-day mortality were no longer significant. The validity of the conclusions derived from this trial was considerably limited by its single-arm design and small sample size. Unknown previous statin use and unclear post-ICH statin use in the matched retrospective cohort further confounded the trial's results. A single-center, prospective, randomized, placebo-controlled phase II trial that intended to compare PHE between simvastatin vs placebo in patients with ICH was initiated in 2008 (ClinicalTrials.gov NCT00718328). However, the study was terminated in 2017 because of poor recruitment, which was largely the result of concurrent, competing trials at that center.
Definitive conclusions regarding statin dose selection for patients with ICH in a clinical trial setting are limited by the heterogeneity of statin use in previous observational studies. Lipophilic statins, such as atorvastatin and simvastatin, at doses approved by the US Food and Drug Administration have shown benefit in preclinical studies and may translate to clinical trials.18 However, preclinical studies have not consistently demonstrated a dose-response effect of statins.4,5,9–11,13 Therefore, it remains unclear whether supraphysiologic doses would be of additional benefit in patients with ICH, particularly when balanced against the increased risk of myopathy and rhabdomyolysis as side effects.e-78
Statins and risk of ICH
Post hoc analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial raised concern regarding a potential association between statin use and the risk of hemorrhagic stroke.e-79,e-80 The mechanism by which statins may increase hemorrhagic stroke risk remains unknown. A correlation between lower cholesterol levels and an increased incidence of hemorrhagic stroke has been suggested by several previous epidemiologic studies, although more recent studies have found no such relationship.e-80—e-84 Alterations in cellular membrane integrity as a result of statin-mediated cholesterol reduction may not fully explain the speculated association between statin use and hemorrhagic stroke risk.30,e-85 Inhibition of platelet activity and the coagulation cascade are other postulated mechanisms of statins that may affect hemorrhage risk.e-86—e-88 The risk of recurrent hemorrhagic stroke during follow-up was found to be >5.5-fold higher with atorvastatin treatment than placebo.e-80 However, patients with hemorrhagic stroke as an entry event comprised only 2% of the SPARCL trial. A Markov decision model recommended avoiding statins in patients with previous ICH, especially in those with previous lobar ICH.e-89 This was based on the relative risk of ICH on statin therapy from the SPARCL trial and risk of recurrent ICH from previous studies.
Contrary to the findings of the SPARCL trial, the vast majority of statin trials have failed to demonstrate an association with hemorrhagic stroke risk.e-90 Occurrence of hemorrhagic stroke was similar between patients randomized to simvastatin 40 mg daily or placebo in the Heart Protection Study (n = 20,536; 0.5% vs 0.5%, p = 0.8).e-91 Although simvastatin-treated patients in the subgroup with previous cerebrovascular disease (n = 3,280) had a higher risk of hemorrhage stroke, this difference was not significant (1.3% vs 0.7%). Subsequent meta-analyses including up to 31 randomized controlled trials with >90,000 patients have also failed to show any effect of statin treatment and associated LDL reduction on ICH risk.e-92,e-93 Furthermore, a meta-analysis of 11 studies with cerebrovascular disease populations, comprising observational studies and the SPARCL trial, demonstrated no association between statin use and increased risk of ICH.e-93 The same meta-analysis also found no association between statin use and recurrent ICH in patients with previous lobar ICH. In addition, the risk of hemorrhagic stroke did not seem to be associated with the intensity or lipophilicity of statin therapy.37,e-94,e-95
No association has been found between post-ICH statin use and recurrent ICH, although the number of studies is limited.21,35 An observational study comprising 79 post-ICH statin users found no association between statin exposure and ICH recurrence in univariate Cox regression analysis, either before (p = 0.56) or after (p = 0.66) adjustment for other predictors.21 Subsequent analysis of statin-naive patients with ICH using Taiwan's National Health Insurance Research Database (n = 8,332) also demonstrated no association between statin use during hospitalization or within 3 months of discharge and ICH recurrence (adjusted hazard ratio = 1.044 [0.812–1.341]).35 Therefore, the association between statin use and increased risk of recurrent ICH remains unclear. In addition, potential benefits from a relatively short duration of statin therapy may outweigh the indeterminate risks of ICH recurrence.
Conclusions
Preclinical and clinical studies support the potential neuroprotective and recovery enhancement effects afforded by statins in the setting of acute ICH. A multitude of proposed mechanisms, including promotion of angiogenesis, increased neurogenesis, inhibition of neuronal apoptosis, acceleration of hematoma resolution, decreased inflammation in the ICH boundary zone, and decreased PHE, could mediate the beneficial effects of statins. The limitations of currently available literature regarding the relationship of statins to post-ICH outcomes have precluded an objective assessment of the potential therapeutic benefits of statins in patients with ICH. Therefore, a prospective, multicenter, randomized trial of statin therapy for patients with ICH is warranted. The mechanisms by which statins may affect ICH patient outcomes remain incompletely defined. As such, a phase II futility analysis or proof-of-concept study using surrogate markers (e.g., PHE) and safety measures (e.g., recurrent ICH) may be necessary ahead of a larger phase III trial.
Glossary
- BBB
blood-brain barrier
- DC
dendritic cell
- eNOS
endothelial nitric oxide synthase
- FPP
farnesyl pyrophosphate
- GGPP
geranylgeranyl pyrophosphate
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- ICH
intracerebral hemorrhage
- IL
interleukin
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- PHE
perihematomal edema
- RBC
red blood cell
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Level
- TNF
tumor necrosis factor
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
C-.J. Chen, D. Ding, N. Ironside, T. Buell, L. Elder, A. Warren, A. Adams, S.J. Ratcliffe, R.F. James, and N.S. Naval report no disclosures relevant to the manuscript. B.B. Worrall reports serving on the editorial board of the journal Neurology. K.C. Johnston reports no disclosures relevant to the manuscript. A.M. Southerland reports serving as past editor of the Neurology Podcast and research support (neuroprotection trial in stroke), Diffusion Pharmaceuticals, Inc. Go to Neurology.org/N for full disclosures.
References
- 1.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 2012;11:720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 2010;92:463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood flow Metab 2007;27:894–908. [DOI] [PubMed] [Google Scholar]
- 4.Jung KH, Chu K, Jeong SW, et al. HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke 2004;35:1744–1749. [DOI] [PubMed] [Google Scholar]
- 5.Seyfried D, Han Y, Lu D, Chen J, Bydon A, Chopp M. Improvement in neurological outcome after administration of atorvastatin following experimental intracerebral hemorrhage in rats. J Neurosurg 2004;101:104–107. [DOI] [PubMed] [Google Scholar]
- 6.Karki K, Knight RA, Han Y, et al. Simvastatin and atorvastatin improve neurological outcome after experimental intracerebral hemorrhage. Stroke 2009;40:3384–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang D, Knight RA, Han Y, et al. Vascular recovery promoted by atorvastatin and simvastatin after experimental intracerebral hemorrhage: magnetic resonance imaging and histological study. J Neurosurg 2011;114:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun HJ, Kim DW, Yi HJ, et al. Effects of statin and deferoxamine administration on neurological outcomes in a rat model of intracerebral hemorrhage. Neurol Sci 2012;33:289–296. [DOI] [PubMed] [Google Scholar]
- 9.Cui JJ, Wang D, Gao F, Li YR. Effects of atorvastatin on pathological changes in brain tissue and plasma MMP-9 in rats with intracerebral hemorrhage. Cell Biochem Biophys 2012;62:87–90. [DOI] [PubMed] [Google Scholar]
- 10.Yang D, Han Y, Zhang J, Chopp M, Seyfried DM. Statins enhance expression of growth factors and activate the PI3K/Akt-mediated signaling pathway after experimental intracerebral hemorrhage. World J Neurosci 2012;2:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewen T, Qiuting L, Chaogang T, et al. Neuroprotective effect of atorvastatin involves suppression of TNF-alpha and upregulation of IL-10 in a rat model of intracerebral hemorrhage. Cell Biochem Biophys 2013;66:337–346. [DOI] [PubMed] [Google Scholar]
- 12.Yang D, Knight RA, Han Y, et al. Statins protect the blood brain barrier acutely after experimental intracerebral hemorrhage. J Behav Brain Sci 2013;3:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang D, Zhang J, Han Y, James E, Chopp M, Seyfried DM. Acute statin treatment improves recovery after experimental intracerebral hemorrhage. World J Neurosci 2013;3:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naval NS, Abdelhak TA, Urrunaga N, Zeballos P, Mirski MA, Carhuapoma JR. An association of prior statin use with decreased perihematomal edema. Neurocrit Care 2008;8:13–18. [DOI] [PubMed] [Google Scholar]
- 15.Naval NS, Abdelhak TA, Zeballos P, Urrunaga N, Mirski MA, Carhuapoma JR. Prior statin use reduces mortality in intracerebral hemorrhage. Neurocrit Care 2008;8:6–12. [DOI] [PubMed] [Google Scholar]
- 16.Leker RR, Khoury ST, Rafaeli G, Shwartz R, Eichel R, Tanne D. Prior use of statins improves outcome in patients with intracerebral hemorrhage: prospective data from the National Acute Stroke Israeli Surveys (NASIS). Stroke 2009;40:2581–2584. [DOI] [PubMed] [Google Scholar]
- 17.Gomis M, Ois A, Rodríguez-Campello A, et al. Outcome of intracerebral haemorrhage patients pre-treated with statins. Eur J Neurol 2010;17:443–448. [DOI] [PubMed] [Google Scholar]
- 18.Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology 2011;76:1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler J, Shoup JP, Czap A, et al. Long-term improvement in outcome after intracerebral hemorrhage in patients treated with statins. J Stroke Cerebrovasc Dis 2013;22:e541-e545. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui FM, Langefeld CD, Moomaw CJ, et al. Use of statins and outcomes in intracerebral hemorrhage patients. Stroke 2017;48:2098–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FitzMaurice E, Wendell L, Snider R, et al. Effect of statins on intracerebral hemorrhage outcome and recurrence. Stroke 2008;39:2151–2154. [DOI] [PubMed] [Google Scholar]
- 22.Eichel R, Khoury ST, Ben-Hur T, Keidar M, Paniri R, Leker RR. Prior use of statins and outcome in patients with intracerebral haemorrhage. Eur J Neurol 2010;17:78–83. [DOI] [PubMed] [Google Scholar]
- 23.Romero FR, Bertolini EdeF, Veloso VN, Venturini L, Figueiredo EG. Outcomes from intracerebral hemorrhage among patients pre-treated with statins. Arq Neuropsiquiatr 2011;69:452–454. [DOI] [PubMed] [Google Scholar]
- 24.Mustanoja S, Strbian D, Putaala J, et al. Association of prestroke statin use and lipid levels with outcome of intracerebral hemorrhage. Stroke 2013;44:2330–2332. [DOI] [PubMed] [Google Scholar]
- 25.Phipps MS, Zeevi N, Staff I, Fortunato G, Kuchel GA, McCullough LD. Stroke severity and outcomes for octogenarians receiving statins. Arch Gerontol Geriatr 2013;57:377–382. [DOI] [PubMed] [Google Scholar]
- 26.Falcone GJ, Brouwers HB, Biffi A, et al. Warfarin and statins are associated with hematoma volume in primary infratentorial intracerebral hemorrhage. Neurocrit Care 2014;21:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei C, Wu B, Liu M, Chen Y. Association between statin use and intracerebral hemorrhage: a systematic review and meta-analysis. Eur J Neurol 2014;21:192–198. [DOI] [PubMed] [Google Scholar]
- 28.Gioia LC, Kate M, McCourt R, et al. Perihematoma cerebral blood flow is unaffected by statin use in acute intracerebral hemorrhage patients. J Cereb Blood flow Metab 2015;35:1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauer A, Greenberg SM, Gurol ME. Statins in intracerebral hemorrhage. Curr Atheroscler Rep 2015;17:46. [DOI] [PubMed] [Google Scholar]
- 30.Sikora Newsome A, Casciere BC, Jordan JD, et al. The role of statin therapy in hemorrhagic stroke. Pharmacotherapy 2015;35:1152–1163. [DOI] [PubMed] [Google Scholar]
- 31.Tapia-Perez H, Sanchez-Aguilar M, Torres-Corzo JG, et al. Use of statins for the treatment of spontaneous intracerebral hemorrhage: results of a pilot study. Cent Eur Neurosurg 2009;70:15–20. [DOI] [PubMed] [Google Scholar]
- 32.Tapia-Perez JH, Rupa R, Zilke R, Gehring S, Voellger B, Schneider T. Continued statin therapy could improve the outcome after spontaneous intracerebral hemorrhage. Neurosurg Rev 2013;36:279–287. [DOI] [PubMed] [Google Scholar]
- 33.Flint AC, Conell C, Rao VA, et al. Effect of statin use during hospitalization for intracerebral hemorrhage on mortality and discharge disposition. JAMA Neurol 2014;71:1364–1371. [DOI] [PubMed] [Google Scholar]
- 34.Pan YS, Jing J, Wang YL, et al. Use of statin during hospitalization improves the outcome after intracerebral hemorrhage. CNS Neurosci Ther 2014;20:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen PS, Cheng CL, Chang YC, Kao Yang YH, Yeh PS, Li YH. Early statin therapy in patients with acute intracerebral hemorrhage without prior statin use. Eur J Neurol 2015;22:773–780. [DOI] [PubMed] [Google Scholar]
- 36.Tapia Pérez JH, Yildiz OC, Schneider T, Nimsky C. Meta-analysis of statin use for the acute therapy of spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2015;24:2521–2526. [DOI] [PubMed] [Google Scholar]
- 37.Tai SY, Lin FC, Lee CY, Chang CJ, Wu MT, Chien CY. Statin use after intracerebral hemorrhage: a 10-year nationwide cohort study. Brain Behav 2016;6:e00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med 2001;7:687–692. [DOI] [PubMed] [Google Scholar]
- 39.Lin YC, Lin JH, Chou CW, Chang YF, Yeh SH, Chen CC. Statins increase p21 through inhibition of histone deacetylase activity and release of promoter-associated HDAC1/2. Cancer Res 2008;68:2375–2383. [DOI] [PubMed] [Google Scholar]
- 40.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci 2005;118:843–846. [DOI] [PubMed] [Google Scholar]
- 41.Yano M, Matsumura T, Senokuchi T, et al. Statins activate peroxisome proliferator-activated receptor gamma through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circ Res 2007;100:1442–1451. [DOI] [PubMed] [Google Scholar]
- 42.Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017;120:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothe G, Herr AS, Stohr J, Abletshauser C, Weidinger G, Schmitz G. A more mature phenotype of blood mononuclear phagocytes is induced by fluvastatin treatment in hypercholesterolemic patients with coronary heart disease. Atherosclerosis 1999;144:251–261. [DOI] [PubMed] [Google Scholar]
- 44.Imanishi T, Ikejima H, Tsujioka H, et al. Association of monocyte subset counts with coronary fibrous cap thickness in patients with unstable angina pectoris. Atherosclerosis 2010;212:628–635. [DOI] [PubMed] [Google Scholar]
- 45.Jaipersad AS, Shantsila E, Blann A, Lip GY. The effect of statin therapy withdrawal on monocyte subsets. Eur J Clin Invest 2013;43:1307–1313. [DOI] [PubMed] [Google Scholar]
- 46.Veillard NR, Braunersreuther V, Arnaud C, et al. Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis 2006;188:51–58. [DOI] [PubMed] [Google Scholar]
- 47.Peng DD, Li ZL. Effect of simvastatin on monocyte CX3CR1 expression in patients with acute coronary syndrome [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao 2008;28:475–477. [PubMed] [Google Scholar]
- 48.Han KH, Ryu J, Hong KH, et al. HMG-CoA reductase inhibition reduces monocyte CC chemokine receptor 2 expression and monocyte chemoattractant protein-1-mediated monocyte recruitment in vivo. Circulation 2005;111:1439–1447. [DOI] [PubMed] [Google Scholar]
- 49.Weber C, Erl W, Weber KS, Weber PC. HMG-CoA reductase inhibitors decrease CD11b expression and CD11b-dependent adhesion of monocytes to endothelium and reduce increased adhesiveness of monocytes isolated from patients with hypercholesterolemia. J Am Coll Cardiol 1997;30:1212–1217. [DOI] [PubMed] [Google Scholar]
- 50.Stulc T, Vrablík M, Kasalová Z, Marinov I, Svobodová H, Ceska R. Leukocyte and endothelial adhesion molecules in patients with hypercholesterolemia: the effect of atorvastatin treatment. Physiol Res 2008;57:185–194. [DOI] [PubMed] [Google Scholar]
- 51.Walter T, Suselbeck T, Borggrefe M, Swoboda S, Hoffmeister HM, Dempfle CE. Effect of atorvastatin on cellular adhesion molecules on leukocytes in patients with normocholesterolemic coronary artery disease. In Vivo 2010;24:189–193. [PubMed] [Google Scholar]
- 52.Yang SS, Li R, Qu X, Fang W, Quan Z. Atorvastatin decreases Toll-like receptor 4 expression and downstream signaling in human monocytic leukemia cells. Cell Immunol 2012;279:96–102. [DOI] [PubMed] [Google Scholar]
- 53.Montecucco F, Burger F, Pelli G, et al. Statins inhibit C-reactive protein-induced chemokine secretion, ICAM-1 upregulation and chemotaxis in adherent human monocytes. Rheumatology (Oxford, England) 2009;48:233–242. [DOI] [PubMed] [Google Scholar]
- 54.Ferro D, Parrotto S, Basili S, Alessandri C, Violi F. Simvastatin inhibits the monocyte expression of proinflammatory cytokines in patients with hypercholesterolemia. J Am Coll Cardiol 2000;36:427–431. [DOI] [PubMed] [Google Scholar]
- 55.Krysiak R, Okopien B. The effect of ezetimibe and simvastatin on monocyte cytokine release in patients with isolated hypercholesterolemia. J Cardiovasc Pharmacol 2011;57:505–512. [DOI] [PubMed] [Google Scholar]
- 56.Frostegård J, Zhang Y, Sun J, Yan K, Liu A. Oxidized low-density lipoprotein (OxLDL)-Treated dendritic cells promote activation of T cells in human atherosclerotic plaque and blood, which is repressed by statins: microRNA let-7c is integral to the effect. J Am Heart Assoc 2016;5:e003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yilmaz A, Reiss C, Weng A, et al. Differential effects of statins on relevant functions of human monocyte-derived dendritic cells. J Leukoc Biol 2006;79:529–538. [DOI] [PubMed] [Google Scholar]
- 58.Yilmaz A, Reiss C, Tantawi O, et al. HMG-CoA reductase inhibitors suppress maturation of human dendritic cells: new implications for atherosclerosis. Atherosclerosis 2004;172:85–93. [DOI] [PubMed] [Google Scholar]
- 59.Leuenberger T, Pfueller CF, Luessi F, et al. Modulation of dendritic cell immunobiology via inhibition of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase. PLoS One 2014;9:e100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youssef S, Stüve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002;420:78–84. [DOI] [PubMed] [Google Scholar]
- Data available from Dryad (Additional References, References 61–95): (links.lww.com/WNL/B13)